Abstract
As current research illuminates the dynamic interplay between the innate and acquired immune responses, the interaction and communication between these two arms has yet to be fully investigated. Polymorphonuclear neutrophils (PMNs) and interferon-γ (IFN-γ) are known critical components of innate and acquired immunity, respectively. However, recent studies have demonstrated that these two components are not entirely isolated. Treatment of PMNs with IFN-γ elicits a variety of responses depending on stimuli and environmental conditions. These responses include increased oxidative burst, differential gene expression, and induction of antigen presentation. Many of these functions have been overlooked in PMNs, which have long been classified as terminal phagocytic cells incapable of protein synthesis. As this review reports, the old definition of the PMN is in need of an update, as these cells have demonstrated their ability to mediate the transition between the innate and acquired immune responses.
Keywords: activation, cytokines, interleukins, neutrophils, phagocytosis
Introduction
Although the interferons were first identified and named for their potent ability to interfere with and inhibit viral infections, it soon became apparent that they could be divided into two categories. Type I interferons, consisting of interferon α and β, have antiviral activity as a primary function. Type II interferon, consisting solely of interferon-γ (IFN-γ), has a multitude of immunoregulatory functions in addition to its antiviral effect.1 Since its discovery, IFN-γ has been shown to be one of the most potent and pleiotrophic cytokines.
Investigations into the functions of IFN-γ have classically focused on the interactions of macrophages and CD4+ T cells. The interaction of a T-cell receptor with an antigen bound to a major histocompatibility complex (MHC) molecule triggers production of IFN-γ by T cells. This IFN-γ then acts to activate macrophages, up-regulating a number of gene products and rendering macrophages additionally cytotoxic by increasing oxidative burst and the production of other oxidants such as nitric oxide. Recently, IFN-γ was shown to be produced by a number of other immune cell types, including natural killer cells (NK) and macrophages; and to regulate the functions of many of these cell types.
The majority of IFN-γ research has focused on IFN-γ's interactions with T cells, NK cells, and activated macrophages; all components of the secondary, acquired response. Research into the primary response, consisting primarily of polymorphonuclear neutrophils and other components of innate immunity, has overlooked the significance IFN-γ. This oversight is unfortunate, as IFN-γ has been shown to be a potent and critical modulator of the innate immune response.
This review will focus on the interactions of IFN-γ and the PMN. This type of interaction between innate and acquired immunity has been overlooked, in part caused by ‘the obsolete concept of the neutrophil as a “terminally differentiated, short-lived, cell devoid of transcriptional activities” found in most biomedical textbooks’.2 As will be demonstrated, PMNs are active, dynamic cells that respond to immunomodulators such as IFN-γ through complex changes in gene expression. This review will explore what is known of gene expression and regulation in response to IFN-γ at a molecular level as well as IFN-γ's effects on the traditional PMN functions of oxidative burst, phagocytosis, and chemotaxis. This discussion should lead to an improved understanding of the roles of both the PMN and IFN-γ in the innate immune response.
Interferon-γ stimulated signal transduction
The primary way in which IFN-γ acts as an immunomodulator is through regulation of gene expression. The signal transduction pathways leading from binding of IFN-γ and its receptor to subsequent activation of gene transcription has been well studied in cell types other than PMNs. These studies have established that the primary method of IFN-γ signal transduction is via a Jak-Stat tyrosine kinase dependent pathway. In this pathway, IFN-γ binding to the receptor triggers the phosphorylation and activation of Jak1, a tyrosine kinase, which then activates signal transducer and activator of transcription 1 (Stat1). Stat1 then forms functionally active homodimers that move into the nucleus and bind to specific DNA sequences.3 New research in non-PMN cell types has indicated that IFN-γ has the ability to activate other signalling pathways as well. These other pathways, such as those involving mitogen-activated protein kinases (Map kinases), and are now being investigated.3
Only recently have the signalling pathways of IFN-γ in PMNs been investigated. Studies have found that resting PMNs express approximately 1000 receptor molecules that can rapidly and stably bind the IFN-γ molecule. After binding, many of these receptors are internalized leading to a subsequent drop in receptor sites on the surface.4,5 Most studies into IFN-γ signalling pathways in PMNs have used the Fc receptor I (FcγRI) gene as a reporter system. The promoter region of the gene encoding FcγRI has been shown to contain an interferon-γ response region, to which IFN-γ-activated transcription factors bound. Using this reporter system, IFN-γ stimulated gene expression in PMNs was demonstrated to utilize the traditional Jak-Stat pathway through the activation of Stat1·6 However, this same pathway has also been shown in PMNs to activate Stat3, which is not usually observed in IFN-γ signalling in other cell types.7
McDonald et al. recently compared the signal transduction pathways of a number of pro-inflammatory cytokines during their activation of FcγRI gene expression in PMNs. This study revealed that these pro-inflammatory cytokines used different transduction pathways to activate expression of the same gene. IFN-γ was shown to use the traditional Jak-Stat1 pathway, while granulocyte–monocyte colony-stimulating factor (GM-CSF) activated Stat5 activity. Tumour necrosis factor-α (TNF-α) and lipopolysaccharide (LPS) were both shown to activate nuclear factor κB (NFκB).8 While IFN-γ utilized a pathway common to a number of cell types, the signalling pathway of GM-CSF through Stat5 had not been previously observed. Therefore, these data indicate that the combination of one cytokine with a specific cell type can result in a unique pattern of gene expression that is triggered by the signal transduction pathway used.
Signalling pathways that utilize Ca2+ flux have recently been shown to be activated in IFN-γ treated PMNs. Simple treatment of PMNs with IFN-γ for short periods elicited a modest Ca2+ signal, and triggered increased sequestration of Ca2+ into intracellular compartments.9,10 This initial response is thought to be involved in the priming of PMNs for a subsequent response. The increased Ca2+ sequestration was shown to prime cellular sensitivity to stimuli, allowing for a subsequent enhancement the next time that signalling pathway is used.10 This modest initial signal has also been shown to be enhanced when IFN-γ treatment is coupled with other stimuli, such as the binding of fibronectin to PMNs.11 Fibronectin, an extracellular matrix protein, would most likely bind to PMNs as they extravasate into the tissues, where full PMN activity would be warranted. These data indicate that IFN-γ may act via a number of signalling pathways to prepare PMNs for subsequent functions and to initiate them.
IFN-γ signalling in PMNs has been shown to use an unusual variety of signalling cascades. Single treatment of these cells with IFN-γ demonstrated that a G-protein dependent pathway was used to elicit Ca2+ flux12 as increased levels of inositol triphosphate were observed.13 However, tyrosine-kinase dependent mechanisms may also be involved, as treatment with genstein, a tyrosine kinase inhibitor, was shown to inhibit Ca2+ flux.12 Increased protein kinase C activity occurred during this Ca2+ flux14 and costimulation with IFN-γ and fibronectin resulted in the activation of sphingosine kinases.11 Sphingosine kinases have not been observed to be activated by IFN-γ in other cell types. Thus, further research into signalling in this cell type is needed to resolve these preliminary findings.
Interferon-γ regulation of gene expression
Historically, PMNs have not been considered capable of responding to stimuli via gene expression and protein synthesis. It was thought that PMNs reacted entirely via the secretion of preformed proteins contained within the cytoplasm and granules at the time of cell migration from the bone marrow into the blood stream. This persistent idea has been disproved repeatedly by research on PMNs. Studies into the PMN response to IFN-γ demonstrate a range of gene products whose expression is modulated by signals sensed in the environment. Table 1 lists the gene products that have been demonstrated to have their expression regulated when PMNs respond to IFN-γ. Most of the genes whose regulation was explored are functionally tied to the immune system. Many of these genes are cytokines or chemokines, indicating that PMNs may play a critical role in signalling and directing other components of the immune response. This list of genes is currently quite limited, as most of these studies occurred prior to global gene expression technology. Recently, gene expression in LPS-treated neutrophils was more fully explored via microarray analysis. These data indicate that in addition to a number of cytokine and oxidative burst-related genes, other genes related to cell growth, cytoskeletal rearrangement, and metabolism are also differentially regulated during the response to LPS.15 Hopefully, as more studies of this kind are performed, the idea of PMNs as cells with a limited functionality will be replaced with the more accurate view that these cells are crucial to signalling and directing a dynamic immune response.
