Abstract
Dendritic cells (DCs) are the only antigen-presenting cell population having a cross-presentation capacity. For cross-presentation, however, the intracellular antigen-processing pathway and its regulatory mechanism have not been defined. Here we report the differences in cross-presentation ability among murine bone marrow-derived immature DC, early immature day8-DC and late immature day10-DC, and fully mature day10 + lipopolysaccharide DC. Day8-DCs and day10-DCs show an immature phenotypic profile but are different in morphology. Day8-DCs can internalize an abundant volume of exogenous soluble ovalbumin (OVA) and result in cross-presentation. In contrast, day10-DCs are not able to cross-present, although they maintain efficient macropinocytosis. Exogenously internalized OVA antigens are stored in the endocytic compartments. The endocytic compartments are temporarily maintained at mildly acidic pH in day8-DCs and are rapidly acidified in day10-DCs after uptake of antigens. We show that OVA antigens accumulated in the endocytic compartments move into the cytosol in day8-DCs but do not in day10-DCs. NH4Cl-treatment, which neutralizes the acidic endocytic compartments and/or delays endosomal maturation, restores day10-DCs for transport the stored OVA antigens from the endocytic compartments into the cytosol. Diphenyleneiodonium chloride-treatment, which acidifies the endocytic compartments, decreases an amount of transported OVA antigen into the cytosol in day8-DCs. These data indicate that only the early immature stage of DC interferes with endosomal maturation, even after uptake of exogenous antigens, and then transports the antigens into the cytosol.
Keywords: dendritic cells, MHC class I, antigen presentation, antigen processing, cross-presentation
Introduction
Major histocompatibility complex (MHC) class I molecules are generally associated with the peptides derived from endogenously synthesized proteins such as tumour proteins and virus-encoded proteins.1 Via MHC class I molecules, tumour cells and virus-infected non-haematopoietic cells can directly present the endogenous antigens to the primed cytotoxic T lymphocytes (CTLs).1,2 Those cells, however, are unable to stimulate naive CD8+ T cells or resting CTLs because of the low expression level of MHC class I, adhesion molecules, and costimulatory molecules on the cell surface.2 Thus, the initial generation of CTL against transplants,3 tumours4 and viral antigens2 requires immune priming by professional antigen-presenting cells (APC), especially dendritic cells (DC).5 Currently several authors describe how DCs can internalize the exogenous antigens and present them via MHC class I molecules to CD8+ T cells. This process is referred to as cross-presentation.5–7 There have been two explanations for the intracellular exogenous antigen-processing pathway for cross-presentation. First, internalized exogenous antigens are transferred from the endocytic compartments (for example, phagosome/endosome) into the cytosol, leading to the conventional MHC class I pathway that is dependent on both the proteasome and the transporter associated with antigen processing (TAP).8–10 Second, soluble ingested proteins can gain access to the lumen of the perinuclear endoplasmic reticulum (ER) in DCs and can be degraded into peptides that can access the MHC class I antigen-processing machinery directly in the ER.11 There may be an irrelevant non-cytosolic pathway in a TAP-independent manner,12,13 in which the antigens are processed into the antigenic peptides and associated with the pre-existing post-Golgi MHC class I molecules, probably in the endocytic compartments. However, little is known about the exact mechanisms of the antigen localization that is crucial for the generation of the antigenic peptides and their binding to MHC class I molecules.
DCs are the most potent professional APC and play a critical role to prime CD8+ CTL responses through cross-presentation in vivo.14,15 The first step of cross-presentation is an uptake of antigen. Immature DCs efficiently endocytose exogenous antigens via several antigen receptors.16–19 Various receptors are involved in antigen uptake in the DCs. DCs express αVβ3, αVβ5 and CD36, which recognize apoptotic cells,16,17 C-type lectin receptors (mannose receptor18 and DEC-20519) for mannans, and Fcγ receptor for antigen–immunoglobulin G (IgG) immune complex.20 Antigen uptake is down-regulated upon maturation of DC,21 although lipopolysaccharide (LPS)-activated DCs transiently up-regulated phagocytosis before down-regulating it.22,23 In vivo, tissue-residing immature DCs, such as Langerhans cells in the skin, possess a high endocytic or phagocytic capacity until they migrate from the periphery into draining lymph nodes upon DC maturation.21,24,25 Although macrophages, rather than DCs, efficiently phagocytose virus-infected apoptotic cells in vitro, they fail to prime the antigen-specific naive CD8+ T cells.16 Thus, the outcome of cross-presentation by DCs would require not only efficient antigen uptake but also the additional regulatory system in the processing and/or the intracellular transport of the internalized antigens.
In immature DCs, internalized antigens are preferentially sorted and accumulated into the distinct compartments. Intermediately mature DCs contain the non-lysosomal class II vesicles (CIIV) which include antigenic peptide–MHC class II complexes, MHC class I, and B7 molecules.26 Lutz et al. reported that the endocytic compartments formed in a splenic immature DC cell line were mildly acidic and exhibited the phenotype such like rab7–, lysosome-associated membrane protein-1 (Lamp-1)+, protease cathepsin D+, and MHC class II molecule+.27 Also, DC maturation induces activation of the vacuolar proton pump that enhanced lysosomal acidification.28 Currently, Delamarre et al. reported that the presentation of exogenous antigens on MHC class I molecules is activated by only some of several signals that activate MHC class II-dependent presentation, indicating that MHC class I-dependent cross-presentation has a distinct pattern from MHC class II-dependent direct presentation.29 It is implicated that the microenvironment in the endocytic compartments may play important roles in MHC class I molecules-mediated exogenous antigen presentation.
The ability of cross-presentation in DC is down-regulated upon maturation of DC. We analysed changes in the pH level of endocytic compartments upon DC maturation after the antigen internalization, and examined the ability of antigen trafficking into the cytosol from the endocytic compartments. We will discuss the mechanisms of regulation for transport of the exogenous antigens from the endocytic compartments into the cytosol, which appears to be essential for cross-presentation.
