Abstract
Objectives
To determine whether muscle weakness is correlated with inflammation, expression of interleukin 1α (IL1α) and major histocompatibility complex (MHC) class I and II antigens on muscle fibres.
Methods
Biopsy specimens from clinically symptomatic (proximal muscles) and asymptomatic (all distal but two proximal) muscles in eight patients with polymyositis, three patients with dermatomyositis and six healthy controls were analysed by immunohistochemistry for the presence of T cells and macrophages, and expression of IL1α and of MHC class I and II antigens. Results were evaluated by conventional light microscopy and by computerised image analysis.
Results
Inflammatory infiltrates with T cells and macrophages were observed to an equal degree in both symptomatic and asymptomatic muscle. The numbers of capillaries with IL1α expression were significantly higher (p<0.05) in the symptomatic and asymptomatic muscles of patients than in controls. The total IL1α expression per tissue section assessed by computerised image analysis was significantly higher in symptomatic muscles but not in asymptomatic muscles compared with that in controls. Neither the number of IL1α‐positive capillaries nor the total IL1α expression differed significantly between symptomatic and asymptomatic muscles. Expression of MHC class I and II antigens on muscle fibres was detected in both symptomatic and asymptomatic muscles but rarely in healthy controls.
Conclusions
Presence of inflammatory infiltrates, T cells and macrophages, and expression of MHC class I and II antigens and of IL1α on muscle fibres were independent of clinical symptoms, and were present to an equal degree in both proximal and distal muscles. Thus, other factors seem to determine the development of clinical symptoms. One such factor could be variations in physical demands.
Polymyositis and dermatomyositis are chronic inflammatory muscle diseases that are clinically characterised by proximal muscle weakness, and dermatomyositis by skin abnormalities. Typical histopathological findings in the muscle tissue are inflammatory infiltrates, mainly composed of T lymphocytes and macrophages.
Inflammation may induce muscle weakness by several mechanisms. Muscle cell necrosis induced by infiltrating inflammatory cells is often proposed. However, there is a lack of correlation between the clinical symptoms and the degree of inflammatory infiltrates in patients with myositis.1,2,3,4 Other phenotypic changes that have been related to muscle weakness include immune function‐related molecules produced locally in muscle tissue, such as major histocompatibility complex (MHC) class I and II antigens and interleukin (IL)1α expressed on muscle fibres and microvessels. MHC class I antigen, which normally is not expressed on muscle fibres, is expressed on muscle fibres independent of inflammatory cell infiltrates in patients with myositis and muscle weakness. This was observed in patients in both early and late chronic phases of the disease.2,5,6,7,8,9,10,11 A role of MHC class I antigens in causing muscle weakness is supported by the observation that transgenic mice with specific up regulation of MHC class I antigens on muscle fibres develop muscle weakness before infiltration of inflammatory cells.12
The most consistently expressed cytokines in muscle tissue of patients with polymyositis and dermatomyositis are the proinflammatory cytokines IL1α and IL1β, which have been found in both the early and the chronic phases, during and after immunosuppressive treatment.2,6,13 Notably, in muscle tissue from patients with muscle weakness but without detectable inflammatory infiltrates, increased IL1α expression was observed in the endothelium of capillaries, suggesting that microvessels with IL1α expression could cause muscle symptoms in patients with polymyositis or dermatomyositis.2,5 Involvement of microvessels in the disease mechanisms of dermatomyositis was already proposed by the observation of a decreased number of capillaries in patients with dermatomyositis, even in patients with early disease without inflammatory infiltrates.14,15,16,17
To further determine the role of the observed phenotypes of microvessels with IL1α expression and that of muscle fibres with MHC class I expression in causing clinical symptoms, we compared the expression of these molecules in two different muscles from the same patient, one from a clinically symptomatic (ie, with subjective muscle weakness) and the other from a clinically asymptomatic muscle. We also investigated histopathological changes, cellular infiltration of T cells and macrophages in the same biopsy specimens.
Patients and methods
Patients
Eleven consecutive patients who were diagnosed with polymyositis (n = 8) or dermatomyositis (n = 3) according to Bohan and Peter's criteria18,19 at the Department of Rheumatology, Karolinska University Hospital, Stockholm, Sweden, between 1994 and 1999, and who agreed to have biopsy specimens taken from two different muscles as part of the diagnostic procedures, were included in the study (table 1). Five patients had definite polymyositis and three had probable polymyositis (all had symmetrical proximal muscle weakness, raised serum creatine kinase levels and muscle biopsy abnormalities typical of polymyositis). One patient had definite dermatomyositis, and two patients had probable dermatomyositis with muscle symptoms and a raised creatine kinase level in addition to the classic rash. No patient fulfilled the diagnosis of inclusion‐body myositis according to Griggs's criteria.20 The mean age at the time of the biopsies was 58 (range 38–76) years.
