Abstract
Both a murine monoclonal antibody to phosphatidylinositol phosphate (PIP) and a human monoclonal antibody (4E10) that is known to have broadly neutralizing capabilities against primary isolates of human immunodeficiency virus type 1 (HIV-1) bound to PIP, as determined by enzyme-linked immunosorbent assay. Each of the antibodies had antigen subsite binding specificities in aqueous medium for small phosphate-containing molecules and for inositol. The anti-PIP monoclonal antibody inhibited infection by two HIV-1 primary isolates in neutralization assays employing primary human peripheral blood mononuclear cells. The data suggest that PIP or related lipids having free phosphates could serve as targets for the neutralization of HIV-1.
Among the most challenging and important unsolved problems in human immunodeficiency virus type 1 (HIV-1) vaccine development is the inability to produce broadly neutralizing antibodies to HIV-1 (11). Antibodies to HIV-1 envelope proteins, including the CD4 and chemokine receptor binding sites, have been produced by HIV infection or vaccination, but because of mutation at critical sites, or because of steric effects, neutralization by antibodies is generally not broadly effective for preventing HIV-1 viral infection. In order to probe the HIV-1 envelope protein for neutralizing sites, a group of rare broadly neutralizing human monoclonal antibodies (MAbs) to HIV-1 serve as critically important models for developing target epitopes in HIV-1 vaccine antigen design (9, 31).
Recently, an important observation was made that two of these neutralizing human gp41 MAbs, known as 4E10 and 2F5, cross-reacted with cardiolipin (CL) and are in the category of antibodies that have lupus anticoagulant-type anti-CL specificities (18, 29). This observation is also consistent with a previous finding that HIV-1 could bind to, and fuse with, CL liposomes and that such binding inhibited infection of A3.01 cells by HIV-1 (20). The latter result suggested that HIV-1 has a binding site for CL. The results from the two laboratories could be interpreted as indicating that CL might serve as a binding site for HIV-1 and that interference with the binding to CL could be exploited for vaccine development (22, 23). However, balanced against this, it is known that CL is not present as a lipid constituent of either HIV-1 or the plasma membrane of any mammalian cell (1), and this therefore raises the question of whether an alternative lipid antigen might actually be the real neutralizing, and perhaps more important, target of 4E10 and 2F5. Reactivity of 4E10 also occurs with other individual phospholipids, including phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, and even phosphatidylcholine liposomes (18, 28). Because of this, it has been suggested that binding of 4E10 to phospholipids is due only to nonspecific hydrophobic interactions of the 4E10 antibody with the fatty acyl regions of the lipid bilayer (28).
Specific polyclonal and monoclonal antibodies to phosphatidylinositol-4-phosphate (PIP) can be readily induced in mice by injection of liposomes containing PIP as an antigen and lipid A as an adjuvant (3, 33). Four complement-fixing murine MAbs to PIP, selected for their abilities to react with liposomes containing PIP but not with liposomes lacking PIP, have been extensively studied (2, 3, 6, 16, 17, 30, 32, 33). The anti-PIP antibodies are characterized by the ability to react with various types of phosphorylated molecules, including certain closely related anionic phospholipids that have charged nonzwitterionic phosphate groups, such as CL (2), and also with denatured DNA (30). Presumably because of cross-reactivity with CL, anti-PIP antibodies gave positive results in clinical assays for lupus anticoagulant activity (2). Anti-PIP antibodies can be inhibited by small soluble phosphorylated molecules, such as inositol hexaphosphate (but not inositol), phosphocholine (but not choline), and nucleotides (but not nucleosides) (3, 30, 33). Because of the phosphate-binding subsite that allows such haptenic inhibition to occur, the antibodies can actually serve as high-affinity carriers and donors for biologically important molecules, as shown by the ability of ATP bound to anti-PIP antibodies to serve as a high-energy phosphate donor for an enzymatic (hexokinase) reaction (32).
In addition to providing information about the molecular architecture of antigen binding subsites, MAbs to PIP are useful probes for exploring potentially important biological binding and receptor activities. Anti-PIP antibodies bind directly to membrane phospholipids on adherent but not on nonadherent macrophages (16). There is also evidence that PIP can be expressed on the cell surface and act as a receptor for diphtheria toxin (6). Antibodies to PIP inhibited diphtheria toxin-induced CHO cell cytotoxicity (17).
