Abstract
Localization of the death receptor Fas to specialized membrane microdomains is crucial to Fas-mediated cell death signaling. Here, we report that the post-translational modification of Fas by palmitoylation at the membrane proximal cysteine residue in the cytoplasmic region is the targeting signal for Fas localization to lipid rafts, as demonstrated in both cell-free and living cell systems. Palmitoylation is required for the redistribution of Fas to actin cytoskeleton-linked rafts upon Fas stimulation and for the raft-dependent, ezrin-mediated cytoskeleton association, which is necessary for the efficient Fas receptor internalization, death-inducing signaling complex assembly and subsequent caspase cascade leading to cell death.
Keywords: cell death, Fas, palmitoylation, rafts, signal transduction
Introduction
The Fas (CD95/APO-1/TNFRSF6) receptor–ligand system is known as one of the key regulators of apoptosis and is particularly important for the maintenance of lymphocyte homeostasis (Bidere et al, 2006). The apoptosis signaling of Fas, a member of the tumor necrosis factor receptor (TNFR) superfamily, is triggered through ligation by its ligand (FasL), either as membrane-associated FasL on FasL-expressing cells or as crosslinked soluble FasL. Following ligand engagement, Fas rapidly recruits Fas-associated death domain protein (FADD) and procaspase-8 to form the death-inducing signaling complex (DISC), which leads to activation of a caspase cascade and ultimately cell death (Kischkel et al, 1995). Two cell types have been defined depending on the requirement (type 2) or not (type 1) for cell death of an additional amplification loop that involves the cleavage by caspase-8 of the Bcl-2-family protein Bid to generate truncated (t) Bid and subsequent tBid-mediated release of cytochrome c from mitochondria, resulting in the activation of procaspase-9, which in turn cleaves downstream effector caspases (Scaffidi et al, 1999).
Recent studies have reported that Fas stimulation could also signal non-apoptotic pathways including NF-κB and MAPK activations, which may lead to the induction of tumorigenic or prosurvival genes (Barnhart et al, 2004; Legembre et al, 2004b). However, the molecular mechanisms of how cell fate is decided are yet to be elucidated.
Although the nature of molecular events downstream of the DISC formation is rather well documented, the knowledge of initial events, particularly at the plasma membrane level, governing the formation of this protein complex remains poorly defined. Several studies have indicated the constitutive and induced association of Fas with membrane microdomains, rafts, in both type I and type II cells (Hueber et al, 2002; Henkler et al, 2005; Miyaji et al, 2005). More recently, Lee et al (2006) defined the clathrin-dependent internalization of the receptor as an additional requirement for Fas-mediated cell death. Nevertheless, although both Fas–raft association and Fas internalization appear essential for Fas-mediated apoptosis (Hueber et al, 2002; Gajate et al, 2004; Gajate and Mollinedo, 2005), the mechanism regulating these two steps and the relationships between them were not known.
To understand the molecular mechanisms that might govern the fate of Fas receptors at the cell surface upon FasL engagement before the DISC formation, we investigated the role of potential post-translational lipid modifications of the Fas cytoplasmic domain. We specifically concentrate on S-palmitoylation, a reversible lipid modification involving the addition of a saturated 16-carbon palmitate moiety to a cysteine residue via a thioester linkage, which is a modification commonly found in transmembrane proteins (Resh, 1999, 2004).
We demonstrate here that Fas is palmitoylated and that this post-translational modification of the receptor is essential for the redistribution of Fas into lipid rafts and its association to ezrin and actin cytoskeleton, which plays an important role in Fas internalization, a step obligatory for the death signal transmission (Lee et al, 2006).
Results
Both mouse and human Fas are palmitoylated at a cysteine residue in the membrane proximal cytoplasmic region
Post-translational modification by lipidation such as palmitoylation, that is, the covalent attachment of a 16-carbon fatty acid to cysteine residues, is one of the mechanisms conferring the localization of many signaling proteins such as Ras GTPases, Fyn and Lck to lipid rafts (see for a review Smotrys and Linder, 2004). As human Fas (hFas) and mouse Fas (mFas) proteins possess a conserved cysteine residue in the membrane proximal cytoplasmic region (Figure 1A), we hypothesized that this residue might be palmitoylated, thus targeting Fas to lipid rafts. To address this issue, we first demonstrated that Fas is indeed palmitoylated. Human embryonic kidney 293 (HEK293) cells transiently expressing hFas or mFas cDNA were metabolically labeled with [3H]palmitate. Immunoprecipitation of Fas followed by immunoblotting and autoradiography demonstrated the incorporation of [3H]palmitic acid into both hFas (Figure 1B) and mFas (Figure 1C). Additionally, following metabolic labeling, the immunoprecipitates were treated with KOH base, which cleaves thioester bonds between proteins and palmitate. Absence of 3H labeling confirmed that the incorporated radiolabel was palmitate (Figure 1B). Fyn served as the control for palmitoylation and 35S as the control for metabolic labeling (Figure 1B).
Figure 1.
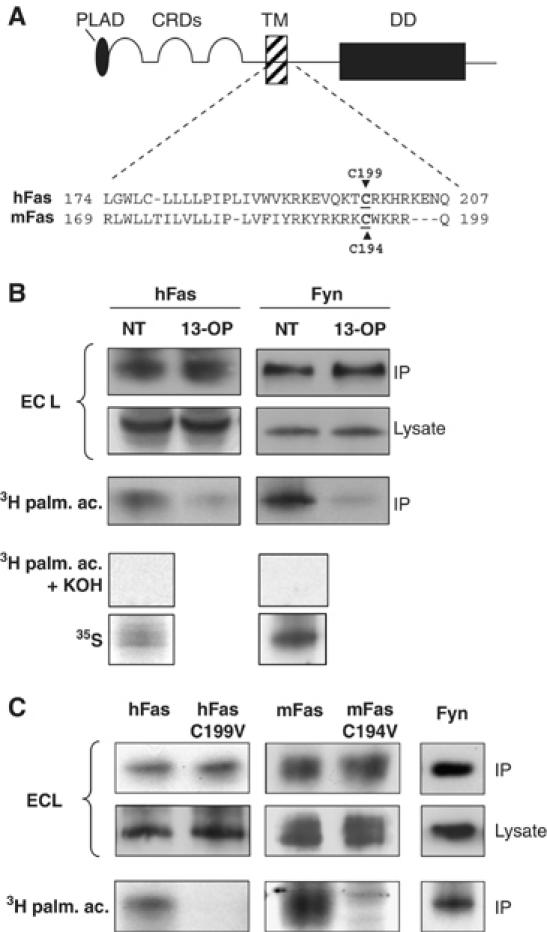
The cytoplasmic domain of Fas is palmitoylated. (A) Diagram and partial protein sequence alignment of human Fas (hFas) and murine Fas (mFas). The conserved cysteine residue (underlined), a putative palmitoylation site, was subjected to site-directed mutagenesis to valine. (B) Fas is palmitoylated. HEK293 cells transiently transfected with wild-type hFas plasmids (pCR3-hFas (PS345)) were labeled with [3H]palmitate or 35S. Cell lysates were immunoprecipitated with anti-hFas and analyzed by both autoradiography and Western blot. Palmitic analogue (13-OP) was used to compete with [3H]palmitate labeling. The immunoprecipitation of Fyn is shown as a [3H]palmitate labeling control. To confirm that the incorporated radiolabel was palmitate, lysates were treated with KOH. (C) Human and murine Fas is palmitoylated at residues C199 and C194, respectively. HEK293 cells transiently transfected with pCR3-hFas (PS345), pCR3.hFas-C199 V, pcDNA3.1.mFas, or pcDNA3.1.mFas-C194V constructs were metabolically labeled and subjected to subsequent procedures as in (B). Results are representative of at least three independent experiments.
