Abstract
It is increasingly becoming clear that various immune cells are infected by the very pathogens that they are supposed to attack. Although many mechanisms for microbial entry exist, it appears that a common route of entry shared by certain bacteria, viruses and parasites involves cellular lipid-rich microdomains sometimes called caveolae. These cellular entities, which are characterized by their preferential accumulation of glycosylphosphatidylinositol (GPI)-anchored molecules, cholesterol and various glycolipids, and a distinct protein (caveolin), are present in many effector cells of the immune system including neutrophils, macrophages, mast cells and dendritic cells. These structures have an innate capacity to endocytoze various ligands and traffic them to different intracellular sites and sometimes, back to the extracellular cell surface. Because caveolae do not typically fuse with lysosomes, the ligands borne by caveolar vesicles are essentially intact, which is in marked contrast to ligands endocytozed via the classical endosome–lysosome pathway. A number of microbes or their exotoxins co-opt the unique features of caveolae to enter and traffic, without any apparent loss of viability and function, to different sites within immune and other host cells. In spite of their wide disparity in size and other structural attributes, we predict that a common feature among caveolae-utilizing pathogens and toxins is that their cognate receptor(s) are localized within plasmalemmal caveolae of the host cell.
Introduction
The ability to seek refuge within effector cells of the immune system appears to be a common trait among several pathogenic micro-organisms. In their intracellular location, the pathogen can escape the antimicrobial actions of other immune cells (and any antibiotics that may be present), and if the immune cell is mobile, the pathogen gets disseminated to deeper niches in the body, as shown recently for human immunodeficiency virus (HIV) and Salmonella.1,2 As immune cells have the intrinsic capacity to kill endocytozed pathogens, the mode of entry appears to be a critical determinant for intracellular survival. Recently, we found that the opportunistic pathogen Escherichia coli was capable of entry into mast cells via an endocytic route that avoided fusion with lysosomes where bacteria are typically killed and degraded.3 Mast cells are inflammatory cells that have recently been shown to play a critical role in the innate immune defence against infectious agents.4,5 The mechanism of bacterial entry into mast cells involved distinct caveolin-containing cellular entities called caveolae.6 This finding is noteworthy for several reasons. First, haematopoietic cells were thought not to possess caveolae,7 and secondly, although caveolae have been shown to be involved in the endocytosis of small macromolecules8,9 their role in the uptake of relatively large particulate bacteria into host cells reveals a much more versatile role in endocytosis than previously imagined. The third, and perhaps most immunologically relevant reason, is that caveolar entities may also be employed by other pathogens to enter and seek refuge within immune and various other host cells. Indeed, a search of the recent literature has revealed several examples where entry of microbial pathogens or their products may be attributable, at least in part, to caveolae or cellular entities closely resembling caveolae. Here, we will explore the notion that co-option of the endocytic and trafficking attributes of caveolae may represent a common but insidious way that intracellular pathogens gain entry into host cells or deliver their toxic products to the host cell.