Table 1.
Gene products induced, up- or down-regulated by IFN-γ in PMNs
| Gene product | Function | Reference |
|---|---|---|
| Gene products induced or up-regulated by PMNs | ||
| BLyS | B Lymphocyte stimulator | 34 |
| C3b | Complement receptor | 42,48 |
| CCL20 | Dendritic chemotactic factor | 119 |
| CCR1 | Chemokine receptor | 44 |
| CCR3 | Chemokine receptor | 44 |
| CCR6 | Chemokine receptor | 22 |
| CD11α | β2 Integrin/Adhesion | 43 |
| CD11β | β2 Integrin/Adhesion | 42 |
| CD14 | LPS binding | 46,47 |
| CD18 | β2 Integrin/Adhesion | 42,43 |
| CD69 | Activation marker | 49 |
| CD80 | Antigen presentation | 18,19,23 |
| CD83 | Dendritic cell marker | 19,22,23 |
| CD86 | Antigen presentation | 18,19,23 |
| gp91-phox | NADPH oxidase subunit | 70 |
| FcγRI | Antibody Fc receptor | 71 |
| FcγRIII | Antibody Fc Receptor | 120 |
| IL-1β | Pro-inflammatory cytokine | 27 |
| IL-1Ra | IL-1 receptor antagonist | 121 |
| IL-6 | Anti-inflammatory cytokine | 33 |
| IP-10 | IFN-γ inducible protein/chemokine | 31,32 |
| I-TAC | T cell chemoattractant | 31 |
| MIG | IFN-γ induced monokine | 31 |
| MHC II | Antigen presentation | 16,17,18,19,20,23 |
| PAF-acether | Platelet activating factor | 122 |
| TNF-α | Pro-inflammatory cytokine | 27 |
| Gene products down-regulated by IFN-γ in PMNs | ||
| CXCR4 | Chemokine receptor | 45 |
| GROα | Chemokine/human KC | 30 |
| IL-8 | Neutrophil chemotactic factor | 25,26,28 |
| MIP-1α | Macrophage inflammatory protein | 28 |
| MIP-1β | Macrophage inflammatory protein | 28 |
| P47-phox | NADPH oxidase subunit | 70,71 |
Mhc ii expression
One of the most unlikely gene products to be induced by IFN-γ in PMNs is MHC II. PMNs have traditionally been considered to be cells solely involved in innate immunity, with no function in antigen presentation or T-cell activation. In vitro experiments with PMNs may be casting doubt on this assumption, as a number of investigators have now reported that PMNs express MHC II on the cell surface when stimulated with IFN-γ or other pro-inflammatory stimuli, such as GM-CSF.16–18 CD80 and CD86, costimulatory molecules required for T-cell activation, have also been shown to be up-regulated under the same conditions as induce MHC II expression.18,19 These expressed MHC II molecules have even been shown to be at least partly functional, as the PMNs have been demonstrated to act as required accessory cells during T-cell activation with staphylococcal enterotoxin, a superantigen that does not require intracellular processing prior to presentation.17 MHC II-expressing PMNs have also been shown to produce interleukin (IL)-8 when stimulated with this same superantigen.20 The ability to fully process and present more fully processed antigens, such as tetanus toxoid, remains controversial. Fanger et al. in a side-by-side comparison of superantigen and tetanus toxoid, found the MHC II-expressing PMNs were only able to effectively present superantigen, and not tetanus toxoid.17 However, Radsak et al. in a later study, were able to induce a low but statistically significant level of activated T cells in response to MHC II-expressing PMNs and tetanus toxoid.18 One explanation for these conflicting results could be a result of genetic polymorphisms in the human population. Reinisch et al. in a study of 55 human subjects, found that only 51% had PMNs that would express MHCII when stimulated.21 This donor-dependence is one possible explanation for the discrepancy in results seen with regard to antigen presentation studies. Regardless, these studies indicate that the idea of PMNs being terminally differentiated cells may need to be reinvestigated as these data indicate an ability to induce previously unknown cell functions.
Further studies of surface marker expression present additional evidence for the ability of PMNs to differentiate after leaving the bone marrow. CD83, a traditional dendritic cell marker, was shown to be expressed on the surface of PMNs stimulated with IFN-γ.22 Further studies of these cells stimulated in vitro have shown that they had altered morphology, lost traditional PMN chemotactic responses, and presented antigen via MHC II; yet maintained phagocytic and oxidative burst capabilities.23 A survey of patients with acute bacterial infections found that over half of the patients tested had circulating PMNs expressing CD83.19 These data indicate that this phenomenon was not simply the result of unlikely in vitro cytokine cocktails, but demonstrated a new functionality for PMNs that should be further explored.
Cytokine and chemokine expression
Neutrophils are usually the first cell type of the immune system to arrive at a site of infection. As such, these cells are critical components of both inflammatory and antimicrobial processes. Both of these processes are tightly regulated through the production of specific cytokines. As the non-phagocytic functions of PMNs have been more fully explored, the crucial ability of PMNs to secrete cytokines and chemokines has been demonstrated clearly. The array of cytokines and chemokines that PMNs are capable of producing has been reviewed by Cassatella.24
The subsets of cytokines and chemokines whose expression in PMNs is modulated by IFN-γ is shown in Table 1. It is interesting to note that IFN-γ treatment of PMNs has been shown to down-regulate IL-8.25,26 IL-8 is a potent chemoattractant of PMNs, indicating that IFN-γ may act as a signal to halt PMN recruitment and infiltration. However, this down-regulation of IL-8 was correlated with an up-regulation of the pro-inflammatory cytokines TNF-α and IL-1β. In vitro experiments have shown that PMNs incubated with IFN-γ demonstrate a transient down-regulation of IL-8 for the first few hours of incubation. Extended incubation of PMNs with IFN-γ leads to production of TNF-α and IL-1β. This new cytokine milieu then acts in an autocrine manner to override the signal from IFN-γ and reactivate IL-8 synthesis.27 A similar pattern of changes in the cytokine network resulting in reactivated gene expression by PMNs was observed with macrophage inflammatory protein (MIP)-1α and MIP-1β expression.28 Given these observations, it appears that PMN responses may change over time as the cytokine milieu changes, and that cytokine production by PMNs may act as much on themselves as on other cells and cell types.
Many of the other signalling molecules produced by IFN-γ-stimulated PMNs are chemokines. It should be noted that IFN-γ appears to down-regulate those chemokines that recruit neutrophils, and up-regulate chemokines that are chemoattractants for components of the acquired immune response, specifically T cells. PMN-derived MIP-1α, MIP-1β, and human growth-regulated oncogene (GRO-α)/murine keratinocyte-derived chemokine were shown to be down-regulated by IFN-γ treatment.28–30 These three chemokines all recruit phagocytic cells such as neutrophils and macrophages. Up-regulated chemokines include IFN-γ inducible protein 10 (IP-10), monokine induced by IFN-γ (MIG), and IFN-inducible T cell α chemoattractant (I-TAC),31,32 all specific for activated T cells. Additionally, IL-6, a molecule thought to be involved in the transition from an innate to an acquired response, and B lymphocyte stimulator (BLyS), a pro-B-cell factor, have also been shown to be up-regulated.33,34 These data all indicate that IFN-γ stimulates PMNs to signal other components of the immune system.