To ensure the specificity of the palmitoylation, the transfected HEK293 cells were also treated with a palmitate analogue, 13-oxypalmitate (13-OP), known to inhibit protein palmitoylation (Hawash et al, 2002). As shown in Figure 1B, the incorporation of [3H]palmitate into Fas was markedly inhibited by the competition with 13-OP, confirming that Fas is indeed palmitoylated.
Using the site-directed mutagenesis approach, we determined whether palmitoylation occurred at the hypothesized cysteine residue (C199 and C194 of the hFas and mFas, respectively). HEK293 cells were transfected with wild-type hFas, C199V human Fas (hFasC199V), wild-type mFas or C194V mouse Fas (mFasC194V). The 5-h incubation in the presence of [3H]palmitate resulted in a significant incorporation of [3H]palmitate into both wild-type Fas expressed in HEK293 cells, whereas the parallel labeling experiments resulted in a lack of [3H]palmitate incorporation into hFasC199V and mFasC194V (Figure 1C). Thus, Fas is palmitoylated in the membrane proximal intracellular region at the cysteine residue 199 for human and 194 for mouse.
Fas palmitoylation is critical for membrane FasL-induced cell death
We then determined whether palmitoylation of Fas at this membrane proximal cysteine residue was directly involved in Fas-mediated cell death. We investigated the Fas-mediated cell death in co-culture experiments, a system closer to the physiological conditions in which we first studied and described both the importance of membrane versus soluble(s) FasL and the relevance of raft association for the Fas cell death signaling in such system. We demonstrate that the membrane FasL was the sole stimulus that activated the Fas receptor, as when the killer cells (J16/Rapo-FasL) were preincubated with the metalloproteinase inhibitor GM6001, which blocks sFasL production by preventing FasL cleavage (Cahuzac et al, 2006), no inhibition of Fas-mediated cell death was observed (Figure 2A). In addition, the supernatant of the killer cell culture, which contains sFasL, did not exert any cell death induction effect (Supplementary Figure 1A).
Figure 2.
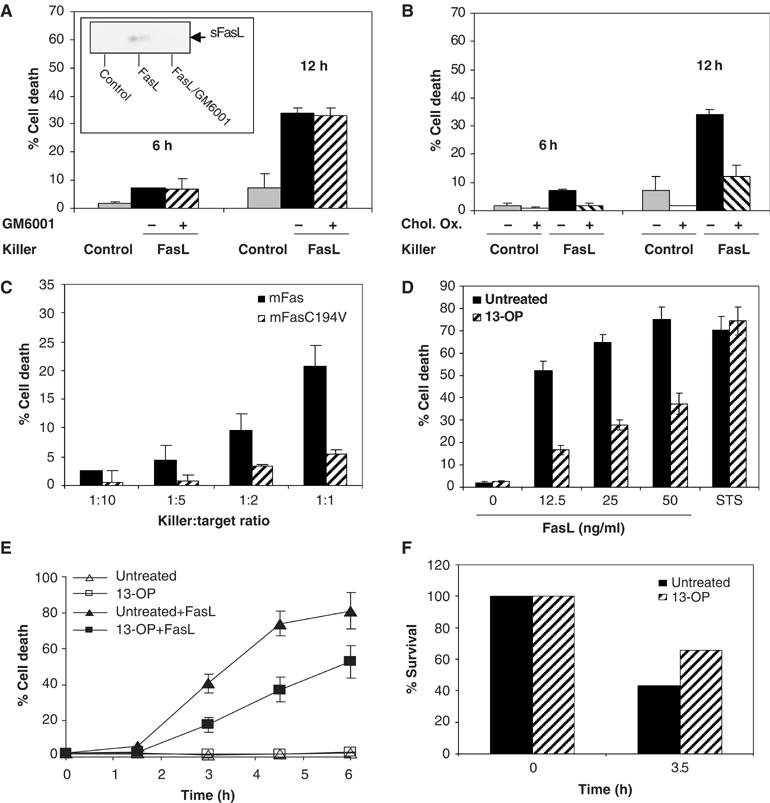
Fas palmitoylation is essential for Fas-induced cell death. (A) L12.10mFas cells were subjected to Fas-induced cell death by co-culture assay. The killer cells expressing FasL on cell surface (J16/Rapo-FasL) or control cells lacking FasL expression (J16/Rapo), pretreated or not with the metalloprotease inhibitor GM6001 (30 μM; 2 h), were incubated with target cells L12.10mFas (killer:target ratio 1:1) for the indicated time. The amount of soluble FasL in the supernatant was examined by Western blot (see the inset). (B) L12.10.mFas cells, pretreated or not with CO (2 h; 4 U/ml), were subjected to Fas-induced cell death by co-culture assay as in (A). (C) L12.10mFas and L12.10mFasC194 V were subjected to Fas-induced cell death by co-culture assay as in (A) with the indicated killer:target ratio for 24 h. (D) Palmitic acid analogue treatment reduced the sensitivity toward Fas-induced cell death. Cells were pretreated with 300 μM 13-OP or left untreated at 37°C, 5% CO2 for 2 h before being subjected to Fas-mediated cell death by incubating with Flag-rhFasL plus 1 μg/ml M2 or to non-Fas-mediated cell death by staurosporine (STS) treatment (2 μM). Cell death was assessed after 5 h. (E) Palmitic acid analogue treatment caused a significant delay in Fas-mediated cell death kinetics. Cells were pretreated with 300 μM 13-OP or left untreated as control before incubating with or without 50 ng/ml Flag-rhFasL plus 1 μg/ml M2 for the indicated time. (F) Palmitic acid analogue inhibited Fas-induced cell death in primary cells. Purified T lymphocytes isolated from the spleen of 6- to 10-week-old mice were stimulated with 50 U/ml IL-2 and 1 μg/ml anti-CD3 antibody for 4 days before plating at 106 cells/ml and treated with 300 μM 13-OP or left untreated at 37°C, 5% CO2 for 2 h before being subjected to Fas killing by incubating with 100 ng/ml Flag-rhFasL plus 1 μg/ml M2 antibody.
Subsequently, we emphasized the importance of Fas association to the rafts in Fas-mediated cell death triggered by membrane FasL by demonstrating that in this co-culture system whose sole stimulus was membrane FasL, preincubation of target cells (L12.10.mFas) with detergent-resistant membrane microdomain (DRM)-disrupting agents such as cholesterol oxidase, which resulted in the loss of Fas–rafts association (Supplementary Figure 2A), led to a dramatic inhibition of Fas-mediated cell death (Figure 2B). We thus concluded that, in contrast to the report by Henkler et al (2005), Fas-mediated cell death induced by the membrane form of FasL was strongly dependent on Fas association to DRMs. Such discrepancies found between the systems reported by us and Henkler et al (2005) are of great interest and warrant further investigation.
We then examined in the same co-culture system the sensitivity of the palmitoylation-deficient Fas-expressing cells to Fas-mediated cell death. Following FasL engagement, L12.10 mouse T cells stably expressing mFasC194V (L12.10mFasC194V) exhibited significantly less cell death than cells expressing wild-type Fas (L12.10mFas) (Figure 2C). The same result was obtained when cell death was triggered by anti-Fas antibody or soluble rhFasL in different cell types and for both mouse and hFas (Figure 3A and B and Supplementary Figure 1C and D).
Figure 3.