CAVEOLAE AS CELLULAR ORGANELLES OF ENDOCYTOSIS and INTRACELLULAR TRAFFICKING
Although originally defined by their distinct morphology (invaginated cave-like structures 50–100 nm in diameter, found on cell surfaces), caveolae are now defined as pleomorphic lateral assemblies (rafts) of glycolipids, cholesterol, various glycosylphosphatidylinositol (GPI)-anchored molecules and a 22 000-MW protein, caveolin-1.10 Caveolae assume a variety of shapes, including flat, vesicular, and tubular forms. They can be either open at the cell surface or closed off, forming a vesicular compartment. Although the main forces driving the formation of these microdomains are lipid–lipid interactions that are dependent on the biophysical characteristics of the lipid components, caveolin protein appears to contribute to the aggregate structure possibly by serving as a scaffolding protein. Although some have believed caveolae to be present in virtually all cell types, their presence in haematopoietic cells has been unclear. Earlier studies employing cell lines of lymphocytes7 and mast cells11 have shown that while microdomains comprising glycolipids, cholesterol and various GPI-anchored molecules were clearly detectable in these cells, the presence of caveolin was not. Hence these structures were referred to as lipid rafts rather than caveolae. Transfection of lymphocyte cell lines with cDNA for caveolin-1 resulted in the migration of caveolin to the lipid rafts, triggering the formation of distinct cave-like caveolae in the plasmalemmal surface of the transfectants.12 Thus, the caveolin protein can also contribute to the morphological appearance of caveolae. Caveolin has the intrinsic ability to form stable homo-oligomers,13,14 and rapid-freeze, deep-etch ultra-electron microscopy of fibroblasts have revealed that these homo-oligomerized caveolin give rise to distinct filamentous striated strands at the base (cytoplasmic face) of each invaginated caveolae.15
Ever since their discovery, the cellular function(s) of caveolae has been the focus of much study. In recent years, caveolae have been implicated as a conduits for transmembrane signal transduction because of the high concentration of receptors and signalling molecules found concentrated within their structure.16,17 Receptors for various growth factors and hormones have been localized to caveolae including epidermal growth factor,18 platelet-derived growth factor19 and insulin.20 Signalling molecules are also specifically localized to these sites including Src family protein tyrosine kinases, protein kinase C isoforms, Ha-Ras and heterotrimeric G protein α subunits.21 Caveolin can directly interact with some of these signalling molecules via a conserved 20 amino acid domain termed the caveolin-scaffolding domain (residues 82–101 of caveolin-1). In addition to serving as a conduit for signalling, caveolae have been implicated in several different endocytic events, including the transcytosis of macromolecules across cells. Caveolae appear to contain all the molecular machinery for docking and fusion that is necessary for a vesicular trafficking system.22 However, the caveolae-mediated endocytic pathway is distinct from the classical endosome–lysosome pathway in a number of ways. For example, in contrast to the classical pathway, the nascent endocytic compartment formed during the uptake of a ligand comprised of lipid-rich elements, often containing caveolin, but is distinctly devoid of clathrin. In the classical pathway, following scission from the plasma membrane, intracellular ligand-containing endosomes typically fuse with lysosomes where the ligand is degraded, whereas there is apparently no fusion of caveolar vesicles with endosomes.10,23 Based mainly on literature obtained from the study of endothelial and epithelial cells, there appears to be several modes of caveolae-mediated entry and intracellular trafficking, depending on the ligand. In certain cases, the entry of the ligand involves a phenomenon called potocytosis, a potential method for ‘pumping’ small molecules and ions from the extracellular medium into the cytoplasm as exemplified by the uptake of folate via its GPI-anchored receptors concentrated within plasmalemmal caveolae.8 In other cases, the ligand is encapsulated and translocated directly to the endoplasmic reticulum as in the case of cholesterol10 or to the nucleus as in the case of some hormones,24 yet in others, the caveolae-bound ligand is translocated across the cell and discharged outside at the basolateral plasma membrane as in the case of albumin,9 immunoglobulin A (IgA) antibodies,25 and certain chemokines.26 Because some caveolae can assume a tubular form (formed by the fusion of multiple swollen caveolae27), there is no scission of the caveolar compartment following binding of the ligand, instead the ligand is funnelled from cell surface to cell surface through the length of the tubular caveolae.28 Regardless of the heterogeneity in intracellular trafficking, ligands endocytozed by caveolae, appear to share at least two common features. The first is that they are not readily degraded following entry into the host cell because the caveolar compartments bearing the ligand fail to fuse with lysosomes, and second, the cognate plasmalemmal receptor for the ligand is an integral component of caveolae. Taken together, caveolae appear to serve a critical physiological function for the cell by internalizing macromolecules (nutrients, growth factors, hormones, antibodies, chemokines, etc.) and trafficking them without degradation and in a functionally active state to various intracellular sites and sometimes, back to the extracellular sites. Obviously, there is selectivity in which macromolecules can utilize caveolae for their entry, because only ligands whose cognate receptors are associated with caveolae are endocytozed in this manner.
