Abstract
Aims—The incidence of lung cancer in Okinawa has been the highest in Japan since 1975, and squamous cell carcinoma (SCC), especially the well differentiated form, is the most prevalent form in Okinawa, although well differentiated SCC is relatively rare in mainland Japan. Furthermore, a high proportion of SCC of the lung in Okinawa was positive for human papillomavirus (HPV). In this study, we report recent striking changes in histological features and in the incidence of HPV infection.
Methods—In Okinawa between 1986 and 1998, 1109 surgically resected lung tumours were examined histopathologically. In addition, human papillomavirus infection was detected by the polymerase chain reaction and Southern blot analysis in SCC cases reported in 1993 and 1995–8. Non-isotopic in situ hybridisation of HPV DNA was also carried out.
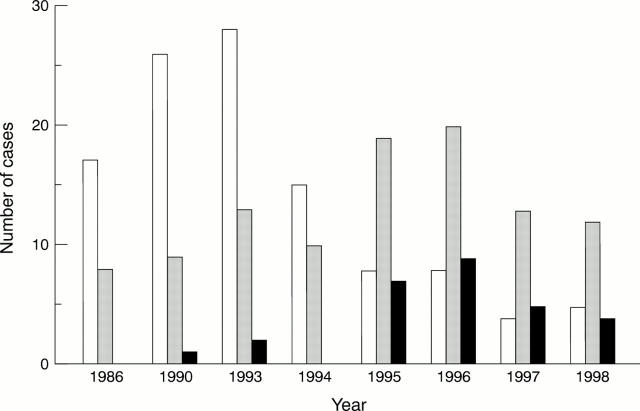
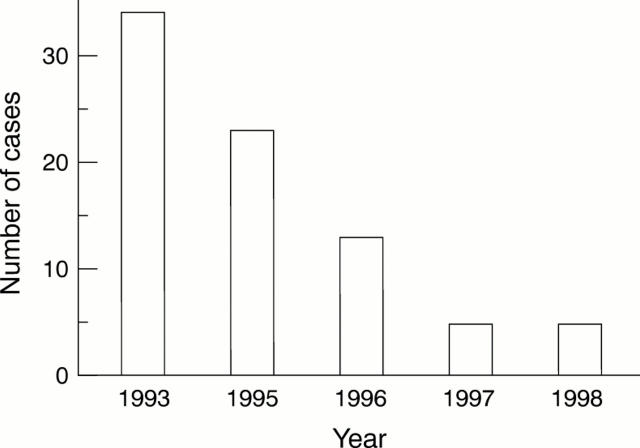
Results—Up until 1994 SCC, especially the well differentiated form, was the most prevalent type of tumour. However, since 1995 the number of such cases has diminished steadily, accompanied by a slight rise in the incidence of adenocarcinoma. Although most present and past patients are heavy smokers, the incidence of SCC, especially the well differentiated form, continues to decrease steadily. Furthermore, in 1993, HPV was detected in 79% of all cases, and was particularly prevalent in the well differentiated form, but the rate fell to 68% in 1995, 35% in 1996, 23% in 1997, and 24% in 1998. The age distribution of patients, the male to female ratio, and the number of tumours overexpressing p53 protein did not change significantly over the study period, and thus did not correlate with changes in the differentiation of SCC.
Conclusions—The decreasing incidence of viral infection correlates strongly with the falling numbers of SCC cases, especially well differentiated cases. These findings suggest that HPV might be involved in the development of SCC of the lung, affecting the histological differentiation of SCC in particular, at least in Okinawa, a subtropical island in southern Japan.
Key Words: squamous cell carcinoma of the lung • histological differentiation • human papillomavirus
Full Text
The Full Text of this article is available as a PDF (241.1 KB).