In view of this, we investigated the potential role that antibodies to PIP might play in the identification of target phospholipid antigens for the induction of effective neutralizing antibodies to HIV. We demonstrate here that not only does the 4E10 antibody resemble anti-PIP antibodies in that it binds to PIP and can be inhibited by small phosphorylated molecules, but specific monoclonal anti-PIP antibodies also resemble 4E10 in that they neutralize primary isolates of HIV-1.
Murine monoclonal immunoglobulin M (IgM) antibodies to PIP were obtained after mice were immunized with liposomes containing PIP as an antigen and lipid A as an adjuvant, as previously described (33). IgM antibody was purified from ascites fluid containing anti-PIP antibody no. 4 by using the protocol supplied with the ImmunoPure IgM purification kit and Slide-A-Lyzer dialysis cassettes (Pierce Chemical Co., Rockville, IL). Monoclonal antibody 4E10 was obtained through the NIH AIDS Research and Reference Reagent Program. The activities of the anti-PIP and 4E10 antibodies were assayed by enzyme-linked immunosorbent assay (ELISA) as described previously (2), with slight modification. Briefly, phospholipid antigen (10 nmol) in 80% ethanol or methanol was dried by evaporation at the bottom of polystyrene ELISA microtiter wells. Plates were blocked with 3% bovine serum albumin in 20 mM Tris-HCl, pH 7.4, and 154 mM NaCl for 2 h; 800 ng/0.1 ml of purified MAb was added per well. Phosphate inhibitors or inositol was used, as indicated, by incubating 30 mM of the compound with the antibody at room temperature for 30 min prior to the addition of the antibody to the antigen. Appropriate goat anti-human IgG or anti-mouse IgM secondary antibody conjugated to alkaline phosphatase was added, and after the addition of substrate, the plates were read at 405 nm. The methods for isolation, propagation, and titration of HIV-1 isolates and the peripheral blood mononuclear cells (PBMC) (10) and pseudovirus/TZM-bl (21) neutralization assays were used as previously described. Briefly, virus was combined with the neutralization reagent for 30 min and incubated at 37°C. Phytohemagglutinin-stimulated PBMC were added and incubated overnight at 37°C. The following day, cells were washed three times and incubated at 37°C. After 4 days, extracellular p24 was measured and percent neutralization was calculated as described previously (10). Included in each experiment was a positive neutralization control (USHIV+). The average neutralization percentage at a 1:40 dilution of USHIV+ was 84%, with a standard deviation of 12%. For the pseudovirus assay, virus and neutralization reagent were combined and incubated for 1 h at 37°C. TZM-bl cells were then added and incubated at 37°C. After 2 days, cells were washed and lysed, luminescence reagent was added, and luminescence was measured. For these assays, USHIV+ at a 1:40 dilution had an average neutralization of 84%, with a standard deviation of 14%.
ELISA titers and affinities of murine and human antibodies that are assayed with different types of phospholipid antigens (for example, murine and human antibody binding titers or affinities to CL and PIP) cannot be meaningfully compared directly from a quantitative standpoint. This is because relatively insoluble lipids often form different or nonhomogenous layering patterns on ELISA wells, and different secondary antibodies are used for detecting murine and human antibodies. Despite this, the subsite binding specificities of the antibodies can be probed by examining the relative patterns of inhibition or enhancement of antibody binding in the presence of soluble haptens in the fluid phase (3). The patterns of effects of soluble haptens containing free phosphate groups or inositol are described below.