CAVEOLAE-MEDIATED UPTAKE OF FimH-EXPRESSING ESCHERICHIA COLI
Escherichia coli is an opportunists pathogen and is a leading cause of extra-intestinal infections in elderly and immunocompromized patients.29 Many of these infections are notable for their persistence even when appropriate antibiotics are applied implying at least a transient intracellular existence.30 Although many host factors contribute to the prevalence of E. coli infections, one microbial determinant appears to be the expression of FimH, a mannose-binding fimbrial adhesin.31 We found that in serum-free conditions, FimH-expressing E. coli can induce uptake by both mast cells3 and macrophages32 via an endocytic route that is distinct from the classical endosome–lysosome pathway and as a result, the bacteria remain in a viable state within morphologically distinct intracellular compartments. Our interest in caveolae as a possible mediator of bacterial uptake into mast cells and macrophages, stemmed from our finding that the receptor on mouse mast cells and macrophages for FimH-expressing bacteria was CD48.33 As this protein has no transmembrane or cytoplasmic domains and is tethered only to the outer leaflet of the plasma membrane by a GPI anchor, it was unclear as to how CD48 was able to induce uptake of bacteria. Interestingly, many GPI-anchored molecules are localized to caveolae34 or migrate to caveolae following ligation,35 and were able to transduce signals by their close proximity to the many signalling molecules aggregated within caveolae.
We sought to investigate if caveolae were involved in the uptake of bacteria by mast cells. Employing a commercial polyclonal antibody against caveolin-1 (used by many laboratories), we probed mast-cell cross-sections and observed the specific localization of caveolin to the plasma membrane and to the intracellular vesicles within mast cells. Interestingly, we did not observe any cave-like structures on the plasmalemmal membrane with our probe. That the caveolin protein was indeed localized within caveolae in the mast cells was indicated by the fact that caveolin localized to the lipid-rich light buoyant fraction following sucrose gradient fractionation of mast cell lysates. It was noteworthy that CD48 was found in the same fraction as caveolin indicating that the bacterial receptor was an integral part of caveolae in mast cells. Thus, although mast cells do not form the classical cave-like structures, plasmalemmal and vesicular caveolae are present. One reason why the earlier studies failed to detect caveolin in haematopoietic cells is because tumour cell lines, rather than primary cells, were employed for the studies and it is now known that caveolin expression can be down-regulated in certain tumour cell lines.21
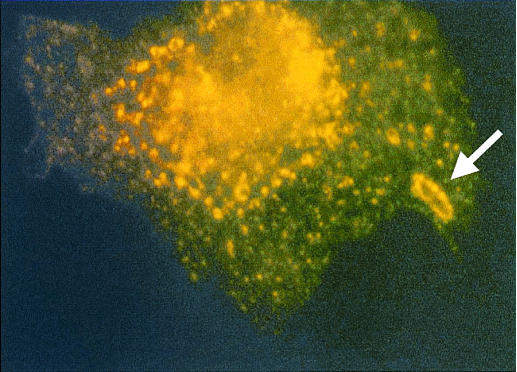
Two independent pieces of data pointed to the involvement of caveolae in bacterial uptake. The first was that immune microscopy and immune electron microscopy revealed that several specific markers of caveolae (caveolin, GM1 and cholesterol) appeared to be recruited to and encapsulate intracellular bacteria.6 Shown in Fig. 1 is an intracellular bacterium within a mast cell. Notice the caveolar vesicle encapsulating the bacteria, revealed by caveolin-specific antibody. Movement of caveolar markers to sites of bacterial entry was also confirmed in cell fractionation studies that allowed us to simultaneously isolate bacterium containing chambers and cellular caveolae. Our cell fractionation and electron microscopic data showed that cellular caveolae (plasmalemmal and vesicular) in mast cells appeared to be recruited to sites of bacterial entry to form distinct bacteria-encapsulating compartments.