Table 1 Clinical and laboratory findings of the patients (n = 11) at the time of muscle biopsies.
| Patient | Age (years) | Sex | Bohan and Peter diagnostic criteria | Biopsy site symptomatic | Biopsy site asymptomatic | Treatment at time of biopsy (symp:asymp) | Anti‐Jo1 antibodies | CK level (μkat/l) |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | f | Definite dermatomyositis | M vastus lat | M tib ant | Pred 15 mg/day: pred 15 mg/day | No | >76.8 |
| 2 | 38 | m | Probable dermatomyositis | M vastus lat | M tib ant | Pred 25 mg/day + Cs 100 mg/day:0 | No | 0.4 |
| 3 | 55 | m | Probable dermatomyositis | M vastus lat | M tib ant | 0 | No | 35.5 |
| 4 | 60 | m | Probable polymyositis | M vastus lat | M tib ant | 0 | Yes | 76.8 |
| 5 | 59 | f | Probable polymyositis | M deltoideus | M vastus lat | Pred 5 mg/day: pred 5 mg/day | No | 5.3 |
| 6 | 53 | f | Probable polymyositis | M vastus lat | M tib ant | 0 | No | 15.0 |
| 7 | 44 | f | Definite polymyositis | M vastus lat | M tib ant | 0 | No | 7.0 |
| 8 | 56 | m | Definite polymyositis | M vastus lat | M tib ant | 0 | No | 23.3 |
| 9 | 72 | f | Definite polymyositis | M vastus lat | M tib ant | Pred 40 mg/day + AZA 100 mg/day: 0 | No | 11.5 |
| 10 | 56 | m | Definite polymyositis | M vastus lat | M deltoideus | 0 | No | 43.8 |
| 11 | 76 | f | Definite polymyositis | M vastus lat | M tib ant | 0 | Yes | 4.3 |
Ant, anterior; asymp, asymptomatic; AZA, azathioprine; CK, creatine kinase (normal levels <2.5 μkat/l); Cs, ciclosporin; f, female; lat, lateralis; m, male; M, musculus; tib, tibialis; Pred, prednisolone; symp, symptomatic.
We aimed to take the biopsy specimens before starting immunosuppressive treatment. Concomitantly, however, treatment had to be started in two patients for clinical reasons before one of the biopsies was carried out (patients 2 and 9 in table 1). Patient 1 had been prescribed a low dose of prednisolone 2 weeks before biopsy sampling and diagnosis by a general practitioner.
Muscle weakness was confirmed by a reduced functional index, performed on all but two patients.21 The median (range) values for the right and left side of the body were 40 (10–63) and 41 (10–63) points, respectively; 64 points indicated maximum capacity.
Controls
Muscle biopsy specimens from five healthy people and one from a patient with myalgia without clinical or histopathological signs of muscle disease were included as controls (4 women and 2 men). The mean age was 51 (range 47–56) years. Muscle biopsy sites were the musculus vastus lateralis in the five controls and the musculus tibialis anterior in the patient with myalgia.
Muscle biopsies
On the basis of where the patients experienced subjective symptoms, primarily muscle weakness, biopsy was carried out on two muscles, one symptomatic and the other asymptomatic. From each site, at least two biopsy specimens were taken, one for diagnosis and the other for research. The biopsy specimens were obtained under local anaesthesia using the semi‐open biopsy technique, and frozen in isopenthane on dry ice and stored at −80°C.22,23 The coded biopsy specimens were analysed for histopathological signs of myositis (inflammation, regenerating and degenerating muscle fibres, muscle fibre atrophy, and central nuclei) by an experienced neuropathologist (IN). Results were assessed on a five‐point scale for inflammation or abnormal fibres: 0, absent/absent; +, scattered inflammatory cells/close to normal; ++; mild inflammation/few; +++, moderate/moderate; ++++, pronounced/many. Degenerating muscle fibres were defined as necrotic fibres invaded by inflammatory cells. Atrophic fibres were defined as muscle fibres with prominent size reduction.
The local ethics committee at Karolinska University Hospital, Solna, Sweden, approved the study, and all patients gave informed consent for participation.
Immunohistochemistry
Table 2 lists the antibodies used for immunohistochemistry. Staining for CD3 (T cells), CD163 (macrophages), human leucocyte antigen (HLA)‐A/B/C (MHC class I) and HLA‐DR (MHC class II) expression was performed with a standard immunohistochemistry protocol.24 Staining with anti‐IL1α antibody was performed according to a protocol described earlier,13 with modifications.2 For all stains, an isotype‐matched irrelevant antibody was included as a negative control. The first and last sections were also stained with haematoxylin–eosin to evaluate the histopathology and to confirm that the histopathological changes remained unchanged throughout the biopsy specimen.
Table 2 Monoclonal antibody panel for immunohistochemistry.
| Antibody | Marker for | Dilution or concentration | Supplier |
|---|---|---|---|
| Anti‐IL1α | IL1α | 5 μg/ml | AMS Biotechnology, Abingdon, UK; lot 130C290300, clone 1277‐89‐7 |
| Anti‐CD3 | T cells | 1:40 | Becton Dickinson, San José, California, USA; lot 61052, clone SK7 |
| Anti‐CD163 | Macrophages | 1:160 | Dako, Glostrup, Denmark; lot 030(401), clone Ber‐MAC 3 |
| Anti‐HLA‐A/B/C | MHC class I | 1:3500 | Dako, Glostrup, Denmark; lot 126, clone W6/32 |
| Anti‐HLA‐DR | MHC class II | 1:320 | Becton Dickinson, San José, California, USA; lot 30443, clone L243 |
HLA, human leucocyte antigen; MHC, major histocompatibility complex.
