Abstract
The polymorphic fungus Candida albicans switches from yeast to filamentous growth in response to a range of genotoxic insults, including inhibition of DNA synthesis by hydroxyurea (HU) or aphidicolin (AC), depletion of the ribonucleotide-reductase subunit Rnr2p, and DNA damage induced by methylmethane sulfonate (MMS) or UV light (UV). Deleting RAD53, which encodes a downstream effector kinase for both the DNA-replication and DNA-damage checkpoint pathways, completely abolished the filamentous growth caused by all the genotoxins tested. Deleting RAD9, which encodes a signal transducer of the DNA-damage checkpoint, specifically blocked the filamentous growth induced by MMS or UV but not that induced by HU or AC. Deleting MRC1, the counterpart of RAD9 in the DNA-replication checkpoint, impaired DNA synthesis and caused cell elongation even in the absence of external genotoxic insults. Together, the results indicate that the DNA-replication/damage checkpoints are critically required for the induction of filamentous growth by genotoxic stress. In addition, either of two mutations in the FHA1 domain of Rad53p, G65A, and N104A, nearly completely blocked the filamentous-growth response but had no significant deleterious effect on cell-cycle arrest. These results suggest that the FHA domain, known for its ability to bind phosphopeptides, has an important role in mediating genotoxic-stress–induced filamentous growth and that such growth is a specific, Rad53p-regulated cellular response in C. albicans.
INTRODUCTION
The polymorphic fungus Candida albicans has proven to be a very useful model for the study of cell morphogenesis (Odds, 1994; Sudbery et al., 2004; Wightman et al., 2004; Zheng et al., 2004). This organism can switch between several morphological forms including yeast, pseudohyphae, and hyphae (Berman and Sudbery, 2002; Sudbery et al., 2004). The yeast–hypha transition has attracted much attention because of its established importance for infection and virulence (Csank et al., 1997; Lo et al., 1997; Gale et al., 1998; Gow et al., 2002; Zheng et al., 2004). Several signal-transduction pathways are known to play roles in growth-form selection (Liu, 2001; Berman and Sudbery, 2002). The cAMP-protein kinase A (PKA) pathway has a key role, because blocking it abolishes true hyphal growth under most experimental conditions (Lo et al., 1997; Stoldt et al., 1997; Rocha et al., 2001). Another important pathway contains a MAP- kinase cascade that seems to have a more important role in regulating pseudohyphal than true hyphal growth (Liu et al., 1994). The two pathways control the transcription factors Efg1p and Cph1p, respectively, which activate the expression of hypha-specific genes for a suite of infection-related functions (Lane et al., 2001).
A diverse range of environmental stimuli can induce C. albicans hyphal or pseudohyphal growth, such as serum, neutral pH, appropriate temperature, certain amino acids and sugars, and some synthetic growth media (Ernst, 2000; Sudbery et al., 2004). These conditions more-or-less mimic certain host conditions. Interestingly, recent experiments have revealed a seemingly new mechanism for induction of filamentous growth that is caused by the perturbation of cell-cycle progression and is, in at least some cases, independent of the cAMP/PKA and MAP-kinase pathways (Bai et al., 2002; Bachewich et al., 2003; Andaluz et al., 2006; Atir-Lande et al., 2005; Bachewich et al., 2005; Bachewich and Whiteway, 2005; Bensen et al., 2005; Chapa y Lazo et al., 2005). An intriguing observation is that interfering with either an early or a late cell-cycle event, or with events thought to activate distinct cell-cycle checkpoints, appears to have strikingly similar effects on cell morphogenesis. For example, C. albicans yeast cells treated with either the DNA-replication inhibitor hydroxyurea (HU) or the microtubule toxin nocodazole exhibited significant cell elongation (Bai et al., 2002; Bachewich et al., 2005). Similar growth also occurs as the result of switching off the expression of either the G1 cyclin Cln3p (Bachewich et al., 2005; Chapa y Lazo et al., 2005) or one of the mitotic cyclins Clb2p and Clb4p (Bensen et al., 2005). Furthermore, deletion of CDC4, which encodes a component of the ubiquitin ligase SCFCdc4p and is thought to have a role in determining cellular events around the G1/S transition, or depleting the polo-like kinase Cdc5p, which is required for mitotic exit, causes constitutive filamentous growth (Bachewich et al., 2003; Atir-Lande et al., 2005). Currently, the signal-transduction pathways that mediate such induction of filamentous growth by cell-cycle perturbations are almost entirely unknown.
In Saccharomyces cerevisiae (Sc), regulated association of different cyclins with the cyclin-dependent protein kinase Cdc28p plays a central role in determining cell morphology: the G1 cyclins (Cln1p and Cln2p) promote bud elongation, whereas the mitotic ones (Clb1p and Clb2p) promote isotropic bud expansion (Lew and Reed, 1993, 1995). Thus, the regulation of activity balance between Clnp/Cdc28p and Clbp/Cdc28p kinases through the cell cycle determines cell morphology. This model might explain some aspects of the induction of filamentous growth by cell-cycle perturbations in C. albicans. However, it does not appear to explain why depleting a G1 or mitotic cyclin can have similar effects on cell morphogenesis in C. albicans.
Cell-cycle checkpoints are biochemical pathways used to monitor and ensure an orderly progression of cell-cycle events such as bud formation, DNA replication, and chromosome segregation (Hartwell and Weinert, 1989; Nyberg et al., 2002). When these events are impaired, the checkpoint pathways temporarily halt cell-cycle progression until the problems are fixed. In S. cerevisiae, cell-cycle arrest is often achieved by altering Cdc28p activity by mechanisms such as Cdc28p phosphorylation and the modulation of cyclin levels (Lew and Reed, 1993, 1995). For example, the morphogenesis-checkpoint kinase Swe1p catalyzes inhibitory phosphorylation of Cdc28p and thus prevents entry into mitosis, leading to the formation of elongated cells (Booher et al., 1993; Wittenberg and La Valle, 2003). In addition, the DNA-replication checkpoint appears to mediate HU-induced pseudohyphal growth (Jiang and Kang, 2003). In C. albicans, the spindle-assembly checkpoint was shown to be required for nocodazole-induced filamentous growth (Bai et al., 2002). Recently, Andaluz et al. (2006) reported that Rad52p depletion triggers the DNA-damage checkpoint and constitutive filamentation. These observations raise the important question of whether the cell-cycle checkpoints may play active roles in promoting filamentous growth under conditions that perturb cell-cycle progression.
Although cell-cycle checkpoints have been extensively investigated in S. cerevisiae and Schizosaccharomyces pombe, reports on the functions of corresponding checkpoints in C. albicans remain limited. In this study, in order to understand the roles of cell-cycle checkpoints in C. albicans morphogenesis, we have identified several conserved key components of the DNA-replication and DNA-damage checkpoints in C. albicans, created deletion mutants, and confirmed the checkpoint functions. We then evaluated their importance in mediating the filamentous growth caused by DNA-replication stress and DNA damage.
MATERIALS AND METHODS
Strains and Growth Conditions
The strains used in this study are listed in Table 1. Except where noted, C. albicans was grown at 30°C in YPD medium (2% yeast extract, 1% bactopeptone, and 2% glucose) or in GMM (2% glucose and 6.79 g/l yeast nitrogen base without amino acids) or in GMM supplemented with the required nutrients for auxotrophic mutants. For hyphal induction, 20% serum was added to liquid YPD medium, and the culture was incubated at 37°C.
Table 1.
C. albicans strains used in this study
| Strain | Relevant genotypea | Source |
|---|---|---|
| SC5314 | Clinical isolate | |
| BWP17 | ura3/ura3 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Wilson et al. (1999) |
| WYRL | rnr2Δ::ARG4/HIS1:P-RNR2 | See text |
| WYS1 | rad9Δ::ARG4/rad9Δ::URA3 | See text |
| WYS1.1 | rad9Δ::ARG4/rad9Δ::URA3 RAD9:HIS1 | See text |
| WYS2 | mrc1Δ::ARG4/mrc1Δ::HIS1 | See text |
| WYS2.1 | mrc1Δ::ARG4/mrc1Δ::HIS1 MRC1:URA3 | See text |
| WYS3 | rad53Δ::ARG4/rad53Δ::URA3 | See text |
| WYS3.1 | rad53Δ::ARG4/rad53Δ::URA3 RAD53:HIS1 | See text |
| rad53-G65A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-R66A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-S81A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-H84A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-N104A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-N109A | rad53Δ::ARG4 rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-G586A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-R587A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-S601A | rad53Δ::ARG4/rad53Δ::URA31 rad53:HIS1 | See text |
| rad53-H604A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-N622A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
| rad53-N627A | rad53Δ::ARG4/rad53Δ::URA3 rad53:HIS1 | See text |
a The strains constructed in this study are all derivatives of BWP17, which is itself a derivative of SC5314 (Enloe et al., 2000). For unknown reasons, ura3 strains like BWP17 are defective in hyphal growth under various inducing conditions (Sharkley et al., 2005; and our unpublished results). Because most of the strains constructed in this study have a reintegrated URA3, which restores wild-type-like hyphal growth (our unpublished results), we used SC5314 rather than BWP17 as the wild-type control strain in most experiments.