Figure 1.
Specific recruitment of the caveolar marker, caveolin at site of bacterial entry (arrow).
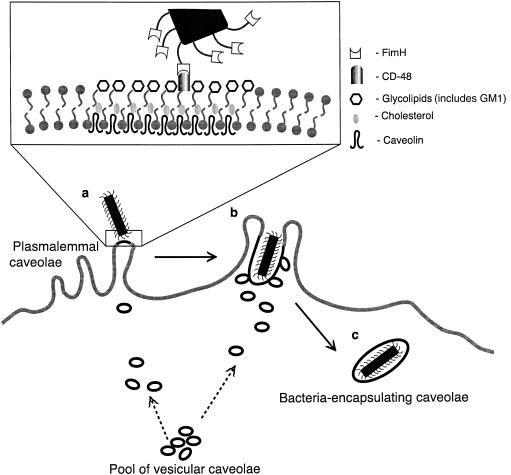
A second and more direct evidence for the role of caveolae was obtained by employing a specific disrupter of caveolae to block uptake of FimH-expressing E. coli by mast cells. β-methyl cyclodextrin, a known disrupter of caveolae (achieved by inducing efflux of cellular cholesterol) inhibited mast cell uptake of FimH-expressing E. coli, but not uptake of isogenic FimH minus E. coli or IgG antibody – opsonized FimH minus E. coli bacteria.6 Cumulatively, these observations suggest that following binding of the FimH adhesins of bacteria to CD48 localized to plasmalemmal caveolae, bacterial uptake occurs via a dynamic process involving remodelling of plasmalemmal caveolae and the recruitment of vesicular caveolae to form large bacteria-encapsulating chambers. A diagrammatic representation of the dynamics of caveolae-mediated uptake of bacteria is provided in Fig. 2.
Figure 2.
Model depicting bacteria-induced mobilization of caveolae in bone marrow-derived mast cell (BMMC). (a) Specific coupling of a FimH-expressing bacterium to CD48 localized within plasmalemmal caveolae. This triggers movement of vesicular caveolae from an intracellular pool to the site of bacterial attachment. The molecular composition of plasmalemmal caveolae is shown in greater detail in the box. (b) The recruited caveolae form plasmalemmal extensions around the adherent bacterium. (c) The plasmalemmal extensions eventually form a distinct intracellular caveolar compartment.
Co-option of endocytic functions of caveolae or lipid rafts by microbes or their products
Interestingly, a review of recent literature has revealed that caveolae or lipid rafts have been implicated in the entry of a small but diverse group of micro-organisms or their products into host cells (A list is provided in Table 1.) For example, it was recently demonstrated that the entry of the classical intracellular bacterium Mycobacterium bovis into macrophages was specifically inhibited by the caveolae disrupter, cyclodextrin.36 The study noted that cholesterol, a major component of caveolae, was critical for the entry of this pathogen into macrophages and by entering at cholesterol-rich domains of the plasma membrane, mycobacteria could ensure their subsequent intracellular survival within the macrophage. The entry of the intestinal pathogen Campylobacter jejuni into host cells has also been shown to be significantly inhibited by disrupters of caveolae.37 Certain viruses require endocytosis for entry and the most frequently described and best-understood pathway is that which occurs constitutively through clathrin-coated vesicles and via the classical endosomal–lysosome route.38 However, simian virus 40 (SV40) appears to enter host cells in non-coated vesicles that have the biochemical and morphological features of caveolae.39 The mode of entry of this virus involves a pathway that delivers the virus directly to the endoplasmic reticulum and completely bypasses the endosomal–lysosmal compartment.40 Although, the receptor for this virus appears to be major histocompatibility complex (MHC) class I, this molecule does not appear to enter the cell with the virus.41 In polarized cells, the capacity of caveolae to traffic directly to the endoplasmic reticulum and to traffic from this site to the basolateral membrane appears to be co-opted by Vibrio cholerae for the activation as well as delivery of cholera toxin to the site of action within the host cell. The specific receptor for cholera toxin is GM1, which is an integral component of caveolae.42 In polarized cells, upon binding of the toxin to its receptor, the toxin/receptor complex is borne via caveolae to the