In all experiments, an isotype‐matched irrelevant antibody (Dako, Glostrup, Denmark) was included as a negative control.
Evaluation of immunohistochemistry
All immunohistochemical stains for patients and controls were analysed on coded slides on whole‐tissue sections by conventional microscopic assessment (Polyvar microscope; Reichert‐Jung, Nussloch, Germany) by two independent observers (CD and IEL). Results are given as the mean of the two assessments. The whole‐tissue section was analysed (median area 4.2 mm2, range 0.1–12.1 mm2). CD3 and CD163 expression is presented as the number of CD3‐positive and CD163‐positive cells/mm2. Expression of MHC class I (HLA‐A/B/C) and class II (HLA DR) antigens was assessed as follows: −, negative fibres, positively stained endothelial cells only; +, 1–10% positively stained fibres; ++, 11–25% positively stained fibres; +++, 26–50% positively stained fibres; ++++, 51–75% positively stained fibres; and +++++, 76–100% positively stained fibres.
IL1α expression was estimated separately in different structures. The numbers of IL1α‐positive infiltrating mononuclear cells, capillaries, larger blood vessels and muscle fibres were calculated per square millimetre. Total IL1α expression was also analysed using a previously described computerised image analysis system5 under 680× magnification. The images were analysed with a Quantimet 600 image analyser (Leica, Cambridge, UK). The image processor was directed by a personal computer, and a special program was written in a high‐level programming language, QUIPS, for this application. Computerised image analysis measures the total positively stained area for IL1α as well as the total area per tissue section. The image analysis results are presented as the percentage positively stained IL1α area in each tissue section.
Statistical analyses
Data were analysed using the software program Statistica V.6.0 from Stat Soft (Tulsa, Oklahoma, USA) Owing to the small number of patients and controls and the non‐normality of the dataset, non‐parametric tests were used to test for significance. We used the Mann–Whitney U test and Wilcoxon's matched pairs test; p<0.05 was considered significant; p>0.05 was considered not significant.
Results
Histopathology
Histopathological changes characteristic of polymyositis or dermatomyositis were observed in muscle biopsy specimens taken from both symptomatic and asymptomatic muscles. The number of specimens showing the presence of histopathological changes did not differ significantly between symptomatic and asymptomatic muscles (table 3, fig 1). These changes included both presence of inflammatory cells and regenerating and degenerating fibres. Muscle fibre atrophy was similarly absent, close to normal or with a few atrophic fibres in both the symptomatic and asymptomatic muscle, with the exception of one patient with polymyositis who had moderate muscle fibre atrophy in the symptomatic muscle and no muscle fibre atrophy in the asymptomatic muscle. We also compared the two samples taken from each biopsy site, in both the symptomatic and asymptomatic muscles (table 3). Regeneration muscle fibres were observed more often in the biopsy specimen taken for diagnosis rather than in the specimen taken for research. We found no difference in histopathological findings between the first and last sections in the consecutive series of sections. We aimed to take the biopsy specimens before starting immunosuppressive treatment, but this was not always feasible for ethical and practical reasons (table 1). In two patients (patients 2 and 9), there was a time lapse of 4 and 2 months respectively between the time points at which the biopsies on the symptomatic and asymptomatic muscle were performed. The difference in time and treatment, however, did not affect the histopathological changes or MHC and IL1α expression. Even so, the patient who was receiving low‐dose prednisolone still had inflammation, and MHC and IL1α were expressed in similar amounts as in the other patients. The biopsy samples that were taken after starting prednisolone treatment still showed histopathological changes, presence of T cells and macrophages, expression of MHC class I and II antigens on muscle fibres, and IL1α expression on capillaries in both symptomatic and asymptomatic muscles in comparably large amounts. Specimens from controls showed no histopathological changes, with the exception of one, which had a few scattered mononuclear inflammatory cells surrounding one fibre and a few atrophic fibres.
Table 3 Number of biopsy specimens with histopathological findings of polymyositis or dermatomyositis in two symptomatic and two asymptomatic muscle biopsy samples from patients with polymyositis or with dermatomyositis, and muscle biopsy specimens from controls.
| Histopathological findings | Controls (n = 6) | Symptomatic muscle (n = 11), biopsy carried out for | Asymptomatic muscle (n = 11), biopsy carried out for | ||
|---|---|---|---|---|---|
| Diagnosis | Research | Diagnosis | Research | ||
| Inflammation | 1* | 9 | 6 | 8 | 6 |
| Degeneration | 0 | 7 | 6 | 5 | 5 |
| Regeneration | 0 | 6 | 2 | 5 | 1 |
| Muscle fibre atrophy | 1 | 8 | 8 | 9 | 5 |
| Central nuclei | 0 | 2 | 5 | 2 | 4 |
*A few scattered inflammatory cells.