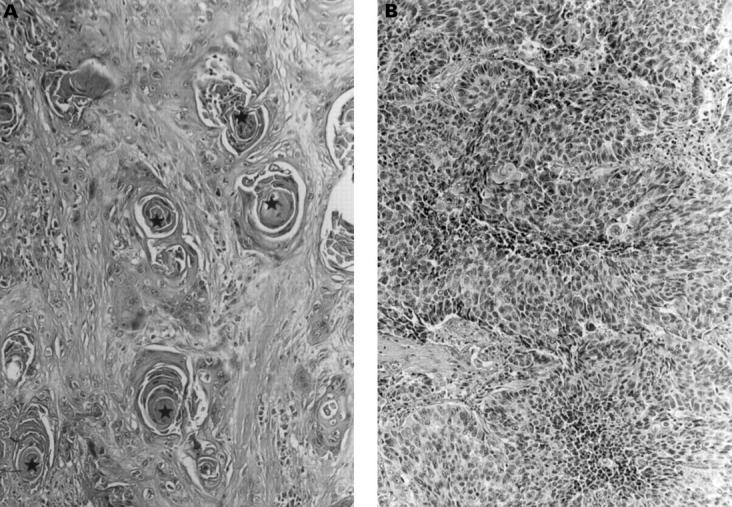
Figure 1 (A) A representative example of well differentiated squamous cell carcinoma of the lung showing common histological features in such tumours in Okinawa (case detected in 1993). Note the presence of keratin pearls (indicated by a star). Haematoxylin and eosin staining; magnification, x150. (B) A representative example of poorly differentiated squamous cell carcinoma of the lung (case detected in 1998). Haematoxylin and eosin staining; magnification, x180.
Figure 2 Squamous cell carcinoma (SCC) of the lung: changes in the histological features. Open bars, well differentiated SCC; shaded bars, moderately differentiated SCC; black bars, poorly differentiated SCC.
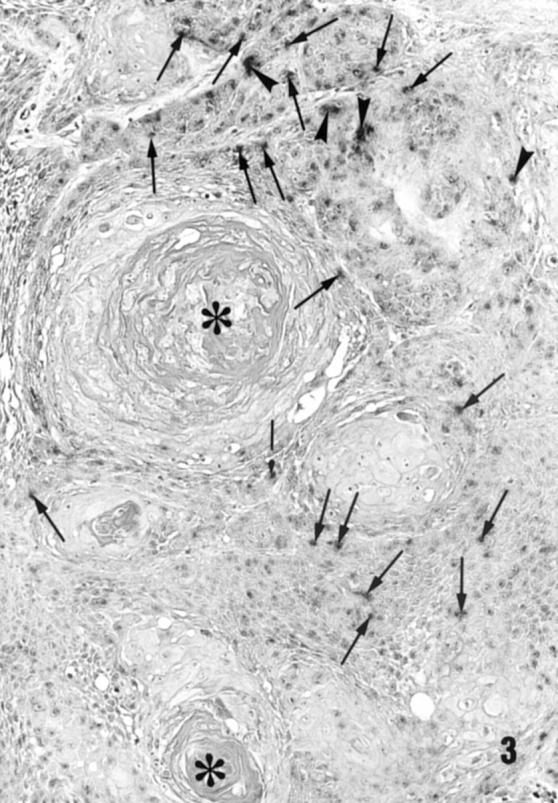
Figure 3 Detection of HPV DNA in squamous cell carcinoma by non-isotopic in situ hybridisation. Note the presence of integrated (arrows) and episomal (arrowheads) forms of HPV in tumour cells (the same well differentiated squamous cell carcinoma case shown in fig 1). Keratin pearls indicated by an asterisk. Magnification, x250
Figure 4 Squamous cell carcinoma of the lung. Changes in the incidence of human papillomavirus positive cases.