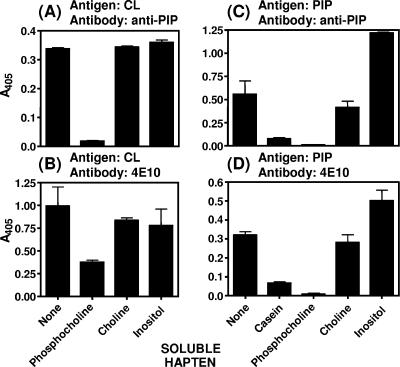
The phosphate-binding subsite of anti-PIP (3, 33) is revealed by the ELISA data in that casein, a highly phosphorylated protein, was inhibitory to binding of anti-PIP both to PIP and to cardiolipin, as was phosphocholine, but no inhibition was found with choline (Fig. 1A and B). The recently reported binding of the 4E10 antibody to CL (18) is confirmed by our data (Fig. 1B), and a new specificity of binding of 4E10 to PIP was also observed (Fig. 1D). The 4E10 antibody, as with anti-PIP, has a similar phosphate-binding subsite in that the binding to CL and PIP was inhibited by phosphocholine but not by choline (Fig. 1B and D).
FIG. 1.
Binding of anti-PIP (A and C) and 4E10 (B and D) antibodies to lipids and effects of soluble haptens on antibody binding, as tested by ELISA with purified CL or PIP as a capture antigen. The data were obtained from a representative experiment, with the mean and standard deviation shown for triplicate points. Longer incubation times in repetitions of the assay gave quantitatively different but qualitatively similar patterns of effects of the indicated haptens.
The binding of the anti-PIP and 4E10 antibodies to PIP was not inhibited by soluble haptenic inositol (Fig. 1C and D), and inositol actually enhanced the binding of the antibodies to the antigen (Fig. 1C and D). No enhancement was observed in the binding of the antibodies to CL in the presence of inositol (Fig. 1A and B). Separate experiments suggested that the enhancement of binding of the antibodies to PIP, but not to CL, probably reflects a low-affinity hydroxyl-hydroxyl association of inositol with the polyhydroxyl head groups on PIP, combined with low-affinity inositol subsites in the anti-PIP and 4E10 antibodies. This was determined by highly reproducible and significant but extremely low counts of association of [3H]inositol with PIP but not with CL coated on ELISA microtiter wells (data not shown).
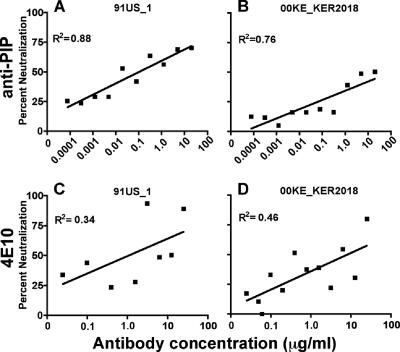
The murine anti-PIP monoclonal antibody was examined for possible HIV-1 neutralizing activity in a model utilizing the inhibition of infection of PBMC. As shown in Fig. 2A and B, the anti-PIP antibody exhibited neutralizing activity that blocked infection of PBMC by both HIV strains 91US_1 (Fig. 2A) and 00KE_KER2018 (Fig. 2B), both of which are primary isolates of HIV-1. For negative controls, we examined the neutralization of 91US_1 with two irrelevant murine IgM MAbs to phosphocholine, MOPC-104E (Sigma, St. Louis, MO) and TEPC 183 (Sigma, St. Louis, MO), as well as a human IgM MAb to lipid A, C5 (ATCC, Chantilly, VA). In two experiments, none of these MAbs mediated neutralization (data not shown). Blinded independent confirmation of the neutralizing activity of the anti-PIP antibody for blocking 91US_1 infection of primary PBMC (both with PBMC from the donor shown in Fig. 2A and with PBMC independently obtained from a separate donor) was kindly provided by John Mascola at the Vaccine Research Center, NIH (data not shown). We conclude that anti-PIP antibodies can neutralize two primary isolates, derived from either clade A (00KE_KER2018) or clade B (91US_1), when PBMC are used as target cells.
FIG. 2.
Anti-PIP neutralizes clade A and B isolates in a PBMC-based assay. Anti-PIP (A and B) and 4E10 (C and D) were tested for neutralization against a clade A (B and D) and a clade B (A and C) isolate. The data presented were derived from the averages for two to three experiments.