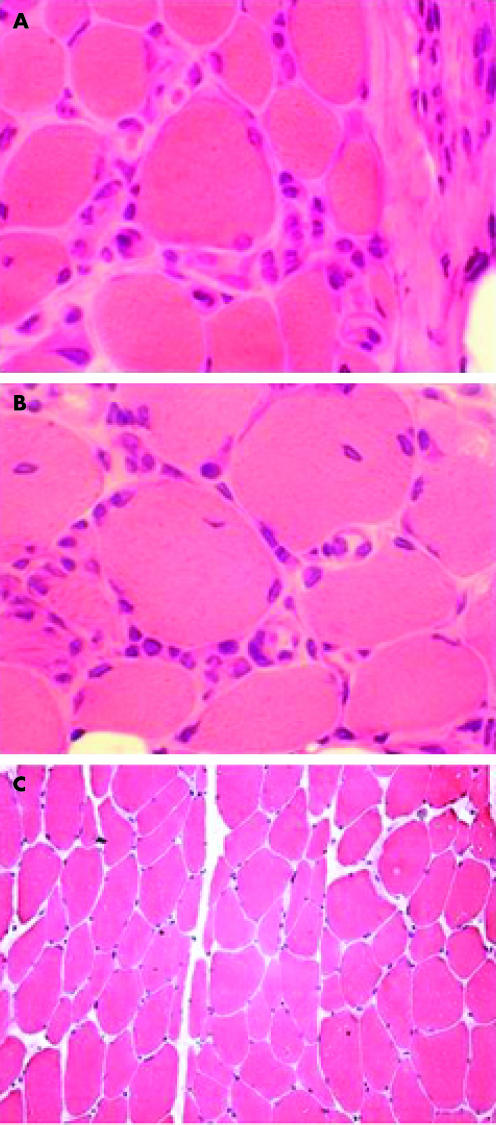
Figure 1 Haematoxylin–eosin staining of a muscle biopsy specimen from a patient with polymyositis and from a healthy control. Infiltration of inflammatory cells in the endomysium in muscle (A) with symptoms (×1130) and (B) without symptoms (×1130). (C) Biopsy specimen from a healthy control (×200). Several inflammatory cells are present in both symptomatic and asymptomatic muscle tissue, but not in healthy muscle tissue.
CD3‐positive T cells and CD163‐positive macrophages
We found a significantly higher number of CD3+ T cells and CD163+ macrophages in biopsy specimens of both symptomatic and asymptomatic muscles than those from the controls. We found no statistically significant difference in the median (range) number of T cells or macrophages/mm2 between symptomatic (T cells: 58.1/mm2, 0.5–159.8/mm2; macrophages: 102.9/mm2, 8.7–146.2/mm2) and asymptomatic (T cells: 55.9/mm2, 1.1–126.8/mm2; macrophages: 54.8/mm2, 8.7–120.4/mm2) muscle tissue. Healthy controls had only a few scattered inflammatory cells identified with anti‐CD3 (1.8/mm2, 0.4–5.0/mm2) and anti‐CD163 (4.9/mm2, 1.8–6.6/mm2) antibodies. These findings are consistent with the histopathological changes described in the previous section.
IL1α expression
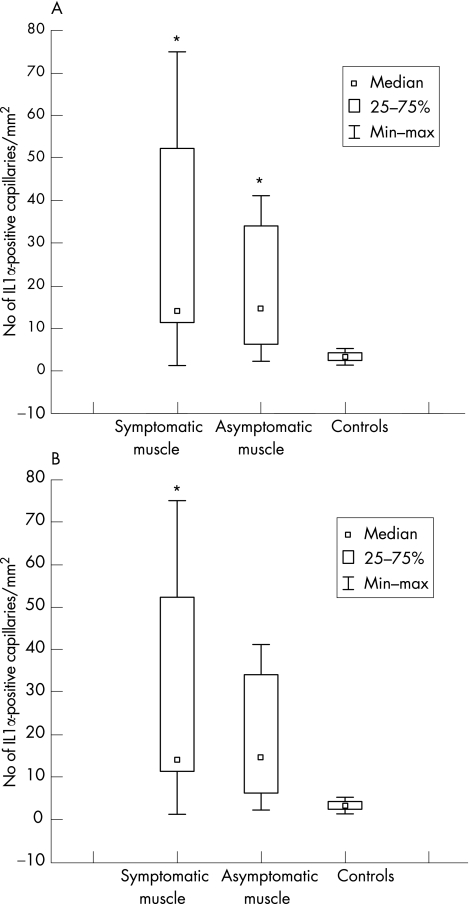
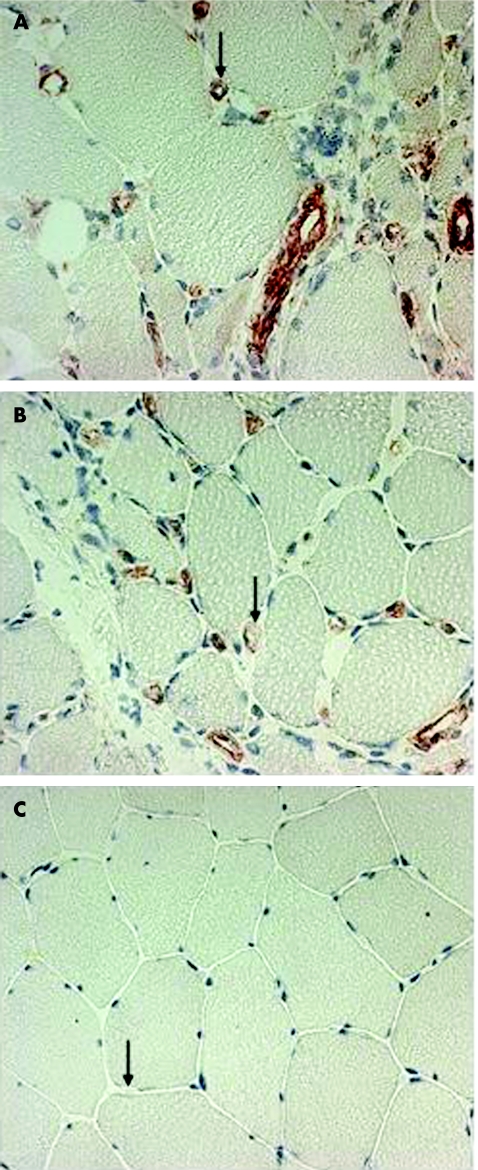
Using conventional microscopy, we recorded a significantly increased number of IL1α‐positive capillaries (median, range) in symptomatic (13.3/mm2, 0.6–76.5/mm2) and asymptomatic (12.4/mm2, 2.3–35.7/mm2) muscles compared with controls (3.4/mm2, 1.4–5.3/mm2; fig 2A). We found no significant difference between the numbers of IL1α‐positive capillaries in symptomatic versus asymptomatic muscles. Some of the IL1α‐expressing capillaries were morphologically changed, with thick, high endothelium venule (HEV)‐like or swollen endothelial cells in both the symptomatic and asymptomatic muscle biopsy specimens (fig 3).
Figure 2 (A) Number of interleukin (IL)1α‐positive capillaries/mm2 in symptomatic, asymptomatic and control muscles by conventional microscopy. p<0.05 v controls. (B) Total IL1α expression in symptomatic, asymptomatic and normal control muscle assessed by computerised image analysis, expressed as the percentage positive staining of the whole‐tissue section. p<0.05 v controls.