trans-Golgi, where it migrates through the Golgi cisternae, and reaches the endoplasmic reticulum where activation of the A-subunit of the toxin occurs.43 Basolaterally targeted vesicles, possibly caveolae, carry the toxin and deliver the A subunit to a site near the Gs/adenyl cyclase complex on the cytosolic surface of the basolateral membrane (while the B-subunit is delivered to the exocytoplasmic surface of the same membrane).44,45 The result is toxin-induced activation of adenyl cyclase, which leads to cellular hypersecretion of chloride ions.46 Parasites such as Toxoplasma gondii and Plasmodium falcipurum enter phagocytic and non-phagocytic cells by active penetration, a rapid process that does not rely on the host-cell endocytic machinery.47 A direct result of this active invasion process is the formation of a specialized compartment called the parasitophorous vacuole that resists fusion with lysosomes.48 Although the underlying mechanism for avoiding lysosomes is still the subject of investigation, it is perhaps significant that the parasitophorous vacuole is preferentially comprised of transmembrane, GPI-anchored and cytoplasmic proteins originating from lipid rafts of the host cell.49
Table 1.
Micro-organisms or their products that utilize caveolae for entry into host cells
Cumulatively, it would appear that different attributes of caveolae-mediated endocytosis are being co-opted by a wide array of micro-organisms. It is noteworthy that all of the cognate receptors of the pathogens and toxins so far known to utilize caveolae have been found to localize to caveolae. Presumably, presence of the cognate receptor within caveolae or lipid rafts is a critical determinant for caveolae-mediated microbial entry into host cells. It is also noteworthy that in addition to co-opting caveolae for their entry, there is also evidence for the involvement of the same structures in the escape of pathogens from host cells. For example, assembling HIV particles appear to require lipid raft components. Indeed, the membrane of HIV comprises of GM1, GPI-anchored proteins, and partitions preferentially into lipid rafts.50 The escape of the malarial parasite from infected erythrocytes also appears to involve active involvement of cellular caveolar components.51
Conclusion
Although several functions have been attributed to caveolae, it is clear that these subcellular entities play a multifaceted role in endocytic processes of immune and other cells. Recently, a number of different pathogens have been described to have the capacity to co-opt caveolae to enter, traffic within, and sometimes re-emerge from host cells. As these pathogens represent only a very small minority of pathogens known to enter host cells, these observations may represent only the tip of the iceberg. Future studies directed at elucidating the molecular events leading to the entry of different pathogens into host cells via caveolae may provide vital clues to (i) the complex and versatile attributes of caveolae as organelles of endocytosis, and (ii) the development of novel strategies to prevent or reverse caveolae-mediated entry of pathogens into host cells.
Acknowledgments
We would like to thank Mathew Duncan for editorial assistance. Our studies were supported by NIH grants AI 35678 and DK 50814.
References
- 1.Dimitrov DS. Cell biology of virus entry. Cell. 2000;101:697–702. doi: 10.1016/s0092-8674(00)80882-x. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Torres A, Jones-Carson J, Baumler AJ, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–8. doi: 10.1038/44593. 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 3.Gao Z, Shin J-S, Malaviya R, Baorto D, Abraham SN. Opsonin-independent phagocytosis of FimH-expressing bacteria by mouse bone marrow derived mast cells. in preparation.
