Abstract
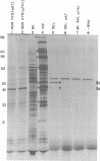
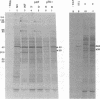
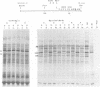
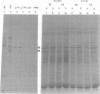
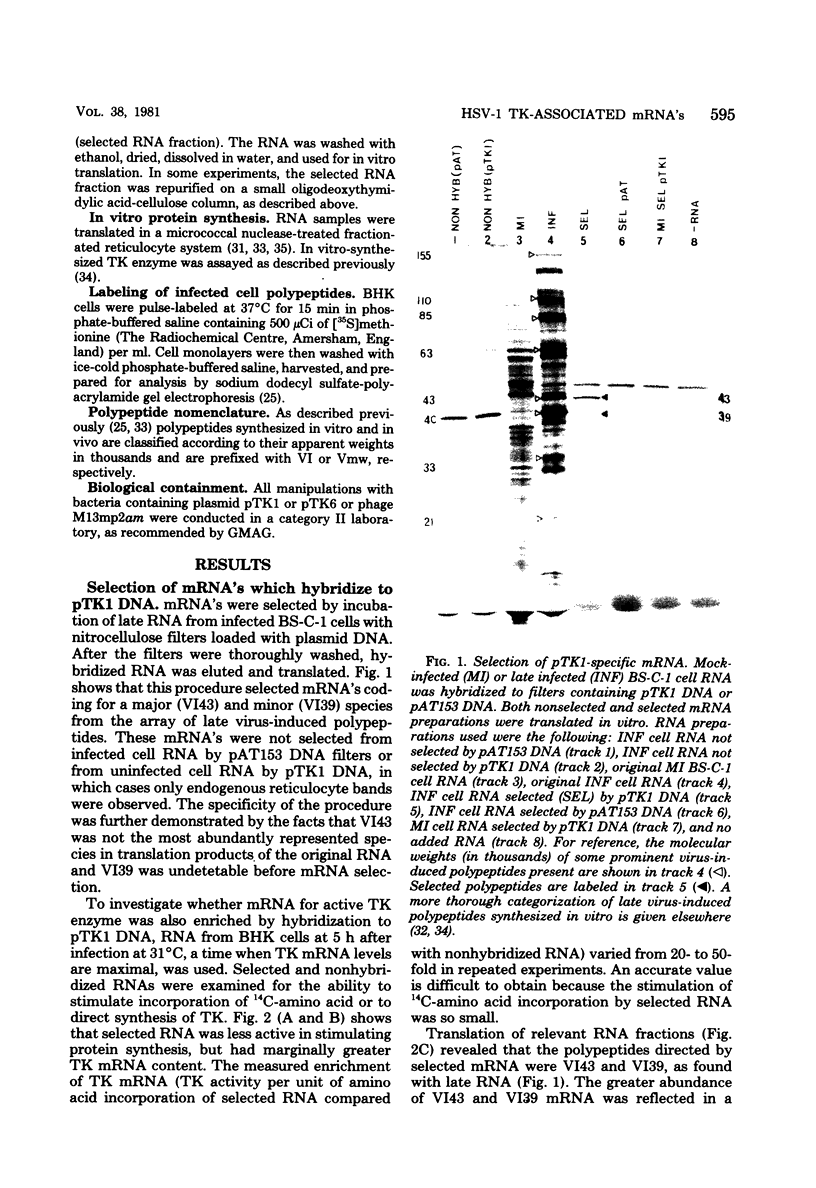
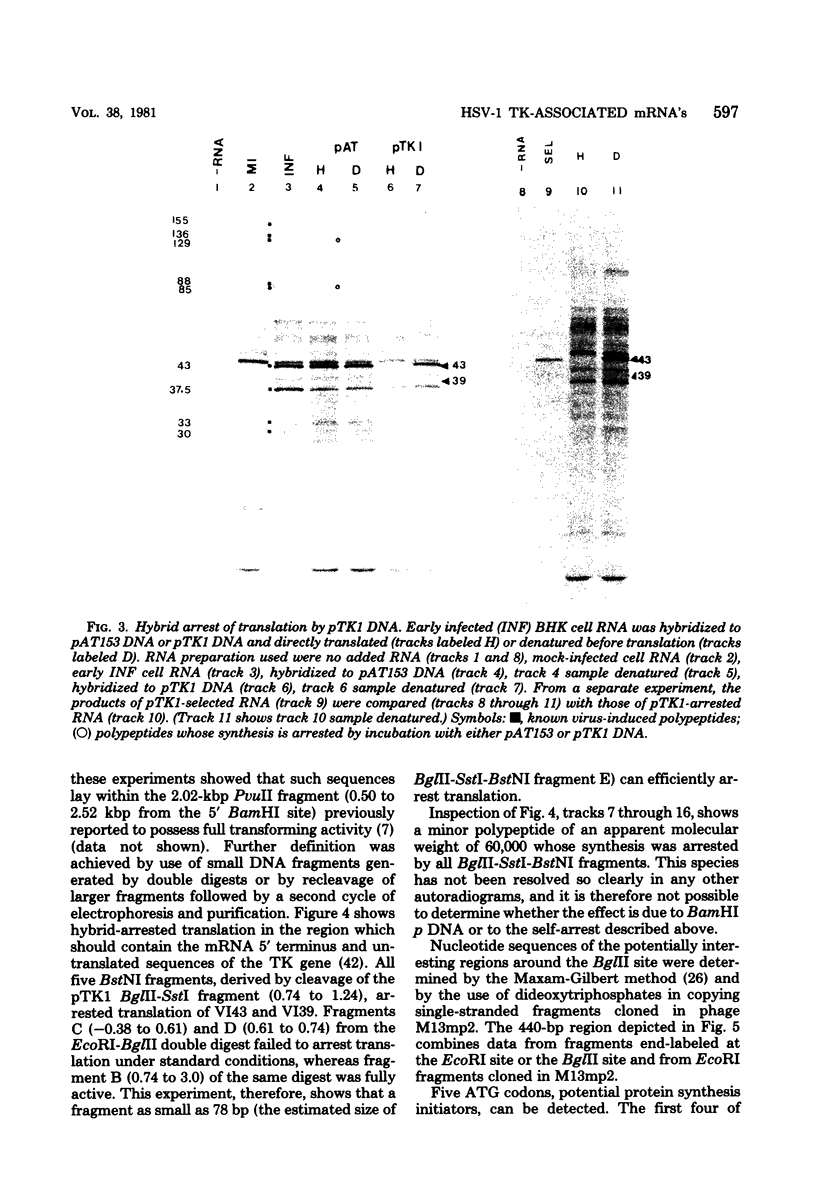
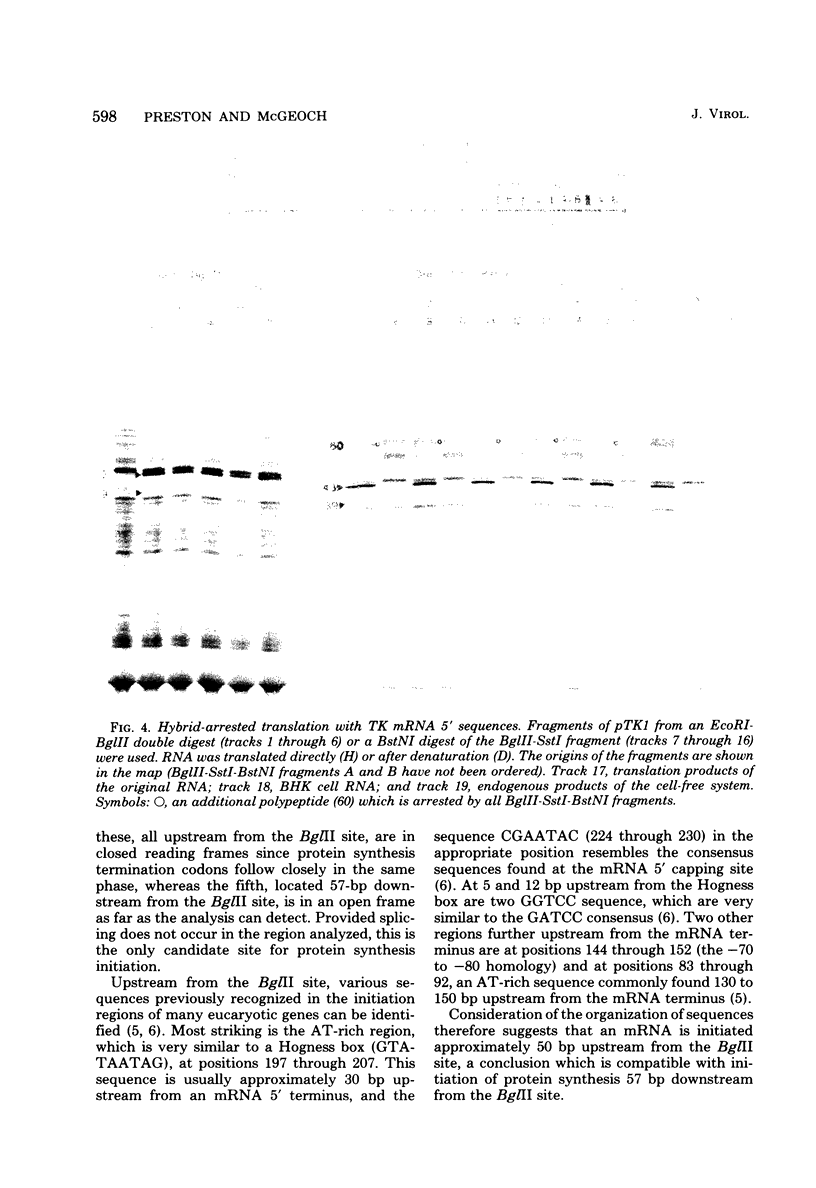
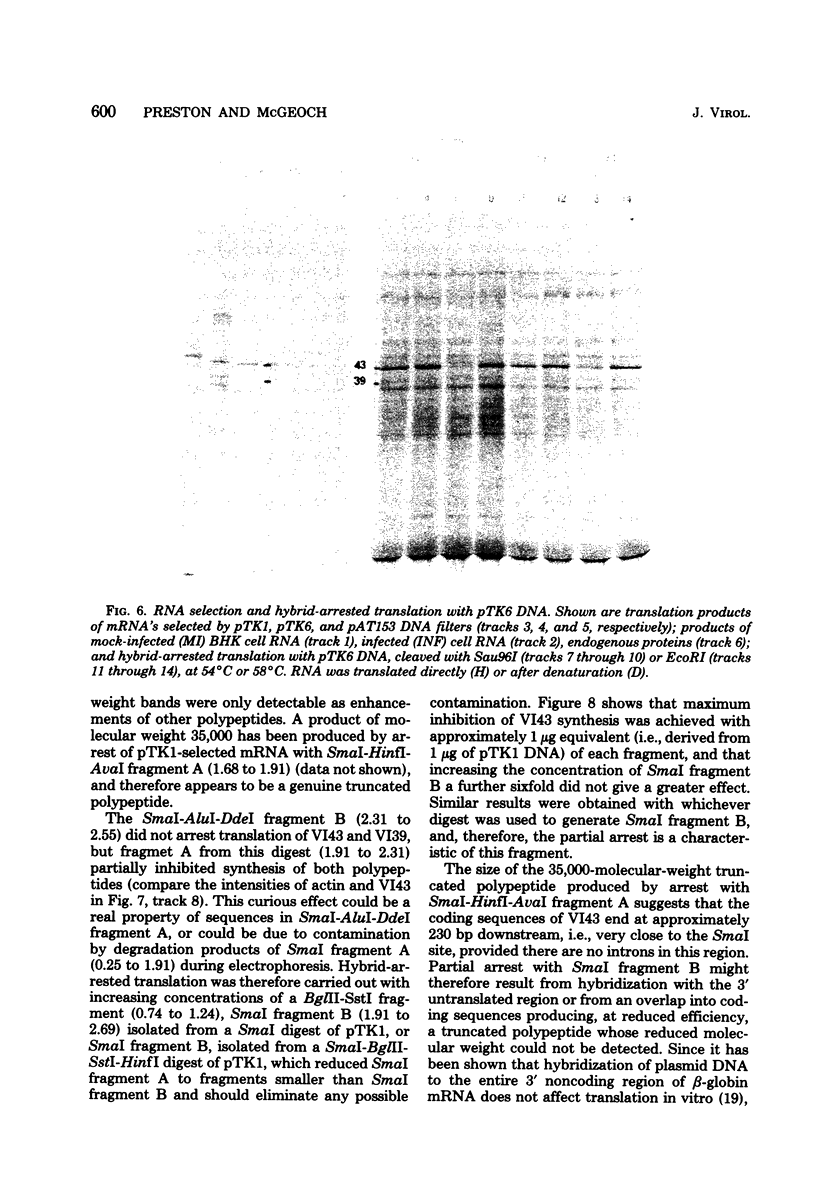
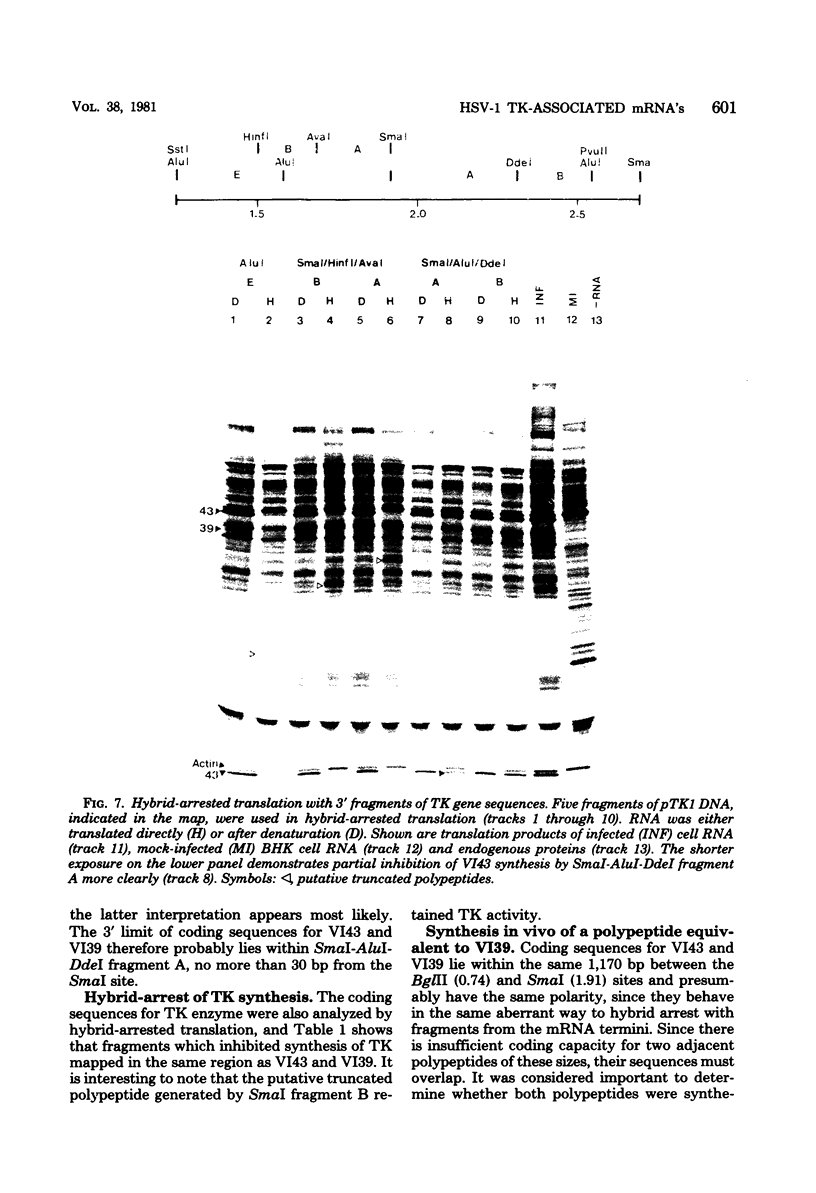
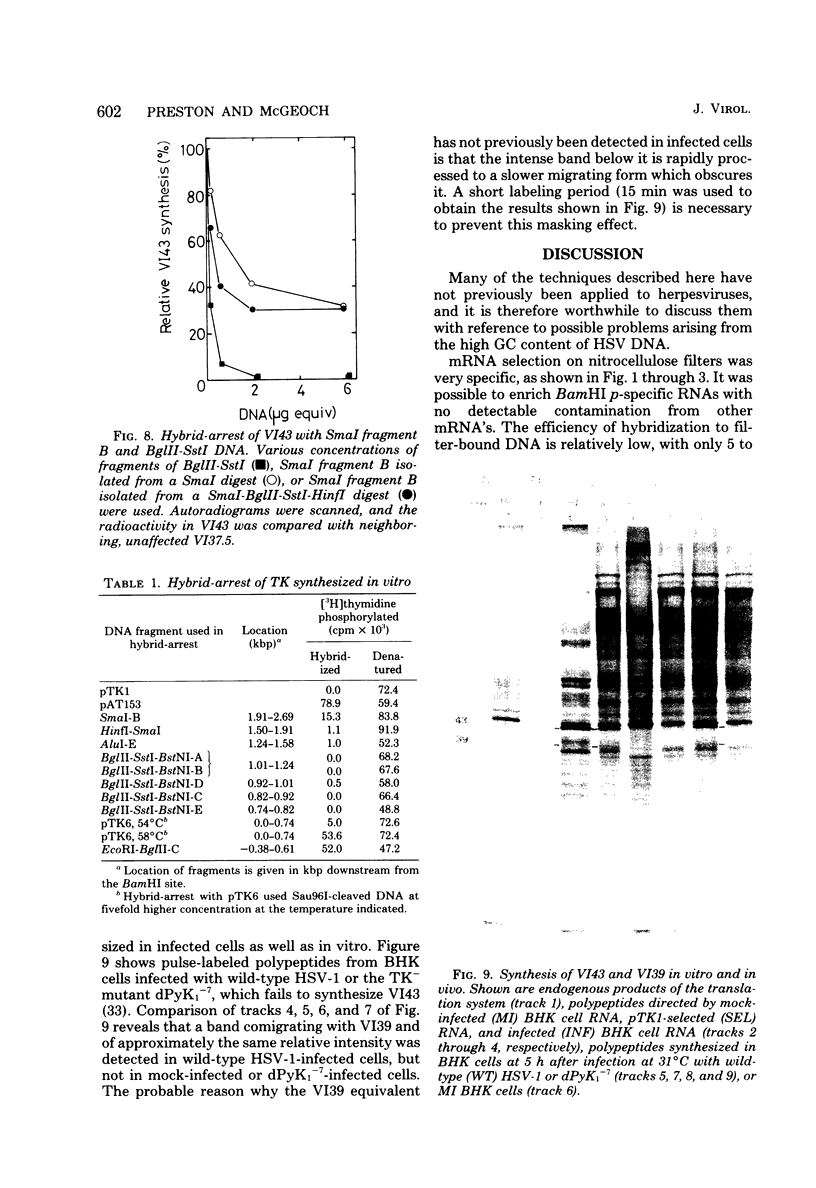
mRNA's homologous to the herpes simplex virus type 1 DNA restriction endonuclease fragment BamHI p, which contains the thymidine kinase gene, have been identified and mapped by hybrid-arrested translation and mRNA selection. Such mRNA's, when translated in vitro, directed the synthesis of polypeptides of apparent molecular weights 43,000 (VI43) and 39,000 (VI39). mRNA for enzymatically active thymidine kinase was enriched by more than 20-fold after selection. Mapping was carried out with restriction endonuclease fragments of BamHI p, and locations of the 5' and 3' termini of VI43 mRNA were deduced. Analysis of nucleotide sequences around the 5' terminus revealed several consensus sequences commonly found at the