Introduction
The impact of the immune system on tumour growth in humans is poorly understood. Several studies have, however, identified T cells, in particular CD8+ cytotoxic T lymphocytes (CTLs), that recognize antigenic peptides expressed on autologous tumour cells.1,2 These tumour antigens include tumour-specific antigens that arise through mutations in normal genes; shared tumour antigens such as cancer–testis antigens; differentiation antigens; and other self-antigens that may be over-expressed in malignant cells. In some cases, where malignancies arise as a result of virus infection, tumour cells may also express viral antigens. Although antigens of each category have been found to act as targets for T-cell responses in human patients, the ability of these T cells to promote tumour rejection remains unproven.3
Experimental evidence obtained using murine models indicates that either CD4+ or CD8+ T-cell responses to certain tumour-associated self-antigens can indeed result in tumour rejection.4–7 Melanocyte differentiation antigens are the best-characterized self-antigens that act as targets for tumour (melanoma) rejection.8 Vigorous immune responses to melanocyte antigens have been shown to result, not only in tumour regression, but also in the development of autoimmune vitiligo. Vitiligo is a disease characterized by skin depigmentation following the destruction of non-malignant melanocytes, often by T cells.9 Overall, these findings indicate that the same effector T cells can be involved in both autoimmunity and tumour immunity. Thus, mechanisms of peripheral tolerance, which inhibit the activation of autoreactive T cells, also impinge upon the induction of T-cell responses to tumour-associated self-antigens.
Several mechanisms account for the lack of reactivity of T cells specific for self-antigens. Many self-reactive T cells are deleted by mechanisms of negative selection in the thymus.10,11 Subsequently, some T cells that escape this process die following encounter with antigen in the periphery, whilst others survive but become functionally inactivated (anergic).12 Other self-reactive T cells, residing in lymphoid tissues, may be oblivious to self-antigens expressed in peripheral sites13 particularly in the absence of any pro-inflammatory stimuli.14 When, however, these cells do meet self-antigens through, for example, infection or tissue injury, other pathways may also be called upon to limit the activation of such cells. An increasing body of evidence indicates that the immunomodulatory potential of a population of CD4+ CD25+ T cells, named CD25+ regulatory cells, represents one such pathway.15,16
CD25+ Regulatory T Cells and Tumour Immunity
CD25+ regulatory T cells comprise 5–10% of peripheral CD4+ T cells in naïve mice, and have been shown in several in vivo murine models to prevent the induction of autoimmune disease17–19 and inflammatory disease.20 Since, as described above, tumour immunity is in part an autoimmune process, it was postulated that CD25+ regulatory cells would also inhibit the generation of immune responses to tumours.21,22 Depletion of these cells, using CD25-specific monoclonal antibodies, has indeed been shown, in a variety of different mouse strains, to promote rejection of several transplantable, murine tumour cell lines including melanoma, fibrosarcoma, leukaemia and myeloma.21–24 These studies imply that CD25+ regulatory cells normally inhibit the generation of effective anti-tumour immune responses in vivo (Fig. 1). Treatment of mice with CD25-specific antibodies may not however, represent the optimal means of inducing tumour immunity through regulatory cell depletion. The CD25 marker is not unique for regulatory T cells since following activation, this molecule, the interleukin-2 (IL-2) receptor α-chain, is also transiently expressed on conventional effector T cells. Antibodies specific for CD25 therefore deplete, not only regulatory cells, but also some effector cells that may be involved in tumour rejection. Although, in the murine models described above the beneficial effects of regulatory cell depletion outweighed the disadvantages of depleting some effector T cells, more specific targeting of the regulatory T-cell population should further improve the efficiency of tumour immunity (see below).
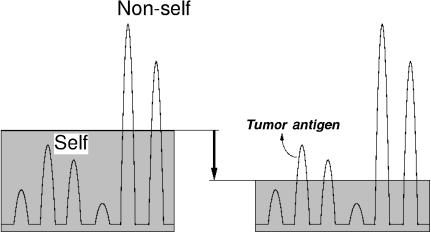
Figure 1.