- 4.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha [see comments] Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 5.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis [see comments] Nature. 1996;381:75–7. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 6.Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells [see comments] Science. 2000;289:785–8. doi: 10.1126/science.289.5480.785. 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- 7.Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–8. [PubMed] [Google Scholar]
- 8.Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–1. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 9.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 11.Field KA, Holowka D, Baird B. Fc epsilon RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Nat Acad Sci USA. 1995;92:9201–5. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21- caveolin. Proc Natl Acad Sci USA. 1995;92:8655–9. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–27. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–11. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 16.Takashi O, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang X, Scherer PE, Lisanti MP. Crowded little caves: structure and function of caveolae. Cell Signal. 1998;10:457–63. doi: 10.1016/s0898-6568(98)00007-2. 10.1016/s0898-6568(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 18.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–5. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10. doi: 10.1074/jbc.271.17.10299. 99. 303. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–8. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 22.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270:14. doi: 10.1074/jbc.270.24.14399. 99. 404. [DOI] [PubMed] [Google Scholar]
- 23.Conrad PA, Smart EJ, Ying YS, Anderson RG, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–33. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada S, Smith RM, Jarett L. Mechanisms of nuclear translocation of insulin. Cell Biochem Biophys. 1999;31:307–19. doi: 10.1007/BF02738245. [DOI] [PubMed] [Google Scholar]
- 25.Hansen GH, Niels-Christiansen LL, Immerdal L, Hunziker W, Kenny AJ, Danielsen EM. Transcytosis of immunoglobulin A in the mouse enterocyte occurs through glycolipid raft- and rab17-containing compartments. Gastroenterology. 1999;116:610–22. doi: 10.1016/s0016-5085(99)70183-6. [DOI] [PubMed] [Google Scholar]
- 26.Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996;59:100–15. [PubMed] [Google Scholar]
- 28.Simionescu N, Siminoescu M, Palade GE. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stapleton A, Stamm WE. Prevention of urinary tract infection. Infect Dis Clin North Am. 1997;11:719–33. doi: 10.1016/s0891-5520(05)70382-2. [DOI] [PubMed] [Google Scholar]
- 30.Kil KS, Darouiche RO, Hull RA, Mansouri MD, Musher DM. Identification of a Klebsiella pneumoniae strain associated with nosocomial urinary tract infection. J Clin Microbiol. 1997;35:2370–4. doi: 10.1128/jcm.35.9.2370-2374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham S, Sharon N, Ofek I. Adhesion of Bacteria to Mucosal Surfaces. In: Ogra PLMJ, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal Immunology. San Diego: Academic Press; 1999. pp. 31–42. [Google Scholar]
- 32.Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–9. doi: 10.1038/39376. 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 33.Malaviya R, Gao Z, Thankavel K, Merwe PA, Abraham SN. The mast cell tumor necrosis factor alpha response to FimH-expressing Excherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Nat Acad Sci USA. 1999;96:8110–5. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–44. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 35.Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–51. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 36.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–50. doi: 10.1126/science.288.5471.1647. 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 37.Wooldridge KG, Williams PH, Ketley JM. Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog. 1996;21:299–305. doi: 10.1006/mpat.1996.0063. 10.1006/mpat.1996.0063. [DOI] [PubMed] [Google Scholar]
- 38.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–51. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–34. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stang E, Kartenbeck J, Parton RG. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norkin LC. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol Rev. 1999;168:13–22. doi: 10.1111/j.1600-065x.1999.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 42.Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–66. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 43.Lencer WI, Hirst TR, Holmes RK. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–90. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 44.Lencer WI, Moe S, Rufo PA, Madara JL. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc Natl Acad Sci USA. 1995;92:10. doi: 10.1073/pnas.92.22.10094. 94. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lencer WI, Constable C, Moe S, et al. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–62. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp GW, Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971;229:266–9. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- 47.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–9. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 48.Mordue DG, Hakansson S, Niesman I, Sibley LD. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 49.Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med. 1999;190:1783–92. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–72. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lauer S, VanWye J, Harrison T, McManus H, Samuel BU, Hiller NL, Mohandas N, Haldar K. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. Embo J. 2000;19:3556–64. doi: 10.1093/emboj/19.14.3556. 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes [published erratum appears in Science 1996; 274:1069] Science. 1996;274:239–42. doi: 10.1126/science.274.5285.239. 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 55.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanous toxins. Nature. 1982;296:651–3. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 56.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–8. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]