IFN-γ has typically been considered to be secreted by and to act on components of the acquire immune system, such as macrophages or T cells. As this review demonstrates, the actions of this potent cytokine are not limited to these cell types but can have dramatic effects on PMNs. Recent research indicated that PMNs may be an important source of this cytokine. In vitro experiments demonstrated that human PMNs stimulated with a combination of LPS, IL-12, and TNF-α secrete low levels of IFN-γ.35 Peritoneal murine PMNs have also been shown to express IFN-γ mRNA in vitro after stimulation with LPS.36In vivo experiments have revealed that PMNs secrete IFN-γ in response to a variety of infectious agents including Nocardia asteroides,37Salmonella typhimurium,38Leishmania major39,40 and Plasmodium berhei.41 During pulmonary infection with Nocardia asteroides, PMNs were found to be the sole source of IFN-γ during the course of infection37 and this response depended on both the viability and growth stage of the organism (unpublished observations). PMN-derived IFN-γ was also found to be required for macrophage control of leishmanial growth and for stimulation of CD4+ T cell migration and cytokine production. In this system, IFN-γ production involved PMN binding of macrophage surface CD28, a molecule usually thought to be involved in T-cell stimulation.40 Further investigations into the role of PMNs as modulators of the immune response are needed to elucidate the frequency of a PMN IFN-γ response and the importance of PMNs as sources of this powerful cytokine.
Other surface markers
In addition to the surface markers already discussed, IFN-γ regulates the expression of a number of other receptors and integrins on the PMN cell surface. Many of those up-regulated are related to PMN adherence and extravasion, such as the integrins CD11α, CD11β and CD18.42,43 Chemokine receptors CCR1, CCR3 and CCR6 are also up-regulated22,44 while CXCR4 is down-regulated45 indicating that IFN-γ may be coupled with other signalling molecules to co-ordinate specific recruitment of cells to the site of infection. Other markers whose expression is enhanced indicate that IFN-γ usually acts as an activating agent for PMNs. These molecules include CD14, the primary binding site for bacterial LPS46,47 and the complement component C3b.48 Recently, IFN-γ treatment was shown to induce CD69 expression on PMNs.49 CD69 is known as an early activation marker for B and T cells. These data suggest that CD69 can be used as a more general marker of activation. Expression of CD69 was observed to correlate with PMN production of IFN-γ (unpublished observations). Interestingly, IFN-γ acts to down-regulate the expression of the IFN-γ receptor on the surface of PMNs.4,5 Given the demonstrated time-dependent nature of the response of PMNs to IFN-γ, this may be a regulatory mechanism used to prevent PMNs from causing damage during the resolution of infection and inflammation.
Priming of neutrophil functions by ifn-γ
While recent investigations demonstrated signal transduction and gene expression in IFN-γ-treated PMNs, most studies have focused on PMN function at a cellular level. Specifically, these studies targeted IFN-γ's action as a priming agent. The term ‘priming’ refers to a stimulus that prepares PMNs for enhanced activity upon secondary stimulation. A variety of traditional PMN functions may be primed, including increased oxidative metabolism, surface receptor expression, degranulation and other functions associated with traditional PMN activities.50 The priming effect of IFN-γ on PMNs include, but is not limited to, these traditional PMN functions. A number of other cytokines have also been shown to act as priming agents for PMNs, including TNF-α and IL-8.51 However, these responses were demonstrated to vary with both the cytokine and the second stimulus involved. A full investigation into the combinatorial effects of priming agent and stimulus could uncover the methods of this finely tuned control of the PMN response.
Oxidative burst
Traditionally, the oxidative burst, or production of reactive oxygen species, has been considered to be a PMN's primary and most important function. With the aim of destroying the foreign invaders, PMNs infiltrate a site of inflammation or infection where they are stimulated to release reactive oxygen species. Hydrogen peroxide (H2O2) and superoxide (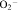 ) are the primary reactive oxygen species produced during oxidative burst. These species can react with other chemical components to create reactive halides, hydroxyl radicals (OH–), and singlet oxygen.52,53 The release of these reactive and toxic compounds results in damage to the targeted cell or object, and often results in irreparable damage and death to the PMN itself.
) are the primary reactive oxygen species produced during oxidative burst. These species can react with other chemical components to create reactive halides, hydroxyl radicals (OH–), and singlet oxygen.52,53 The release of these reactive and toxic compounds results in damage to the targeted cell or object, and often results in irreparable damage and death to the PMN itself.
A multitude of signals, both foreign and host cell derived, have been demonstrated to stimulate oxygen metabolism and oxidative burst. While not considered a traditional PMN activator, IFN-γ has been demonstrated to enhance, or prime, increased reactive oxygen species production in combination with a secondary stimulus.54,55 Berton et al. were the first to observe the priming effect of IFN-γ on PMN oxidative burst. When pretreated with IFN-γ, PMNs stimulated with either f-methionine-leucine-phenylalanine (fMLP), concanavalin A (Con A), or LPS demonstrated increased O2 consumption and 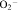 production.56 A number of later studies confirmed these observations, documenting increased H2O2 production,
production.56 A number of later studies confirmed these observations, documenting increased H2O2 production, 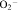 production, and increased reducing power in response to a variety of chemical stimuli including fMLP, Con A, LPS, and zymosan.42,57–62 Interestingly, IFN-γ did not enhance the PMN response to the stimulus phorbol 12-myristate acetate, which stimulates cells without the use of a surface membrane receptor.57,63,64 These results indicated that the IFN-γ priming effect may be specific to stimuli that act via membrane associated receptors. The IFN-γ priming response was demonstrated in a number of species including humans,56 cows,62 mice65 and rats.66
production, and increased reducing power in response to a variety of chemical stimuli including fMLP, Con A, LPS, and zymosan.42,57–62 Interestingly, IFN-γ did not enhance the PMN response to the stimulus phorbol 12-myristate acetate, which stimulates cells without the use of a surface membrane receptor.57,63,64 These results indicated that the IFN-γ priming effect may be specific to stimuli that act via membrane associated receptors. The IFN-γ priming response was demonstrated in a number of species including humans,56 cows,62 mice65 and rats.66
The IFN-γ priming effect was found to be dose and time dependent. Doses as low as 2 U/ml enhanced production while doses ranging from 50 to 1000 U/ml elicited an optimal response, dependent on the incubation period.58,64 While preincubation of PMNs with IFN-γ for a period as short as 10 min was shown to enhance oxidative burst60,67 incubation for an hour or longer was shown to have a maximal effect on oxidative burst.56,68 Incubation of IFN-γ-treated PMNs with protein synthesis inhibitors such as cyclohexamide (an inhibitor of translation), or actinomycin D (an inhibitor of RNA synthesis), inhibited oxidative burst. These results indicate that the enhancement of oxidative burst by PMNs is dependent on new protein synthesis.56,58,64
The primary enzyme involved in the production of reactive oxygen species is reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. NADPH oxidase is a multiunit enzyme complex that fully assembles upon cellular stimulation to catalyze the formation of superoxide anion. The main regulated subunit of NADPH oxidase is the membrane protein gp91-phox.55 Mutations in this gene were correlated with chronic granulomatous disease.55 IFN-γ treatment was demonstrated to up regulate the expression of the gp91-phox subunit in a protein synthesis dependent manner.69,70 However, the p47-phox subunit, which is constitutively expressed in resting PMNs, was shown to be down-regulated by IFN-γ exposure.71 While the regulation of these subunits remains a complex process that is not fully understood, the end result of IFN-γ treatment has been clearly demonstrated to be priming of the oxidative response.
Nitric oxide production
The production of nitric oxide (NO) during the oxidative burst of PMNs has been a subject of some debate. Inducible nitric oxide synthase (iNOS), the enzyme involved, had only recently been observed to be expressed by PMNs of any species. Inducible nitric oxide synthase expression and NO production were shown to be induced by IFN-γ. McCall et al. first observed that iNOS expression in rat PMNs was enhanced in a cyclohexamide-dependent manner by IFN-γ.72 This observation has since been extended to human PMNs, with the demonstration that iNOS expression is IFN-γ dose dependent73 and that the gene product colocalizes with myeloperoxidase in the primary granules.74 This colocalization suggests that iNOS is directly involved with the enhanced oxidative burst and cytotoxicity of IFN-γ treated PMNs, as myeloperoxidase catalyses the reaction of H2O2 into more toxic intermediates. This colocalization of these two enzymes may result in enhanced reaction of H2O2 with NO to form the highly reactive and toxic peroxynitrite (ONOO–) molecule.