Materials and methods
Mice
C57BL/6 mice were purchased from Nihon SLC (Hamamatsu, Japan). TAP-deficient mice were bred in the animal facilities at Yokohama City University School of Medicine, Yokohama, Japan. Mice were used at 4–12 weeks old. They were maintained according to institutional guidelines under approved protocols in the Institute of Laboratory Animal Science (School of Medicine, Yokohama City University).
Reagents
Chicken ovalbumin (OVA, grade IV) was purchased from Sigma Chemicals (St. Louis, MO). The culture medium was RPMI-1640 (Gibco BRL, Rockville, MD) supplemented with 10% fetal calf serum (FCS), 200 U/ml penicillin (Banyu Pharmaceutical, Tokyo, Japan), 200 µg/ml streptomycin sulphate (Meiji Seika, Tokyo, Japan), 2 mm l-glutamine (Wako Pure Chemical Industries, Osaka, Japan), and 10 mmN-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES; Dojindo Laboratories, Kumamoto, Japan). Murine recombinant granulocyte–macrophage colony-stimulating factor (rGM-CSF) was kindly provided by Kirin Brewery (Gumma, Japan). Murine recombinant interleukin-4 (IL-4) was purchased from BD PharMingen (San Diego, CA). NH4Cl, diphenyleneiodonium chloride (DPI), leupeptin, and pepstatin A were purchased from Sigma.
Cell lines
RF33-70 is a CD8+ T hybridoma specific for OVA257−264, SIINFEKL bound to H-2Kb, which was a gift from Dr Rock KL. CTLL-2 was purchased from the American Type Culture Collection (Rockville, MD).
Antibodies
The antibodies used for fluorescence-activated cell sorter (FACS) analyses were fluorescein isothiocyanate (FITC)-conjugated anti-H2-KbDb monoclonal antibodiy (mAb), CTDb (Cedarlane Laboratories, Hornby, Ontario, Canada), phycoerythrin (PE)-conjugated anti-mouse MHC class II mAb, NIMR-4 (Southern Biotechnology Associates, Birmingham, AL), biotin-conjugated anti-CD11c mAb, HL-3 (BD PharMingen), and PE-conjugated anti-mouse CD80 mAb, 16-10A1 (BD PharMingen), FITC-conjugated anti-mouse CD86 mAb, GL-1 (eBioscience, Nagoya, Japan), biotin-conjugated anti-mouse CD40 mAb, 1C10 (eBioscience), biotin-conjugated mouse anti-H2-Kb-SIINFEKL mAb, 25-D1.16 (a kind gift of Dr Germain RN). FITC-conjugated ultra-avidin (Leinco Technologies, St. Louis, MO), PC5-conjugated streptavidin (Coulter-Immunotech, Marseille, France), FITC-conjugated goat immunoglobulins (H+L) (Southern Biotechnology Associates), PE-conjugated rat IgG1κ (BD PharMingen) were used in these experiments. The antibody used for immunoprecipitation and Western blot analyses was biotin-conjugated rabbit anti-OVA polyclonal antibody, which was made in our laboratory. We confirmed the OVA specificity of this antibody by Western blot and enzyme-linked immunosorbent assay.
Bone marrow-derived dendritic cells
Bone marrow (BM)-derived DCs were obtained according to the modified method of Lutz.30 Briefly, femurs and tibiae from 4–12-week-old female mice were removed and BM cells were flushed with the culture medium using a 26 gauge needle (Terumo, Tokyo, Japan). Cells were incubated in 0·83 m ammonium chloride tris(hydroxymethyl)aminomethane (Tris) buffer for 2 min to remove erythrocytes and washed twice. BM cells (4–6 × 107) were obtained per mouse. BM cells (4–6 × 106) were seeded per 100 mm dish in culture medium supplemented with 200 U/ml murine GM-CSF and 0·45–1·35 × 102 U/ml murine IL-4 (BD PharMingen). On days 3 and 6, fresh culture medium was added to the plates. On day 8, 0·9–1·2 × 107 weakly adherent cells were collected by gentle pipetting and were used as immature day8-DCs. Day8-DCs were transferred into new plates and cultured again for two more days in the presence of GM-CSF and IL-4. The harvested cells were used as day10-DCs. We prepared day10+LPS-DCs from the culture of day8-DCs with 1 µg/ml LPS during the last 24 hr of culture for two more days. Approximately 80% of day8-DCs and 95% of day10-DCs were MHC class II molecule+ and CD11c+ cells.
FACS analysis
The antibodies used for FACS analyses were CTDb, NIMR-4, 16-10A1, HL-3, 1C10, GL-1, and 25-D1.16. For the direct immuno-staining of MHC class I molecules, MHC class II molecules, CD80, and CD86, 1 × 106 cells were incubated with each mAb on ice for 30 min. Immuno-staining for CD11c and CD40 was performed by an indirect immuno-staining method using FITC-conjugated streptavidin (for 1C10 and HL-3) as secondary antibody. For the detection of intracellular H-2Kb-OVA257−264 (SIINFEKL) complexes, the cells were fixed with 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) on ice for 20 min, washed twice with PBS, and then permeabilized with 0·1% saponin, followed by immuno-staining with anti-H2-Kb-SIINFEKL complex mAb, 25-D1.16. For negative controls, the cells were incubated with FITC-conjugated goat immunoglobulins (for CTDb and GL-1), PE-conjugated rat IgG1κ (for NIMR-4 and 16-10A1), ultraavidin-FITC (for 25-D1.16), and streptavidin-PC5 (for 1C10 and HL-3). Fluorescence staining was analysed with a FACScan flow cytometer and CELLQuest Software (Becton Dickinson Immunocytometry Systems, San Jose, CA). In some experiments, data are expressed as mean fluorescence intensity (MFI) values after subtraction of the MFI obtained with the control mAb.