To prepare synchronized cultures by centrifugal elutriation, cells were grown overnight at 30°C in a medium containing 2% galactose and 6.79 g/l yeast nitrogen base supplemented with amino acids. G1 cells were obtained using a Beckman (Fullerton, CA) JE or J6-MC elutriator. G1 cells prepared using different elutriators can differ in average size and uniformity, which may significantly influence the timing of entry into the cell cycle, leading to variation between experiments. However, cells to be compared directly for cell-cycle–related functions were always prepared using the same elutriator.
Sensitivity to DNA-damaging agents was tested on solid or liquid medium. For the former, cells were grown in YPD overnight at 30°C and serially diluted into fresh YPD at concentrations of 5 × 106, 5 × 105, and 5 × 104 cells/ml; after 2 h of growth at 30°C, 5 μl of each culture was dropped onto YPD plates containing different concentrations of HU or methylmethane sulfonate (MMS) and incubated at 30°C. Plates were photographed at 12 and 24 h. For tests in liquid medium, cells were grown in YPD at 30°C overnight and diluted into fresh YPD at a concentration of 5 × 106 cells/ml. After 2 h of growth at 30°C, HU or MMS was added to a final concentration of 50 mM or 0.02%, respectively, for further growth. Aliquots of cells were collected at 2-h intervals, and cell viability was evaluated by spreading diluted samples onto YPD plates and counting colony-forming units after 2 d of incubation at 30°C. Aphidicolin (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO at 10 mg/ml (∼30 mM), stored at −20°C, and diluted appropriately into liquid growth medium.
Gene Deletion and Mutagenesis
C. albicans deletion mutants were constructed by sequentially deleting the two copies of the target gene from BWP17 (Wilson et al., 1999). The deletion cassettes were constructed by flanking a selectable marker gene (ARG4, HIS1, or URA3) with the AB and CD fragments, which correspond to the 5′- and 3′-untranslated regions (UTRs) of the target gene, respectively. Strain genotypes were verified by Southern blot analysis or PCR. To reintegrate wild-type RAD53 into rad53Δ cells (also see Figure 8, A and B), the genomic region from nucleotide (nt) −1106-2890 (the first nt of the start codon is 1), which contains the promoter, coding sequence, and 3′-UTR, was PCR-amplified using the forward primer 5′-GGGCTCGAGTGACAGTTTTTGACAGTG-3′ (underlined letter is an added XhoI site) and the reverse primer 5′-GGGATCGATTAAGAGATCGAATGATAT G-3′ (underlined letter is a ClaI site). The PCR fragment was cloned between XhoI and ClaI sites in a CIp10-based plasmid (Murad et al., 2000) containing HIS1 as selectable marker. The resultant plasmid was linearized at a unique NdeI site (−857) in the promoter region and transformed into rad53Δ cells (strain WYS3). The same integration strategy was used to introduce various FHA-domain-mutation alleles of RAD53 into rad53Δ cells. Correct integration was verified by PCR using two primers targeting nt −1161 to −1142 (5′-AACATGTTTGTTAGTTCTG-3′) and 53-71 (5′-GTTTGCGTCTGAGTTGTC-3′). The sequence targeted by the first primer is outside of the promoter region included in the integration construct.
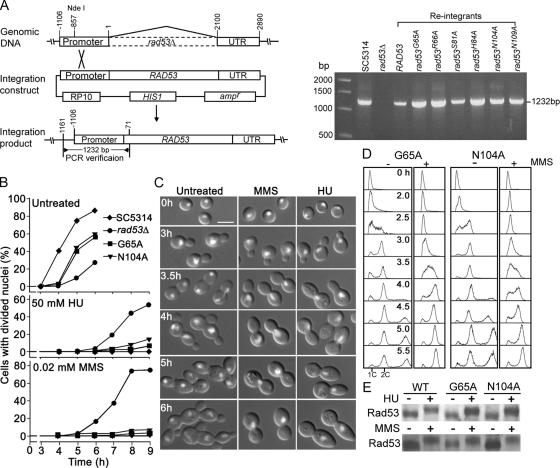
Figure 8.
Functional analyses of rad53-G65A and rad53-N104A alleles. (A) Schematic description of the strategy for integrating RAD53 wild-type and mutant alleles at the RAD53 chromosomal locus (left panel; for details, see Materials and Methods) and PCR verification of correct integration (right panel). (B–E) Analyses of phenotypes in strains SC5314 (wild type), WYS3 (rad53Δ), rad53-G65A, and rad53-N104A. (B) rad53-G65A and rad53-N104A cells are delayed in nuclear division under unperturbed conditions but able to arrest nuclear division in response to HU or MMS. Elutriated G1 cells were released into YPD containing 50 mM HU or 0.02% MMS for growth at 30°C. Aliquots of cells were collected at intervals for DAPI staining, and the fractions of cells with divided nuclei were counted. (C) rad53-G65A cells are normal in arresting nuclear division in response to MMS or HU, but fail to elongate. rad53-G65A G1 cells were released into YPD containing 50 mM HU or 0.02% MMS and photographed at the indicated times. rad53-N104A cells behaved very similarly. Bar, 5 μm. (D) The G65A and N104A mutations have no obvious deleterious effects on cell-cycle arrest in response to MMS. Elutriated rad53-G65A and rad53-N104A G1 cells were released into YPD containing no MMS or 0.02% MMS for growth at 30°C, and aliquots were collected at the indicated times for flow cytometry. (E) The G65A and N104A mutations do not affect Rad53p hyperphosphorylation in response to DNA damage. Cells were grown in YPD containing 50 mM HU or 0.02% MMS for 2 h, and Western blot analysis was performed using anti-CaRad53p antibodies.
A similar strategy was used to reintroduce wild-type RAD9 into the rad9Δ mutant and wild-type MRC1 into the mrc1Δ mutant. For RAD9, the promoter and complete coding region were PCR-amplified from nt −995-3535 and cloned into a CIp10-based, HIS1-marked plasmid. The resulting plasmid was linearized at the unique AatII site at nt −785 and transformed into WYS1 cells. For MRC1, the genomic region from nt −872-3750 was PCR-amplified and cloned into a CIp10-based, URA3-marked plasmid. The resulting plasmid was linearized at the unique HpaI site at nt −227 and transformed into WYS2 cells.
To construct the RNR2-shut-off strain WYRL, one copy of RNR2 was first replaced with ARG4 using the strategy described above. To replace the promoter of the second copy with the MET3 promoter (Care et al., 1999), the selectable marker HIS1 was first inserted upstream of the 5′ end of the MET3 promoter in pGEM-Teasy plasmid (Promega, Singapore). The HIS1-PMET3 fragment was then flanked with AB and CD fragments at the 5′- and 3′-ends, respectively; these fragments corresponded to nt −833 to −327 and nt 1-386, respectively. The AB-HIS1-PMET3-CD cassette was cloned into a plasmid for amplification, cut out, and used to transform the rnr2Δ::ARG4/RNR2 heterozygous mutant.
Site-directed mutagenesis of amino acid residues in the FHA domains of Rad53p was performed using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA).
Protein Methods
To extract proteins, cells were harvested by brief centrifugation, and ∼100 mg of cell pellet was resuspended in 300 μl of ice-cold lysis buffer containing 9 M urea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (Sigma-Aldrich, St. Louis, MO), 1.5 M Tris (pH 9.5), 1 mg/ml Pefabloc (Roche, Basel, Switzerland), 1 mg/ml leupeptin (Sigma-Aldrich), 1 mg/ml pepstatin (Sigma-Aldrich), and 0.15% DTT. After adding an equal volume of acid-washed glass beads (Sigma-Aldrich), the cells were broken by three rounds of 45-s beating at 5000 rpm in a MicroSmash MS-100 bead beater (TOMY Medico, Minato-ku, Japan) with 2 min of cooling on ice between rounds. After centrifugation in a microfuge at 13,000 rpm for 10 min at 4°C, the supernatant was collected. The protein concentration of the supernatant was determined using the Bradford protein assay (Bio-Rad, Hercules, CA) after neutralizing the pH using 0.1 M HCl. Approximately 30 μg of protein was separated by 10% SDS-PAGE and transferred to Hybond-C nylon membrane (Amersham, Buckinghamshire, United Kingdom). For Western blot analysis, the membrane was first immersed with gentle shaking in phosphate-buffered saline containing 0.1% Tween-20 (PBST) and 5% nonfat dry milk for 1 h at room temperature; then the membrane was incubated in PBST containing 1% milk and the primary antibody followed by incubation in PBST containing 1% milk and the horseradish peroxidase–coupled secondary antibody. The membrane was developed using the ECL system (Amersham). Anti-CaRad53p antibody was raised against the peptide LRPLDSERNRKSSKQ (amino acids 485-499) by Biogenes GmbH (Berlin, Germany).