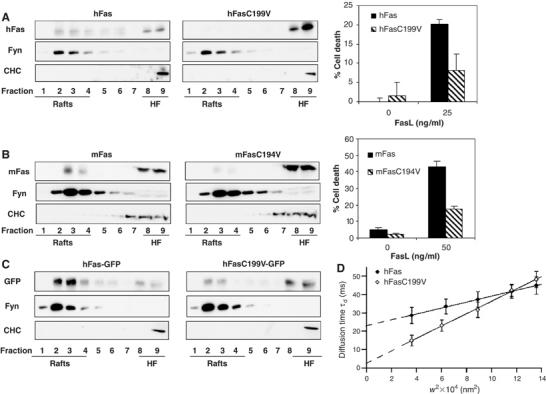
Palmitoylation is required for the constitutive Fas-to-rafts localization. PNS from HEK293 cells transiently expressing hFas or hFasC199V (A), L12.10 cells stably expressing mFas or mFasC194 V (B) or HEK293 cells transiently expressing hFas-GFP or hFasC199V-GFP (C) were subjected to biochemical DRM preparation and sucrose gradient fractions were immunoblotted with the indicated antibodies. Fas-mediated cell death was performed in parallel by incubating the cells with Flag-rhFasL plus 1 μg/ml M2 for 5 h. (D) The diffusion behavior of hFas-GFP and hFasC199V-GFP in COS-7 cell was established in living cells by FCS. FCS diffusion laws were derived by plotting the diffusion time (td) versus the transversal area of the confocal volume. A positive deviation of the t0 y-intercept from the origin indicates that molecular diffusion was hindered by a microdomain compartmentalization of the plasma membrane (Wawrezinieck et al, 2005; Lenne et al, 2006).
We also used a pharmacological approach to provide additional evidence for a critical role of palmitoylation in Fas-mediated cell death. Pretreating L12.10 (Figure 2D and E) or HEK293 (Supplementary Figure 1F) cells expressing mFas with 13-OP led to a marked reduction in cell death after FasL engagement. Cells expressing mFasC194V did not exhibit additional cell death inhibition after 13-OP treatment (Supplementary Figure 1F). The inhibition of cell death by 13-OP was specific for Fas-mediated cell death as cell death by other apoptotic stimuli, for example, staurosporine, was not affected (Figure 2D). A significant decrease in Fas-mediated cell death was also observed in peripheral T lymphocytes (Figure 2F) and in Jurkat T cells (data not shown) treated with 13-OP, confirming the importance of palmitoylation in Fas-induced cell death in both primary cells and cells endogenously expressing Fas protein. Mutation at the C194 residue or treatment with 13-OP (Supplementary Figure 1B and E) also impaired the activation of caspase-8 and caspase-9 as well as the downstream cleavage of PARP. Altogether, these findings emphasized the specificity of Fas palmitoylation at C194 or C199 residue (for mFas and hFas, respectively) and indicated its role in Fas-mediated cell death.
Palmitoylation is required for the constitutive Fas-to-rafts localization
Studies from several groups, including ours, have provided evidence for a crucial role of Fas association with rafts in the initiation of Fas cell death signaling (Gajate and Mollinedo, 2001, 2005; Algeciras-Schimnich et al, 2002; Hueber et al, 2002; Scheel-Toellner et al, 2002; Aouad et al, 2004; Muppidi and Siegel, 2004; Henkler et al, 2005; Miyaji et al, 2005; Rotolo et al, 2005). In the light of these findings, we investigated whether palmitoylation is involved in localizing Fas to lipid rafts. First, using a biochemical approach, we subjected the post-nuclear supernatant (PNS) of HEK293 transiently expressing hFas or hFasC199V (Figure 3A) and L12.10 cells stably expressing mFas or mFasC194V (Figure 3B) to solubilization with polyethylene ether Brij 98 followed by sucrose gradient ultracentrifugation, after which rafts were found as low-density, detergent-resistant fractions. We showed that a significant proportion of both human and murine wild-type Fas were constitutively partitioned in the DRM as previously reported (Hueber et al, 2002) (19 and 42%, respectively; based on densitometric analysis), whereas a much smaller proportion of the C194V murine Fas or the C199V hFas were localized into the DRM (0.5 and 5.3%, respectively) (Figure 3A and B). Correspondingly, the dislocation from DRM due to the loss of palmitoylation could therefore be estimated as 97% for hFas and 87% for mFas. The dually acylated Fyn and clathrin heavy chain served as raft and non-raft markers, respectively.
In addition to this biochemical approach, we employed fluorescence correlation spectroscopy (FCS) to investigate the lateral diffusion of wild-type human Fas-GFP (hFas-GFP) and hFasC199V-GFP molecules in the surface membrane of living cells (Kahya et al, 2004; Gosch and Rigler, 2005). We first verified that the chimeric hFas-GFP and its palmitoylation-deficient mutant (hFasC199V-GFP) behaved as the non-chimeric counterparts in terms of DRM localization (Figure 3C; 63% dislocation for hFasC199V-GFP when compared to hFas-GFP) and in terms of Fas killing (data not shown). The FCS method records measurements at different spatial scales of observations and allows the submicron organization of cell membranes to be studied less invasively and more reliably than other classical techniques. The FCS methodology measures the apparent diffusion time (τd), which provides information on the diffusion mode of the molecule of interest. If a molecule follows Brownian motion, the average time τd a molecule stays within an illuminated area is strictly proportional to the square of the beam radius (w2) (Wawrezinieck et al, 2005; Lenne et al, 2006). Our results showed that the diffusion behavior of hFas-GFP clearly differed from a free diffusion mode for which the y-intercept (t0) equals zero (Figure 3D). The τd of hFas-GFP increased linearly with w2, but t0 was strictly and significantly positive with a value of 22.9±0.52 ms (Figure 3D). This diffusion behavior was closely comparable to that of the raft marker BODIPY-C5-ganglioside-GM1 (t0=25±3 ms) (Cahuzac et al, 2006). On the other hand, a Brownian-like diffusion behavior was observed for hFasC199V-GFP, as the y-intercept t0 is nearly null (2.5±1.25 ms) (Figure 3D). Such a diffusion mode is typically observed for molecules such as BODIPY-C5-phosphatidylcholine analogue or for molecules that are dislocated from rafts following their disruption, for example, following cholesterol oxidase treatment (Wawrezinieck et al, 2005; Cahuzac et al, 2006; Lenne et al, 2006). Thus, through biochemical and biophysical approaches, our results from both cell-free and living cell systems show for the first time that palmitoylation of Fas is required for its localization in lipid rafts.
Fas–ezrin association depends on Fas palmitoylation but is not required for Fas–DRM association
It has been reported that Fas associates and colocalizes with ezrin, a cytoskeletal protein of the 4.1 protein family, and that this Fas–ezrin association is essential for Fas-mediated cell death in T lymphocytes (Parlato et al, 2000). Nevertheless, the mechanism governing this association upon Fas triggering is poorly understood. We observed that the disruption of Fas palmitoylation, by C194V mutation as well as competition with palmitic analogues 2-BrP and 13-OP, not only inhibited DISC formation but also decreased the association of Fas to ezrin following FasL-triggered activation (Figure 4A). To place the palmitoylation-mediated Fas–ezrin association in the molecular sequence of Fas-triggered apoptosis, we investigated the effect of the loss of Fas–ezrin association on DISC formation by using an RNAi approach. We established L12.10.mFas cells stably expressing short hairpin ezrin RNA, in which expression of ezrin was efficiently inhibited when compared to cells expressing control shRNA (Figure 4B and C). DISC was isolated after stimulation of the cells with rhFasL crosslinked with anti-Flag antibody. The inhibition of ezrin expression and thus the loss of Fas–ezrin association resulted in blockage of DISC formation (Figure 4B). In contrast, cells expressing control shRNA did not lose association between Fas and ezrin nor the ability to form DISC.
Figure 4.