Antigen processing and presentation assays
DCs were seeded at 1–5 × 106 cells/well into 6-well flat bottom tissue culture plates (Sumitomo Bakelite, Tokyo, Japan) and incubated with NH4Cl (0, 10, 20, 50, and 100 mm) for 30 min at 37°. Leupeptin, pepstatin A, or DPI was used instead of NH4Cl in some experiments. Then, OVA protein was added at a final concentration of 1 mg/ml, and the DCs were incubated for either an additional 4 or 6 hr at 37° in the continued presence or absence of NH4Cl. The DCs were washed twice with ice-cold culture medium to remove OVA and NH4Cl. The DCs were fixed with 2% PFA in PBS for 10 min on ice, and then washed. DCs (1 × 104) and RF33-70 (5 × 104) were incubated in 200 µl of culture medium in 96-well flat bottom culture plates (Becton Dickinson Labware, Franklin Lakes, NJ). After 20–24 hr, 100 µl of supernatants was harvested and the IL-2 content was assessed with CTLL-2 cell line. CTLL-2 cells (1 × 104) were added to test supernatants and were cultured at 37° for 20–24 hr. [Methyl-3H]thymidine (ICN Pharmaceuticals, Costa Mesa, CA) was added at 0·5 µCi/well during the final 6 hr of culture and thymidine incorporation was measured as counts per minute (c.p.m.). As a control for the toxicity of reagents, we used the OVA257−264 peptide at a final concentration of 1 µg/ml instead of OVA protein.
Preparation of endocytic compartment fraction from DCs and detection of OVA and its degraded products
Day8-DCs and day10-DCs were treated with NH4Cl or DPI for 30 min. The DCs were pulsed with 1 mg/ml soluble biotin-conjugated OVA for various times (1, 2 and 4 hr) and washed. The DCs were collected in the homogenization buffer (250 mM sucrose, 3 mM Tris-HCl (pH 7·4), 0·1 mM ethylenediaminetetraacetic acid (EDTA)) and homogenized by passages through a 26G needle on ice until Trypan Blue stained 80–90% of the cells. The postnuclear supernatant was ultracentrifuged to identify the cytosol fraction at 100 000 g for 1 hr at 4°. Then the pellet was used as the endocytic compartment enriched fraction. The contamination of endocytic compartment fraction into the cytosol fraction was examined by β-hexosaminidase activity, which is specifically localized in the endosome and the lysosome. We confirmed that the β-hexosaminidase activity in the cytosol fraction was less than 10% of that in whole cell lysate. Internalized OVA-biotin in each fraction was immunoprecipitated by rabbit anti-OVA polyclonal Ab and protein G Sepharose™ (Amersham Pharmacia Biotech AB, Uppsala, Sweden). Samples of each fraction were loaded on a 12·5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel, and detected by Western blot using peroxidase-conjugated Streptavidin (Beckman Coulter, Hialeah, FL).
pH indicator
To qualitatively assess the pH level in the endocytic compartments, DCs were grown on micro slide glasses (Matsunami Glass Ind., Osaka, Japan) for 1 hr in culture medium, and then pulsed for 4 hr at 37° in the presence of the pH sensitive fluorescence dye, LysoSensor Yellow/Blue DND-160® (L-7545; Molecular Probes, Eugene, OR) at a final concentration of 5 µM. DCs were washed twice with complete medium and twice with cold PBS to remove the LysoSensor®, and then fixed with 2% PFA for 20 min on ice. The endocytic compartments were visualized using an Olympus Provis microscope (Olympus, Tokyo, Japan) using a triple pass (blue/green/red) cube, which allows the excitation at 384 nm and the collection at 540 nm. In some experiments, the neutralization of endocytic compartments in day10-DCs was determined. DCs were incubated with 50 mm NH4Cl at 37° for 30 min before and during LysoSensor® pulsing.
Results
Day8-DCs cross-present the exogenous antigens via MHC class I molecules but day10-DCs do not, although they can efficiently internalize
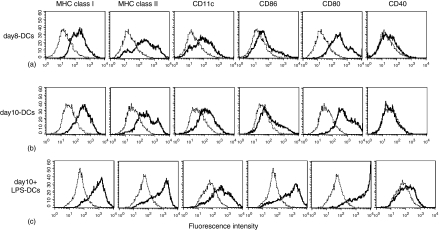
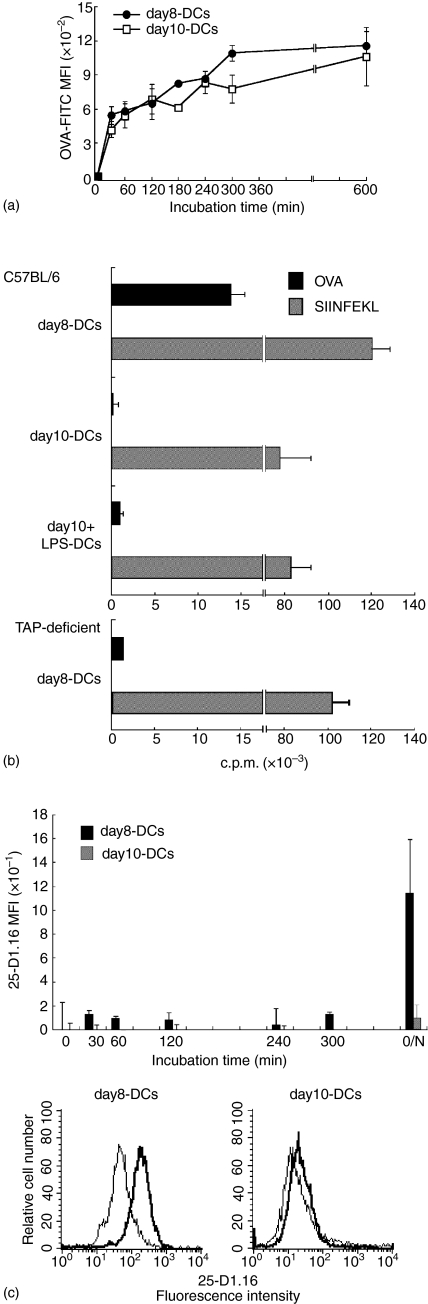
We prepared three types of bone marrow-derived DCs to examine the antigen-processing pathway of cross-presentation upon DC maturation. Immature DCs were obtained by culturing BM cells with GM-CSF and IL-4 for 8 days (day8-DCs) and for 10 days (day10-DCs). LPS-stimulated fully mature DCs (day10+LPS-DCs) were generated as described in Materials and Methods. We examined the expression of phenotypic markers on these DCs (Fig. 1). Day8-DCs and day10-DCs expressed comparably MHC class I, MHC class II, CD11c, CD80, CD86 and showed no expression of CD40. We compared the cell size and the morphology between day8-DCs and day10-DCs. Day10-DCs exhibited a larger cell size, a higher granularity, and more strongly adherent veils than day8-DCs (data not shown). Day10+LPS-DCs expressed all molecules at higher levels than both day8-DCs and day10-DCs. The flow cytometric analysis showed that the surface expression on day8-DCs and day10-DCs showed the immature DC maturation stage phenotype, because immature DCs express a low level of MHC molecules, CD11c, CD80, CD86, and very little CD40.16,18–20 In contrast, day10+LPS-DCs exhibited a fully matured phenotype, which was induced by the addition of maturation stimulus of LPS. Next, we compared the ability of antigen uptake in day8-DCs and day10-DCs. Both day8-DCs and day10-DCs were able to efficiently take up FITC-labelled soluble OVA by macropinocytosis (Fig. 2a). Mature day10+LPS-DCs were defective in their antigen uptake ability (data not shown). We observed that the amount of internalized OVA antigens reached to a plateau at 4 hr in day8-DCs and day10-DCs.