start of eucaryotic mRNA's and which are presumably involved in initiation of transcription by RNA polymerase II. Translation of mRNA's for VI43, VI39, and the thymidine kinase enzyme was arrested only by a 1,170-base-pair region of BamHI p. Since this region is insufficient for adjacent genes, coding sequences for VI43 and VI39 must overlap; the possible relationship of these two polypeptides is discussed. A virus-induced product equivalent to VI39 was detected in infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Costa R. H., Holland L. E., Wagner E. K. Characterization of herpes simplex virus type 1 RNA present in the absence of de novo protein synthesis. J Virol. 1980 Apr;34(1):9–27. doi: 10.1128/jvi.34.1.9-27.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Holland L. E., Gaylord B. H., Wagner E. K. Isolation and translation of mRNA encoded by a specific region of the herpes simplex virus type 1 genome. J Virol. 1980 Feb;33(2):749–759. doi: 10.1128/jvi.33.2.749-759.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Gait M. J., Mayol L., Young I. G. A short primer for sequencing DNA cloned in the single-stranded phage vector M13mp2. Nucleic Acids Res. 1980 Apr 25;8(8):1731–1743. doi: 10.1093/nar/8.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Portmann R., Irminger J. C., Birnstiel M. L. Ubiquitous and gene-specific regulatory 5' sequences in a sea urchin histone DNA clone coding for histone protein variants. Nucleic Acids Res. 1980 Mar 11;8(5):957–977. doi: 10.1093/nar/8.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. Translation of specific vaccinia virus RNAs purified as RNA-DNA hybrids on potassium iodide gradients. Nucleic Acids Res. 1979 Aug 10;6(11):3599–3612. doi: 10.1093/nar/6.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K., Bodemer M., Summers W. C. Characterization of the mRNA for herpes simplex virus thymidine kinase by cell-free synthesis of active enzyme. Nucleic Acids Res. 1978 Jul;5(7):2333–2344. doi: 10.1093/nar/5.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Morse L. S., Roizman B., Quinn K. E. Mapping of the thymidine kinase genes of type 1 and type 2 herpes simplex viruses using intertypic recombinants. J Gen Virol. 1980 Aug;49(2):235–253. doi: 10.1099/0022-1317-49-2-235. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Shipman C., Jr, Wagner E. K. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology. 1980 Feb;101(1):10–24. doi: 10.1016/0042-6822(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Cohn R. H., Kedes L. H., Davidson N. Positions of sea urchin (Strongylocentrotus purpuratus) histone genes relative to restriction endonuclease sites on the chimeric plasmids pSp2 and pSp17. Biochemistry. 1977 Apr 5;16(7):1504–1512. doi: 10.1021/bi00626a040. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R. Regulation of herpesvirus thymidine kinase activity in LM(TK) cells transformed by ultraviolet light-irradiated herpes simplex virus. Virology. 1977 Jan;76(1):331–340. doi: 10.1016/0042-6822(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. N., Roberts B. E., Efstratiadis A. The 3' noncoding region of beta-globin mRNA is not essential for in vitro translation. Nucleic Acids Res. 1979 Jan;6(1):153–166. doi: 10.1093/nar/6.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W., Chanock R. M., Lai C. J. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1857–1861. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. C. Evidence for a herpes simplex virus-specific factor controlling the transcription of deoxypyrimidine kinase. J Virol. 1978 Aug;27(2):269–274. doi: 10.1128/jvi.27.2.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Wildy P., Buchan A., Darby G. Introduction of the herpes simplex virus thymidine kinase gene into mouse cells using virus DNA or transformed cell DNA. Cell. 1978 Mar;13(3):581–587. doi: 10.1016/0092-8674(78)90331-8. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Abnormal properties of an immediate early polypeptide in cells infected with the herpes simplex virus type 1 mutant tsK. J Virol. 1979 Nov;32(2):357–369. doi: 10.1128/jvi.32.2.357-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Cell-free synthesis of herpes simplex virus-coded pyrimidine deoxyribonucleoside kinase enzyme. J Virol. 1977 Sep;23(3):455–460. doi: 10.1128/jvi.23.3.455-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. The cell-free synthesis of herpesvirus-induced polypeptides. Virology. 1977 May 1;78(1):349–353. doi: 10.1016/0042-6822(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Rapp F., Turner N., Schaffer P. A. Biochemical transformation by temperature-sensitive mutants of herpes simplex virus type 1. J Virol. 1980 Jun;34(3):704–710. doi: 10.1128/jvi.34.3.704-710.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Smiley J. R., Wagner M. J., Summers W. P., Summers W. C. Genetic and physical evidence for the polarity of transcription of the thymidine kinase gene of herpes simplex virus. Virology. 1980 Apr 15;102(1):83–93. doi: 10.1016/0042-6822(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Davison A., Chartrand P., Stow N. D., Preston V. G., Timbury M. C. Recombination in herpes simplex virus: mapping of mutations and analysis of intertypic recombinants. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):827–840. doi: 10.1101/sqb.1979.043.01.089. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Eglin R. P., Sanders P. G., Clements J. B. The association of herpes simplex virus with squamous carcinoma of the cervix, and studies of the virus thymidine kinase gene. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1180):411–421. doi: 10.1098/rspb.1980.0143. [DOI] [PubMed] [Google Scholar]