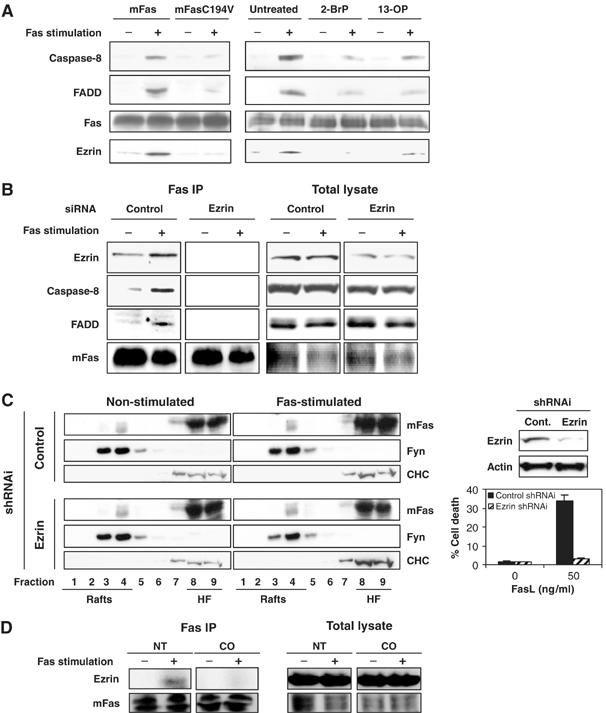
Palmitoylation is required for Fas–ezrin association, which is essential for DISC formation but not for Fas–raft association. (A) DISC isolation by biotinylated anti-Fas antibody followed by strepavidin-conjugated beads was performed on L12.10.mFas or L12.10.mFasC194V cells (108) pretreated or not with 100 μM 2-bromopalmitate (2-BrP) overnight or 300 μM 13-OP for 2 h, and subsequently stimulated with 50 ng/ml Flag-rhFasL plus 1 μg/ml M2 for 10 min or left unstimulated at 37°C. DISC isolation (B) and sucrose gradient (C) were performed on L12.10.mFas cells stably expressing short hairpin ezrin RNA or control RNA. In parallel, Fas-mediated cell death was examined after an additional 3 h incubation with 50 ng/ml Flag-rhFasL plus 1 μg/ml M2 (C, bottom right). Immunoblots show the efficiency of the transient ezrin knockdown (C, top right). (D) DISC isolation was performed as in (A) on L12.10.mFas cells pretreated or not with 4 U/ml CO for 2 h.
We next investigated whether the presence of ezrin was also critical in localizing Fas to lipid rafts. For this purpose, DRMs were isolated from L12.10 cells stably expressing either ezrin shRNA or control shRNA. No difference in terms of Fas localization in DRM was detectable in the absence or presence of ezrin, whereas Fas-mediated cell death was profoundly affected (Figure 4C). Therefore, this ruled out the requirement of ezrin association for Fas localization to lipid rafts. In marked contrast, we demonstrate that rafts integrity was required for the association of ezrin to Fas. Indeed, preincubation of L12.10.mFas cells with cholesterol oxidase prevented the recruitment of ezrin to Fas upon FasL engagement (Figure 4D).
Our findings demonstrate that palmitoylation of Fas is essential for its association with ezrin. This association is indispensable for the DISC formation step but not for Fas–raft association.
Fas associates with cytoskeleton-linked rafts upon activation
Given the importance of ezrin-dependent association of Fas to the actin cytoskeleton in Fas-mediated apoptosis (Parlato et al, 2000; Luciani et al, 2004) and of interactions between lipid rafts and the cytoskeleton in numerous cellular processes including platelet activation (Bodin et al, 2005), neutrophil polarization (Seveau et al, 2001), chemotaxis and chemokinesis (Bodin and Welch, 2005), and immunological synapse formation in T cells (Villalba et al, 2001), we set out to investigate the relationship between Fas–cytoskeleton and Fas–raft associations.
We first asked whether the palmitoylation-targeted raft localization of Fas was involved in its connection with the actin cytoskeleton. As we aimed to isolate the cytoskeleton fraction from HEK293 cells expressing mFas or mFasC194V before and after stimulation with anti-Fas antibody using Triton X-100 resistance fractionation (Bodin et al, 2005), we first confirmed that both the Fas–DRM association and the dislocation of Fas from DRM in the absence of palmitoylation were also found when Triton X-100 was used as detergent (Figure 5A).
Figure 5.
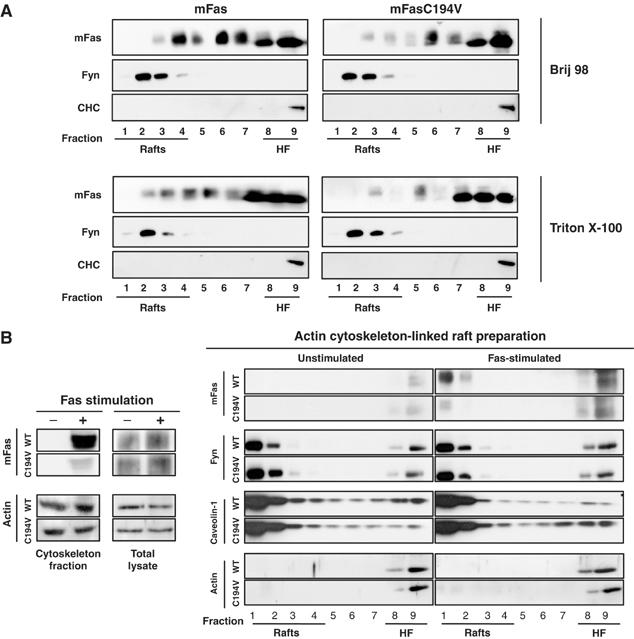
Fas associates with cytoskeleton-linked rafts upon activation. (A) PNS from 293 cells stably expressing mFas or 293 mFasC194V was subjected to the DRM preparation using either 1% Brij 98 or 0.5% Triton X-100 for solubilization. The sucrose gradient fractions were subjected to immunoblotting. (B) Total lysate and actin cytoskeleton fraction were prepared from HEK293 cells (2.5 × 107) stably expressing mFas or mFasC194V before and after 25-min activation with 100 ng/ml anti-Fas (JO2) crosslinked with 100 ng/ml protein A and then subjected to SDS–PAGE and immunoblotting or to cytoskeleton-linked DRM isolation (Bodin et al, 2005). The resulting sucrose gradient fractions were subjected to SDS–PAGE and immunoblotting.
Having found a similar raft localization behaviour with both Triton X-100 and Brij 98 (Figure 5A), we proceeded to cytoskeleton isolation using Triton X-100, with which actin cytoskeleton could be obtained as insoluble pellet. We found that wild-type Fas co-isolated with the actin cytoskeleton following stimulation. Importantly, a much smaller proportion of C194V Fas was associated with the cytoskeleton following Fas activation (Figure 5B). The targeting of Fas to lipid rafts by palmitoylation thus seems to play an important role in dictating the extent of association of Fas with the actin cytoskeleton. To further investigate this, we isolated DRM that are connected to actin cytoskeleton from non-stimulated and Fas-stimulated 293mFas or 293mFasC194V cells. The packed actin cytoskeleton fraction, isolated by fractionation with Triton X-100, was depolymerized by potassium iodide treatment (Bodin et al, 2005) and then subjected to sucrose gradient ultracentrifugation. Using this method, actin was found exclusively in the high-density fraction of the gradient. On the contrary, the majority of caveolin-1 and Fyn was found in the low-density fraction, as expected for markers of lipid rafts, and their distribution appeared unchanged following activation, implicating their constitutive and constant level of association with the cytoskeleton-linked rafts (Figure 5B). Upon activation, more Fas was recovered in the sucrose gradient fractions, in agreement with the increase in Fas association with the actin cytoskeleton (Figure 5B). While an equivalent amount of wild-type and C194V Fas was recovered in the high-density fraction, remarkably, following Fas activation, a substantial proportion of wild-type Fas was partitioned into the cytoskeleton-linked DRM whereas the C194V Fas was not (Figure 5B). Thus, Fas palmitoylation is not only required for its localization in rafts, but is also, even more specifically, instrumental in targeting Fas to the actin cytoskeleton-linked rafts where the association between Fas and the dynamic actin cytoskeleton, an essential step for the transduction of death signal (Fais et al, 2005), is likely to occur.