Figure 1.
FACS analysis reveals the surface profile of day8-DCs, day10-DCs, and day10+LPS-DCs. BM-DCs were generated in vitro in culture medium containing GM-CSF and IL-4 for 8 days (a, day8-DCs), for 10 days (b, day10-DCs), and for 10 days with 1 µg/ml LPS during the last 24 hr (c, day10+LPS-DCs). Cell surface expressions of MHC class I, MHC class II, CD11c, CD86, CD80, and CD40 molecules were analysed with fluorescein-labelled antibodies by FACS analysis as described in Materials and Methods. Dotted line histograms representing controls were stained with isotype-matched antibodies.
Figure 2.
Day8-DCs can efficiently cross-present exogenous OVA antigens via MHC class I molecules, that is a TAP-dependent conventional MHC class I processing pathway. (a) DCs (1 × 104) derived from C57BL/6 (upper panel), TAP–/– (lower panel) mice were cocultured with RF33-70 (5 × 104) in the presence of 1 mg/ml soluble OVA or 1 µg/ml OVA257−264 peptide (SIINFEKL) for 24 hr. Culture supernatants were harvested and assessed for IL-2 content using the CTLL-2 proliferation assay. [3H]thymidine was added for measurement of CTLL-2 proliferation at 0·5 µCi/well during the final 6 hr of culture. Data represent one of more than two independent experiments and the error bars express the SD of data from triplicate wells. (b) DCs were incubated at 37° with 1 mg/ml OVA–FITC for described times. The harvested DCs were washed, fixed with 2% PFA, and used for FACS analysis. MFI were calculated based on MFI of OVA–FITC ingested by DCs. Data represent a mean of MFI of three independent experiments ± SEM. DCs were pulsed with 1 mg/ml soluble OVA for described time at 37°, fixed with 2% PFA, permeabilized with 0·1% saponin, and stained with 25.D1-16 to examine the intracellular expression of H2-Kb-SIINFEKL complex in DCs. Upper panel represents a mean of MFI of three independent experiments ± SEM. Lower panels show histogram profiles of the intracellular expression of H2-Kb-SIINFEKL complexes in DCs after the incubation with OVA for 24 hr. Dotted line histograms representing controls were stained with isotype-matched antibodies.
We compared the cross-presentation ability of day8-DCs and day10-DCs. It was determined by IL-2 secretion from RF33-70, which is a CD8+ T-cell hybridoma recognizing H-2Kb-OVA257−264 antigenic peptide (SIINFEKL) complex. We had confirmed that the cross-presentation by these DCs was dependent on the concentration of OVA and the response of RF33-70 reached the plateau at the concentration of 1 mg/ml OVA (data not shown). DCs were incubated with RF33-70 in the presence of 1 mg/ml soluble OVA protein or 1 µg/ml SIINFEKL for 24 hr. Day8-DCs could efficiently cross-present OVA antigens. In contrast, day10-DCs failed to do as well as day10+LPS-DCs (Fig. 2b, upper panel). It should be noted that day10-DCs and day10+LPS-DCs could efficiently stimulate RF33-70 in the addition of SIINFEKL. Also, the response of RF33-70 was indeed negligible without addition of DCs even in the presence of SIINFEKL (data not shown). Thus, RF33-70 hybridoma cells could not present SIINFEKL to each other.
Although day10-DCs can internalize exogenous antigens, they are unable to form MHC class I molecules and OVA peptide complex
Day8-DCs processed exogenous antigens for cross-presentation in a TAP-dependent manner, because day8-DCs from TAP-deficient mice could not cross-present exogenous OVA antigen to RF33-70 (Fig. 2b, lower panel). This TAP-dependent presentation system is known as the one of intracellular exogenous antigen-processing pathways for cross-presentation.8–10 In the TAP-dependent pathway, after the exogenous antigens are internalized into the endocytic compartments (macropinosomes and/or antigen retention compartments27), some of them would be exported into the cytosol and processed into antigenic peptides via proteasome to load the MHC class I molecules in ER through TAP. To confirm the processing of internalized OVA antigens in day8-DCs and day10-DCs, we examined the formation of MHC class I and SIINFEKL complexes intracellularly. DCs were incubated with 1 mg/ml soluble OVA for 30 min-18 hr at 37°, fixed with PFA, and stained with 25-D1·16 mAb recognizing the H2-Kb-SIINFEKL complex. In day8-DCs, we detected the expression of H2-Kb-SIINFEKL complexes on the cell surface (data not shown) as well as inside the cells (Fig. 2c) after only 30 min of OVA pulse. However, there was little intracellular expression of H2-Kb-SIINFEKL complexes even after 24 hr of pulse in day10-DCs. Taken together, day10-DCs could considerably maintain the ability to capture exogenous soluble antigens by macropinocytosis, but could not form the MHC–antigenic peptide complex intracellularly.