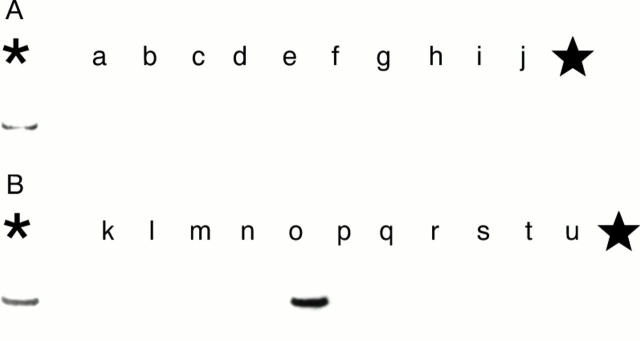
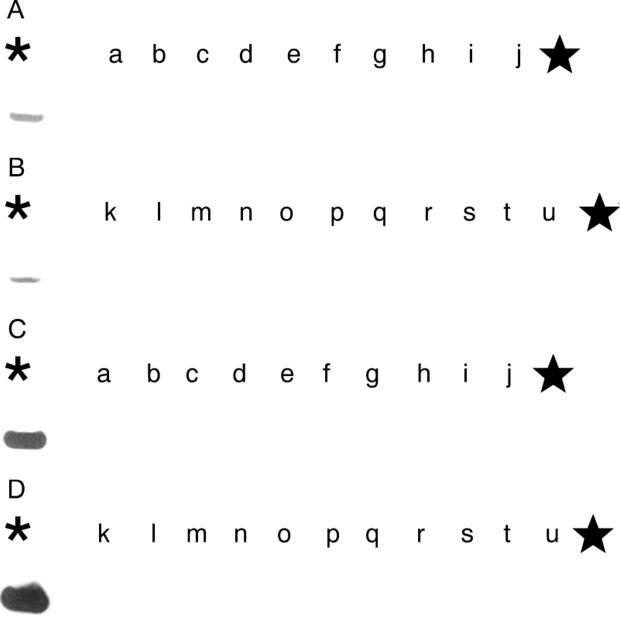
Figure 5 Demonstration of human papillomavirus (HPV) DNA in the squamous cell carcinoma cases in 1998. Demonstration of the HPV-6 E6 region by the polymerase chain reaction and Southern blot analysis. The 189 bp band is shown in lane o (case 13 (1998) in table 5). The asterisk denotes the positive control (HPV-6 in plasmid pML); the star denotes the negative control (distilled water).
Figure 6 Demonstration of the human papillomavirus 11 (HPV-11) E6 region in the squamous cell carcinoma cases in 1998 by the polymerase chain reaction (PCR) and Southern blot analysis. Positive bands (230 bp) are shown in lanes a, d, and e (cases 1, 2, and 7 (1998) in table 5). The asterisk denotes the positive control (HPV-11 in plasmid pBR 322); the star denotes the negative control (distilled water).
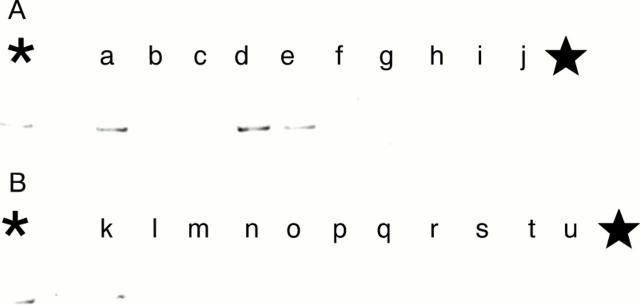
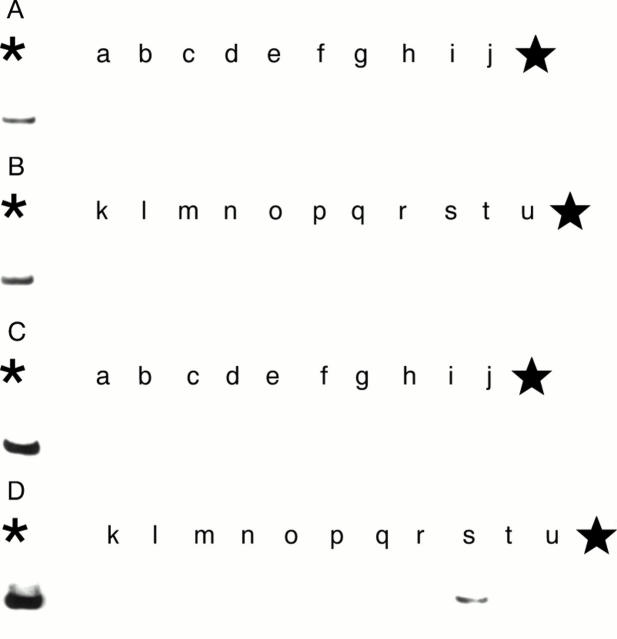
Figure 7 (A and B) Demonstration of the human papillomavirus 16 (HPV-16) E6 region in the squamous cell carcinoma cases in 1998 by the polymerase chain reaction (PCR) and Southern blot analysis. No positive bands (240 bp) are shown. (C and D) Demonstration of the HPV-16 E7 region by PCR and Southern blot analysis. No positive bands (171 bp) are shown. The asterisk denotes the positive control (HPV-16 in plasmid pBR 322). The star denotes the negative control (distilled water).