Protein dephosphorylation was carried out using the method described by Vialard et al. (1998), and λ-phosphatase was purchased from New England Biolabs (Ipswich, MA).
Microscopy and Flow Cytometry
Staining of nuclei and actin structures was carried out as previously described (Zheng et al., 2003). A Leica DMR fluorescence microscope (Deerfield, IL) interfaced with METAMORPH software (Molecular Devices, Sunnyvale, CA) was used for imaging.
For flow cytometry, ∼107 cells were fixed in 70% ethanol for 1 h at room temperature. Cells were then centrifuged, washed twice with a buffer containing 50 mM Tris-HCl (pH 8.0) and 5 mM EDTA, and then treated with 1 mg/ml RNase in the same buffer for 3 h at 37°C. The cells were then harvested by centrifugation, washed twice with PBS, and incubated overnight at 4°C in 50 μg/ml propidium iodide in PBS. The cells were then diluted 10-fold with PBS and sonicated briefly to break up cell aggregates. The DNA contents of 1 × 104 cells were analyzed in a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
RESULTS
Both DNA-Replication Stress and DNA Damage Cause Constitutive Filamentous Growth of C. albicans
As a preliminary step to determine whether DNA checkpoints are involved in the filamentous growth induced by cell-cycle perturbations in C. albicans, we tested the effects of a variety of genotoxic insults. Although HU has been reported to cause filamentous growth (Bachewich et al., 2005), it remains unclear whether cell elongation is triggered by the inhibition of DNA synthesis or by disturbance of other cellular processes. To ask if alternative means of inhibiting DNA replication would also trigger filamentous growth, we examined the effects of the distinct DNA-synthesis toxins HU and aphidicolin (AC), which inhibit ribonucleotide reductase and DNA polymerase α, respectively, and of shutting off the expression of RNR2, which encodes a ribonucleotide-reductase subunit.
The high concentration of HU (200 mM) used by Bachewich et al. (2005) proved to be lethal. However, we found that at lower HU concentrations, the buds of yeast cells first elongated significantly during cell-cycle arrest; later, the cells were able to re-enter the cell cycle and continue to grow in the filamentous form with apparently normal cell and nuclear division, although with a longer cell-cycle time (Figure 1, A–F). Fluorescence-activated cell sorter (FACS) analysis showed that cells treated with 50 mM HU in liquid medium had arrested with a 1C DNA content by 1 h and that the arrest continued for >5 h (Figure 1A). By 7 h, however, the cell cycle had resumed, as shown by the presence of divided nuclei and new buds (Figure 1, B and D). Similar results were obtained on solid medium (Figure 1, E and F) and with 75 mM HU, although the arrest was longer in this case (Figure 1D). The tubular buds were much wider in diameter than hyphae (Figure 1, B and F) and contained clear constrictions at septal junctions (Figure 1C), a feature typical of pseudohyphae (Sudbery et al., 2004). New rounds of nuclear division and bud formation occurred in both the apical and subapical cells, forming branched filaments (Figure 1, B, C, and E). Despite its many normal-looking features, the cell cycle was much slower after its reinitiation. By counts of new bud formation, the first cell cycle after exit from the arrest in 50 mM HU was estimated to be ∼190 min, compared with ∼90 min in HU-free medium.
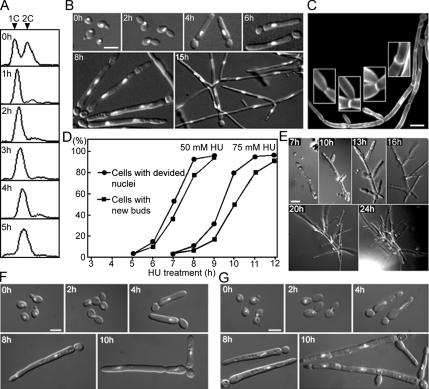
Figure 1.
HU and AC induce constitutive filamentous growth in wild-type cells. Strain SC5314 was grown on liquid (A–D and G) or solid (E and F) YPD at 30°C. Micrographs show overlaid fluorescence and DIC images. (A) Asynchronous cells were treated with 50 mM HU, and aliquots were collected at 1-h intervals for FACS analysis. (B) Samples of the HU-treated population were stained with DAPI and examined for cell morphology and nuclear distribution. (C) Cells treated with 50 mM HU for 15 h were costained with DAPI and Calcofluor White to reveal cell-wall features. Insets show constricted septa and branch points at higher magnification. (D) Cells were treated with 50 or 75 mM HU, and aliquots were harvested at intervals for DAPI staining. The fractions of cells with divided nuclei and with new buds at the tips of the long daughter cells (see Figure 1B, 8 h) were determined. (E) Time-lapse observation of a cell treated with 50 mM HU. (F) Cells were treated with 50 mM HU and washed off the plates at the indicated times for DAPI staining. (G) Cells were treated with 100 μM AC, and aliquots were collected at intervals for DAPI staining. A control experiment showed that the DMSO used as solvent for AC had no effect on its own at this concentration. Bars, 5 μm.
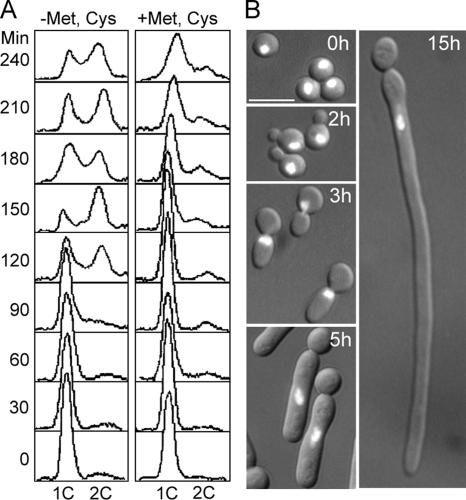
In parallel experiments, we tested the effects of AC and depletion of Rnr2p. At 100 μM, AC produced effects very similar to those of 50 mM HU: nuclear division was arrested transiently, and bud elongation began during arrest and continued after reinitiation of the cell cycle (Figure 1G). Because RNR2 is essential, we constructed a strain in which one copy of the gene was deleted and the other was placed under control of the MET3 promoter (Care et al., 1999). G1 cells were inoculated into GMM medium or into GMM containing 1 mM each of methionine and cysteine to switch off RNR2 expression. These conditions effectively blocked DNA replication as evaluated by both flow cytometry and microscopic examination: cells maintained a 1C DNA content for at least 4 h (Figure 2A; compare left and right panels) and arrested with a single nucleus for extended periods (Figure 2B). During the arrest, buds elongated dramatically (Figure 2B). However, like wild-type cells treated with high concentrations of HU, the Rnr2p-depleted cells could not exit from the arrest, probably because they have no other means to raise cellular dNTP levels in the absence of ribonucleotide reductase. Taken together, the results above strongly suggest that DNA-replication stress triggers filamentous growth in C. albicans.
Figure 2.
Depletion of Rnr2p leads to cell elongation. G1 cells of the RNR2-shut-off strain WYRL were prepared by elutriation and released into GMM or into GMM containing 1 mM each of methionine (Met) and cysteine (Cys) for growth at 30°C. Aliquots were harvested at intervals for flow cytometry and microscopic examination after staining with DAPI. (A) FACS profiles from the two cultures. (B) Cell morphologies and nuclear status of cells in the repressing medium at the indicated time points. Bar, 5 μm.
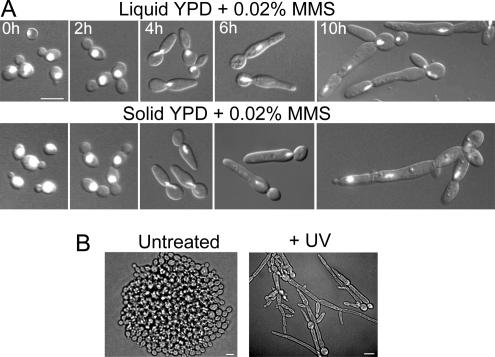
To test whether DNA damage also causes filamentous growth, we examined the effects of the DNA-alkylating agent MMS and of UV radiation. Cells were either inoculated into liquid or spread onto solid YPD medium supplemented with 0.01–0.03% MMS. In both cases, the cells were found to switch to filamentous growth (Figure 3A). After arrest with a single nuclear mass for ∼6 h, the cells re-entered the cell cycle, but they continued to produce elongated cells, although these were less elongated than those produced in response to HU or AC. To test the effect of UV radiation, cells were spread onto YPD plates and exposed to a 3-s pulse of UV radiation. Strikingly, this treatment caused extensive cell elongation (Figure 3B). Taken together, the results show that C. albicans can also switch to filamentous growth when DNA damage occurs.
Figure 3.