The endocytic compartments maintain a mildly acidic pH level only in day8-DCs after antigen uptake
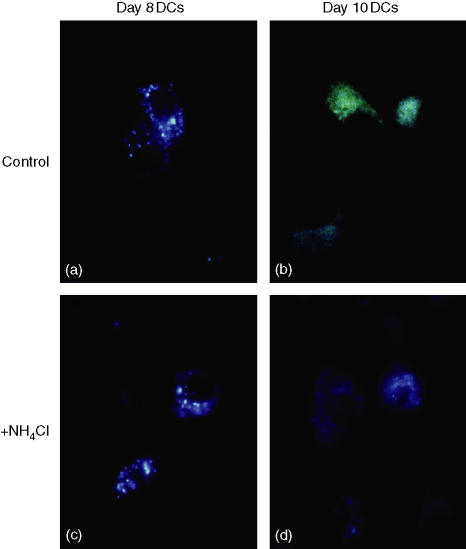
The internalized exogenous antigens are accumulated into the endocytic compartments before the antigens were exported into the cytosol.27 The endocytic compartments formed by endocytosis, in general, exhibit primarily a neutral pH level similar to that of outer environment, and rapidly gain the molecules associated with acidification and proteolysis by the repeated fusion to lysosome.31,32 DCs were reported to maintain a neutral pH level in the endocytic compartments and the internalized antigens had been accumulated without proteolysis during immature DC stage.27,28 We examined an internal pH level of the endocytic compartments in day8-DCs and day10-DCs by using LysoSensor®, pH indicator. LysoSensor® is a soluble acid-tropic probe representing pH and increases its fluorescence intensity upon acidification. The dye emits a prominently yellow fluorescence when it is accumulated in acidic vesicles, while it reveals a blue fluorescence in neutral organelles. LysoSensor® pulsed to day8-DCs and day10-DCs could be internalized as exogenous soluble antigens via macropinocytosis. We observed that LysoSensor® was accumulated into the endocytic compartments in more than 90% of DCs (data not shown). The endocytic compartments in day8-DCs remained blue-green-coloured at least for 4 hr after antigen uptake (Fig. 3a). However, Fig. 3(b) shows the green-coloured fluorescent vesicles dispersed around the nucleus in day10-DCs, that is evidently at a lower pH than that in the day8-DCs. The endocytic compartments were almost synchronized to be acidic only for 4 hr of LysoSensor® pulse. Taken together, although day10-DCs exhibited the immature phenotype with a lower expression of MHC and CD80, CD86 (Fig. 1) and the efficient ability of antigen uptake, their endocytic compartments could be acidified immediately after antigen uptake, like mature DC. Thus, day8-DCs are at an early phase and day 10-DCs are at a late phase in the immature DC stage.
Figure 3.
Day8-DCs exhibit a mildly acidic pH level in the endocytic compartments while day10-DCs show an acidic pH level. Upper panels; day8-DCs (a) and day10-DCs (b) were incubated with 5 µM LysoSensor® Yellow/Blue DND-160® for 4 hr at 37°. The DCs internalized LysoSensor® as exogenous soluble antigens. Lower panels; day8-DCs (c) and day10-DCs (d) were pretreated with 50 mm NH4Cl for 30 min and then incubated with LysoSensor® in presence of NH4Cl for 4 hr. The DCs were fixed with 2% PFA on ice for 20 min and visualized by fluorescence microscopy. Photographs shown are of typical cells.
Internalized OVA antigens are exported from the endocytic compartments into the cytosol in day8-DCs but not in day10-DCs
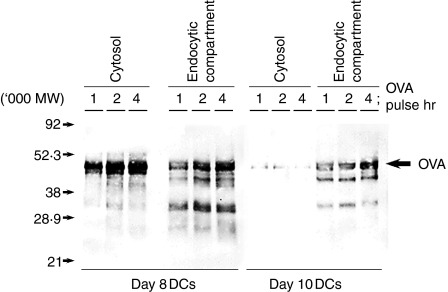
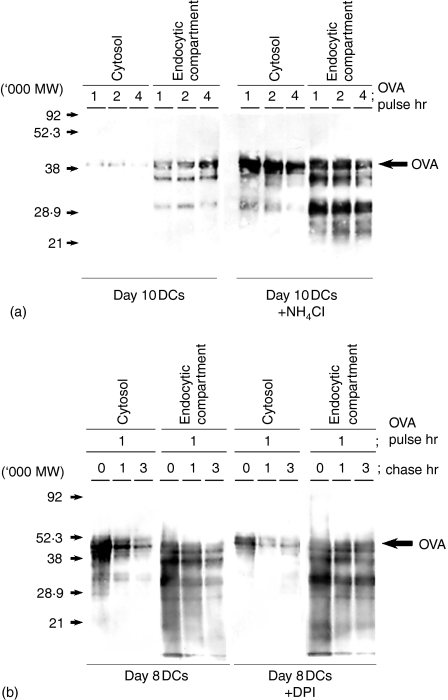
We examined the fate of the OVA antigens in DCs after the antigen uptake. Day8-DCs and day10-DCs were pulsed with 1 mg/ml soluble biotin-conjugated OVA antigen for 1–4 hr. We prepared an endocytic compartment fraction and a cytosolic fraction to determine the localization of OVA and its degraded products. OVA and its degraded products in each fraction were immunoprecipitated with anti-OVA polyclonal antibody and detected by Western blot with avidin-peroxidase. OVA and its degraded products were observed in the endocytic compartment fractions in day8-DCs and day10-DCs after 1 hr of antigen pulse. In the cytosolic fraction, a high amount of OVA and its products were detected after 1 hr of OVA pulse in day8-DCs but not in day10-DCs (Fig. 4). Thus, day8-DCs could internalize and accumulate the exogenous antigen in the endocytic compartments and then export them into the cytosol without degradation. In contrast, day10-DCs showed rapid acidification of the endocytic compartments and a defect in export of internalized exogenous antigen into the cytosol.