Control of immune responses to self-antigens, tumour antigens, and non-self antigens depicted as a continuum. Ordinate denotes immune responsiveness. The horizontal line indicates the level of T-cell-mediated immunoregulation. Each peak denotes individual T-cell clones; the peaks above the line represent overt immune responses to the antigens. When the level goes down (e.g. the CD25+ regulatory T-cell-mediated immunoregulation is reduced by removing the regulatory T cells), immune responses to certain tumour antigens or self-antigens become apparent, presumably because the activation thresholds of effector T cells become lowered.
Several studies indicate that CD25+ regulatory cells inhibit the activation of both CD4+ and CD8+ T cells in vitro and that rejection of primary tumours in mice depleted of CD25+ regulatory cells is T-cell-dependent. In some cases (melanoma and a fibrosarcoma), both CD4+ and CD8+ T cells were found to be required for tumour rejection whilst only CD8+ T cells were found to be required for rejection of a murine myeloma.21,23 It is possible that the role of CD4+ T cells is simply to behave as helper cells for the activation of CD8+ CTLs, particularly in the case where the tumour cells are poorly immunogenic. In tumour-inoculated mice depleted of CD25+ regulatory T cells, CD8+ T cells and CD4– CD8– cells exhibit tumour-specific and non-specific killing activity, respectively.22 The majority of such tumour non-specific CD4– CD8– effector cells are natural killer (NK) cells that kill a broad spectrum of tumour cell lines in vitro.22 Their cytotoxic action may be critical for tumour rejection since, at least in the case of melanoma, mice depleted of both CD25+ regulatory cells and NK1.1+ cells are unable to reject the tumours (A. Gallimore, unpublished data). NK cells can also be produced in vitro from normal spleen cells by simply culturing them after depleting CD25+ regulatory T cells.22 Depletion of these cells leads to spontaneous activation and proliferation of self-reactive CD4+ T cells recognizing self-peptides/major histocompatibility complex (MHC) on antigen-presenting cells. The large amount of IL-2 thus produced by CD4+ T cells as the result of this autologous mixed lymphocyte reaction leads to the development of NK cells as lymphokine-activated killer (LAK) cells from the CD4– CD8– population in the spleen. It is also possible that CD25+ regulatory cells directly inhibit NK-cell activation. NK cells, and perhaps other cells of the innate immune system, activated as a result of CD25+ regulatory cell depletion could further augment tumour-specific CD4+ and CD8+ T-cell responses through tumour destruction and by promoting inflammatory conditions at the site of tumour challenge.
Several studies indicate that rejection of primary tumours inoculated into mice depleted of CD25+ regulatory cells is accompanied by the ability to reject second tumours inoculated up to at least 3 months later.21,23,24 As described above, in the case of melanoma, CD4+, CD8+ T cells and NK1.1+ cells are required for rejection of primary tumours, although CD4+ T cells alone mediate rejection of the same tumour when inoculated 2–3 months later.23 These results indicate that at least in this particular experimental system, different types of effector cells are involved in rejection of primary and secondary tumours.
The studies described above indicate that removal of CD25+ regulatory cells can provoke tumour-specific immune responses. Two separate studies have shown that immune responses to melanocyte differentiation antigens are more easily detected in CD25+ regulatory cell-depleted, melanoma-immune mice than in normal, tumour-bearing mice.23,24 A proportion of the former but not the latter group of mice, also develop signs of autoimmune vitiligo. It is not yet clear, however, whether or not specific responses to melanocyte self-antigens are responsible for tumour rejection. As discussed below, the suppression exerted by CD25+ regulatory cells is thought to be largely antigen non-specific, suggesting that depletion of the regulatory T cells could uncover responses to a spectrum of antigens expressed by the tumour cells. In addition, the depletion could, as described above, promote pro-inflammatory conditions at the site of tumour challenge, facilitating induction of immune responses to many types of tumour antigens.