DNA damage by MMS or UV causes filamentous growth in wild-type cells. (A) SC5314 cells were grown on liquid or solid YPD containing 0.02 mM MMS at 30°C and collected at intervals for staining with DAPI and examination by DIC and fluorescence microscopy. The cells on solid medium were washed off the agar surface for staining. (B) SC5314 cells were spread onto YPD plates and irradiated with UV (80 J/m2) for 3 s followed by incubation at 30°C for 16 h. Bars, 5 μm.
Identification of the MRC1, RAD53, and RAD9 Genes of the C. albicans DNA-Replication and DNA-Damage Checkpoint Pathways
In eukaryotes, DNA-replication stress and DNA damage activate, through respective checkpoint pathways, a range of cellular responses including cell-cycle arrest, activation of repair-gene transcription, stabilization of replication forks, and delay of late-origin firing (Zhou and Elledge, 2000). To ask if the induction of filamentous growth by genotoxic stress is another of the cellular responses regulated by the checkpoint pathways, we first needed to identify the C. albicans checkpoint genes, which have been little studied to date.
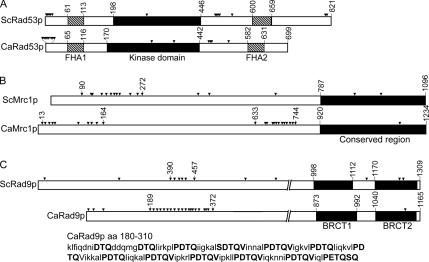
In S. cerevisiae, the serine/threonine protein kinase Rad53p is the downstream effector of the DNA-replication checkpoint (Sanchez et al., 1999), Mrc1p is the signal transducer that transmits the DNA-replication-stress signal to Rad53p (Katou et al., 2003; Osborn and Elledge, 2003), and Rad9p (Weinert and Hartwell, 1989; Zhou and Elledge, 2000) is the signal transducer for the DNA-damage checkpoint. The C. albicans genome (http://www.candidagenome.org) contains candidate orthologues of RAD53 (Contig: Chr3_ Ctg10259, orf 19.6936), MRC1 (Contig: Chr1_ctg10064, orf19. 658), and RAD9 (Contig: Chr5_Ctg10202, IPF8741.3f and IPF8741.5f). CaRAD53 encodes a 699-amino acid (aa) protein that has 46% identity with ScRad53p. Each protein contains a cluster of serine/glutamine (SQ) and threonine/glutamine (TQ) pairs near the N-terminus and two forkhead-associated (FHA) domains at equivalent positions; these flank a highly conserved kinase domain (Figure 4A). CaMRC1 encodes a protein of 1234 aa, which is 138 aa longer than ScMrc1p. The two proteins have only 17% overall sequence identity, but higher identity (32%) is found in the C-terminal third of the proteins. A cluster of 12 [S/T]Q residues, which are known to be the phosphorylation sites of the upstream PI3-family protein kinases Mec1p and Tel1p (Sun et al., 1998; Osborn and Elledge, 2003), exists from aa 90-272 in ScMrc1p. Interestingly, CaMrc1p contains two [S/T]Q clusters, one between aa 1 and 185 with 10 and the other between aa 630 and 742 with 12 (Figure 4B). CaRAD9 encodes an 1165-aa protein that has significant sequence identity (∼30%) to ScRad9p only over the C-terminal 400 aa, which contains two BRTC domains (Figure 4C), which are found predominantly in proteins involved in cell-cycle checkpoints responsive to DNA damage (Glover, 2006). In the N-terminal two thirds of each protein, multiple [S/T]Q residues are present. Strikingly, CaRad9p contains a total of 24 such pairs, 20 of which are located between aa 180 and 373, whereas ScRad9 contains only 10 such pairs in the region from aa 1-690. Between aa 180 and 310 of CaRad9p are 13 TQ pairs, each embedded in extended, conserved motifs of PDTQV/I, which are regularly spaced by 5-7 aa.
Figure 4.
Domain organizations of C. albicans Rad53p (A), Mrc1p (B), and Rad9p (C) compared with their S. cerevisiae orthologues. Arrowheads mark [S/T]Q amino acid pairs, and amino acids at domain boundaries are indicated by numbers. The sequence of CaRad9p from aa 180-310 is also shown. Conserved [S/T]Q-containing motifs are indicated by bold letters.
Functional Characterization of RAD53, MRC1, and RAD9
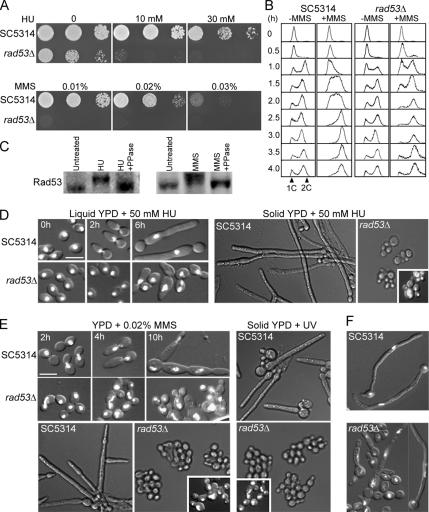
To investigate the roles of the putative DNA-checkpoint genes in cellular responses to DNA damage and replication stress, we constructed deletion mutants of RAD53, MRC1, and RAD9 (see Materials and Methods and Table 1). None of the heterozygous mutants showed increased sensitivity to HU or MMS or any other discernible defects (data not shown). Interestingly, in contrast to the lethality of Scrad53Δ, homozygous Carad53Δ cells were viable, although they grew noticeably more slowly than wild-type cells and exhibited hypersensitivity to both HU and MMS (Figure 5A). Flow cytometry showed that in contrast to wild type, the mutant cells were unable to slow down DNA replication in response to MMS treatment (Figure 5B, 0.5- to 2.0-h samples), consistent with a defect in the intra-S checkpoint. Because mother and daughter cells of the mutant often failed to separate during MMS treatment (see Figure 5E), the flow cytometry data did not reveal whether the mutant might also be defective in G2/M arrest (see, however, below). Western-blot analysis of wild-type cells treated with HU or MMS showed that CaRad53p exhibited a retarded electrophoretic mobility that was abolished by λ-phosphatase treatment of the cell lysates (Figure 5C), indicating that CaRad53p, like ScRad53p, becomes hyperphosphorylated in response to DNA damage. Taken together, the results suggest that CaRad53p is indeed the orthologue of ScRad53p.
Figure 5.
Functional characterization of the rad53Δ mutant. (A) rad53Δ cells grow more slowly than wild-type cells and are hypersensitive to HU and MMS. SC5314 and WYS3 cells were serially diluted, spotted onto YPD plates containing different concentrations of HU or MMS, and incubated at 30°C for 24 h. (B) rad53Δ cells are unable to slow down DNA synthesis when treated with MMS. Elutriated SC5314 and WYS3 G1 cells were released into YPD containing 0 or 0.02% MMS for growth at 30°C. Aliquots were harvested at intervals for flow cytometry. (C) Rad53p undergoes hyperphosphorylation in response to HU and MMS. SC5314 cells were grown in YPD containing 50 mM HU or 0.02% MMS at 30°C for 2 h. Untreated cells were included as a control. Cell lysates were prepared for Western blot analyses with (+PPase) or without prior treatment with λ-phosphatase. The blot was probed with a polyclonal antibody against CaRad53p. (D) rad53Δ cells fail to arrest cell-cycle progression and are impaired for filamentous growth upon HU treatment. SC5314 and WYS3 cells were grown in liquid YPD containing 50 mM HU at 30°C and stained with DAPI at the indicated times. The cells were also spread onto YPD plates containing 50 mM HU and incubated for 16 h at 30°C. The mutant cells were washed off the plates for DAPI staining (inset). (E) rad53Δ cells are completely blocked for filamentous growth in response to MMS and UV. SC5314 and WYS3 cells were grown in liquid (top) or on solid (bottom) YPD medium containing 0.02% MMS. The cells in liquid medium were stained with DAPI at the indicated times. The plates were incubated at 30°C for 16 h, and the mutant cells were washed off for DAPI staining (inset). The cells were also spread onto YPD plates followed by UV radiation and incubation at 30°C for 16 h. The mutant cells were stained with DAPI (inset). (F) rad53Δ cells are defective in hyphal growth. SC5314 and WYS3 yeast cells were inoculated into YPD containing 20% bovine serum and grown at 37°C for 4 h. Bars, 5 μm.
We next examined the rad53Δ cells for filamentous growth in reaction to various genotoxic stresses. In liquid YPD medium containing 50 mM HU, ∼90% of rad53Δ cells grew in yeast form, whereas the rest showed some elongation that was greatly reduced in comparison with wild-type cells (Figure 5D, left half); also, nuclear division was not arrested in the mutant cells, indicating a defect in G2/M arrest. The lack of HU-induced filamentous growth was also observed on solid medium (Figure 5D, right half). In addition, rad53Δ cells grew exclusively in yeast form upon MMS or UV treatment (Figure 5E). The lack of cell elongation did not seem to be the result of cell death, because most cells could go through more than one budding cycle, producing microcolonies on plates (Figure 5, D and E), and methylene-blue staining showed no increase in the number of dead rad53Δ cells compared with wild-type cells after 12 h of HU or MMS treatment (data not shown). Thus, Rad53p is critically required for the filamentous growth induced by either DNA-replication stress or DNA damage. All the defects of the rad53Δ cells were fully corrected by reintegrating a copy of wild-type CaRAD53 at the RAD53 locus as described in Materials and Methods and Figure 8A (data not shown).