Figure 3 Immunohistochemical localisation of interleukin (IL)1α in endothelial cells, visualised as red staining, in muscle tissue from a patient with polymyositis and from a healthy control. (A) Biopsy specimen from a symptomatic muscle with swollen IL1α‐positive capillaries (arrow). (B) Biopsy specimen from an asymptomatic muscle from the same patient, also with swollen IL1α‐positive capillaries (arrow). (C) Biopsy specimen from healthy control with IL1α‐negative capillaries (arrow). Magnification ×340.
The number of larger blood vessels with positive IL1α expression was significantly increased in symptomatic muscles (4.9/mm2, 0.1–25/mm2) compared with healthy control muscles. We found no significant difference between symptomatic and asymptomatic muscles (4.1/mm2, 0.9–8.2/mm2) or between asymptomatic and control muscles (1.9/mm2, range: 0.6–6.0/mm2). IL1α was not expressed on muscle fibres in any of the three groups.
Total IL1α expression per tissue section analysed by computerised image analysis was significantly higher in biopsy specimens of symptomatic muscle than in those of controls, but when comparing specimens of asymptomatic muscle and normal controls, the difference did not reach significance (p = 0.05). We found no significant difference in total IL1α expression in the symptomatic muscles compared with asymptomatic muscles (fig 2B).
Expression of MHC class I and II antigens in muscle fibres
MHC class I (HLA‐A/B/C) expression was similarly strongly (75–100% positively stained area per muscle tissue section) expressed in muscle fibres in eight specimens each from symptomatic and asymptomatic muscles, and one patient did not express any MHC class I antigen on muscle fibres in either biopsy specimen. Although this patient did not have any inflammatory cell infiltrates in the biopsy specimens, they nonetheless had muscle and skin symptoms, electromyography (EMG) and creatine kinase results typical of dermatomyositis. There was strong agreement of expression of MHC class I antigens between symptomatic and asymptomatic muscles, with a few exceptions; one patient expressed it strongly only in the asymptomatic muscle (table 4) and another patient moderately in the symptomatic muscle biopsy and strongly in the asymptomatic muscle biopsy specimen. Six patients had a weak to moderate expression of MHC class II (HLA‐DR) in muscle fibres both in the symptomatic and asymptomatic muscles. One patient had stronger expression in the symptomatic than in the asymptomatic muscle. No MHC class II antigens were expressed on muscle fibres in four patients. Neither MHC class I nor class II antigens were expressed on muscle fibres in controls, so we did not think it necessary to carry out statistical analyses comparing patients and controls (table 4).