The antigen-specificity of the regulatory T cells engaged in suppressing tumour immunity remains to be determined. Recent studies indicate that CD25+ regulatory cells, at least some of them, develop in the thymus following high-avidity interactions with self-peptide/MHC ligands expressed on cortical epithelial cells.25,26 The cells also display various accessory molecules at high levels, being phenotypically similar to antigen-primed cells.27 The high avidity of CD25+ regulatory cells to the selecting ligands due to the high affinity of T-cell receptors or high-level expression of accessory molecules, or both, is thought to render the cells highly responsive to further stimulation by self-antigens and thereby facilitate their rapid mobilization and effector function (suppression) at the site of tissue damage.28 The physiological trigger(s) for activation of regulatory cells have not been identified in vivo although it is tempting, in the case of the tumour models described above, to speculate that the tumour cells may themselves be involved. Given that normal T-cell repertoire contains tumour-reactive T cells as well as self-reactive T cells, it should not be surprising that regulatory T cells also recognize a broad range of antigens including tumour-specific as well as tumour-associated antigens. The trigger for CD25+ regulatory cell activity may not reside exclusively (if at all) in specificity for tumour antigens but rather in the context in which any antigen is presented to the immune system. The tumour environment, which is thought to be immunosuppressive due to high local concentrations of anti-inflammatory cytokines such as transforming growth factor-β, may lend itself to the activation of CD25+ regulatory T cells, perhaps through preventing activation of dendritic cells (DCs) and therefore favouring activation of the regulatory cells over conventional effector cells. Indeed the results of several in vitro studies imply that immature but not mature DCs promote regulatory T-cell activation29,30 (Fig. 2). It remains to be determined, however, whether such regulatory T cells are activated from the naturally present CD25+ regulatory T cells or have differentiated from naive T cells on immature DCs.
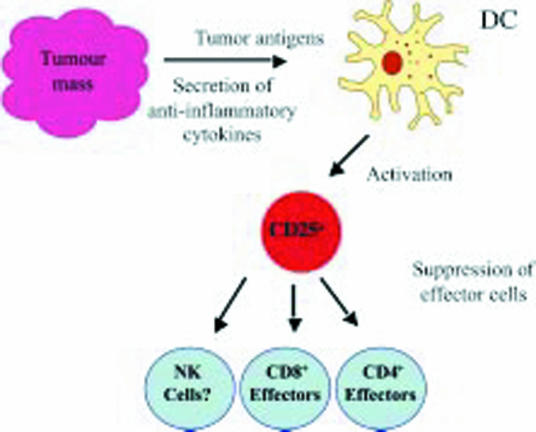
Figure 2.
Model for the control of tumour immunity by CD25+ regulatory T cells. Tumour cells create an immunosuppressive environment through the production of anti-inflammatory mediators. As a result of this, antigen-presenting cells, such as dendritic cells (DCs), fail to mature and either promote stimulation of naturally occurring CD25+ regulatory cells specific for tumour-derived antigens or induce CD25+ regulatory cells from naive T cells. In either case, these CD25+ regulatory cells inhibit effector cells such as CD4+ and CD8+ T cells (and possibly NK cells) in an antigen-non-specific manner. In this way, effective tumour immunity fails to develop. Removal of the regulatory T cells therefore promotes development of tumour immunity.
As mentioned above, in vitro data indicate that once activated, the suppression CD25+ regulatory T cells exert is antigen-non-specific,31,32 and these cells could therefore inhibit immune responses to all tumour antigens regardless of whether these antigens are self-antigens or not. The precise mechanism(s) of action of CD25+ regulatory cells are unclear although experimental evidence exists to suggest that their inhibitory effects are mediated by the production of immunosuppressive cytokines such as transforming growth factor-β and IL-1033,34 whilst other studies argue that the inhibitory effects of regulatory T cells are cell-contact dependent.31,32,35 Neither mechanism has so far been investigated in the context of inhibition of tumour immunity.