When rad53Δ cells were examined for serum-induced hyphal growth, we unexpectedly observed a significant defect. Yeast cells were inoculated into YPD containing 20% serum and incubated at 37°C. By 1 h, 70% of wild-type cells but only 1.4% of rad53Δ cells (n = 100) had grown germ tubes with average lengths of 2.8 μm and 1.7 μm, respectively. By 3 h, 86% of wild-type cells had developed true hyphae, with an average length of ∼23.5 μm, whereas only 9% of rad53Δ cells had grown true hyphae, with an average length of 9.3 μm. Could the reduced hyphal growth of rad53Δ cells be the result of their slow growth rate? In S. cerevisiae, Rad53p regulates the availability of dNTPs during S phase (Zhao et al., 1998), so that slow DNA synthesis is one of the reasons for the slow proliferation of rad53Δ cells. Furthermore, Scrad53 cells are delayed in the completion of mitosis in an unperturbed cell cycle (Pike et al., 2003). Therefore, we thought that a comparison of germ-tube formation in G1 cells might better indicate whether Carad53Δ mutants have a specific defect in hyphal growth. We prepared G1 cells and first released them into YPD at 30°C for yeast growth to determine budding indices. We found that both wild-type and rad53Δ cells started to bud at ∼2 h (data not shown), indicating similar cell growth rates in G1 phase. We then subjected the same G1 cells to hyphal-induction conditions. Strikingly, 25 and 82% of wild-type cells had grown germ tubes at 1 and 2 h, respectively, whereas only 2 and 10% of rad53Δ cells had done so. By 4 h, 18% of the mutant cells had formed hyphae, whereas the rest either remained as yeast or exhibited varied degrees of abnormal elongation (Figure 5F). Together, these data reveal that Rad53p contributes to true hyphal morphogenesis.
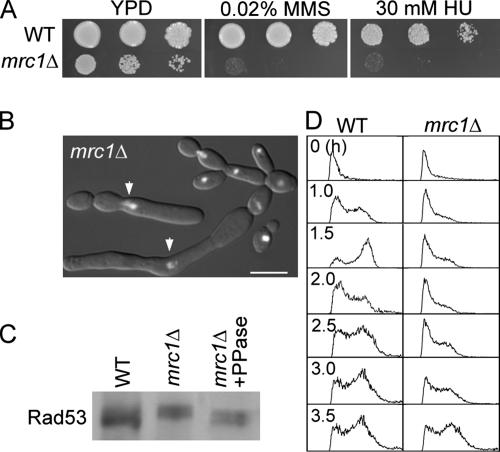
Camrc1Δ cells grew significantly more slowly than wild-type cells and were sensitive to HU and MMS (Figure 6A), consistent with defects in DNA checkpoints. Even under unperturbed conditions, 30% of the mutant cells were highly elongated (Figure 6B) and were similar in morphology to cells treated with HU or AC. Interestingly, a fraction of the elongated cells contained a single nucleus (Figure 6B), suggesting cell-cycle arrest caused by checkpoint pathways that do not depend on Mrc1p. It is known that DNA-damage-like structures are generated during DNA synthesis in Scmrc1Δ cells, which causes cell-cycle arrest via the DNA-damage checkpoint (Alcasabas et al., 2001; Katou et al., 2003). Consistent with the hypothesis that the same events occur in Camrc1Δ cells, Rad53p was hyperphosphorylated in these cells (Figure 6C), and flow cytometry analysis of mrc1Δ G1 cells released into YPD at 30°C showed a much slower S phase than in wild-type cells (Figure 6D), reminiscent of the defect reported for Scmrc1Δ cells (Alcasabas et al., 2001; Katou et al., 2003; Calzada et al., 2005; Szyjka et al., 2005). Thus, the Camrc1Δ mutant exhibits phenotypes similar to those of Scmrc1Δ mutants, supporting their orthologous relationship. The observed constitutive activation of Rad53p and cell elongation in the CamrcΔ mutant is consistent with our hypothesis that activation of DNA-checkpoint pathways leads to filamentous growth.
Figure 6.
Characterization of mrc1Δ cells. (A) The mrc1Δ mutant grows slowly and is sensitive to HU and MMS. SC5314 and WYS2 yeast cells were serially diluted and spotted onto YPD plates containing 0.02% MMS or 30 mM HU for growth at 30°C for 24 h. (B) mrc1Δ cells exhibit elongation and cell separation/cytokinesis defects under unperturbed conditions. WYS2 cells were grown in YPD at 30°C and stained with DAPI. Arrows mark the elongated cells with a single nuclear mass. Bar, 5 μm. (C) Rad53p is hyperphosphorylated in mrc1Δ cells under unperturbed conditions. SC5314 and WYS2 cells were grown in YPD at 30°C before Western blot analysis using anti-CaRad53p antibodies. Half of the lysate was pretreated with λ-phosphatase (PPase). (D) mrc1Δ cells exhibit slow DNA synthesis. Elutriated SC5314 and WYS2 G1 cells were released into YPD at 30°C, and aliquots were collected at the indicated times for flow cytometry.
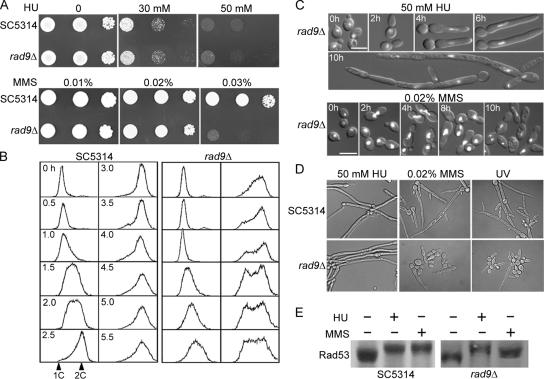
Carad9Δ cells showed a normal growth rate and resistance to HU but were hypersensitive to MMS (Figure 7A). Flow cytometry showed that the rad9Δ cells were impaired in arresting cells at G2/M upon MMS treatment (Figure 7B). These results are consistent with Rad9p's expected role in the DNA-damage checkpoint. We next examined whether rad9Δ cells displayed filamentous growth in response to various inducing agents. The mutant showed normal cell-cycle arrest and filamentous growth in both liquid and solid media containing HU (Figure 7C), as well as true hyphal growth when induced by serum (data not shown). However, the rad9Δ cells were significantly compromised for filamentous growth in response to MMS or UV radiation (Figure 7D), demonstrating that Rad9p has a specific role in the DNA-damage checkpoint and is critically required for DNA-damage–induced filamentous growth. Notably, ∼15% of the MMS- or UV-treated rad9Δ cells exhibited various degrees of elongation, which might be due to the presence of alternative signaling pathways that activate Rad53p in C. albicans. In S. cerevisiae, two partially overlapping Rad53p-activation pathways function in response to DNA damage, one containing Rad9p and the other Rad17p, Rad24p, Mec3p, and Ddc1p (Kondo et al., 1999). Consistent with this explanation, we found a lower level of Rad53p phosphorylation in rad9Δ cells than in wild-type cells upon MMS treatment (Figure 7E).
Figure 7.
Characterization of the rad9Δ mutant. (A) The rad9Δ mutant grows and responds normally to HU but is sensitive to MMS. SC5314 and WYS1 cells were serially diluted, spotted onto YPD plates containing 0, 30, or 50 mM HU or 0.01, 0.02, or 0.03% MMS, and incubated at 30°C for 24 h. (B) rad9Δ cells are defective in cell-cycle arrest upon MMS treatment. Elutriated SC5314 and WYS1 G1 cells were released into YPD containing 0.02% MMS for growth at 30°C, and aliquots were collected at the indicated times for flow cytometry. (C) rad9Δ cells undergo normal HU-induced filamentous growth but are severely impaired in MMS-induced filamentous growth. WYS1 cells were grown in YPD containing 50 mM HU or 0.02% MMS at 30°C, and aliquots were stained with DAPI at the indicated times (cf. the wild-type cells in Figure 3A). Bars, 5 μm. (D) rad9Δ cells are defective in MMS- and UV-induced filamentous growth on solid medium. SC5314 and WYS1 cells were spread onto YPD plates containing 50 mM HU or 0.02% MMS or were exposed to UV radiation on YPD plates; the plates were then incubated at 30°C for 16 h. (E) Rad53p undergoes partial phosphorylation upon MMS treatment of the rad9Δ strain. SC5314 and WYS1 cells were grown in YPD containing 50 mM HU or 0.02% MMS at 30°C for 2 h before Western blot analysis with anti-CaRad53p antibodies.