Phagocytosis and cytocidal effects
Treatment of PMNs with IFN-γ was demonstrated to have significant effects on the functions of phagocytosis and cell killing. Short-term treatment of PMNs with IFN-γ (20–30 min) was shown by Shalaby et al. to increase the phagocytosis of latex beads75 and other studies demonstrated increased phagocytosis of Plasmodium falciparum merozoites induced by IFN-γ treatment.76 Studies with IFN-γ knockout mice have also demonstrated that PMNs from these mice exhibit a twofold reduction in phagocytosis. Because the primary function of PMNs is to damage and destroy foreign microbes, numerous studies have investigated how IFN-γ treatments modulate this ability. IFN-γ has been shown to be a potent stimulator of cytocidal activity, as shown in Table 2.
Table 2.
IFN-γ treatment enhances PMN killing of the following organisms
| Organism | Reference |
|---|---|
| Bacteria | |
| Brucella abortus | 90 |
| Enterococcus faecalis | 92 |
| Legionella pneumophila | 89 |
| Mycobacterium fortuitum | 91 |
| Mycobacterium tuberculosis | 93 |
| Staphylococcus aureus | 67 |
| Fungi | |
| Aspergillus fumigatus | 88 |
| Blastomyces dermatitidis | 81–83 |
| Candida albicans | 59,65,77,78,79,80 |
| Candida parasilosis | 80 |
| Candida tropicalis | 80 |
| Paracoccdioides brasiliensis | 85–87 |
| Pencilillium marneffi | 84 |
| Protozoa | |
| Entamoeba histolytica | 94 |
| Plasmodium falciparum | 95 |
| Other | |
| Gastric endothelial cells | 63 |
| Litomosoides sigmodontis (filaria worm) | 123 |
| Tumour cells | 73,96,124 |
The effect of IFN-γ on the PMN response to a variety of fungi has been thoroughly investigated. In general these studies found some form of enhanced anti-fungal activity from PMNs treated with IFN-γ. Studies of the PMN response to Candida revealed an IFN-γ dose dependent inhibition of growth, hyphal damage, or hyphal killing with both human peripheral blood and mouse peritoneal PMNs.65,77–80 Studies with Blastomyces dermatitidis have shown an enhanced oxidative burst within the first 6 hr post-treatment with IFN-γ that changed the effect of PMN attack from fungistatic to fungicidal.81–83 This shift from a fungistatic to a fungicidal effect with IFN-γ treatment was further demonstrated with Penicillium marneffei84 and Paracoccidioides brasiliensis.85–87 Increased oxidative burst was also observed in response to Aspergillus fumigatus. This IFN-γ triggered response was shown to be to be dependent on new transcription and protein synthesis.88
IFN-γ has also been demonstrated to enhance the bactericidal activity of PMNs towards a variety of bacterial species, including Brucella abortus, Legionellla pneumophila, Enterococcus faecalis and Mycobacterium fortuitum.89–92 However, not all species investigated used oxidative burst to enhance bactericidal activity. Responses to Brucella abortus and Enterococcus were demonstrated to be the result of increased superoxide anion or hydrogen peroxide secretion.90,92 However, the bactericidal response to Mycobacterium fortuitum was observed to result from nonoxidative mechanisms.91 This non-oxidative response to Mycobacterium fortuitum coincided with an increased time to killing (18 hr) as compared to those studies linked to oxidative burst. The effect of IFN-γ on PMN responses to Mycobacterium tuberculosis has also been investigated, with IFN-γ being observed to inhibit the bactericidal activity of PMNs.93 These data indicate that IFN-γ-primed responses to different bacterial species vary widely and may be the result of PMN recognition of species-specific surface molecules.
IFN-γ-primed PMN killing of non-fungal eukaryotic targets has also been observed. IFN-γ treatment was shown to enhance contact-dependent killing of Entamoeba histolytica, with production of hydrogen peroxide shown to be required.94 IFN-γ-treated PMNs have also been demonstrated to inhibit the growth of Plasmodium falciparum and to kill the parasite via a phagocytic mechanism.95 IFN-γ-primed PMNs have also been shown to effect the killing of tumour cells. Interestingly, the cytocidal effect of these PMNs was found to be bimodal, with an initial (5 min post incubation), trypsin-sensitive cytocidal mechanism, followed after 180 min of IFN-γ incubation by a second cytocidal mechanism that is trypsin-insensitive.96 This two-phase effect may be similar to or linked with to the bimodal regulation of IL-8 expression described earlier. The changing stimulatory environment and cytokine milieu may result in changes of PMN functions over time. These studies of cellular killing mechanisms of IFN-γ primed PMNs suggest that PMNs exhibit multiple mechanisms of killing elicited by differences in the target cell components.
Fc receptor expression and antibody-dependent cellular cytotoxicity (adcc)
ADCC was shown to be enhanced by IFN-γ treatment of PMNs. This effect was demonstrated in both human and bovine cells to require a 2–4 hr incubation period with IFN-γ.75,97,98 However, ADCC did not require RNA or protein synthesis. Using bovine PMNs, Steinbeck et al. demonstrated that IFN-γ enhanced ADCC of chicken erythrocytes even in the presence of RNA and protein synthesis inhibitors.99 Non-specific cell cytotoxicity, in which IgG activated PMNs kill non-opsonized bystander cells, was not affected by IFN-γ treatment, although IgG treatment stimulated increased production of superoxide and hydrogen peroxide.100
Enhanced ADCC in IFN-γ-primed PMNs was closely correlated with expression of FcγRI by the PMN.101 The high affinity receptor for monomeric IgG1, FcγRI (CD64), is not found on the surface of resting PMNs. FcγRII, the receptor for polymeric IgG1 and IgG2, and FcγRIII, both low-affinity receptors for various forms of IgG, are expressed constitutively at low levels on resting PMNs.102 Fc receptor I (FcγRI), but not FcγRII or FcγRIII, was shown to be induced in both human and bovine PMNs by IFN-γ treatment.101,103–105 This induction required an extended exposure time to IFN-γ, as treatments for less than 1 hr have no effect on FcγRI expression.106 Treatment with 100 U/ml IFN-γ for 4–5 hr was shown by Hoffmeyer et al. to induce an average 15 000 FcγRI molecules on the surface of each PMN107 with mRNA of FcγRI induced by IFN-γ 1 hr post treatment.108 FcγRI expression has been correlated with increased ADCC,102,104,109 increased microbicidal activity110 and increased oxidative burst via the activation of NADPH oxidase.107 At the molecular level, the binding of neutrophil FcγRI to IgG1 was shown to activate similar signal transduction pathways as seen on monocytic cells, including Ca2+ flux, and required tyrosine kinase activation.107 However, the expression of FcγRI on PMNs, unlike on monocytic cells was inhibited by the immunosuppresant dexamethazone111 and was not affected by inhibitors of Na+/H+ antiporters, as was observed in monocytic cells.108 These data, like those seen during investigations into Ca2+ flux signalling, indicate that PMNs may use novel pathways of signalling to control gene expression, and to elicit the appropriate response.
Chemotaxis and apoptosis
IFN-γ has never been demonstrated to have a chemotactic effect on PMNs. In the human, bovine and murine systems, IFN-γ was found not to have a chemotactic effect on PMNs, but rather inhibited both random and directed migration.112–114 This suppression of cellular migration was observed both in in vitro experiments and in vivo in the mouse peritoneal cavity.113 Additionally, IFN-γ suppressed the chemotactic migration of PMNs toward fMLP. This inhibitory effect was demonstrated to be independent of protein synthesis or tyrosine kinase activity.114In vitro, IFN-γ increased the rate of PMN adherence.115 These data indicate that IFN-γ may act as a signal of arrival to a site of inflammation allowing for the accumulation of PMNs.