Table 4 Expression of major histocompatibility complex (MHC) class I (HLA‐A/B/C) and MHC class II (HLA‐DR) antigens on muscle fibres in patients with polymyositis or dermatomyositis, and in controls.
| Diagnosis | HLA‐A/B/C | HLA‐DR | |||
|---|---|---|---|---|---|
| Symptomatic muscle | Asymptomatic muscle | Symptomatic muscle | Asymptomatic muscle | ||
| Patient | |||||
| 1 | DM | +++++ | ++++ | − | − |
| 2 | DM | − | +++++ | − | − |
| 3 | DM | − | − | − | − |
| 4 | PM | +++ | +++++ | − | − |
| 5 | PM | +++++ | +++++ | ++ | ++ |
| 6 | PM | +++++ | +++++ | ++ | ++ |
| 7 | PM | +++++ | +++++ | +++++ | ++ |
| 8 | PM | +++++ | +++++ | ++ | ++ |
| 9 | PM | +++++ | +++++ | + | ++ |
| 10 | PM | +++++ | +++++ | + | + |
| 11 | PM | +++++ | +++++ | ++ | ++ |
| Controls | |||||
| 1 | Healthy | − | − | ||
| 2 | Myalgia | − | − | ||
| 3 | Healthy | − | − | ||
| 4 | Healthy | − | − | ||
| 5 | Healthy | − | − | ||
| 6 | Healthy | + | + | ||
DM, dermatomyositis; HLA, human leucocyte antigen; PM, polymyositis.
Scoring for HLA‐A/B/C and HLA‐DR: −, negative fibres, positively stained endothelial cells only; +, 1–10% positive fibres; ++, 11–25% positive fibres; +++, 26–50% positive fibres; ++++, 51–75% positive fibres; and +++++, 76–100% positive fibres.
Discussion
We investigated muscle biopsy specimens from two different sites, one from symptomatic and the other from asymptomatic muscle, around the time of diagnosis in 11 patients with polymyositis or dermatomyositis. One major finding was recorded: histopathological changes, including inflammatory infiltrates, as well as expression of MHC class I and II antigens on muscle fibres, were observed to an equal degree in both symptomatic and asymptomatic muscles. This was also true for the number of IL1α‐expressing microvessels, which was increased in both symptomatic and asymptomatic muscles compared with that in control specimens.
To our knowledge, this is the first study to investigate two different muscles from the same patient with myositis, one from a clinically symptomatic (all proximal) and the other from an asymptomatic (mostly distal) muscle. As the histopathological changes and the immunohistochemistry data including T cells and macrophages were similar in the two biopsies from each patient, we believe that the data are valid and may be generalised despite the limited number of patients; it would be unethical to include more patients in the study. To further strengthen our results, we analysed two biopsy samples from each site. Thus, four samples were analysed from each patient, all with similar histopathological findings, two from symptomatic and two from asymptomatic muscles, with the exception of the numbers of regenerating fibres, which are often scattered and unevenly distributed. We found that the presence and degree of muscle fibre atrophy, with one exception, was similar in both the symptomatic and symptomatic muscle, and thus could not explain the clinical symptoms. The choice of biopsy location in each patient was, in our study, based on where the patients experienced subjective clinical symptoms such as weakness, tenderness and pain. Magnetic resonance imaging (MRI) was not included in the protocol, and was performed on only five patients for clinical purposes and did not correspond to muscle biopsy sites. Two patients nonetheless had MRI results typical of myositis. EMG was performed on all patients (seven had EMG results typical of myositis), but even so, muscles analysed did not correspond to muscle biopsy sites. It is therefore not possible to draw any conclusion between MRI and EMG results and findings in the muscle biopsy specimens. To confirm impaired muscle function, we used the functional index test. A limitation of this test is that the tasks in the functional index do not correspond exactly to the specific muscles that we have investigated. All specimens from symptomatic muscle were taken from proximal muscles and those from asymptomatic muscles were taken from distal muscles. This corresponds well to the classical distribution according to textbooks on muscle weakness in patients with myositis.25 Our results, however, suggest that both proximal and distal muscles are affected by infiltration of inflammatory cells in polymyositis and dermatomyositis, and, thus, as previously reported, the degree of inflammatory infiltrates does not correspond to the localisation of the patient's subjective muscle symptoms. One of the controls had a few mononuclear inflammatory cells surrounding one muscle fibre, but T cells and macrophages were present in equal proportions as in other normal controls. This control patient had no clinical muscle symptoms, thus we believe that this finding could be within normal limits.