Role of FHA Domains in Rad53p-mediated Cellular Functions
The results above are consistent with a role for Rad53p as the effector kinase in both the DNA-damage and DNA-replication checkpoint pathways and in mediating the induction of filamentous growth by genotoxic stress. The two FHA domains of ScRad53p are known to interact with different upstream and downstream proteins, resulting in different responses to different genotoxic insults (Durocher et al., 1999; Durocher and Jackson, 2002; Emili et al., 2001; Duncker et al., 2002; Tsai, 2002; Leroy et al., 2003; Schwartz et al., 2003). Thus, we asked whether the FHA domains of CaRad53p are required for the filamentous growth induced by genotoxins. To address this, we selected six highly conserved amino acids in each FHA domain for alanine substitution. Each mutant rad53 allele was transformed into rad53Δ cells by integration at the RAD53 chromosomal promoter (Figure 8A). A wild-type copy was similarly integrated as a control (strain WYS3.1). The transformants were then examined for cell-cycle arrest, filamentous growth, and survival upon exposure to HU and MMS as described in Table 2. Among the 12 single-residue mutations, N109A was the only one that had no discernible deleterious effect on any of the Rad53p functions examined. The H84A substitution was similar to N109A except that it considerably reduced the number of elongated cells upon HU or MMS treatment. All other mutations, to widely varied extents, impaired multiple Rad53p-mediated functions. The G65A and N104A mutations are interesting in that they only moderately impaired cell-cycle arrest and survival upon exposure to HU or MMS but almost completely abrogated cell elongation, indicating that the mutations resulted in a genetic separation of cell-cycle arrest and cell elongation. Similarly, several mutant alleles, such as rad53-R586A, were able to support both cell-cycle arrest and cell elongation without providing resistance to HU or MMS, consistent with a separation of the cell-cycle arrest and DNA-damage-repair functions of Rad53p.
Table 2.
Effects of the FHA-domain mutations on DNA checkpoint-mediated functions
| Arrest (%)a |
Cell elongation (%)b |
Resistancec |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | HU | MMS | HU | MMS | |
| rad53Δ | 8 | 9 | 10 | 9 | 3 | <1 | + | + |
| RAD53 | 11 | 93 | 95 | 90 | 91 | 83 | ++++ | ++++ |
| FHA1 mutation | ||||||||
| rad53-G65A | 12 | 63 | 80 | 50 | 6 | <1 | ++ | +++ |
| rad53-R66A | 9 | 85 | 78 | 51 | 48 | 40 | + | + |
| rad53-S81A | 7 | 53 | 66 | 42 | 12 | 8 | + | + |
| rad53-H84A | 10 | 24 | 92 | 89 | 46 | 40 | ++++ | ++++ |
| rad53-N104A | 7 | 53 | 82 | 54 | 6 | <1 | ++ | +++ |
| rad53-N109A | 8 | 30 | 93 | 91 | 89 | 81 | ++++ | ++++ |
| FHA2 mutation | ||||||||
| rad53-G586A | 16 | 90 | 67 | 28 | 62 | 57 | + | + |
| rad53-R587A | 11 | 78 | 77 | 45 | 16 | 11 | + | + |
| rad53-S601A | 9 | 40 | 45 | 18 | 13 | 9 | + | + |
| rad53-H604A | 15 | 65 | 57 | 26 | 22 | 18 | + | + |
| rad53-N622A | 17 | 68 | 58 | 22 | 21 | 16 | + | + |
| rad53-N627A | 15 | 90 | 78 | 43 | 58 | 53 | ++ | ++ |
a Stationary-phase yeast cells (∼90% G1 cells) were treated with 50 mM HU in fresh YPD, and the percentages of budded cells containing a single nuclear mass were counted at 2-h intervals.
b Stationary-phase yeast cells were treated with 50 mM HU or 0.02 mM MMS in fresh YPD for 5 h, and the fractions of cells with an elongated bud (length of bud ≥1.5 times that of the mother) were counted.
c Equivalent numbers of yeast cells were treated with 50 mM HU or 0.02 mM MMS for 2 h and then serially diluted before being spread onto YPD plates for counting colony-forming units [from few (+) to many (++++)] after a 2-d incubation at 30°C.
Because it is important to understand the causal relationship between cell-cycle arrest and cell elongation, we carried out more detailed analysis of the rad53-G65A and rad53-N104A alleles using elutriated G1 cells. On release into YPD, the cells of wild-type, rad53Δ, rad53-G65A, and rad53-N104A strains started to bud at a similar time of ∼2 h (data not shown), but nuclear division was significantly delayed in rad53Δ relative to wild-type cells (Figure 8B), a phenotype also observed in Scrad53Δ cells (Pike et al., 2003). rad53-G65A and rad53-N104A cells underwent nuclear division at a time earlier than rad53Δ but later than wild-type cells, indicating that the mutant rad53 alleles are partially functional in regulating nuclear division kinetics. In YPD containing HU or MMS, rad53Δ cells started to show divided nuclei between 4 and 5 h, and nearly 50% of HU-treated and 80% of MMS-treated cells had completed nuclear division by 8–9 h (Figure 8B). In contrast, more than 80% of wild-type, rad53-G65A, and rad53-N104A cells still contained a single nuclear mass at 9 h. The percentages of cells with divided nuclei were slightly higher in the mutants than in wild-type cells, indicating that the G65A and N104A mutations compromise slightly the cell-cycle-arrest function of Rad53p. However, in marked contrast to the highly elongated wild-type cells, rad53-G65A, and rad53-N104A cells exhibited little cell elongation in response to HU or MMS (Figure 8C). The results confirm that the G65A and N104A mutations preferentially impair the function of Rad53p required for cell elongation without significantly compromising its function in mediating cell-cycle arrest. The rad53-G65A and rad53-N104A cells also showed defects in cell separation, which were especially prominent in fresh cultures started from elutriated G1 cells (Figure 8C). However, the defect became less severe when the cultures reached higher density, when many separate cells were present (data not shown).
To evaluate MMS-induced cell-cycle arrest in rad53-G65A and rad53-N104A cells, elutriated G1 cells were grown in the presence of 0.02% MMS, and their DNA contents were examined by flow cytometry. Figure 8D shows that MMS-treated rad53-G65A and rad53-N104A cells exhibited much slower S-phase progression than untreated cells and then arrested in G2/M phase with a 2C DNA content, indicating a functional checkpoint response for cell-cycle arrest. The flow-cytometry profiles of the untreated cells started to show an additional peak after 4 h because of the formation of cell aggregates. Western blot analyses showed that cellular levels of the mutant versions of Rad53p were comparable to that in wild-type cells, and the mutant proteins underwent similar hyperphosphorylation in response to HU or MMS (Figure 8E), indicating that the defects of the mutants are not due to failure in Rad53p hyperphosphorylation.
In summary, mutation analyses of Rad53p functions identified two mutations, G65A and N104A in the FHA1 domain, that nearly completely abolish filamentous growth in response to either DNA-replication inhibition or DNA damage without significantly impairing cell-cycle arrest, demonstrating a critical role of the FHA1 domain in mediating the DNA-damage–induced filamentous growth. Because all the mutations in the FHA2 domain also compromise filamentous growth to varied extents (Table 2), both FHA domains contribute to the induction of filamentous growth by genotoxic stress. Our results also demonstrate that both FHA domains play essential roles in the cell's resistance to genotoxins.
DISCUSSION
In this study, we have shown that a diverse range of genotoxic insults leads to filamentous growth in C. albicans. In all eukaryotic cells, one universal, immediate consequence of insults to DNA is the activation of DNA checkpoints, which trigger a repertoire of cellular responses including cell-cycle arrest, activation of repair and adaptation mechanisms, stabilization of replication forks, and a delay of late-origin firing (Zhou and Elledge, 2000). Collectively, these checkpoint-mediated functions ensure damage repair, faithful DNA replication and chromosome segregation, and ultimately the survival of the organisms. In regard to the filamentous growth induced by genotoxic stress in C. albicans, two questions are of central importance. Are DNA checkpoints required for this response? If so, might this growth be one of the cellular responses activated directly by the checkpoint pathways? In this study, we have shown that intact DNA-replication and DNA-damage checkpoint pathways are indeed required for the filamentous growth. Moreover, we have found that FHA1-domain mutations in Rad53p can preferentially block cell elongation without significantly impairing cell-cycle arrest in response to genotoxic insults, thus providing the first evidence that the polarized growth is mediated by the checkpoint functions rather than being the result of the cell-cycle arrest. Unexpectedly, we also found that RAD53 is required for normal hyphal growth, suggesting a general role for Rad53p in polarized morphogenesis.