Interestingly, we found that following cell fractionation with Triton X-100 to obtain cytoskeleton, Fas could only be found associated to the DRM from the cytoskeleton pellet but not the DRM from non-cytoskeleton-associated supernatant (data not shown). Indeed, we observed that without prior mechanical disruption of the cells, which would inevitably disrupt the actin cytoskeleton (e.g., by sonication or homogenization), using Triton X-100 solubilization alone (which was the case of cytoskeleton isolation method employed here) rafts could not be isolated from the non-cytoskeleton-associated solubilization supernatant, as raft materials remained with the cytoskeleton pellet (as assessed by the presence of raft markers, e.g., Fyn, caveolin-1 and GM1, in the fractions of DRM preparation; data not shown). This observation has been reported previously (Draberova et al, 1996).
Fas palmitoylation plays an important role in receptor internalization
Previous studies have proposed that initial Fas-induced cell death events involve caspase-independent formation of Fas microaggregates and caspase-8-dependent formation of larger Fas aggregates, which are subsequently internalized via an actin-dependent endosomal pathway, and that the disruption of the actin filament hinders the formation of DISC (Algeciras-Schimnich et al, 2002). It was reported that this actin-dependent receptor internalization occurred only in type I cells (Algeciras-Schimnich and Peter, 2003; Lee et al, 2006) and not in type II cells (Algeciras-Schimnich et al, 2002). Importantly, recent studies by Lee et al (2006) have reported a general requirement of receptor internalization after Fas activation for DISC amplification, caspase activation and cell death in type I cells. On the other hand, Fas is recruited to rafts domains independently of the death domain and the DISC formation (Eramo et al, 2004; our unpublished results) and Fas internalization upon engagement by its ligand takes place in rafts (Eramo et al, 2004). Therefore, although several studies have provided support for the essential role of Fas-to-rafts localization, Fas–actin cytoskeleton interaction, DISC formation and receptor internalization in the transduction of a death signal by Fas, the molecular mechanisms underlying the exact connections and sequences among these early events are still not completely understood.
To shed some light into the issues surrounding these events, we first investigated some of the relevant aspects of stimulation-induced Fas internalization following FasL engagement in our experimental system. To quantitatively assess the extent of receptor internalization, we synchronized Fas activation by raising the temperature to 37°C after incubation of L12.10.mFas cells at 4°C with rhFasL-Flag crosslinked with mouse anti-Flag IgG (M2) antibody. The amount of FasL remained on the cell surface, representing the uninternalized Fas–FasL complexes, was then quantified by surface staining, followed by flow cytometric analysis. Accordingly, we first investigated the role of lipid rafts in the internalization of Fas after stimulation. Disrupting raft formation by pretreating the cells with cholesterol oxidase, methyl-β-cyclodextrin (MβCD) or lovastatin before FasL engagement resulted in a significant delay in FasL-induced internalization of Fas (Figure 6A), Fas–rafts association and cell death (Supplementary Figure 2A), supporting the earlier proposal by Eramo et al (2004) that lipid rafts are the sites of Fas internalization. In agreement with previous studies reporting that Fas internalization was dependent on actin polymerization (Algeciras-Schimnich et al, 2002), and was upstream of optimal DISC formation and caspase activation (Lee et al, 2006), we showed that pretreating the cells with an inhibitor of actin polymerization, latrunculin A, significantly delayed Fas internalization (Figure 6B) and inhibited cell death (Supplementary Figure 2B), whereas a treatment with the pan-caspase inhibitor, zVAD, which effectively inhibited cell death (Supplementary Figure 2C), failed to inhibit the FasL-induced Fas internalization (Figure 6B).
Figure 6.
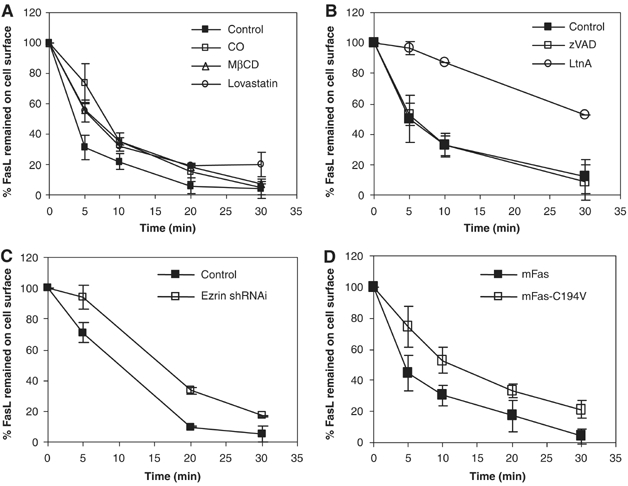
Fas palmitoylation is required for efficient caspase-independent, raft- and actin-dependent FasL-induced internalization. (A) Raft integrity is essential for efficient Fas internalization. L12.10.mFas cells (106) untreated or pretreated in the absence of serum with 4 U/ml CO for 2 h, 10 mM MβCD for 12 min or 5 μM lovastatin for 24 h were subjected to synchronized activation and internalization assessment. (B) Effective Fas internalization depends on actin polymerization but not caspase activities. L12.10.mFas cells were untreated or pretreated with 5 μM latrunculin A (LtnA) or 10 mM zVAD-fmk for 30 min before the internalization assay. (C) FasL-induced Fas internalization is ezrin-dependent. Internalization assay was performed on L12.10.mFas cells stably expressing ezrin shRNA or control shRNA. (D) L12.10.mFas and L12.10.mFas-C194V cells were analyzed for Fas internalization.
We then investigated the role of ezrin in Fas internalization. L12.10.mFas cell lines stably expressing ezrin short hairpin (sh) RNA or control shRNA, which expressed equivalent levels of Fas, were subjected to stimulation-induced internalization. Suppressing the expression of ezrin significantly reduced the ability of Fas to be internalized after FasL engagement (Figure 6C) and the susceptibility toward Fas-induced cell death (Figure 4C). Thus, ezrin plays an important role in efficient FasL-induced Fas internalization and in turn in Fas-mediated cell death. Last but not the least, we found that L12.10.mFasC194V cells exhibited a marked delay in Fas internalization when compared to L12.10.mFas cells (Figure 6D), thus demonstrating that effective receptor internalization was significantly dependent on Fas palmitoylation.
Discussion
Our studies identify new molecular steps occurring upstream of the DISC formation upon FasL binding. We indeed reported here for the first time that the receptor Fas is post-translationally modified through palmitoylation at the membrane proximal cysteine residue in the cytoplasmic region and that this modification is required for the raft-dependent, ezrin-mediated cytoskeleton association, which in turn is necessary for efficient Fas receptor internalization (Figure 7).
Figure 7.
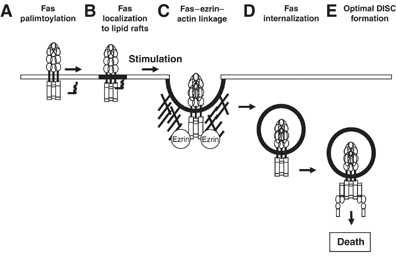
Schematic representation of the new molecular steps occurring upstream of Fas internalization reported in this paper: a post-translational modification of the death receptor Fas by palmitoylation (A) allows the targeting of the Fas receptor to lipid membrane microdomains (B) where following stimulation by binding to membrane FasL or crosslinked soluble FasL the connection between Fas receptor and actin cytoskeleton occurs via the association of Fas with ezrin (C). The ezrin-mediated cytoskeleton association initiates receptor internalization (D), a prerequisite step for the intracellular optimal formation of DISC (E), which leads to an efficient caspase activation and cell death.