Figure 4.
Internalized OVA antigens are exported into the cytosol from the endocytic compartments in day8-DCs but not in day10-DCs. Day8-DCs and day10-DCs were pulsed with 1 mg/ml soluble biotin-conjugated OVA for various times (1, 2 and 4 hr). The endocytic compartment fraction and cytosol fraction were prepared from these DCs. Internalized OVA-biotin in each fraction was immunoprecipitated by rabbit anti-OVA polyclonal antibody. Samples of each fraction were loaded on a 12·5% SDS–PAGE gel, and detected by Western blot using peroxidase-conjugated streptavidin.
NH4Cl-treatment of day10-DCs restores cross-presentation ability
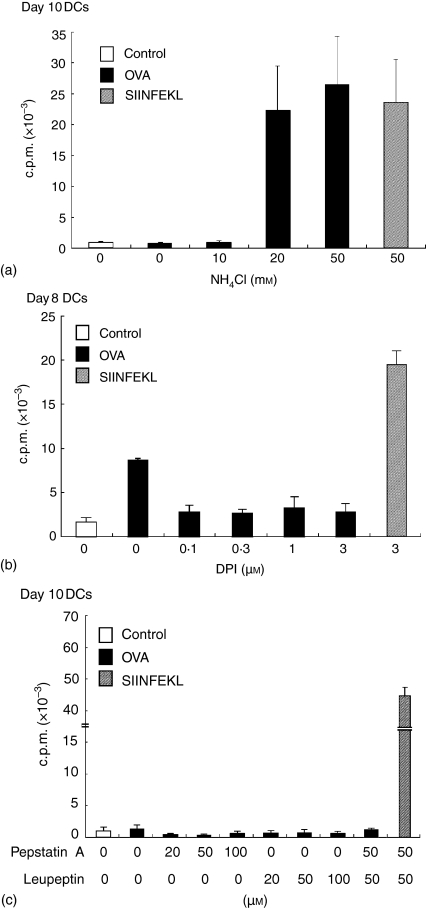
Acidification and maturation of the endocytic compartment is required to activate the proteases processing exogenous antigens presented by MHC class II molecules.33–35 Does the environment in the endocytic compartments play any roles on antigen processing pathway in cross-presentation? To address this question, we examined the cross-presentation ability of day10-DCs treated with 50 mm NH4Cl, which increases the pH in the vesicles36,37(Fig. 3d). We had confirmed that OVA uptake ability of NH4Cl-treated day10-DCs was comparable with that of untreated day10-DCs (data not shown). The DCs treated with NH4Cl were pulsed with 1 mg/ml soluble OVA for 4 hr and then fixed with PFA. As shown in Fig. 2(b), day10-DCs without NH4Cl treatment could do little cross-presentation of soluble OVA to RF33-70. However, NH4Cl-treated day10-DCs recovered the ability of cross-presentation (Fig. 5a). We also examined the effect of cross-presentation in day8-DCs treated with DPI, which is an inhibitor of NADPH oxidase, to acidify the compartments.38 Day8-DCs were treated with 10 µm DPI for 30 min and then were pulsed with OVA for 6 hr in the presence of DPI to test for cross-presentation. DPI treatment resulted in suppression of the effect of cross-presentation in day8-DCs (Fig. 5b). We examined the effects of protease inhibitors, leupeptin and pepstatin A on cross-presentation by day10-DCs. These reagents are the inhibitors of lysosomal cysteine protease (cathepsin B, L, and S) and aspartyl protease (cathepsin D), and do not change the pH level in the endocytic compartments of DCs (data not shown). As shown in Fig. 5(c), the treatment with leupeptin or pepstatin A could not recover the cross-presenting ability of day10-DCs. DCs treated with these reagents could efficiently present SIINFEKL to RF33-70 hybridoma. Therefore, treatment with these reagents was not toxic to DCs.
Figure 5.
Day10-DCs recover their ability of cross-presentation by the treatment with NH4Cl, and while day8-DCs lose the ability by the treatment with DPI. Day10-DCs (a) were pretreated with NH4Cl (0, 10, 20, 50, and 100 mM) for 30 min at 37°. Day10-DCs (c) were pretreated with pepstatin A (0, 20, 50, and 100 µM) and/or leupeptin (0, 20, 50, and 100 µM) for 30 min at 37°. These DCs were then pulsed for 4 hr with or without 1 mg/ml soluble OVA or 1 µg/ml SIINFEKL in the continued presence of each regent. Day8-DCs (b) were pretreated with DPI (0, 0·1, 0·3, 1, and 3 µm) for 30 min at 37°. The DCs were then pulsed for 6 hr with or without 1 mg/ml soluble OVA or 1 µg/ml SIINFEKL in the continued presence of DPI. These DCs were washed and fixed with 2% PFA on ice for 10 min, and tested for their cross-presentation ability to RF33-70 as described in the legend for Fig. 2.
NH4Cl-treatment of day10-DCs restores the export of OVA from the endocytic compartments to the cytosol
We examined the trafficking of exogenous antigen from the endocytic compartment into the cytosol in NH4Cl-treated day10-DCs and DPI-treated day8-DCs (Fig. 6). Day10-DCs were pulsed with OVA for 1–4 hr in the presence of NH4Cl. Day8-DCs were pulsed with OVA for 1 hr and chased for 1–3 hr in the presence of DPI. We detected intact molecular size and the degraded products of OVA in the cytosolic fraction in untreated day8-DCs (Fig. 6b) and also in NH4Cl-treated day10-DCs (Fig. 6a). DPI treatment decreased the amount of OVA detected in the cytosol in day8-DCs at 1 hr of OVA pulse (Fig. 6b). Thus, the physiological conditions in the endocytic compartments influences the transport of internalized exogenous antigens into the cytosol and the cross-presentation ability.
Figure 6.