PMNs, as terminally differentiated cells, are short-lived and readily undergo apoptosis, or programmed cell death. Stimulation of PMNs with a number of activating substances, including IFN-γ, has been shown to extend the life span and functional activity of these effector cells. Colotta et al. found that IFN-γ treatment reduced the number of PMNs with apoptotic morphology by 10-fold after 48 hr in culture, and that the in vitro life span of PMNs could be extended from 48 hr to beyond 96 hr via IFN-γ treatment.116 This extension of the lifespan of PMNs has also been observed in vivo in cells from bacterial sepsis patients.117 Suppression of apoptosis was also correlated with other functions induced by IFN-γ, such as FcγRI expression and enhanced oxidative burst.107,118 Similarly to what has been reported in other cell types, suppression of apoptosis in PMNs involved tyrosine-kinase dependent pathways.117
Conclusions
PMNs are the first cells to respond to an infection. As such it is logical to think of these cells as evaluators of the scene at hand, dynamically responding to the environmental conditions and directing the subsequent response of other immune cell types such as T cells, B cells, and macrophages. Research on the non-phagocytic roles of PMNs has been hampered by both the persistent belief that PMNs have a limited range of functionality and difficulties in culturing this cell type for in vitro experimentation. However, as we understand better the crosstalk and integrated nature of the innate and acquired immune responses, the ‘non-traditional’ role of PMNs to respond to stimuli via gene expression and secretion may prove to be of critical importance. Indeed, the data discussed in this review demonstrate that this is the case, as IFN-γ-induced gene expression has been seen to be critical for enhanced ADCC and effective cytotoxic mechanisms against a wide variety of microbial pathogens. The production of IFN-γ, and other cytokines, by PMNs clearly illustrates the ability of these cells to stimulate and direct an appropriate immune response. Even though IFN-γ and PMNs might seem an unlikely combination, this review shows it is an important and dynamic one.
Acknowledgments
Public Health Service grant R01-HL69426 from the National Heart, Lung and Blood Institute funded this work.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- BlyS
B lymphocyte stimulator
- Con A
concanavalin A
- FcγRI or CD64
Fc receptor I
- fMLP
f-methionine-leucine-phenylalanine
- GM-CSF
granulocyte monocyte colony-stimulating factor
- GRO-α
human growth-regulated oncogene
- H2O2
hydrogen peroxide
- I-TAC
interferon-inducible T-cell α chemoattractant
- IP-10
interferon-γ inducible protein 10
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- MIP
macrophage inflammatory protein
- MHC
major histocompatability complex
- Map kinases
mitogen activated protein kinases
- MIG
monokine induced by interferon-γ
- NK cells
natural killer cells
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- NFκB nuclear factor κB PMNs
polymorphonuclear neutrophils
- Stat1
signal transducer and activator of transcription 1
- TNF-α
tumour necrosis factor-α
References
- 1.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.Cassatella MA. The neutrophil: an emerging regulator of inflammatory and immune response. In: Cassatella MA, editor. Chemical Immunology and Allergy. Vol. 83. Basel: Karger; 2003. p. XI. [Google Scholar]
- 3.Ramana CV, Gil P, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-γ dependent signaling. Trends Immunol. 2002;23:96. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 4.Finbloom DS. The interferon-γ receptor on human monocytes, monocyte-like cell lines and polymorphonuclear leukocytes. Biochem Soc Trans. 1990;18:222. doi: 10.1042/bst0180222. [DOI] [PubMed] [Google Scholar]
- 5.Hansen BD, Finbloom DS. Characterization of the Interaction between recombinant human interferon-γ and its receptor on human polymorphonuclear leukocytes. J Leukocyte Biol. 1990;47:64. doi: 10.1002/jlb.47.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Bovolenta C, Gasperini S, McDonald PP, Cassatella MA. High affinity receptor for IgG (FcγRI/CD64) gene and STAT protein binding to the IFN-γ response region (GRR) are regulated differentially in human neutrophils and monocytes by IL-10. J Immunol. 1998;160:911. [PubMed] [Google Scholar]
- 7.Caldenhoven E, Buitenhuis M, Dijk T Bv, Raaijmakers JAM, Lammers J-WJ, Koenderman L, Groot RPD. Lineage-specific activation of STAT3 by interferon-γ in human neutrophils. J Leukocyte Biol. 1999;65:391. doi: 10.1002/jlb.65.3.391. [DOI] [PubMed] [Google Scholar]
- 8.McDonald PP, Bovolenta C, Cassatella MA. Activation of distinct transcription factos in neutrophils by bacterial LPS, interferon-γ, and GM-CSF and the necessity to overcome the action of endogenous proteases. Biochemistry. 1998;37:13165. doi: 10.1021/bi972539o. [DOI] [PubMed] [Google Scholar]
- 9.Rotnes JS, Aas V, Iversen JG. Interferon-gamma modulates cytosolic free calcium in human neutrophilic granulocytes. Eur J Haematol. 1994;53:65. doi: 10.1111/j.1600-0609.1994.tb01867.x. [DOI] [PubMed] [Google Scholar]
- 10.Aas V, Larsen K, Iversen JG. IFN-γ induces calcium transients and increases the capacitative calcium entry in human neutrophils. J Interferon Cytokine Res. 1998;18:197. doi: 10.1089/jir.1998.18.197. [DOI] [PubMed] [Google Scholar]
- 11.Aas V, Algeroy S, Sand KL, Iversen J-G. Fibronectin promotes calcium signalling by interferon-γ in human neutrophils via G-protein and sphingosine kinase-dependent mechanisms. Cell Comm Adhesion. 2001;8:125. doi: 10.3109/15419060109080712. [DOI] [PubMed] [Google Scholar]
- 12.Aas V, Larsen K, Iversen J-G. Interferon-γ elicits a G-protein-dependent Ca2+ signal in human neutrophils after depletion of intracellular Ca2+ stores. Cellular Signaling. 1999;11:101. doi: 10.1016/s0898-6568(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 13.Chavez MM, Novato-Silva E, Gomez MV, Limae-Silva FC, Nogueira-Machado JA. Effect of gamma interferon and interleukin 10 on phosphoinositol turnover by human neutrophils in vitro. Braz J Med Biol Research. 1996;29:1389. [PubMed] [Google Scholar]
- 14.Aas V, Torjesen P, Iversen J-G. Interferon-γ affects protein kinase C activity in human neutrophils. J Interferon Cytokine Res. 1995;15:777. doi: 10.1089/jir.1995.15.777. [DOI] [PubMed] [Google Scholar]
- 15.Malcolm KC, Arndt PG, Manos EJ, Jones DA, Worthen GS. Microarray analysis of lipopolysaccharide treated human neutrophils. Am J Physiol Lung Cell Mol Physiol. 2003;284:L663. doi: 10.1152/ajplung.00094.2002. [DOI] [PubMed] [Google Scholar]
- 16.Gosselin EJ, Wardwell K, Rigby WFC, Guyre PM. Induction of MHC II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ and IL-3. J Immunol. 1993;151:1482. [PubMed] [Google Scholar]
- 17.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatability complex class II expressing neutrophils. proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128. [PubMed] [Google Scholar]
- 18.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation. major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iking-Konert C, Wagner C, Denefleh B, Hug F, Schneider M, Andrassy K, Hansch GM. Up-regulation of the dendritic cell marker CD83 on polymorphonuclear neutrophils (PMN): divergent expression in acute bacterial infections and chronic inflammatory disease. Clin Exp Immunol. 2002;130:501. doi: 10.1046/j.1365-2249.2002.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei L, Altstaedt J, Ohe Mvd, Proft T, Gross U, Rink L. Induction of interleukin-8 in human neutrophils after MHC class II cross-linking with superantigens. J Leukocyte Biol. 2001;70:80. [PubMed] [Google Scholar]