Fas palmitoylation is required for Fas–raft association
We show that Fas palmitoylation is not only required for Fas localization to lipid rafts (Figure 3), but also constitutes a targeting signal for the redistribution of Fas to actin cytoskeleton-linked rafts upon Fas stimulation (Figure 5). One might imagine that the variable amount of Fas residing within the raft according to the cell type might be due to the differential ability of the receptor to be palmitoylated, with the protein pamitoylation process being a highly dynamic and reversible process (Resh, 2004).
Importantly, our results bring the first genetic proof of Fas localization to lipid rafts, as opposed to all the previous data using drugs to demonstrate Fas–rafts association. In addition, we demonstrated this association by analyzing the lipid microdomains in living cells through an ultra-resolution imaging technique using the FCS approach (Figure 3D), which records measurements at different spatial scales of observation (Marguet et al, 2006).
Also of interest is our observation that from the cytoskeleton isolation using only Triton X-100 solubilization (without prior mechanical disruption of the cells), DRMs were co-isolated only with the cytoskeleton fraction. This finding, which agrees with earlier report (Draberova et al, 1996), emphasizes the importance of considering the roles of association of rafts to the actin cytoskeleton as well as the organization/reorganization of these entities in various cell signaling pathways, whose regulation of signals has been shown to be dependent on dynamic association/dissociation of signaling molecules to/from lipid rafts and actin cytoskeleton, such as that of Fas.
Fas–raft association is a prerequisite for efficient Fas internalization, an obligatory process for DISC formation
Our finding that activation of caspases was not required for receptor internalization is in concert with the reports by Eramo et al (2004) and Lee et al (2006). This strengthens the idea that the extensive DISC formation and caspase-8 activation take place downstream of receptor internalization. Studies by Lee et al suggested that this internalization occurs through a clathrin-mediated pathway and allows the targeting of the receptor to early endosomes where an optimal DISC formation takes place. The prerequisite role of death receptor internalization in the initiation of signaling has been first demonstrated for TNFR1 (Schneider-Brachert et al, 2004). Using a genetic approach, Schneider-Brachert et al (2004) have shown that for the NF-κB signaling pathway, the formation of TNFR1–TRADD, RIP-1 and TRAF complex occurred at the level of the plasma membrane. In contrast, in the proapoptotic pathway, the formation of TNFR1-associated DISC, which includes TRADD, FADD and caspase-8, required receptor internalization and occurred in the endocytic vesicles. These endocytic vesicles containing the activated TNFR1 were termed receptosomes and were shown to be the site where caspase-8 was extensively activated.
Our results demonstrating the involvement of rafts in Fas internalization might appear at first in contradiction with those by Lee et al (2006) showing that following engagement by the ligand, Fas is internalized into an EEA-1-positive endosomal compartment via a clathrin-dependent mechanism, as the clathrin-mediated endocytosis has usually been found to associate with non-raft rather than raft molecules, as is the case for the non-signaling receptors such as transferring receptor (TfR). However, several recent studies have shown that raft-associated and signaling-competent receptors can recruit necessary machinery for their endocytosis via clathrin-coated pits upon ligand engagement (Stoddart et al, 2002; Puri et al, 2005). We are conducting several studies that should allow us to analyze whether an endocytosis scenario similar to that found for BCR (for instance, initial DRM recruitment of phosphorylated clathrin before internalization) can also be applied to the Fas receptor.
Based on the data of Lee et al (2006) and our unpublished observations (K Chakrabandhu and AO Hueber), the formation of DISC that triggers apoptosis signaling pathway occurs within the endosomal compartments. However, at present, there are no mechanisms to explain the role of endosome location. Based on the results of Ehehalt et al (2003), it is possible to propose the following scenario: Fas associates with the small elementary rafts in the plasma membrane in the absence of ligand binding (this is supported by our FCS analysis); Fas ligation triggers its internalization together with the associated raft lipids into the endosome where large raft platforms are formed probably by small raft coalescence. This will in turn allow assembly of Fas oligomers of higher order, which efficiently recruit other DISC components FADD and caspase-8 to initiate apoptosis signaling.
Ezrin recruitment to Fas is critical for efficient Fas internalization but not for Fas–raft association
Although the presence of ezrin was reported to be critical for Fas-mediated cell death 6 years ago (Parlato et al, 2000), the molecular mechanism underlying this requirement is not known until now. We demonstrate here that whereas ezrin was dispensable for Fas–raft association (Figure 4C), its interaction with the receptor Fas upon FasL engagement requires raft integrity and is necessary for an optimal DISC formation. All these results allow us, for the first time, to position the function of ezrin between Fas–raft localization at the plasma membrane and DISC formation.
As we have shown that palmitoylation is required for the targeting of Fas to actin cytoskeleton-linked rafts, we hypothesized that this targeting is closely involved in the mechanism of Fas–ezrin association. Indeed, we observed that ezrin, while not detectable in the rafts of total cell post-nuclear supernatant, was constitutively localized to the actin cytoskeleton-linked DRMs, whereas Fas only partitioned to the actin cytoskeleton-linked DRMs after Fas triggering (data not shown). Thus, it is highly likely that these cytoskeleton-linked rafts are the sites where Fas–ezrin–actin cytoskeleton linkage occurs following Fas stimulation.
Fas palmitoylation as a bifurcation switch for the cellular fate of Fas-stimulated cells
Fas, a closely related family member of TNFR1, is also able to transduce both NF-κB and apoptotic signals depending on cell types (Barnhart et al, 2004; Legembre et al, 2004a, 2004b, 2004c). Interestingly, the non-apoptotic pathways generated by Fas have been reported independent of its ability to internalize (Lee et al, 2006), therefore leading to the hypothesis that the internalization process might play an essential role in defining the cellular fate of the Fas receptor. In this context, it is likely that differential post-translational modifications of the receptor may play important and decisive roles in the selective receptor compartmentalization and formation of the protein complexes, and thus, the regulation of death receptor signaling and the ultimate fate of the cell. Experiments that are currently in progress in our laboratory aimed to investigate the possibility that Fas palmitoylation status could be one of the decisive conditions dictating whether cells should undergo apoptosis or activate non-apoptotic signal.
Altogether, our results pointed out the essential role of protein–lipid interaction in the initial molecular events of Fas-mediated cell death where the post-translational modification of the death receptor Fas by palmitoylation is identified as the key in targeting the receptor to the membrane microdomains where the crucial protein–protein interaction, namely the connection between the Fas receptor and actin cytoskeleton, occurs via the association of Fas with ezrin. This palmitoylation-directed, raft-dependent, ezrin-mediated cytoskeleton association is required for efficient caspase-8-independent receptor internalization, which we propose to be indispensable for the intracellular amplification of Fas signaling brought about by an extensive DISC formation along the endocytic pathway, leading to efficient activation of the caspase cascade and, ultimately, cell death.
Materials and methods
Constructs
Human Fas C199V (pCR3.hFasC199V) and mFas C194V (pcDNA3.1.mFasC194V) constructs were obtained using the Quikchange site-directed mutagenesis kit (Invitrogen) with pCR3-hFas (PS345) (a kind gift from P Schneider) and pcDNA3.1.mFas as respective templates. Human Fas-EGFP (hFas-GFP) was generated by cloning the full-length Fas insert, excised from pCR3-hFas into pEGFP-N1 plasmid (Clontech). The hFasC199V-GFP constructs were then obtained by the Quikchange site-directed mutagenesis kit using hFas-GFP as the template. Primers used are listed in Supplementary data.