Conservation of the DNA-Replication and DNA-Damage Checkpoint Pathways
Using the completed C. albicans genome database, we identified candidate genes for several key proteins of the DNA-replication and DNA-damage checkpoints, including the Chk2-family kinase Rad53p and the signal transducers Mrc1p and Rad9p. CaRad53p is highly similar to ScRad53p in both sequence and domain organization. ScRad53p is activated by phosphorylation and acts as a downstream effector of both the DNA-replication and DNA-damage checkpoints (Zhou and Elledge, 2000). Our data show that a Carad53Δ mutant, like Scrad53 mutants, is hypersensitive to genotoxins and fails to arrest cell-cycle progression, and CaRad53p is also hyperphosphorylated upon DNA damage. These results indicate that CaRad53p is an orthologous Chk2 kinase. However, unlike the lethality of Scrad53Δ mutations, CaRAD53 is not essential for cell survival. Although the candidates for CaMrc1p and CaRad9p only share localized sequence similarity with ScMrc1p and ScRad9p, their relatedness was supported by the presence of similar functional domains and motifs such as the [S/T]Q clusters in both proteins and the BRCT repeats in Rad9p. Moreover, Camrc1Δ and Carad9Δ mutants exhibited a range of defects, many of which are also found in the corresponding S. cerevisiae mutants, supporting their roles in the DNA-replication and DNA-damage checkpoints, respectively. Identification of these key components of the checkpoint pathways in C. albicans allowed us to address the role of each pathway in mediating the filamentous growth induced by genotoxic stress, as discussed below.
S. cerevisiae contains another conserved checkpoint kinase, Chk1p, which primarily mediates response to DNA damage (Sanchez et al., 1999). Using the ScChk1p sequence to BLAST-search the C. albicans genome database, we were not able to identify a candidate Chk1p. Similar scores of e-31 to e-34 were obtained for several protein kinases with similar domain organizations, suggesting either that one of these kinases is the true orthologue of ScChk1p or that functionally redundant kinases are present for Chk1p functions in C. albicans. We are currently investigating these possibilities.
Role of the DNA Checkpoints in the Filamentous Growth Induced by Genotoxic Stress
The observation that a multitude of conditions that exert genotoxic stress via different means and cellular targets all lead to filamentous growth in C. albicans suggests that a common mechanism may be involved. The DNA checkpoints seemed likely to be part of this mechanism, because they are activated under these various circumstances. We tested this hypothesis by blocking the checkpoint pathways by gene deletion. Deletion of RAD53 nearly completely blocked the filamentous growth caused by either inhibition of DNA replication or DNA damage. In contrast, deletion of RAD9 blocked the filamentous growth caused by DNA-damaging agents but not that caused by DNA-replication inhibitors, consistent with the evidence that CaRad9p functions specifically in the DNA-damage checkpoint. The lack of filamentous growth in the checkpoint mutants is unlikely to be the result of cell death, for two reasons. First, methylene-blue staining did not reveal increased cell death in the mutants relative to wild-type cells. Second, the mutant cells treated with HU or MMS could undergo multiple cell cycles to produce microcolonies on plates. Taken together, the data indicate that intact DNA checkpoints are indeed required for the filamentous growth induced by genotoxic stress in C. albicans.
Role of Rad53p in True Hyphal Growth
A rather unexpected finding was the impaired ability of rad53Δ cells in true hyphal growth. Initially, we thought that this effect might reflect the slow growth of the mutant cells. Previous studies of Scrad53 mutant cells indicated that the slow growth is attributable to several defects: limitation of dNTPs during S phase (Zhao et al., 1998), accumulation of excess histones (Gunjan and Verreault, 2003), and slower nuclear-division kinetics (Pike et al., 2003). An important feature of C. albicans is the inducibility of germ-tube formation in early-G1 yeast cells. This allowed us to compare the responses of mutant and wild-type cells to hyphal induction before the G1/S transition, thus avoiding the phases presumably responsible for the slow growth of rad53Δ mutants. We found that elutriated G1 cells of both wild-type and rad53Δ strains indeed started to bud at about the same time. However, the rad53Δ cells exhibited a significant decrease in the number of germ tubes formed. Moreover, the majority of the rad53Δ cells remained as yeast or exhibited abnormal elongation after prolonged hyphal induction. These data suggest a role for Rad53p in true hyphal development. Although how loss of Rad53p function causes the hyphal defects remains unclear, several studies in S. cerevisiae have suggested possible functional connections of Rad53p with cell morphogenesis. Pike et al. (2004) reported the identification of a new checkpoint protein, Mdt1p, and showed that even under unperturbed conditions, mdt1Δ cells exhibited a delay in anaphase completion, which causes cell elongation. Interestingly, Mdt1p is constitutively threonine-phosphorylated and binds to the FHA1 domain of Rad53p. In addition, Wu and Jiang (2005) recently found that a S. cerevisiae mutant expressing cdc42-Y40F was specifically defective in HU-induced filamentous growth and thus proposed a direct interaction between Rad53p and Cdc42p, a Rho GTPase essential for polarized cell growth (Etienne-Manneville, 2004). Moreover, ScRad53p activation is known to lead to G2/M arrest by inhibiting the Polo-like kinase Cdc5p (Sanchez et al., 1999), and depletion of CaCdc5p has been shown to cause cell elongation (Bachewich et al., 2003). Further studies of the molecular events linking CaRad53p to cell morphogenesis might well follow these leads from S. cerevisiae.
Genetic Separation of Rad53p-mediated Responses to Genotoxic Stress
Activation of ScRad53p results in diverse cellular responses, partly through FHA-domain–mediated interactions with different upstream and downstream proteins (Durocher et al., 1999; Pike et al., 2004). The two FHA domains of ScRad53p are known to mediate associations with different partners (Durocher and Jackson, 2002; Tsai, 2002; Schwartz et al., 2003), and FHA-domain mutations can alter their substrate specificity (Durocher et al., 2000; Yongkiettrakul et al., 2004). We reasoned that if CaRad53p promotes filamentous growth through FHA-domain–mediated interactions with other proteins, it might be possible to find FHA-domain mutations that block or compromise the genotoxic-stress–induced filamentous growth without affecting other Rad53p-controlled responses. Indeed, we found two single-amino-acid mutations in FHA1 that almost completely blocked the filamentous growth induced by HU, MMS, or UV but left the cell-cycle-arrest function largely intact. Similar functional separation was also observed between cell-cycle arrest, filamentous growth, and resistance to genotoxic insults. For example, rad53-G586A cells exhibited largely normal cell-cycle arrest and significant cell elongation but were as sensitive to HU and MMS as rad53Δ cells. One plausible explanation of such separation of function is that the different substitutions differentially affect Rad53p's interactions with various downstream effector proteins. In this case, the filamentous growth might constitute a specific Rad53p-mediated response to genotoxic stress. Although we have shown that cell-cycle arrest can occur without concurrent cell elongation in some RAD53 mutant cells, it remains unclear whether the genotoxic-stress–induced filamentous growth can happen in the complete absence of cell-cycle arrest.
ACKNOWLEDGMENTS
Y.W. is an adjunct associate professor in the Department of Microbiology, National University of Singapore. This work was funded by the Agency for Science, Technology and Research (A*STAR) of Singapore. We thank all members of the Y.W. laboratory for comments and suggestions.
Abbreviations used:
- AC
aphidicolin
- FHA
fork-head association
- HU
hydroxyurea
- MMS
methylmethane sulfonate
- PKA
protein kinase A.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0442) on December 20, 2006.