The generalised histopathological muscle involvement of both proximal and distal muscle groups was also supported by the extensive expression of MHC class I and II antigens on muscle fibres in both symptomatic and asymptomatic muscles, but controls were all negative. In one patient, the symptomatic muscle specimen did not express MHC class I antigens on muscle fibres, but the asymptomatic muscle expressed it strongly. This could be due to sampling error, as the symptomatic muscle biopsy specimen was small; this strengthens the need for adequate size of the biopsy specimen. One patient with typical dermatomyositis did not express any MHC class I antigen on muscle fibres in any biopsy sample. Although expression of MHC class I antigens on muscle fibres is a common finding in patients with polymyositis or dermatomyositis, it was not present in 38% of patients with polymyositis or dermatomyositis at the time of diagnosis in a recent study.26 Expression of MHC class II antigens on muscle fibres was less often seen in our study, but still without difference between symptomatic and asymptomatic muscles. The persisting expression of MHC class I antigens on muscle fibres, despite immunosuppressive treatment given to a few of our patients before carrying out biopsy, is consistent with a previous finding in patients with polymyositis and dermatomyositis.2,6,26
The number of capillaries expressing IL1α was also increased to a similar extent compared with the control biopsy specimens in both the symptomatic and asymptomatic muscles. We have previously reported an increased number of IL1α‐expressing microvessels independent of closely localised inflammatory infiltrates in both the early and the late chronic phases of polymyositis and dermatomyositis.2,5,6 Those specimens were obtained mainly from symptomatic thigh muscles. To those previous observations, we can now add an increased number of IL1α‐positive capillaries in distal and asymptomatic muscle. We believe that the increased number of ILα‐expressing capillaries in both symptomatic and asymptomatic muscles indicates a general involvement of the microvessels in muscles of patients with polymyositis or dermatomyositis. The larger and thick, high endothelium venule (HEV)‐like IL1α‐positive capillaries indicate that the endothelial cells of the capillaries are activated. This is a prerequisite to allow extravasation of inflammatory cells into the muscle tissue, and is found to an equal degree in both symptomatic and asymptomatic muscles. Other earlier findings to support the hypothesis that the endothelial cells might have an important role in the pathogenesis of myositis are that intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 have been detected on endothelial cells in all three subtypes of the disease (polymyositis, dermatomyositis and inclusion‐body myositis).27,28
We could not detect any difference in IL1α expression, the presence of inflammatory cell infiltrates with T cells and macrophages, or expression of MHC class I or II antigens, that could explain the muscle symptoms. Another possibility is that the clinical symptoms, which are predominantly localised to proximal muscles in patients with myositis, are caused by different physical demands on proximal and distal muscles or by different characteristics of muscle fibres in different muscle groups. Similar mechanisms could also account for the distribution of muscle weakness in other myopathies such as muscular dystrophies.29 The physical demands of, for example, endurance and strength and the physiological demand of oxygen supply are higher in thigh muscles than in calf muscles. Thus, a local tissue hypoxia, which could be a consequence of inflammation, could make the thigh muscles more prone to clinical symptoms than the distal muscles.30,31,32 Interestingly, IL1α and as other molecules previously reported to be up regulated in myositis, such as transforming growth factor β, intercellular adhesion molecule 1 and vascular cell adhesion molecule 1, could all be up regulated by hypoxia.13,27,33 In addition, the previously reported reduced levels of adenosine triphosphate in muscle of patients with myositis support the hypotheses of local muscle tissue hypoxia.30 Thus, an increased physical demand in a muscle tissue with hypoxia could lead to a further reduction in oxygen tension, which could possibly explain the reduced functional capacity and pain that the patients experience.34
In conclusion, we have shown that, independent of clinical symptoms, inflammatory cells, expression of MHC class I and II antigens on muscle fibres and IL1α‐expressing microvessels are present in both proximal and distal muscles. These findings indicate that the inflammatory changes constitute a general phenotype in skeletal muscle tissue of patients with polymyositis or dermatomyositis, and suggest that other factors are more important in causing the clinical symptoms.
Acknowledgements
We thank Professor Kristian Borg for providing muscle biopsy samples from healthy people, Eva Lindroos for laboratory technical assistance, and Professor Lars Klareskog and Cecilia Grundtman for critically reviewing the manuscript.
Abbreviations
EMG - electromyography
HLA - human leucocyte antigen
MHC - major histocompatibility complex
MRI - magnetic resonance imaging
Footnotes
Funding: This study was supported by grants from the Swedish Rheumatism Association, King Gustaf Vth 80‐year Foundation, the Swedish Research Council 2002‐74X‐14045‐02A, Professor Nanna Svartz Foundation, Magnus Bergvalls Foundation, Börje Dahlin Foundation, Stiftelsen Clas Groschinskys Minnesfond, Karolinska Institutet Foundation and the Vardal Foundation.
Competing interests: None.
References
- 1.Bunch T W, Worthington J W, Combs J J, Ilstrup D M, Engel A G. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 198092365–369. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg P, Wikman A L, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol 200027940–948. [PubMed] [Google Scholar]