T-cell accessory molecules play distinctive roles in activating CD25+ regulatory T cells or modifying their functions. For example, these cells constitutively express cytotoxic T-lymphocyte antigen-4 (CTLA-4; CD152). CTLA-4 is required for activation of the regulatory T cells, as blockade of CTLA-4 expressed on them abrogates suppression in vivo and in vitro.20,36 CTLA-4 is also known to transduce negative signals in activated T cells, thereby leading to attenuation of T-cell responses. It is therefore likely that in vivo blockade of CTLA-4 may enhance tumour immunity by augmenting the activity of effector T cells as well. Glucocorticoid-induced tumour necrosis factor (TNF) receptor superfamily related gene (GITR), a member of the TNF receptor superfamily, is also predominantly expressed on CD25+ regulatory T cells.37 In contrast to CTLA-4, ligation but not blockade of GITR molecules abrogates CD25+ T-cell-mediated suppression. GITR ligation also has co-stimulatory activity, enhancing effector activities of T cells. Thus, in contrast to anti-CD25 antibody treatment, which may eliminate not only regulatory T cells but also tumour effector T cells (see above), an advantage of anti-CTLA-4 antibody or anti-GITR antibody treatment is that it can attenuate T-cell regulation and enhance T-cell effector activity, synergistically promoting tumour immunity.
Concluding Remarks
CD4+ CD25+ T cells with phenotypic and functional properties similar to those described for murine regulatory cells have been identified in humans.38–42 It will be very interesting therefore to discover whether or not these cells also prevent the generation of effective anti-tumour immune responses in cancer patients. The clinical benefits of treating patients with antibodies specific for CD25 must however, be approached with caution. Depletion of CD25+ cells could, for example, result in depletion of effector T cells important for controlling chronic infections with pathogens. Of greater concern is the possibility that depletion of regulatory cells could trigger the development of autoimmunity. So far, in the murine models described above, treatment with CD25-specific monoclonal antibodies, did not, in the majority of cases, result in the development of autoimmune disease. Development of autoimmunity following removal of regulatory cells may however, depend on several factors such as the individuals' genetic background, and/or infection with certain pathogens. Thus, as outlined above, many important questions regarding CD25+ regulatory cells remain to be answered before the immunotherapeutic potential of manipulating these cells in cancer treatment can be fully exploited.
References
- 1.Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol. 1997;9:681–3. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 2.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–93. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–70. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 4.Xiang R, Lode HN, Chao TH, et al. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492–7. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overwijk WW, Lee DS, Surman DR, et al. Vaccination with a recombinant vaccinia virus encoding a ‘self’ antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of ‘self’-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Apolloni E, Ronca R, Zamboni P, Overwijk WW, Surman DR, Restifo NP, Zanovello P. Genetic vaccination with ‘self’ tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 2000;60:253–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton AN, Gold JS, Blachere NE. Immunity against cancer: lessons learned from melanoma. Curr Opin Immunol. 2001;13:134–40. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 9.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192:1637–44. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 11.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990;248:1342–8. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 12.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–8. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 13.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 17.Furtado GC, Olivares-Villagomez D, De Curotto Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001;182:122–34. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- 18.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 20.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 22.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–18. [PubMed] [Google Scholar]
- 23.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immunity. 2002;2:1. [serial online] http://www.cancerimmunity.org/v2p1/020201.htm. [PubMed] [Google Scholar]
- 24.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 27.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 28.Sakaguchi S. Policing the regulators. Nat Immunol. 2001;2:283. doi: 10.1038/86283. [DOI] [PubMed] [Google Scholar]
- 29.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 32.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 38.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 40.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]