REFERENCES
- Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P.J.H., Bousset K., Furuya K., Diffley J.F.X., Carr A. M., Elledge S. J. Mrc1 transduces signals of DNA replication to activate Rad53. Nat. Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- Andaluz E., Ciudad T., Gómez-Raja J., Calderone R., Larriba G. Rad52 depletion in Candida albicans triggers both the DNA-damage checkpoint and filamentation accompanied by but independent of expression of hypha-specific genes. Mol. Microbiol. 2006;59:1452–1472. doi: 10.1111/j.1365-2958.2005.05038.x. [DOI] [PubMed] [Google Scholar]
- Atir-Lande A., Gildor T., Kornitzer D. Role for the SCFCDC4 ubiquitin ligase in Candida albicans morphogenesis. Mol. Biol. Cell. 2005;16:2772–2785. doi: 10.1091/mbc.E05-01-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachewich C., Thomas D. Y., Whiteway M. Depletion of a polo-like kinase in Candida albicans activates cyclase-dependent hyphal-like growth. Mol. Biol. Cell. 2003;14:2163–2180. doi: 10.1091/mbc.02-05-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachewich C., Nantel A., Whiteway M. Cell cycle arrest during S or M phase generates polarized growth via distinct signals in Candida albicans. Mol. Microbiol. 2005;57:942–959. doi: 10.1111/j.1365-2958.2005.04727.x. [DOI] [PubMed] [Google Scholar]
- Bachewich C., Whiteway M. Cyclin Cln3p links G1 progression to hyphal and pseudohyphal development in Candida albicans. Eukaryot. Cell. 2005;4:95–102. doi: 10.1128/EC.4.1.95-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C., Ramanan N., Wang Y. M., Wang Y. Spindle assembly checkpoint component CaMad2p is indispensable for Candida albicans survival and virulence in mice. Mol. Microbiol. 2002;45:31–44. doi: 10.1046/j.1365-2958.2002.02995.x. [DOI] [PubMed] [Google Scholar]
- Bensen E. S., Clemente-Blanco A., Finley K. R., Correa-Bordes J., Berman J. The mitotic cyclins Clb2p and Clb4p affect morphogenesis in Candida albicans. Mol. Biol. Cell. 2005;16:3387–3400. doi: 10.1091/mbc.E04-12-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J., Sudbery P. E. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002;3:918–930. doi: 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- Booher R. N., Deshaies R. J., Kirschner M. W. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 2005;19:1905–1919. doi: 10.1101/gad.337205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care R. S., Trevethick J., Binley K. M., Sudbery P. E. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 1999;34:792–798. doi: 10.1046/j.1365-2958.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- Chapa y Lazo B., Bates S., Sudbery P. The G1 cyclin Cln3 regulates morphogenesis in Candida albicans. Eukaryot. Cell. 2005;4:90–94. doi: 10.1128/EC.4.1.90-94.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csank C., Makris C., Meloche S., Schröppel K., Röllinghoff M., Dignard D., Thomas D. Y., Whiteway M. Derepressed hyphal growth and reduced virulence in a VH1 family-related protein phosphatase mutant of the human pathogen Candida albicans. Mol. Biol. Cell. 1997;8:2539–2551. doi: 10.1091/mbc.8.12.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D., Henckel J., Fersht A. R., Jackson S. P. The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell. 1999;4:387–394. doi: 10.1016/s1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- Durocher D., Jackson S. P. The FHA domain. FEBS Lett. 2002;513:58–66. doi: 10.1016/s0014-5793(01)03294-x. [DOI] [PubMed] [Google Scholar]
- Durocher D., Taylor I. A., Sarbassova D., Haire L. F., Westcott S. L., Jackson S. P., Smerdon S. J., Yaffe M. B. The molecular basis of FHA domain: phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol. Cell. 2000;6:1169–1182. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Duncker B. P., Shimada K., Tsai-Pflugfelder M., Pasero P., Gasser S. M. An N-terminal domain of Dbf4p mediates interaction with both origin recognition complex (ORC) and Rad53p and can deregulate late origin firing. Proc. Natl. Acad. Sci. USA. 2002;99:16087–16092. doi: 10.1073/pnas.252093999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emili A., Schieltz D. M., Yates J. R., 3rd, Hartwell L. H. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol. Cell. 2001;7:13–20. doi: 10.1016/s1097-2765(01)00150-2. [DOI] [PubMed] [Google Scholar]
- Ernst J. F. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology. 2000;146:1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42–the centre of polarity. J. Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Gale C. A., Bendel C. M., McClellan M., Hauser M., Becker J. M., Berman J., Hostetter M. K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- Glover J. N. Insights into the molecular basis of human hereditary breast cancer from studies of the BRCA1 BRCT domain. Fam. Cancer. 2006;5:89–93. doi: 10.1007/s10689-005-2579-z. [DOI] [PubMed] [Google Scholar]
- Gow N. A., Brown A. J., Odds F. C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Gunjan A., Verreault A. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell. 2003;115:537–549. doi: 10.1016/s0092-8674(03)00896-1. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Jiang Y. W., Kang C. M. Induction of S. cerevisiae filamentous differentiation by slowed DNA synthesis involves Mec1, Rad53 and Swe1 checkpoint proteins. Mol. Biol. Cell. 2003;14:5116–5124. doi: 10.1091/mbc.E03-06-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., Ashikari T., Sugimoto K., Shirahige K. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- Kondo T., Matsumoto K., Sugimoto K. Role of a complex containing Rad17, Mec3, and Ddc1 in the yeast DNA damage checkpoint pathway. Mol. Cell. Biol. 1999;19:1136–1143. doi: 10.1128/mcb.19.2.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S., Birse C., Zhou S., Matson R., Liu H. DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J. Biol. Chem. 2001;276:48988–48996. doi: 10.1074/jbc.M104484200. [DOI] [PubMed] [Google Scholar]
- Leroy C., Lee S. E., Vaze M. B., Ochsenbien F., Guerois R., Haber J. E., Marsolier-Kergoat M.-C. PP2C phosphatases Ptc2 and Ptc3 are required for DNA checkpoint inactivation after a double-strand break. Mol. Cell. 2003;11:827–835. doi: 10.1016/s1097-2765(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I. Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet. Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Liu H., Kohler J., Fink G. R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Liu H. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 2001;4:728–735. doi: 10.1016/s1369-5274(01)00275-2. [DOI] [PubMed] [Google Scholar]
- Lo H.-J., Köhler J. R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G. R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Murad A.M.A., Lee P. R., Broadbent I. D., Barelle C. J., Brown A.J.P. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast. 2000;16:325–327. doi: 10.1002/1097-0061(20000315)16:4<325::AID-YEA538>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nyberg K. A., Michelson R. J., Putnam C. W., Weinert T. A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Candida species and virulence. Am. Soc. Microbiol. News. 1994;60:313–318. [Google Scholar]
- Osborn A. J., Elledge S. J. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 2003;17:1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Yongkiettrakul S., Tsai M.-D., Heierhorst J. Diverse but overlapping functions of the two forkhead-associated (FHA) domains in Rad53 checkpoint kinase activation. J. Biol. Chem. 2003;278:30421–30424. doi: 10.1074/jbc.C300227200. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Yongkiettrakul S., Tsai M.-D., Heierhorst J. Mdt1, a novel Rad53 FHA1 domain-interacting protein, modulates DNA damage tolerance and G2/M cell cycle progression in Saccharomyces cerevisiae. Mol. Cell. Biol. 2004;24:2779–2788. doi: 10.1128/MCB.24.7.2779-2788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha C.R.C., Schröppel K., Harcus D., Marcil A., Dignard D., Taylor B. N., Thomas D. Y., Whiteway M., Leberer E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Bachant J., Wang H., Hu F., Liu D., Tetzlaff M., Elledge S. J. Control of the DNA damage checkpoint by Chk1 and Rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- Schwartz M. F., Lee S. J., Duong J. K., Eminaga S., Stern D. F. FHA domain-mediated DNA checkpoint regulation of Rad53. Cell Cycle. 2003;2:384–396. [PubMed] [Google Scholar]
- Sharkey L. L., Liao W. L., Ghosh A. K., Fonzi W. A. Flanking direct repeats of hisG alter URA3 marker expression at the HWP1 locus of Candida albicans. Microbiology. 2005;151:1061–1071. doi: 10.1099/mic.0.27487-0. [DOI] [PubMed] [Google Scholar]
- Stoldt V. R., Sonneborn A., Leuker C. E., Ernst J. F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P., Gow N., Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Sun Z., Hsiao J., Fay D. S., Stern D. F. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998;281:272–274. doi: 10.1126/science.281.5374.272. [DOI] [PubMed] [Google Scholar]
- Szyjka S. J., Viggiani C. J., Aparicio O. M. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell. 2005;19:691–697. doi: 10.1016/j.molcel.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tsai M.-D. FHA: a signal transduction domain with diverse specificity and function. Structure. 2002;10:887–888. doi: 10.1016/s0969-2126(02)00795-5. [DOI] [PubMed] [Google Scholar]
- Vialard J. E., Gilbert C. S., Green C. M., Lowndes N. F. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 1998;17:5679–5688. doi: 10.1093/emboj/17.19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T., Hartwell L. Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae. J. Cell Sci. Suppl. 1989;12:145–148. doi: 10.1242/jcs.1989.supplement_12.12. [DOI] [PubMed] [Google Scholar]
- Wightman R., Bates S., Amornrrattanapan P., Sudbery P. In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization. J. Cell Biol. 2004;164:581–591. doi: 10.1083/jcb.200307176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. B., Davis D., Mitchell A. P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg C., La Valle R. Cell-cycle-regulatory elements and the control of cell differentiation in the budding yeast. Bioessays. 2003;25:856–867. doi: 10.1002/bies.10327. [DOI] [PubMed] [Google Scholar]
- Wu X., Jiang Y. W. Possible integration of upstream signals at Cdc42 in filamentous differentiation of S. cerevisiae. Yeast. 2005;22:1069–1077. doi: 10.1002/yea.1294. [DOI] [PubMed] [Google Scholar]
- Yongkiettrakul S., Byeon I. J., Tsai M.-D. The ligand specificity of yeast Rad53 FHA domains at the +3 position is determined by nonconserved residues. Biochemistry. 2004;43:3862–3869. doi: 10.1021/bi036195f. [DOI] [PubMed] [Google Scholar]
- Zhao X., Muller E.G.D., Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- Zheng X.-D., Wang Y.-M., Wang Y. CaSPA2 is important for polarity establishment and maintenance in Candida albicans. Mol. Microbiol. 2003;49:1391–1405. doi: 10.1046/j.1365-2958.2003.03646.x. [DOI] [PubMed] [Google Scholar]
- Zheng X., Wang Y., Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. B., Elledge S. J. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]