- 21.Reinisch W, Lichtenberger C, Steger G, Tillinger W, Scheiner O, Gangl A, Maurer D, Willheim M. Donor dependent, interferon-γ induced HLA-DR expression on human neutrophils in vivo. Clin Exp Immunol. 2003;133:476. doi: 10.1046/j.1365-2249.2003.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashiro S, Wang J-M, Yang D, Gong W-H, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958. [PubMed] [Google Scholar]
- 23.Iking-Konert C, Cseko C, Wagner C, Stegmaier S, Andrassy K, Hansch GM. Transdifferentiation of polymorphonuclear neutrophils. acquisition of CD83 and other functional characteristics of dendritic cells. J Mol Med. 2001;79:464. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- 24.Cassatella MA. Neutrophil derived proteins: Selling cytokines by the pound. Adv Immunol. 1999;73:369. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 25.Cassatella MA, Guasparri I, Ceska M, Bazzoni F, Rossi F. Interferon-gamma inhibits interleukin-8 production by human polymorphonuclear leukocytes. Immunology. 1993;78:177. [PMC free article] [PubMed] [Google Scholar]
- 26.Cassatella MA, Aste M, Calzetti F, Constantin G, Guasparri I, Ceska M, Rossi F. Studies on the regulatory mechanisms of interleukin-8 gene expression in resting and IFN-γ treated neutrophils: Evidence on the capability of staurosporine of inducing the production of interleukin-8 by human neutrophils. Biochem Biophys Res Commun. 1993;190:660. doi: 10.1006/bbrc.1993.1099. [DOI] [PubMed] [Google Scholar]
- 27.Meda L, Gasperini S, Ceska M, Cassatella MA. Modulation of proinflammatory cytokine release from human polymorphonuclear leukocytes by gamma interferon. Cell Immunol. 1994;157:448. doi: 10.1006/cimm.1994.1241. [DOI] [PubMed] [Google Scholar]
- 28.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interferon gamma modulated the expression of neutrophil-derived chemokines. J Invest Med. 1995;43:58. [PubMed] [Google Scholar]
- 29.Cassatella MA. Interferon-γ inhibits the lipopolysaccharide-induced macrophage inflammatory protein 1α gene transcription in human neutrophils. Immunol Lett. 1996;49:79. doi: 10.1016/0165-2478(95)02484-0. [DOI] [PubMed] [Google Scholar]
- 30.Gasperini S, Calzetti F, Russo MD, De Gironcoli M, Cassatella MA. Regulation of GROα production in human granulocytes. J Inflamm. 1995;45:143. [PubMed] [Google Scholar]
- 31.Gasperini S, Marchi M, Calzetti F, et al. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell α chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928. [PubMed] [Google Scholar]
- 32.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-γ-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 33.Jablonska E, Kiluk M, Markiewicz W, Jablonski J. Priming effects of GM-CSF, IFN-γ and TNF-α on human neutrophil inflammatory cytokine production. Melanoma Res. 2002;12:123. doi: 10.1097/00008390-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-γ is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145. [PubMed] [Google Scholar]
- 36.Yu C-L, Sun K-H, Tsai C-Y, Tsai Y-Y, Tsai S-T, Huang D-F, Han S-H, Yu H-S. Expression of Th1/Th2 cytokine mRNA in peritoneal exudative polymorphonuclear neutrophils and their effects on mononuclear cell Th1/Th2 cytokine production in MRL-lpr/lpr mice. Immunology. 1998;95:480. doi: 10.1046/j.1365-2567.1998.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-γ in response to pulmonary infection with Nocardia asteroides. J Leukocyte Biol. 2002;72:373. [PubMed] [Google Scholar]
- 38.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 39.Venuprasad K, Banerjee PP, Chattopadhyay S, Sharma S, Pal S, Parab PB, Mitra D, Saha B. Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-γ secretion and restriction of Leishmania growth. J Immunol. 2002;169:920. doi: 10.4049/jimmunol.169.2.920. [DOI] [PubMed] [Google Scholar]
- 40.Venuprasad K, Chattopadhyay S, Saha B. CD28 signaling in neutrophil induces T-cell chemotactic factor (s) modulating T-cell response. Hum Immunol. 2003;64:38. doi: 10.1016/s0198-8859(02)00689-4. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Sendo F. Cytokine and chemokine mRNA expression in neutrophils from CBA/NSlc mice infected with Plasmodium berghei ANKA that induces experimental cerebral malaria. Parasitol Int. 2001;50:139. doi: 10.1016/s1383-5769(01)00063-0. [DOI] [PubMed] [Google Scholar]
- 42.Klebanoff SJ, Olszowski S, Voorhis WCv, Ledbetter JA, Waltersdorph AM, Schlechte KG. Effects of γ-interferon on human neutrophils: protection from deterioration on storage. Blood. 1992;80:225. [PubMed] [Google Scholar]
- 43.Leite F, O'Brien S, Sylte MJ, Page T, Atapattu D, Czuprynski CJ. Inflammatory cytokines enhance the interaction of Mannheima haemolytica leukotoxin with bovine peripheral blood neutrophils in vitro. Infect Immun. 2002;70:4336. doi: 10.1128/IAI.70.8.4336-4343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonecchi R, Polentarutti N, Luini W, et al. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC Chemokines by IFN-γ in human neutrophils. J Immunol. 1999;162:474. [PubMed] [Google Scholar]
- 45.Nagase H, Miyamasu M, Yamaguchi M, et al. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukocyte Biol. 2002;71:711. [PubMed] [Google Scholar]
- 46.Buckle AM, Jayaram Y, Hogg N. Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol. 1990;81:339. doi: 10.1111/j.1365-2249.1990.tb03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeshita S, Nakatani K, Takata Y, Kawase H, Sekine I, Yoshioka S. Interferon-gamma and tumor necrosis factor-alpha enhance lipopolysaccharide binding to neutrophils via CD14. Inflamm Res. 1998;47:101. doi: 10.1007/s000110050290. [DOI] [PubMed] [Google Scholar]
- 48.Livingston DH, Appel SH, Sonnenfeld G, Malagoni MA. The effect of tumor necrosis factor-α and interferon-γ on neutrophil function. J Surg Res. 1989;46:322. doi: 10.1016/0022-4804(89)90195-9. [DOI] [PubMed] [Google Scholar]
- 49.Atzeni F, Schena M, Ongari AM, Carrabba M, Bonara P, Minonzio F, Capsoni F. Induction of CD69 activation molecule on human neutrophils by GM-CSF, IFN-γ, and IFN-α. Cell Immunol. 2002;220:20. doi: 10.1016/s0008-8749(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 50.Walker BA, Ward PA. Priming and signal transduction on neutrophils. Biol Signals. 1992;1:237. doi: 10.1159/000109329. [DOI] [PubMed] [Google Scholar]
- 51.Galligan C, Yoshimura T. Phenotypic and functional changes of cytokine-activated neutrophils. Chem Immunol Allergy. 2003;83:24. doi: 10.1159/000071555. [DOI] [PubMed] [Google Scholar]
- 52.Beaman L, Beaman BL. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbol. 1984;38:27. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- 53.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 54.Watson DA, Musher DM, Hamill RJ. Interferon-gamma and polymorphonuclear leukocytes. Ann Int Med. 1988;109::250. doi: 10.7326/0003-4819-109-3-250_2. [DOI] [PubMed] [Google Scholar]
- 55.Berton G, Cassatella MA. Modulation of neutrophil functions by Interferon-γ. Immunol Series. 1992;57:437. [PubMed] [Google Scholar]
- 56.Berton G, Zeni L, Cassatella MA, Rossi F. Gamma Interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986;138:1276. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- 57.Kowanko IC, Ferrante A. Stimulation of neutrophil respiratory burst and lysosomal enzyme release by human interferon-gamma. Immunology. 1987;62:149. [PMC free article] [PubMed] [Google Scholar]
- 58.Cassatella MA, Capelli R, Bianca VD, Grzeskowiak M, Dusi S, Berton G. Interferon-gamma activates human neutrophil oxygen metabolism and exocytosis. Immunology. 1988;63:499. [PMC free article] [PubMed] [Google Scholar]