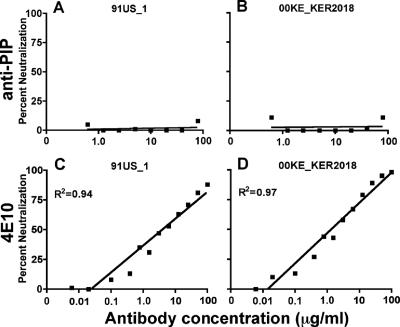
Interestingly, when the anti-PIP and 4E10 antibodies were tested against pseudoviruses from the same clades in the TZM-bl cell line model system, the 4E10 antibody exhibited neutralization, but the anti-PIP antibody did not (Fig. 3). Neutralization by 4E10 is less potent with the PBMC assay than with the pseudovirus/TMZ-bl assay, and as shown in Fig. 3, the variability around the data points is more tightly spaced in the latter assay. This observation is compatible with previous findings that the pseudovirus/TZM-bl assay shows more neutralization than PBMC-based assays when 4E10 is used. In contrast, with X5, a broadly neutralizing gp120 antibody, more neutralization is observed in the PBMC assay than in the TZM-bl-based assay (9, 12). Although the reasons for the discordances between the two assays are still undetermined, TZM-bl (also known as JC-53BL) is a HeLa cell-based cell line that contains levels of CCR5 that may be at least 10-fold higher than those in PBMC (25). It has been reported that high concentrations of CCR5 in HeLa lines are associated with lower neutralization efficacy with X5 antibody (12), and anti-PIP antibodies may be similar to X5 in this respect. It is well known that high protein/lipid ratios can sterically inhibit binding of antibodies to lipids on cells or lipoproteins (4, 5, 13, 16). In a striking example of the absence of binding to whole cells, antibodies to PIP did not bind to nonadherent cultured macrophages, as determined by indirect immunofluorescence (16). We have also found that anti-PIP antibodies do not bind to PBMC, as determined by fluorescence-activated cell sorter analysis (data not shown). Based on this, it would seem unlikely that anti-PIP is directly reacting with the target cell prior to binding of HIV-1. However, one simple hypothesis to explain the poor neutralization by anti-PIP in the pseudovirus/TZM-bl system compared to that in the PBMC assay is that the anti-PIP antibodies might neutralize HIV-1 by binding to viral lipids when the lipid bilayer of the virus directly interacts with the lipid bilayer of the target cell. A further possibility is that anti-PIP reacts with phosphatidylinositol-4,5-bisphosphate during viral assembly and budding (27). In either case, such binding of anti-PIP to the viral lipids might be sterically hindered by excessively high levels of adjacent CCR5 protein on the TZM-bl target cell when the viral and plasma membrane lipid bilayers are closely apposed.
FIG. 3.
Anti-PIP does not neutralize clade A and B isolates in a TZM-bl pseudovirus assay. Anti-PIP (A and B) and 4E10 (C and D) were tested for neutralization against a clade A (B and D) and a clade B (A and C) pseudovirus. The data presented were derived from the averages for two to five experiments.
Our data with murine MAb anti-PIP and human MAb 4E10, each of which binds to PIP and CL and neutralizes HIV-1, suggest that cell surface or viral PIP, or related inositol phosphatides, could play a role in the interaction of HIV-1 with target cells. The inositol phosphatides, which comprise a family of eight chemical species with different combinations of phosphate groups arranged around the polyhydroxyl inositol ring, are highly versatile signaling molecules, with key roles in receptor-mediated signal transduction, signal-induced actin assembly and remodeling, and membrane trafficking (14). PIP, an intermediate in the synthesis of phosphatidylinositol-4,5-bis-phosphate from phosphatidylinositol (14), is synthesized by a phosphatidylinositol-4-kinase that is located in the lipid rafts and caveola-like vesicles of the plasma membrane of eukaryotic cells (7, 34). A huge and sometimes confusing array of proteins bind to inositol phosphatides, perhaps the most well-known of which are glycosylphosphatidylinositol (GPI)-anchored proteins (19). Certain GPI-anchored proteins, such as Thy-1 and CD59, are incorporated into the HIV-1 virion during budding of the virus from lipid rafts (24). In addition, non-protein-linked (i.e., free) GPI is displayed on the surface of mammalian cells (8), and free GPI would presumably be incorporated into HIV-1. GPI-anchored proteins were originally discovered on the surface of Trypanosoma brucei (15), and antibodies to phosphatidylinositol and PIP were induced in rabbits infected with Trypanosoma rhodesiense (26). Thus, PIP in an infectious organism can be immunogenic, and it appears possible that PIP in the lipid bilayer of the HIV-1 virion or in the host cell, or in other membrane lipids with free phosphate, could represent a target for neutralizing antibodies to HIV-1.