- 3.Olsen N J, Park J H. Inflammatory myopathies: issues in diagnosis and management. Arthritis Care Res 199710200–207. [DOI] [PubMed] [Google Scholar]
- 4.Plotz P H, Dalakas M, Leff R L, Love L A, Miller F W, Cronin M E. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med 1989111143–157. [DOI] [PubMed] [Google Scholar]
- 5.Englund P, Nennesmo I, Klareskog L, Lundberg I E. Interleukin‐1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum 2002461044–1055. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg I, Kratz A K, Alexanderson H, Patarroyo M. Decreased expression of interleukin‐1alpha, interleukin‐1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum 200043336–348. [DOI] [PubMed] [Google Scholar]
- 7.Emslie‐Smith A M, Arahata K, Engel A G. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell‐mediated cytotoxicity in myopathies. Hum Pathol 198920224–231. [DOI] [PubMed] [Google Scholar]
- 8.Appleyard S T, Dunn M J, Dubowitz V, Rose M L. Increased expression of HLA ABC class I antigens by muscle fibres in Duchenne muscular dystrophy, inflammatory myopathy, and other neuromuscular disorders. Lancet 19851361–363. [DOI] [PubMed] [Google Scholar]
- 9.Bartoccioni E, Gallucci S, Scuderi F, Ricci E, Servidei S, Broccolini A.et al MHC class I, MHC class II and intercellular adhesion molecule‐1 (ICAM‐1) expression in inflammatory myopathies. Clin Exp Immunol 199495166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inukai A, Kuru S, Liang Y, Takano A, Kobayashi Y, Sakai M.et al Expression of HLA‐DR and its enhancing molecules in muscle fibers in polymyositis. Muscle Nerve 200023385–392. [DOI] [PubMed] [Google Scholar]
- 11.Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 19882364–72. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon P J, Lee E.et al Conditional up‐regulation of MHC class I in skeletal muscle leads to self‐sustaining autoimmune myositis and myositis‐specific autoantibodies. Proc Natl Acad Sci USA 2000979209–9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg I, Ulfgren A K, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum 199740865–874. [DOI] [PubMed] [Google Scholar]
- 14.Carry M R, Ringel S P, Starcevich J M. Distribution of capillaries in normal and diseased human skeletal muscle. Muscle Nerve 19869445–454. [DOI] [PubMed] [Google Scholar]
- 15.Casademont J, Grau J M, Estruch R, Pedro‐Botet J C, Urbano‐Marquez A. Relationship between capillary and muscle damage in dermatomyositis. Int J Dermatol 199029117–120. [DOI] [PubMed] [Google Scholar]
- 16.Emslie‐Smith A M, Engel A G. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 199027343–356. [DOI] [PubMed] [Google Scholar]
- 17.Estruch R, Grau J M, Fernandez‐Sola J, Casademont J, Monforte R, Urbano‐Marquez A. Microvascular changes in skeletal muscle in idiopathic inflammatory myopathy. Hum Pathol 199223888–895. [DOI] [PubMed] [Google Scholar]
- 18.Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975292344–347. [DOI] [PubMed] [Google Scholar]
- 19.Bohan A, Peter J B. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975292403–407. [DOI] [PubMed] [Google Scholar]
- 20.Griggs R C, Askanas V, DiMauro S, Engel A, Karpati G, Mendell J R.et al Inclusion body myositis and myopathies. Ann Neurol 199538705–713. [DOI] [PubMed] [Google Scholar]
- 21.Josefsson A, Romanus E, Carlsson J. A functional index in myositis. J Rheumatol 1996231380–1384. [PubMed] [Google Scholar]
- 22.Dorph C, Nennesmo I, Lundberg I E. Percutaneous conchotome muscle biopsy. A useful diagnostic and assessment tool. J Rheumatol 2001281591–1599. [PubMed] [Google Scholar]
- 23.Henriksson K G. “Semi‐open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 197959317–323. [PubMed] [Google Scholar]
- 24.Frostegard J, Ulfgren A K, Nyberg P, Hedin U, Swedenborg J, Andersson U.et al Cytokine expression in advanced human atherosclerotic plaques: dominance of pro‐inflammatory (Th1) and macrophage‐stimulating cytokines. Atherosclerosis 199914533–43. [DOI] [PubMed] [Google Scholar]
- 25.Engel A G, Franzini‐Armstrong C.Myology: basic and clinical. London: McGraw‐Hill, 1994
- 26.van der Pas J, Hengstman G J, ter Laak H J, Borm G F, van Engelen B G. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry 200475136–139. [PMC free article] [PubMed] [Google Scholar]
- 27.De Bleecker J L, Engel A G. Expression of cell adhesion molecules in inflammatory myopathies and Duchenne dystrophy. J Neuropathol Exp Neurol 199453369–376. [DOI] [PubMed] [Google Scholar]
- 28.Cid M C, Grau J M, Casademont J, Tobias E, Picazo A, Coll‐Vinent B.et al Leucocyte/endothelial cell adhesion receptors in muscle biopsies from patients with idiopathic inflammatory myopathies (IIM). Clin Exp Immunol 1996104467–473. [PubMed] [Google Scholar]
- 29.Tidball J G, Wehling‐Henricks M. Damage and inflammation in muscular dystrophy: potential implications and relationships with autoimmune myositis. Curr Opin Rheumatol 200517707–713. [DOI] [PubMed] [Google Scholar]
- 30.Park J H, Vital T L, Ryder N M, Hernanz‐Schulman M, Partain C L, Price R R.et al Magnetic resonance imaging and P‐31 magnetic resonance spectroscopy provide unique quantitative data useful in the longitudinal management of patients with dermatomyositis. Arthritis Rheum 199437736–746. [DOI] [PubMed] [Google Scholar]
- 31.Taylor P C. VEGF and imaging of vessels in rheumatoid arthritis. Arthritis Res 20024(Suppl 3)S99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blake D R, Merry P, Unsworth J, Kidd B L, Outhwaite J M, Ballard R.et al Hypoxic‐reperfusion injury in the inflamed human joint. Lancet 19891289–293. [DOI] [PubMed] [Google Scholar]
- 33.Shreeniwas R, Koga S, Karakurum M, Pinsky D, Kaiser E, Brett J.et al Hypoxia‐mediated induction of endothelial cell interleukin‐1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest 1992902333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niinikoski J, Paljarvi L, Laato M, Lang H, Panelius M. Muscle hypoxia in myositis. J Neurol Neurosurg Psychiatry 1986491455. [DOI] [PMC free article] [PubMed] [Google Scholar]