- 59.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Neutrophil oxidative burst in response to blastoconidia and pseudohyphae of Candida albicans: Augmentation by granulocyte colony-stimulating factor and interferon-γ. J Infect Dis. 1992;166:668. doi: 10.1093/infdis/166.3.668. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki K, Furui H, Kaneko M, Takagi K, Satake T. Priming effect of recombinant human interleukin-2 and recombinant human interferon-γ on human neutrophil superoxide production. Drug Res. 1990;40:1176. [PubMed] [Google Scholar]
- 61.Chavez MM, Silvestrini AA, Silva-Teixeira DN, Nogueira-Machado JA. Effect in vitro of gamma interferon and interleukin-10 on generation of oxidizing species by human granulocytes. Inflamm Res. 1996;45:313. doi: 10.1007/BF02252942. [DOI] [PubMed] [Google Scholar]
- 62.Sample AK, Czuprynski CJ. Recombinant bovine interferon-gamma, but not interferon-alpha, potentiates bovine neutrophil oxidative responses in vitro. Vet Immunol Immunopathol. 1990;25:23. doi: 10.1016/0165-2427(90)90107-4. [DOI] [PubMed] [Google Scholar]
- 63.Lieser MJ, Kozol RA, Tennenberg SD. Interferon-γ primes neutrophil mediated gastric surface cell cytotoxicity. Am J Physiol. 1995;268:G843. doi: 10.1152/ajpgi.1995.268.5.G843. [DOI] [PubMed] [Google Scholar]
- 64.Tennenberg SD, Fey DE, Lieser MJ. Oxidative priming of neutrophils by interferon-gamma. J Leukocyte Biol. 1993;53:301. doi: 10.1002/jlb.53.3.301. [DOI] [PubMed] [Google Scholar]
- 65.Tansho S, Abe S, Yamaguchi H. Inhibition of Candida albicans growth by murine peritoneal neutrophils and augmentation of the inhibitory activity by bacterial lipopolysaccharide and cytokines. Microbiol Immunol. 1994;38:379. doi: 10.1111/j.1348-0421.1994.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 66.Slater AD, Klein JB, Czarniecki CW, Sonnenfeld G. The effect of interferon-γ on rejection and neutrophil function following transplantation. J Interferon Res. 1993;13:359. doi: 10.1089/jir.1993.13.359. [DOI] [PubMed] [Google Scholar]
- 67.Edwards SW, Say JE, Hughes V. Gamma interferon enhances the killing of Staphylococcus aureus by human neutrophils. J Gen Microbiol. 1988;134:37. doi: 10.1099/00221287-134-1-37. [DOI] [PubMed] [Google Scholar]
- 68.Lappegaard KT, Benestad HB, Rollag H. Interferons affect oxygen metabolism in human neutrophil granulocytes. J Interferon Res. 1988;8:665. doi: 10.1089/jir.1988.8.665. [DOI] [PubMed] [Google Scholar]
- 69.Newburger PE, Ezekowitz RAB, Whitney C, Wright J, Orkin SH. Induction of phagocyte cytochrome b heavy chain gene expression by interferon γ. Proc Natl Acad Sci U S A. 1988;85:5215. doi: 10.1073/pnas.85.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassatella MA, Bazzoni F, Flynn RM, Dusi S, Trinichieri G, Rossi F. Molecular basis of Interferon-γ and Lipopolysaccharide enhancement of phagocyte respiratory burst capability. J Biol Chem. 1990;265:20241. [PubMed] [Google Scholar]
- 71.Cassatella MA, Bazzoni F, Calzetti F, Guasparri I, Rossi F, Trinchieri G. Interferon-γ transcriptionally modulates the expression of the genes for the high affinity IgG-Fc receptor and the 47-kDa cytosolic component of NADPH oxidase in human polymorphonuclear leukocytes. J Biol Chem. 1991;266:22079. [PubMed] [Google Scholar]
- 72.McCall TB, Palmer RMJ, Moncada S. Induction of nitric oxide synthase in rat peritoneal neutrophils and its inhibition by dexamethasone. European J Immunol. 1991;21:2523. doi: 10.1002/eji.1830211032. [DOI] [PubMed] [Google Scholar]
- 73.Yamashita T, Uchida T, Araki A, Sendo F. Nitric oxide is an effector molecule in inhibition of tumor cell frowth by rIFN-γ activated rat neutrophils. Int J Cancer. 1997;71:223. doi: 10.1002/(sici)1097-0215(19970410)71:2<223::aid-ijc17>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 74.Evans TJ, Buttery LDK, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci U S A. 1996;93:9553. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shalaby MR, Aggarwal BB, Rinderknecht E, Svedersky LP, Finkle BS, Palladino MA., Jr Activation of human polymorphonuclear neutrophil functions by Interferon-γ and tumor necrosis factor. J Immunol. 1985;135:2069. [PubMed] [Google Scholar]
- 76.Kumaratilake LM, Ferrante A, Jaeger T, Rzepczyk CM. Effects of cytokines, complement, and antibody on the neutrophil respiratory burst and phagocytic response to Plasmodium falciparum merozoites. Infection Immunity. 1992;60:3731. doi: 10.1128/iai.60.9.3731-3738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Djeu JY, Blanchard DK. Regulation of human Polymorphonuclear neutrophils (PMN) activity against Candida albicans by large granular lymphocytes via release of a PMN-activating factor. J Immunol. 1987;139:2761. [PubMed] [Google Scholar]
- 78.Djeu JY, Blanchard DK, Halkias D, Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by Interferon-γ and tumor necrosis factor. J Immunol. 1986;137:2980. [PubMed] [Google Scholar]
- 79.Diamond RA, Lyman CA, Wysong DR. Disparate effects if interferon-γ and tumor necrosis factor-α on early neutrophil respiratory burst and fungicidal responses to Candida albicans hyphae in vitro. J Clin Invest. 1991;87:711. doi: 10.1172/JCI115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Effects of granulocyte colony-stimulating factor and interferon-γ on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukocyte Biol. 1995;57:651. doi: 10.1002/jlb.57.4.651. [DOI] [PubMed] [Google Scholar]
- 81.Morrison CJ, Brummer E, Isenberg RA, Stevens DA. Activation of murine polymorphonuclear neutrophils for fungicidal activity by recobinant gamma interferon. J Leukocyte Biol. 1987;41:434. doi: 10.1002/jlb.41.5.434. [DOI] [PubMed] [Google Scholar]
- 82.Morrison CJ, Brummer E, Stevens DA. In vivo activation of peripheral blood polymorphonuclear neutrophils by gamma interferon results in enhanced fungal killing. Infection Immunity. 1989;57:2953. doi: 10.1128/iai.57.10.2953-2958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison CJ, Stevens DA. Enhanced killing of Blastomyces dermatiditis by gamma interferon-activated murine peripheral blood polymorphonuclear neutrophils. Int J Immunopharmacol. 1989;11:855. doi: 10.1016/0192-0561(89)90106-9. [DOI] [PubMed] [Google Scholar]
- 84.Kudeken N, Kawakami K, Saito A. Cytokine-induced fungicidal activity of human polymorphonuclear leukocytes against Penicillium marneffei. FEMS Immunol Med Microbiol. 1999;26:115. doi: 10.1111/j.1574-695X.1999.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 85.Kurita N, Biswas SK, Oarada M, Sano A, Nishimura K, Miyaji M. Fungistatic and fungicidal activities of murine polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol. 1999;37:19. [PubMed] [Google Scholar]
- 86.Kurita N, Oarada M, Ito E, Miyaji M. Antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol. 1999;37:261. [PubMed] [Google Scholar]
- 87.Kurita N, Oarada M, Miyaji M, Ito E. Effect of cytokines on antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol. 2000;38:177. doi: 10.1080/mmy.38.2.177.182. [DOI] [PubMed] [Google Scholar]
- 88.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanchard DK, Friedman H, Klein TW, Djeu JY. Induction of interferon-gamma and tumor necrosis factor by Legionella pneumophila: Augmentation of human neutrophil bactericidal activity. J Leukocyte Biol. 1989;45:538. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- 90.Canning PC, Roth JA. Effects of in vitro and in vivo administration of recombinant bovine Interferon-γ on bovine neutrophil responses to Brucella abortus. Vet Immunol Immunopathol. 1989;20:119. doi: 10.1016/0165-2427(89)90093-7. [DOI] [PubMed] [Google Scholar]