The present data suggest that 4E10 initially binds specifically to the head groups of the phospholipids, particularly with phosphate groups, and this occurs prior to nonspecific hydrophobic sequestration of 4E10 among the buried phospholipid fatty acids (28). Regardless of the exact mechanism of neutralization by anti-PIP and 4E10 antibodies, it does appear evident that the phosphate-binding subsite, and possibly the inositol-binding subsite, of each antibody might play a role. The data suggest that the initial neutralizing effects of anti-PIP, and perhaps 4E10, may be more strongly associated with the head groups of the phospholipids than with hydrophobic interactions with the hydrophobic regions of either HIV-1 or plasma membrane lipid bilayers.
Acknowledgments
We thank Lindsay Wiesczorek, Kara Lombardi, and Erik Odom (HMJF) and Anna Sambor (Vaccine Research Center, NIH) for technical assistance and Andrew Rosa Borges (HMJF) for helpful comments regarding the manuscript. We also thank John Mascola (VRC, NIH) for the confirmatory work cited in the text.
This work was supported through Cooperative Agreement no. DAMD17-93-V-3004 between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the U.S. Army Medical Research and Material Command, working together with the Division of AIDS, National Institute for Allergy and Infectious Diseases, NIH, Bethesda, MD.
The views and opinions expressed herein are the private opinions of the authors and do not necessarily reflect the views of the U.S. Army or the U.S. Department of Defense.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Aloia, R. C., H. Tian, and F. C. Jensen. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA 90:5181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alving, B. M., B. Banerji, W. E. Fogler, and C. R. Alving. 1987. Lupus anticoagulant activities of murine monoclonal antibodies to liposomal phosphatidylinositol phosphate. Clin. Exp. Immunol. 69:403-408. [PMC free article] [PubMed] [Google Scholar]
- 3.Alving, C. R. 1986. Antibodies to liposomes, phospholipids and phosphate esters. Chem. Phys. Lipids 40:303-314. [DOI] [PubMed] [Google Scholar]
- 4.Alving, C. R. 2006. Antibodies to lipids and liposomes: immunology and safety. J. Liposome Res. 16:157-166. [DOI] [PubMed] [Google Scholar]
- 5.Alving, C. R., and N. M. Wassef. 1999. Naturally occurring antibodies to cholesterol: a new theory of LDL cholesterol metabolism. Immunol. Today 20:362-366. [DOI] [PubMed] [Google Scholar]
- 6.Alving, C. R., B. Iglewski, K. A. Urban, J. Moss, R. L. Richards, and J. C. Sadoff. 1980. Binding of diphtheria toxin to phospholipids in liposomes. Proc. Natl. Acad. Sci. USA 77:1986-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoyagi, K., T. Sugaya, M. Umeda, S. Yamamoto, S. Terakawa, and M. Takahashi. 2005. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 280:17346-17352. [DOI] [PubMed] [Google Scholar]
- 8.Baumann, N. A., J. Vidugiriene, C. E. Machamer, and A. K. Menon. 2000. Cell surface display and intracellular trafficking of free glycosylphosphatidylinositols in mammalian cells. J. Biol. Chem. 275:7378-7389. [DOI] [PubMed] [Google Scholar]
- 9.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, B. K., J. M. Darden, S. Tovanabutra, T. Oblander, J. Frost, E. Sanders-Buell, M. S. de Souza, D. L. Birx, F. E. McCutchan, and V. R. Polonis. 2005. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J. Virol. 79:6089-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 12.Choudhry, V., M. Y. Zhang, I. Harris, I. A. Sidorov, B. Vu, A. S. Dimitrov, T. Fouts, and D. S. Dimitrov. 2006. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem. Biophys. Res. Commun. 348:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijkstra, J., G. M. Swartz, Jr., J. J. Raney, J. Aniagolu, L. Toro, C. A. Nacy, and S. J. Green. 1996. Interaction of anti-cholesterol antibodies with human lipoproteins. J. Immunol. 157:2006-2013. [PubMed] [Google Scholar]