Figure 8 (A and B) Demonstration of the human papillomavirus 18 (HPV-18) E6 region in the squamous cell carcinoma cases in 1998 by the polymerase chain reaction (PCR) and Southern blot analysis. No positive bands (160 bp) are demonstrated in the cases. (C and D) Demonstration of the HPV-18 E7 region by PCR and Southern blot analysis. A positive band (152 bp) is shown in lane s (case 5 (1998) in table 5). The asterisk denotes the positive control (HPV-18 in plasmid pBR 322); the star denotes the negative control (distilled water).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asato T., Nakajima Y., Nagamine M., Nakashima Y., Takei H., Maehama T., Yamashiro T., Higashi M., Nakayama M., Kanazawa K. Correlation between the progression of cervical dysplasia and the prevalence of human papillomavirus. J Infect Dis. 1994 Apr;169(4):940–941. doi: 10.1093/infdis/169.4.940. [DOI] [PubMed] [Google Scholar]
- Auerbach O., Garfinkel L., Parks V. R. Histologic type of lung cancer in relation to smoking habits, year of diagnosis and sites of metastases. Chest. 1975 Apr;67(4):382–387. doi: 10.1378/chest.67.4.382. [DOI] [PubMed] [Google Scholar]
- Béjui-Thivolet F., Liagre N., Chignol M. C., Chardonnet Y., Patricot L. M. Detection of human papillomavirus DNA in squamous bronchial metaplasia and squamous cell carcinomas of the lung by in situ hybridization using biotinylated probes in paraffin-embedded specimens. Hum Pathol. 1990 Jan;21(1):111–116. doi: 10.1016/0046-8177(90)90082-g. [DOI] [PubMed] [Google Scholar]
- Cason J., Kaye J. N., Jewers R. J., Kambo P. K., Bible J. M., Kell B., Shergill B., Pakarian F., Raju K. S., Best J. M. Perinatal infection and persistence of human papillomavirus types 16 and 18 in infants. J Med Virol. 1995 Nov;47(3):209–218. doi: 10.1002/jmv.1890470305. [DOI] [PubMed] [Google Scholar]
- Chang K. W., Chang C. S., Lai K. S., Chou M. J., Choo K. B. High prevalence of human papillomavirus infection and possible association with betel quid chewing and smoking in oral epidermoid carcinomas in Taiwan. J Med Virol. 1989 May;28(1):57–61. doi: 10.1002/jmv.1890280113. [DOI] [PubMed] [Google Scholar]
- Chen B., Yin H., Dhurandhar N. Detection of human papillomavirus DNA in esophageal squamous cell carcinomas by the polymerase chain reaction using general consensus primers. Hum Pathol. 1994 Sep;25(9):920–923. doi: 10.1016/0046-8177(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Cooper K., Herrington C. S., Stickland J. E., Evans M. F., McGee J. O. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol. 1991 Dec;44(12):990–996. doi: 10.1136/jcp.44.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Kern W. H. Adenosquamous carcinoma of the lung: a clinical and pathologic study of seven cases. Hum Pathol. 1985 May;16(5):463–466. doi: 10.1016/s0046-8177(85)80083-6. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V. G., Zacharatos P., Kotsinas A., Kyroudi A., Rassidakis A. N., Ikonomopoulos J. A., Barbatis C., Herrington C. S., Kittas C. Human papilloma virus (HPV) is possibly involved in laryngeal but not in lung carcinogenesis. Hum Pathol. 1999 Mar;30(3):274–283. doi: 10.1016/s0046-8177(99)90005-9. [DOI] [PubMed] [Google Scholar]
- Hirayasu T., Iwamasa T., Kamada Y., Koyanagi Y., Usuda H., Genka K. Human papillomavirus DNA in squamous cell carcinoma of the lung. J Clin Pathol. 1996 Oct;49(10):810–817. doi: 10.1136/jcp.49.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impraim C. C., Saiki R. K., Erlich H. A., Teplitz R. L. Analysis of DNA extracted from formalin-fixed, paraffin-embedded tissues by enzymatic amplification and hybridization with sequence-specific oligonucleotides. Biochem Biophys Res Commun. 1987 Feb 13;142(3):710–716. doi: 10.1016/0006-291x(87)91472-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita I., Dosaka-Akita H., Shindoh M., Fujino M., Akie K., Kato M., Fujinaga K., Kawakami Y. Human papillomavirus type 18 DNA and E6-E7 mRNA are detected in squamous cell carcinoma and adenocarcinoma of the lung. Br J Cancer. 