- 91.Geertsma MF, Nibbering PH, Pos O, Furth R. v. Interferon-γ-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than normal granulocytes. Eur J Immunol. 1990;20:869. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- 92.Onyeji CO, Nicolau DP, Nightingale CH, Bow L. Interferon-γ effects on activities of gentamicin and vancomycin against Enterococcus faecalis resistant to the drugs: an in vitro study with human neutrophils. Int J Antimicrob Agents. 1999;11:31. doi: 10.1016/s0924-8579(98)00085-5. [DOI] [PubMed] [Google Scholar]
- 93.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70:4591. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denis M, Chadee K. Human neutrophils activated by Interferon-γ and tumor necrosis factor-α kill Entamoeba histolytica trophozoites in vitro. J Leukocyte Biol. 1989;46:270. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- 95.Kumaratilake LM, Ferrante A, Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum: Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-γ: comparisons with tumor necrosis factor effects. J Immunol. 1991;146:762. [PubMed] [Google Scholar]
- 96.Miyake Y, Ajitsu S, Yamashita T, Sendo F. Enhancement by recombinant interferon-γ of spontaneous tumor cytostasis by human neutrophils. Mol Biotherapy. 1988;1:37. [PubMed] [Google Scholar]
- 97.Basham TY, Smith WK, Merigan TC. Interferon enhances antibody-dependent cellular cytotoxicity when suboptimal concentration of antibody are used. Cell Immunol. 1984;88:393. doi: 10.1016/0008-8749(84)90172-2. [DOI] [PubMed] [Google Scholar]
- 98.Steinbeck MJ, Roth JA, Kaeberle ML. Activation of bovine neutrophils by recombinant interferon-γ. Cell Immunol. 1986;98:137. doi: 10.1016/0008-8749(86)90274-1. [DOI] [PubMed] [Google Scholar]
- 99.Steinbeck MJ, Webb DSA, Roth JA. Role for arachidonic acid metabolism and protien synthesis in recombinant bovine interferon-γ induced activation of bovine neutrophils. J Leukocyte Biol. 1989;46:450. doi: 10.1002/jlb.46.5.450. [DOI] [PubMed] [Google Scholar]
- 100.Geffner JR, Minnucci F, Isturiz MA. Interferon-γ is unable to increase monocyte and neutrophil-mediated nonspecific cytotoxicity induced by immune complexes. Immunol Lett. 1992;33:21. doi: 10.1016/0165-2478(92)90088-6. [DOI] [PubMed] [Google Scholar]
- 101.Petroni KC, Shen L, Guyre PM. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-γ and glucocorticoids. J Immunol. 1988;140:3467. [PubMed] [Google Scholar]
- 102.Shen L, Guyre PM, Fanger MW. Polymorphonuclear leukocyte function triggered through the high affinity Fc receptor for monomeric IgG. J Immunol. 1987;139:534. [PubMed] [Google Scholar]
- 103.Perussia B, Dayton ET, Lazarus R, Fanning V, Trinchieri G. Immune Interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983;158:1092. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perussia B, Kobayashi M, Rossi ME, Anegon I, Trinchieri G. Immune interferon enhances functional properties of human granulocytes. role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulation factor. J Immunol. 1987;138:765. [PubMed] [Google Scholar]
- 105.Worku M, Paape MJ, Marquardt WW. Modulation of Fc receptors for IgG on bovine polymorphonuclear neutrophils by interferon-γ through de novo RNA transcription and protein synthesis. Am J Vet Res. 1994;55:234. [PubMed] [Google Scholar]
- 106.Capsoni F, Bonara P, Minonzio F, Ongari AM, Colombo G, Rizzardi GP, Zanussi C. The effect of cytokines on human neutrophil Fc receptor-mediated phagocytosis. J Clin Lab Immunol. 1991;34:115. [PubMed] [Google Scholar]
- 107.Hoffmeyer F, Witte K, Schmidt RE. The high affinity FcγRI on PMN. regulation of expression and signal transduction. Immunology. 1997;92:544. doi: 10.1046/j.1365-2567.1997.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cassatella MA, Flynn RM, Amezaga MA, Bazzoni F, Vicentini F, Trinchieri G. Interferon gamma induces in human neutrophils and macrophages expression of the mRNA for the high affinity receptor for monomeric IgG (FcγR-I or CD64) Biochem Biophys Res Comms. 1990;170:582. doi: 10.1016/0006-291x(90)92131-i. [DOI] [PubMed] [Google Scholar]
- 109.Matsumoto S, Takei M, Moriyama M, Imanishi H. Enhancement of IA-like antigen expression by interferon-gamma in polymorphonucear neutrophils. Chem Pharmacol Bull. 1987;35:436. doi: 10.1248/cpb.35.436. [DOI] [PubMed] [Google Scholar]
- 110.Brar DW, Borden EC, Proctor RA. Recombinant Interferon-γ preserves human granulocyte bactericidal and chemoluminescence activities. J Infect Dis. 1993;168:128. doi: 10.1093/infdis/168.1.128. [DOI] [PubMed] [Google Scholar]
- 111.Pan L, Mendel DB, Zurlo J, Guyre PM. Regulation of the steady state level of FcγRI mRNA by IFN-γ and dexamethasone in human monocytes, neutrophils, and U-937 cells. J Immunol. 1990;145:267. [PubMed] [Google Scholar]
- 112.Ohmann HB, Babiuk LA. Alteration of some leukocyte functions following in vivo and in vitro exposure to recombinant bovine alpha- and gamma- interferon. J Interferon Res. 1986;6:123. doi: 10.1089/jir.1986.6.123. [DOI] [PubMed] [Google Scholar]
- 113.Canono BP, Middleton MH, Campbell PA. Recombinant mouse interferon-γ is not chemotactic for macrophages or neutrophils. J Interferon Res. 1989;9:79. doi: 10.1089/jir.1989.9.79. [DOI] [PubMed] [Google Scholar]
- 114.Bignold LP, Ferrante A, Haynes DR. Studies of chemotactic, chemotactic movement-inhibiting and random movement-inhibiting effects if interleukin-1 alpha and beta, tumor necrosis factors alpha and beta and interferon gamma on human neutrophils in assays using ‘Sparse-Pore’ polycarbonate (nuclepore) membranes in Boyden Chamber. Int Arch Allergy Appl Immunol. 1990;91:1. doi: 10.1159/000235081. [DOI] [PubMed] [Google Scholar]
- 115.Seow WK, Thong YH. Augmentation of human polymorphonuclear leukocyte adherence by Interferon. Int Arch Allergy Appl Immunol. 1986;79:305. doi: 10.1159/000233991. [DOI] [PubMed] [Google Scholar]
- 116.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012. [PubMed] [Google Scholar]
- 117.Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trenz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356. [PubMed] [Google Scholar]
- 118.Moulding DA, Walter C, Hart CA, Edwards SW. Effects of Staphylococcal enterotoxins on human neutrophil functions and apoptosis. Infect Immun. 1999;67:2312. doi: 10.1128/iai.67.5.2312-2318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scapini P, Crepaldi L, Pinardi C, Calzetti F, Cassatella MA. CCL20/macrophage inflammatory protein-3α production in LPS-stimulated neutrophils is enhanced by the chemoattractant formyl-methionyl-leucyl-phenylalanine and IFN-γ through independent menchanisms. Eur J Immunol. 2002;32:3515. doi: 10.1002/1521-4141(200212)32:12<3515::AID-IMMU3515>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 120.Buckle A-M, Hogg N. The effect of IFN-γ and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol. 1989;143:2295. [PubMed] [Google Scholar]
- 121.McDonald PP, Gasperini S, Calzetti F, Cassatella MA. Modulation by interferon-γ of the production and gene expression of IL-1 receptor agtagonist in human neutrophils. Cell Immunol. 1998;184:45. doi: 10.1006/cimm.1998.1255. [DOI] [PubMed] [Google Scholar]
- 122.Geffner JR, Schattner MA, Lazzari MA, Isturiz MA. Interferon-gamma enhances PAF-acether production by stimulated human polymorphonuclear leukocytes. Scand J Immunol. 1991;33:575. doi: 10.1111/j.1365-3083.1991.tb02528.x. [DOI] [PubMed] [Google Scholar]
- 123.Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud K, Fleischer B, Hoerauf A. Lack of interferon-γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]
- 124.Uchida T, Yamashita T, Araki A, Watanabe H, Sendo F. rIFN-γ-activated rat neutrophils induce tumor cell apoptosis by nitric oxide. Int J Cancer. 1997;71:231. doi: 10.1002/(sici)1097-0215(19970410)71:2<231::aid-ijc18>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]