Day10-DCs recover their ability transport of OVA antigen into the cytosol by the treatment with NH4Cl, and while day8-DCs lose the ability by the treatment with DPI. Day10-DCs (a) were pretreated with or without 20 mm NH4Cl for 30 min and pulsed with 1 mg/ml soluble biotin-conjugated OVA for various times (1, 2 and 4 hr). Day8-DCs (b) were pretreated with or without 10 µM DPI for 30 min, pulsed with 1 mg/ml soluble biotin-conjugated OVA for 1 hr, and chased at various times (0, 1 and 3 hr). The endocytic compartment fraction and cytosol fraction were prepared from these DCs. Immunoprecipitation and Western blot analysis as described in the legend for Fig. 4.
Discussion
We have shown tight correlation between DC maturation and the intracellular exogenous antigen-processing pathway, which is important for cross-presentation. We showed that only early immature DCs could transport ingested antigens from the endocytic compartments into the cytosol and perform cross-presentation. In our experiments, we used two phases of immature DCs. Both day8-DCs and day10-DCs showed a low expression of MHC and accessory molecules on the cell surface (Fig. 1) and had an efficient ability of antigen uptake (Fig. 2a). Immature DCs exhibit lower MHC class I, MHC class II, and CD80/86 expression levels on the cell surface and more efficient ability of antigen uptake than mature DCs.16,18–20 Therefore, both day8-DCs and day10-DCs used in our experiments are at an immature stage on DC maturation. Day10-DCs showed more rapid acidification of the endocytic compartments after antigen uptake (Fig. 3b) than that of day8-DCs (Fig. 3a). Lutz et al. reported that the acidification of the endocytic compartments formed by endocytosis is repressed in the immature DC cell line.27 Trombetta et al. reported that DC maturation induces activation of the vacuolar proton pump that enhances the acidification of lysosome.28 In our experiment, day10-DCs were generated by pipetting and replating of day8-DCs. Delamarre et al. reported that two stimuli are able to induce functional maturation; these are CD40 ligation and the physical stimulation by disruption of cell-cell contact.29 Furthermore, day10-DCs exhibited a larger cell size, a higher granularity, and more strongly adherent veils than day8-DCs (data not shown). Therefore day10-DCs are partially maturing in contrast to day8-DCs. Thus, day8-DCs are at an early phase in the immature stage, and day10-DCs remain an immature phenotypic feature but have already started on maturation pathway.
We showed that cross-presentation was observed in early immature day8-DCs but not in late immature day10-DCs (Fig. 2b), although both DCs were able to uptake the exogenous antigen (Fig. 2a). Here, we demonstrated that day8-DCs could transport the ingested antigen from the endocytic compartments into the cytosol, but day10-DCs could not do (Fig. 4). We conclude that the cross-presentation pathway requires two steps, the first step is antigen internalization and the second is antigen transport into the cytosol. Both steps associate tightly with DC maturation stage. Cross-presentation of exogenous antigen is reported to access the several antigen processing pathways, most of which are dependent on both proteasome and TAP (Fig. 2b) in the cytosol.8–11 Thus, it is essential for cross-presentation to transport ingested antigens into the cytosol and access the conventional MHC class I pathway. The endocytic compartments in day8-DCs maintained neutral or mildly acidic pH even after uptake of antigens (Fig. 3a). In immature DCs, internalized antigens are preferentially sorted and accumulated into the distinct endocytic compartments, which are termed to ‘the retention compartments’ by Lutz et al.27 The compartments include antigen peptide–MHC class II complexes, MHC class I, B7 molecules, and Lamp-1. Maturation of the endocytic compartment in DCs is in parallel with DC maturation and is dependent on repeated fusions to late endosome and/or lysosome, which lowers a pH with an activation of vacuolar H+–ATPase.28,29 Then, the low pH activates the vesicular acid proteases in the endocytic compartments to induce the proteolysis of antigens.28,29 What differences between day8-DC and day10-DCs could determine the ability of antigen transport into the cytosol? There are three possibilities to explain the export of internalized exogenous antigens into the cytosol. The first possibility is that activation of acid protease in the endocytic compartments is important for transport of antigens into the cytosol. Maturation of endosome/phagosome is known to induce the acidification, and followed by the activation of the lysosomal acidic proteases such as cathepsin D, B, L, and S.31–35 It is possible that acid proteases are inactive in the mildly acidic endocytic compartments in both day8-DCs and NH4Cl-treated day10-DCs, which may protect the ingested antigen from the complete processing, and then the antigen is exported into the cytosol. However, this possibility is unlikely, because the treatment of day10-DCs with inhibitors of proteases, leupeptin and pepstatin A, could not restore the cross-presentation (Fig. 5c). Also, when protease-insensitive dextran–FITC was used as a probe, day8-DCs could transport them into the cytosol but day10-DCs could not do. NH4Cl treatment of day10-DCs could restore the dextran-FITC antigen export into the cytosol (data not shown).
Our data might support two other possibilities, not mutually exclusive, to explain mechanisms of regulation for export of ingested antigens into the cytosol. One possibility is that mildly acidic pH in the endocytic compartments plays an important role for the transport of ingested antigens, and the others is that the delayed fusion with late endosome/lysosome is important for the transport. Day8-DCs maintained mildly acidic pH in the endocytic compartment even after the uptake of antigen (Fig. 3a) and then could transport these antigens into the cytosol (Fig. 4). Na+/H+ exchange, Na+/K+ vacuolar-type ATPase, and NADPH oxidase associate with the acidification of phagosomes.36–39 NH4Cl inhibits the acidification of the endocytic compartments as acidotropic agents.37 DPI is an inhibitor of NADPH oxidase to acidify the compartments.38 NH4Cl treatment of day10-DCs changed the acidic pH into mild acidic pH (Fig. 3d) and then restored the transport of the internalized exogenous antigens from the endocytic compartments into the cytosol (Fig. 6a). DPI treatment of day8-DCs lost this ability (Fig. 6b). Thus, it is possible that antigen transport is dependent on mildly acidic pH-inducible and/or fully acidic pH-resistant machinery in the endocytic compartments of DCs. However, we cannot exclude the other possibility that the transport of ingested antigen might require the physiological environment rather than the pH in the endocytic compartments without repeated fusion with late endosome/lysosome. In the endocytic compartments in day8-DCs, transferrin receptor, early endosome marker, was strongly positive, and Lamp-1 and MHC class II molecules were weakly positive (data not shown). Thus, the endocytic compartments in day8-DCs, which are able to transport the ingested antigens into the cytosol, are immature. Treatment with NH4Cl inhibits not only the intravesicular acidification but also the phagosome–lysosome fusion.40 Therefore, it is also possible that transport of ingested antigen into the cytosol is dependent on a slower transition of early endosomes to later endosomes or delayed fusion with lysosomes.