Cell transfection
HEK293 cells were transfected as previously described (Herincs et al, 2005). Stable L12.10 cells expressing mFas (L12.10.mFas) or mFasC194V (L12.10.mFasC194V) were established by transfection with pcDNA3.1.mFas or pcDNA3.1.mFasC194V constructs using the Nucleofector system (Amaxa) and selecting for stable cells with neomycin. L12.10.mFas cells expressing ezrin shRNA or control shRNA were established by transfecting cells with mouse ezrin shRNAi (pTER-mEzrin) or control shRNAi (pTER-SIMA) plasmids and selected for stable cells with Zeocin. Transient transfections in COS-7 cells were performed with ExGen 500 (Euromedex). Oligonucleotides used to create shRNAi constructs are listed in Supplementary data.
T-cell isolation and purification
T cells were prepared from the spleen of 4- to 8-week-old mice by grinding the organ through a Scrynel nylon 100-μm mesh (VWR International) in serum-free RPMI (Gibco BRL) supplemented with 10% fetal bovine serum (FBS), 50 μM β-mercaptoethanol and 10 mM HEPES (Gibco BRL). Mature T cells were isolated from splenocytes using mouse T cell negative isolation kit (Dynal). The purity was confirmed by FACS analysis with an anti-CD4/anti-CD8 staining to be >90%.
Metabolic [3H]palmitate labeling
[9,10(n)-3H] palmitic acid (specific activity 60 Ci/mmol) (Amersham Biosciences) or in vitro Cell Labeling Pro Mix 35S (specific activity 50 Ci/mmol) (Amersham Biosciences) was added to the medium of transiently Fas- or Fyn-transfected HEK293 cells at 0.2 mCi/ml in the absence of serum and incubated for 5 h at 37°C. Cells were washed in PBS and lysates were subjected to immunoprecipitation with anti-Fas or anti-Fyn. After SDS–PAGE of the immunoprecipitates, gels were fixed and enhanced using Amplify (Amersham) and exposed for autoradiography.
DISC isolation
DISC isolation was performed on L12.10 cells (1 × 108) stimulated with 50 ng/ml Flag-rhFasL plus 1 μg/ml M2 antibody for 10 min or left unstimulated at 37°C. The PNS was prepared and solubilized in buffer A (25 mM HEPES, 150 mM NaCl, 1 mM EGTA, protease inhibitors cocktail) containing 0.5% Nonidet P-40 (NP-40) and 10% glycerol (lysis buffer) at 4°C and then immunoprecipitated with biotinylated JO2 anti-Fas antibody coupled to streptavidin–agarose beads at 4°C, overnight. The beads were washed four times with lysis buffer and the immunoprecipitates were eluted from beads with Laemmli buffer at 95°C for 5 min and subjected to SDS–PAGE, followed by immunoblotting.
DRM separation
PNS from HEK293 (2.5 × 107) or L12.10 (10 × 107) cells was solubilized in 1 ml buffer A containing 1% Brij 98 (Aldrich) for 1 h on ice, followed by addition of 2 ml of 2 M sucrose in buffer A before being placed at the bottom of a step sucrose gradient (1.33–0.9–0.8–0.75–0.7–0.6–0.5–0.4–0.2 M) in buffer A. Gradients were centrifuged at 38 000 r.p.m. for 16 h in an SW41 rotor (Beckman Coulter) at 4°C. One-milliliter fractions were harvested from the top, except for the bottom fraction (no. 9), which contained 3 ml.
Cell death assays
The sensitivity of Fas-expressing target cells L12.10.mFas was determined by co-culture assays with FasL-expressing cells, J16/Rapo-FasL (Cahuzac et al, 2006) (killer), or the FasL negative cells, J16/Rapo (control), in a 1:1 ratio or as indicated. Killer and control cells were prestained with 5 μM diacetate succinimidyl ester (CFSE) before the co-culture to distinguish them from target cells during FACS analysis. In other experiments, Fas-mediated cell death was accomplished by incubating the cells with the indicated amount of Flag-rhFasL plus 1 μg/ml anti-Flag M2 antibody or with JO2 anti-Fas antibody plus protein A, at 37°C. After the indicated time, the apoptosis in the target cells was detected by flow cytometric analysis. Briefly, cells were fixed in ice-cold 70% ethanol, washed in 38 mM sodium citrate (pH 7.4) and stained for 20 min at 37°C in 38 mM sodium citrate (pH 7.4) with 69 μM propidium iodide (Sigma) and 5 μg/ml RNase A (Sigma). Cells were analyzed with a flow cytometer (FACSCalibur; Becton Dickinson), and the proportion of apoptotic cells represented by the sub-G1 peak was determined (Hueber et al, 2002).
Cholesterol depletion treatment
Cells were incubated in 37°C serum-free DMEM with 10 mM HEPES containing either 10 mM MβCD (Sigma-Aldrich) at 37°C for 12 min or 4 U/ml of cholesterol oxidase (CO; Calbiochem) at 37°C for 2 h. For lovastatin treatment, cells were cultured for 24 h in serum-free DMEM containing 5 μM lovastatin (Calbiochem). Following drug treatment, cells were washed twice before performing experiments.
Fluorescence correlation spectroscopy
Measurements were performed as previously described (Wawrezinieck et al, 2005). Briefly, FCS data were collected using an Axiovert 200M microscope equipped with a Zeiss C-Apochromat × 40 NA 1.2, water-immersion objective and with excitation from a 488 nm line of an Ar+ ion laser. The laser waist was set by selecting the lateral extension of the laser beam falling into the back aperture of the objective with a diaphragm. Fluorescence was collected through the same objective, separated from the excitation light by a dichroic mirror and sent onto avalanche photodiodes through a 525–565 nm bandpass filter. FCS measurements were performed in HANK's buffered solution with 10 mM HEPES pH 7.4 by illuminating the sample with an excitation power of <4 μW at the back aperture of the objective lens. Autocorrelations were processed by a hardware correlator (ALV-GmBH) and data were analyzed with built-in functions of IgorPro (Wavemetrics).
Cytoskeleton fraction isolation
Actin cytoskeleton isolation was carried out as previously described (Bodin et al, 2005) with slight modifications. Briefly, cells were solubilized for 10 min in cytoskeleton lysis buffer (0.5% Triton X-100 (v/v), protease inhibitors cocktail, 1 mM Na3VO4, 20 mM EGTA and 100 mM Tris pH 7.4) and then centrifuged (15 000 g, 15 min, 4°C). The Triton X-100-insoluble pellet was washed once with the lysis buffer and then with the lysis buffer without Triton X-100 to obtain the cytoskeleton fraction. To obtain the cytoskeleton-linked DRM, the isolated cytoskeleton fraction was incubated with 1 ml of 0.6 M KI for 30 min at 4°C to depolymerize the actin filaments (Payrastre et al, 1991; Bodin et al, 2005) before being subjected to the DRM preparation as described above.