1995 Feb;71(2):344–349. doi: 10.1038/bjc.1995.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., leRiche J. C., Zheng Y., Coldman A., MacAulay C., Hawk E., Kelloff G., Gazdar A. F. Sex-related differences in bronchial epithelial changes associated with tobacco smoking. J Natl Cancer Inst. 1999 Apr 21;91(8):691–696. doi: 10.1093/jnci/91.8.691. [DOI] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P., Paraskevas M., Guijon F. Variability of polymerase chain reaction-based detection of human papillomavirus DNA is associated with the composition of vaginal microbial flora. J Med Virol. 1994 Jun;43(2):194–200. doi: 10.1002/jmv.1890430218. [DOI] [PubMed] [Google Scholar]
- Nakazato I., Hirayasu T., Kamada Y., Tsuhako K., Iwamasa T. Carcinoma of the lung in Okinawa, Japan: with special reference to squamous cell carcinoma and squamous metaplasia. Pathol Int. 1997 Oct;47(10):659–672. doi: 10.1111/j.1440-1827.1997.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulou K., Labropoulou V., Davaris P., Mavromara P., Tsimara-Papastamatiou H. Detection of human papillomaviruses in squamous cell carcinomas of the lung. Virchows Arch. 1998 Jul;433(1):49–54. doi: 10.1007/s004280050215. [DOI] [PubMed] [Google Scholar]
- Peters E. J., Morice R., Benner S. E., Lippman S., Lukeman J., Lee J. S., Ro J. Y., Hong W. K. Squamous metaplasia of the bronchial mucosa and its relationship to smoking. Chest. 1993 May;103(5):1429–1432. doi: 10.1378/chest.103.5.1429. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schneider A. Natural history of genital papillomavirus infections. Intervirology. 1994;37(3-4):201–214. doi: 10.1159/000150378. [DOI] [PubMed] [Google Scholar]
- Sugimura H., Wakai K., Genka K., Nagura K., Igarashi H., Nagayama K., Ohkawa A., Baba S., Morris B. J., Tsugane S. Association of Ile462Val (Exon 7) polymorphism of cytochrome P450 IA1 with lung cancer in the Asian population: further evidence from a case-control study in Okinawa. Cancer Epidemiol Biomarkers Prev. 1998 May;7(5):413–417. [PubMed] [Google Scholar]
- Suzuk L., Noffsinger A. E., Hui Y. Z., Fenoglio-Preiser C. M. Detection of human papillomavirus in esophageal squamous cell carcinoma. Cancer. 1996 Aug 15;78(4):704–710. doi: 10.1002/(SICI)1097-0142(19960815)78:4<704::AID-CNCR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Syrjänen K. J., Syrjänen S. M. Human papillomavirus DNA in bronchial squamous cell carcinomas. Lancet. 1987 Jan 17;1(8525):168–169. doi: 10.1016/s0140-6736(87)92010-1. [DOI] [PubMed] [Google Scholar]
- Travis W. D., Lubin J., Ries L., Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996 Jun 15;77(12):2464–2470. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Tsuhako K., Nakazato I., Hirayasu T., Sunakawa H., Iwamasa T. Human papillomavirus DNA in adenosquamous carcinoma of the lung. J Clin Pathol. 1998 Oct;51(10):741–749. doi: 10.1136/jcp.51.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R. G., Pickren J. W., Lane W. W., Bross I., Takita H., Houten L., Gutierrez A. C., Rzepka T. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977 Apr;39(4):1647–1655. doi: 10.1002/1097-0142(197704)39:4<1647::aid-cncr2820390439>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wilczynski S. P., Bergen S., Walker J., Liao S. Y., Pearlman L. F. Human papillomaviruses and cervical cancer: analysis of histopathologic features associated with different viral types. Hum Pathol. 1988 Jun;19(6):697–704. doi: 10.1016/s0046-8177(88)80176-x. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Ries L. A., Giovino G. A., Miller D. S., Rosenberg H. M., Shopland D. R., Thun M. J., Edwards B. K. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999 Apr 21;91(8):675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- Yousem S. A., Ohori N. P., Sonmez-Alpan E. Occurrence of human papillomavirus DNA in primary lung neoplasms. Cancer. 1992 Feb 1;69(3):693–697. doi: 10.1002/1097-0142(19920201)69:3<693::aid-cncr2820690316>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]