- 14.Downes, C. P., A. Gray, and J. M. Lucocq. 2005. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 15:259-268. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson, M. A., M. G. Low, and G. A. Cross. 1985. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J. Biol. Chem. 260:14547-14555. [PubMed] [Google Scholar]
- 16.Fogler, W. E., G. M. Swartz, Jr., and C. R. Alving. 1987. Antibodies to phospholipids and liposomes: binding of antibodies to cells. Biochim. Biophys. Acta 903:265-272. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, R. L., B. H. Iglewski, F. Roerdink, and C. R. Alving. 1982. Suppression of cytotoxicity of diphtheria toxin by monoclonal antibodies against phosphatidylinositol phosphate. Biophys. J. 37:23-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes, B. F., J. Fleming, E. W. St. Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 19.Ikezawa, H. 2002. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 25:409-417. [DOI] [PubMed] [Google Scholar]
- 20.Konopka, K., B. R. Davis, C. E. Larsen, D. R. Alford, R. J. Debs, and N. Duzgunes. 1990. Liposomes modulate human immunodeficiency virus infectivity. J. Gen. Virol. 71:2899-2907. [DOI] [PubMed] [Google Scholar]
- 21.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montefiori, D. C. 2005. Neutralizing antibodies take a swipe at HIV in vivo. Nat. Med. 11:593-594. [DOI] [PubMed] [Google Scholar]
- 23.Nabel, G. J. 2005. Immunology. Close to the edge: neutralizing the HIV-1 envelope. Science 308:1878-1879. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, D. H., and J. E. K. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards, R. L., J. Aronson, M. Schoenbechler, C. L. Diggs, and C. R. Alving. 1983. Antibodies reactive with liposomal phospholipids are produced during experimental Trypanosoma rhodesiense infections in rabbits. J. Immunol. 130:1390-1394. [PubMed] [Google Scholar]
- 27.Saad, J. S., J. Miller, J. Tai, A. Kim, R. H. Ghanam, and M. F. Summers. 2006. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. USA 103:11364-11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Martínez, S., M. Lorizate, H. Katinger, R. Kunert, and J. L. Nieva. 2006. Membrane association and epitope recognition by HIV-1 neutralizing anti-gp41 2F5 and 4E10 antibodies. AIDS Res. Hum. Retrovir. 22:998-1006. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Martínez, S., M. Lorizate, H. Katinger, R. Kunert, G. Basanez, and J. L. Nieva. 2006. Specific phospholipid recognition by human immunodeficiency virus type-1 neutralizing anti-gp41 2F5 antibody. FEBS Lett. 580:2395-2399. [DOI] [PubMed] [Google Scholar]
- 30.Stollar, B. D., T. McInerney, T. Gavron, N. M. Wassef, G. M. Swartz, Jr., and C. R. Alving. 1989. Cross-reactions of nucleic acids with monoclonal antibodies to phosphatidylinositol phosphate and cholesterol. Mol. Immunol. 26:73-79. [DOI] [PubMed] [Google Scholar]
- 31.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 32.Wassef, N. M., G. M. Swartz, B. M. Alving, and C. R. Alving. 1993. ATP specifically bound as a hapten to a monoclonal anti-phospholipid antibody retains phosphate donor activity. Biochem. Biophys. Res. Commun. 190:582-588. [DOI] [PubMed] [Google Scholar]
- 33.Wassef, N. M., F. Roerdink, G. M. Swartz, Jr., J. A. Lyon, B. J. Berson, and C. R. Alving. 1984. Phosphate binding specificities of monoclonal antibodies against phosphoinositides in liposomes. Mol. Immunol. 21:863-868. [DOI] [PubMed] [Google Scholar]
- 34.Waugh, M. G., D. Lawson, S. K. Tan, and J. J. Hsuan. 1998. Phosphatidylinositol 4-phosphate synthesis in immunoisolated caveolae-like vesicles and low buoyant density non-caveolar membranes. J. Biol. Chem. 273:17115-17121. [DOI] [PubMed] [Google Scholar]