In the splenic immature DC cell line, exogenous antigens in the endocytic compartments are transported into the cytoplasm, but not in macrophages.8 Macrophages, rather than DCs, efficiently phagocytose virus-infected apoptosis cells, but fail to cross-present to prime the antigen-specific naïve CD8+ T cells.2 We could not observe the restored cross-presentation ability in macrophage by NH4Cl treatment (data not shown). We now predict the presence of the delivery machinery of exogenous antigens into the cytosol, which is DC specific and associated with the physiological conditions in the endosomal compartments.
Taken together, cross-presentation requires at least two steps that are antigen internalization and antigen transport into the cytosol. Our data show that the ability for the transport of intravesicular exogenous antigen into the cytosol is restricted to the early immature stage of DC. Also, physiological environments in the endocytic compartments are important for the transport of antigens into the cytosol. However, regulators for the transport are still unclear. Further studies will be required to elucidate the precise mechanism.
Acknowledgments
This work was supported by the Yokohama Foundation for Advancement of Medical Science, Japan, and Yokohama Academic Foundation, Japan. We appreciate Dr Holden T. Maecker and Dr Smita A. Ghanekar from BD Bioscience for critically reviewing the manuscript. We thank Ms. Leiko Minami for reading and comments.
Abbreviations
- DCs
dendritic cells
- OVA
ovalbumin
- MHC
major histocompatibility complex
- CTLs
cytotoxic T lymphocytes
- APC
antigen-presenting cell
- TAP
transporter associated with antigen processing
- CIIV
class II vesicles
- Lamp-1
lysosome lysosomeassociated membrane protein-1
- FCS
fetal calf serum
- HEPES
N–2–hydroxyethylpiperazine–N–2–ethanesulphonic acid
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- DPI
diphenyleneiodonium chloride
- mAb
monoclonal antibody
- FACS
fluorescence-activated cell sorter
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- BM
bone marrow
- LPS
lipopolysaccharide
- Tris
tris(hydroxymethyl)aminomethane
- PFA
paraformaldehyde
- PBS
phosphate-buffered saline
- MFI
mean fluorescence intensity
- c.p.m.
counts per minute
- EDTA
ethylenediaminetetraacetic acid
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
- 1.Rock KL, Goldberg AL. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol. 1999;17:739–9. doi: 10.1146/annurev.immunol.17.1.739. [DOI] [PubMed] [Google Scholar]
- 2.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-hematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 3.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson BWS, Lake RA, Nelson DJ, Scott BA, Marzo AL. Cross-presentation of tumour antigens: evaluation of threshold, duration, distribution and regulation. Immunol Cell Biol. 1999;77:552–8. doi: 10.1046/j.1440-1711.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 5.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–73. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 7.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo. implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 9.Norbury CC, Chambers BJ, Prescott AR, Ljunggren H-G, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 10.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–13. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 12.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann MF, Oxenius A, Pircher H, Hengartner H, Ashton-Richardt PA, Tonegawa S, Zinkernagel RM. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739–43. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–7. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 15.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 16.Albert ML, Pearce SFA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belz GT, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone FR, Heath WR. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol. 2002;168:6066–70. doi: 10.4049/jimmunol.168.12.6066. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 20.Regnault A, Lankar D, Lacabanne V, et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett WS, Chen L-M, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galán JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–34. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 22.West MA, Wallin RPA, Matthews SP, Svensson HG, Zaru R, Ljunggren H-G, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 23.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;13:839–48. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Pack M, Inaba K. Dendritic cell development and maturation. Adv Exp Med Biol. 1997;417:1–6. doi: 10.1007/978-1-4757-9966-8_1. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–7. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 27.Lutz MB, Rovere P, Kleijmeer MJ, et al. Intracellular routes and selective retention of antigens in mildly acidic cathepsin D/lysosome-associated membrane protein-1/MHC class II-positive vesicles in immature dendritic cells. J Immunol. 1997;159:3707–16. [PubMed] [Google Scholar]
- 28.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–3. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 29.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–22. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz MB, Kukutsch N, Ogilvie ALJ, Rößner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 31.Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113:1515–24. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- 32.Van Dyke RW. Acidification of lysosomes and endosomes. Subcell Biochem. 1996;27:331–60. doi: 10.1007/978-1-4615-5833-0_10. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandra L, Song R, Harding CV. Phagosomes are fully competent antigen-processing organelles that mediate the formation of peptide: class II MHC complexes. J Immunol. 1999;162:3263–72. [PubMed] [Google Scholar]
- 34.Driessen C, Bryant RAR, Lennon-Duménil A-M, Villadangos JA, Bryant PW, Shi G-P, Chapman HA, Ploegh HL. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol. 1999;147:775–90. doi: 10.1083/jcb.147.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 37.Cardelli JA, Richardson J, Miears D. Role of acidic intracellular compartments in the biosynthesis of Dictyostelium lysosomal enzymes. J Biol Chem. 1989;264:3454–63. [PubMed] [Google Scholar]
- 38.Mayer SJ, Keen PM, Craven N, Bourne FJ. Regulation of phagolysosome pH in bovine and human neutrophils. the role of NADPH oxidase activity and an Na+/H+ antiporter. J Leukoc Biol. 1989;45:239–48. doi: 10.1002/jlb.45.3.239. [DOI] [PubMed] [Google Scholar]
- 39.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–3. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 40.Hart PD, Young MR. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic Mycobacterium and a nonpathogenic Yeast. J Exp Med. 1991;174:881–9. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]