Internalization assays
Assessment was performed after synchronized stimulation. L12.10.mFas or L12.10.mFas-C194V cells (106) were incubated with 200 ng/ml rhFasL with 1 μg/ml M2 or with M2 only (control for background binding) in DMEM supplemented with 5% FBS and 10 mM HEPES on ice for 45 min (except for CO, MβCD and lovastatin experiments, which were performed without serum), followed by two washes with ice-cold medium to remove unbound FasL. After adding cold medium (0.5 ml), cells were warmed and kept at 37°C, 5% CO2 for the indicated time to trigger synchronized Fas stimulation or were kept on ice (control, 0 min activation). The activation was stopped by adding ice-cold medium. Cells were then washed and incubated for 30 min on ice with goat anti-mouse Ig-FITC. Surface-bound FasL was analyzed by flow cytometry. To assess the degree of internalization of Fas–FasL complexes, which corresponded to the decrease in surface-bound FasL, the mean fluorescence intensity (MFI) of the background control cells (incubated with M2 only) was subtracted from the MFI of FasL-bound cells at each time point to obtain MFIabsolute. The MFIabsolute of stimulated cells was divided by the MFIabsolute of control non-activated cells (t=0) to obtain the percentage of FasL remaining on the cell surface.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Material
Acknowledgments
We thank M Sekoni for technical assistance and AM Bernard for advices in rafts preparation. This work was supported by institutional funds from the Centre National de la Recherche Scientifique (CNRS), and by grants from the Ligue nationale contre le cancer (LNCC), the Association pour la Recherche contre le Cancer (ARC), the Emerald Foundation, Canceropole-PACA ACI 2004. KC and ZH are supported by a postdoctoral fellowship from the Susan G Komen Breast Cancer Foundation and a doctoral fellowship from the Ligue nationale contre le cancer (LNCC), respectively.
References
- Algeciras-Schimnich A, Peter ME (2003) Actin dependent CD95 internalization is specific for type I cells. FEBS Lett 546: 185–188 [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME (2002) Molecular ordering of the initial signaling events of CD95. Mol Cell Biol 22: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouad SM, Cohen LY, Sharif-Askari E, Haddad EK, Alam A, Sekaly RP (2004) Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death. J Immunol 172: 2316–2323 [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Legembre P, Pietras E, Bubici C, Franzoso G, Peter ME (2004) CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J 23: 3175–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidere N, Su HC, Lenardo MJ (2006) Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol 24: 321–352 [DOI] [PubMed] [Google Scholar]
- Bodin S, Soulet C, Tronchere H, Sie P, Gachet C, Plantavid M, Payrastre B (2005) Integrin-dependent interaction of lipid rafts with the actin cytoskeleton in activated human platelets. J Cell Sci 118: 759–769 [DOI] [PubMed] [Google Scholar]
- Bodin S, Welch MD (2005) Plasma membrane organization is essential for balancing competing pseudopod- and uropod-promoting signals during neutrophil polarization and migration. Mol Biol Cell 16: 5773–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahuzac N, Baum W, Kirkin V, Conchonaud F, Wawrezinieck L, Marguet D, Janssen O, Zornig M, Hueber AO (2006) Fas ligand is localized to membrane rafts, where it displays increased cell death-inducing activity. Blood 107: 2384–2391 [DOI] [PubMed] [Google Scholar]
- Draberova L, Amoui M, Draber P (1996) Thy-1-mediated activation of rat mast cells: the role of Thy-1 membrane microdomains. Immunology 87: 141–148 [PMC free article] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Sargiacomo M, Ricci-Vitiani L, Todaro M, Stassi G, Messina CG, Parolini I, Lotti F, Sette G, Peschle C, De Maria R (2004) CD95 death-inducing signaling complex formation and internalization occur in lipid rafts of type I and type II cells. Eur J Immunol 34: 1930–1940 [DOI] [PubMed] [Google Scholar]
- Fais S, De Milito A, Lozupone F (2005) The role of FAS to ezrin association in FAS-mediated apoptosis. Apoptosis 10: 941–947 [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F (2001) The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood 98: 3860–3863 [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F (2005) Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem 280: 11641–11647 [DOI] [PubMed] [Google Scholar]
- Gajate C, Del Canto-Janez E, Acuna AU, Amat-Guerri F, Geijo E, Santos-Beneit AM, Veldman RJ, Mollinedo F (2004) Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J Exp Med 200: 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosch M, Rigler R (2005) Fluorescence correlation spectroscopy of molecular motions and kinetics. Adv Drug Deliv Rev 57: 169–190 [DOI] [PubMed] [Google Scholar]
- Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML (2002) The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling. Biochim Biophys Acta 1589: 140–150 [DOI] [PubMed] [Google Scholar]
- Henkler F, Behrle E, Dennehy KM, Wicovsky A, Peters N, Warnke C, Pfizenmaier K, Wajant H (2005) The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL–Fas clusters of high stability. J Cell Biol 168: 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herincs Z, Corset V, Cahuzac N, Furne C, Castellani V, Hueber AO, Mehlen P (2005) DCC association with lipid rafts is required for netrin-1-mediated axon guidance. J Cell Sci 118: 1687–1692 [DOI] [PubMed] [Google Scholar]
- Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT (2002) An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep 3: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya N, Scherfeld D, Bacia K, Schwille P (2004) Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J Struct Biol 147: 77–89 [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14: 5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC (2006) The role of receptor internalization in CD95 signaling. EMBO J 25: 1009–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legembre P, Barnhart BC, Peter ME (2004a) The relevance of NF-kappaB for CD95 signaling in tumor cells. Cell Cycle 3: 1235–1239 [DOI] [PubMed] [Google Scholar]
- Legembre P, Barnhart BC, Zheng L, Vijayan S, Straus SE, Puck J, Dale JK, Lenardo M, Peter ME (2004b) Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep 5: 1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legembre P, Schickel R, Barnhart BC, Peter ME (2004c) Identification of SNF1/AMP kinase-related kinase as an NF-kappaB-regulated anti-apoptotic kinase involved in CD95-induced motility and invasiveness. J Biol Chem 279: 46742–46747 [DOI] [PubMed] [Google Scholar]
- Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D (2006) Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J 25: 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani F, Matarrese P, Giammarioli AM, Lugini L, Lozupone F, Federici C, Iessi E, Malorni W, Fais S (2004) CD95/phosphorylated ezrin association underlies HIV-1 GP120/IL-2-induced susceptibility to CD95(APO-1/Fas)-mediated apoptosis of human resting CD4(+)T lymphocytes. Cell Death Differ 11: 574–582 [DOI] [PubMed] [Google Scholar]
- Marguet D, Lenne PF, Rigneault H, He HT (2006) Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J 25: 3446–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji M, Jin ZX, Yamaoka S, Amakawa R, Fukuhara S, Sato SB, Kobayashi T, Domae N, Mimori T, Bloom ET, Okazaki T, Umehara H (2005) Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med 202: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Siegel RM (2004) Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol 5: 182–189 [DOI] [PubMed] [Google Scholar]
- Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S (2000) CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J 19: 5123–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payrastre B, van Bergen en Henegouwen PM, Breton M, den Hartigh JC, Plantavid M, Verkleij AJ, Boonstra J (1991) Phosphoinositide kinase, diacylglycerol kinase, and phospholipase C activities associated to the cytoskeleton: effect of epidermal growth factor. J Cell Biol 115: 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, Luzzi P, Di Fiore PP, Tacchetti C (2005) Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol Biol Cell 16: 2704–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451: 1–16 [DOI] [PubMed] [Google Scholar]
- Resh MD (2004) Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem 37: 217–232 [DOI] [PubMed] [Google Scholar]
- Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R (2005) Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem 280: 26425–26434 [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274: 22532–22538 [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM (2002) The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun 297: 876–879 [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S (2004) Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21: 415–428 [DOI] [PubMed] [Google Scholar]
- Seveau S, Eddy RJ, Maxfield FR, Pierini LM (2001) Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol Biol Cell 12: 3550–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys JE, Linder ME (2004) Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem 73: 559–587 [DOI] [PubMed] [Google Scholar]
- Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM (2002) Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17: 451–462 [DOI] [PubMed] [Google Scholar]
- Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A (2001) Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol 155: 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrezinieck L, Rigneault H, Marguet D, Lenne PF (2005) Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys J 89: 4029–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Material


