Abstract
Human immunodeficiency virus (HIV)-associated dementia is a neurodegenerative syndrome characterized by cognitive decline, personality change, and motor deficits. HIV-associated encephalitis (HAE), the neuropathology responsible for HIV-associated dementia, involves the formation of multinucleated giant cells or syncytia. In this article we describe the apoptotic pathways activated in the brains of HAE-affected patients. Approximately 50% of multinuclear giant cells exhibited apoptotic DNA fragmentation as detected by the terminal dUTP nick-end labeling technique. In addition, the presence of syncytia in the frontal cortex of ∼35% of HAE patients correlated with the number of cells expressing the HIV-1 protein p24. Histochemical and immunohistochemical analyses revealed that HAE-associated syncytia underwent apoptosis through a mitochondrial pathway previously delineated for HIV-1 envelope-elicited syncytia in vitro. We observed over-expression of the mammalian target of rapamycin (mTOR), a kinase that mediates activation of the pro-apoptotic transcription factor p53, and p53-dependent up-regulation of two effectors of mitochondrial apoptosis, namely the BH3-only proteins Puma and transglutaminase type 2 (TG2). Interestingly, although mTOR activation and Puma induction were observed in dying syncytia and neurons, IkB phosphorylation and TG2 up-regulation were only found in syncytia. These findings provide substantial new information on the cell death mechanisms that regulate HAE, suggesting an important pathogenetic role of syncytia in the disease.
Infection with the human immunodeficiency virus-1 (HIV-1) is associated with a progressive decrease in CD4 T-cell number and a consequent impairment in host immune defenses. It has been recently estimated that ∼10% of HIV-infected patients develop HIV-associated dementia, a neurodegenerative syndrome characterized by cognitive decline, personality change, and motor deficits.1 The neuropathology, particularly that associated with HIV-associated dementia, is characterized by a subacute HIV-associated encephalitis (HAE) with a variable degree of perivascular inflammation.2 Mononuclear (microglial) cells are present throughout gray and white matter and tend to cluster in loose aggregates in association with occasional reactive astrocytes. A mild gliosis is found in the gray matter, both in the cortex and in the central gray structures. The white matter is also punctuated by small foci of active demyelination, usually with a paravascular or perivascular distribution. Reactive astrocytosis may extend into subcortical gray structures. In milder cases, the inflammatory changes consist only in a few perivascular lymphocytes and brown-pigmented macrophages. In more severe cases, the infiltrates consist of perivascular and parenchymal macrophages and multinucleated cells of macrophage origin. These infiltrates are also found in the white matter and in deep gray structures, especially the basal ganglia and thalamus, as well as in the brain stem.3–5
The pathogenetic mechanism(s) at the basis of HIV-induced neuronal injury is not fully elucidated.1,6,7 HIV enters the central nervous system (CNS) early in the course of infection through infected macrophages and resides both in macrophages and microglia. Considering that neurons are not productively infected by HIV, it appears that the damage8–11 affecting neurons is due to the action of toxic products (such as cytokines, chemokines, and endothelial adhesion molecules) released by infected macrophage-microglial cells. However, it has also been proposed that HIV-1-encoded proteins (gp120, Vpr, and Tat) might participate in the neurodegenerative process.1,12–14 In transgenic animals, gp120 (which is one of the proteins derived from the env gene of HIV-1) is neurotoxic and activates caspase-dependent apoptosis.15 Accordingly, active caspase-316 and p5317 have been detected in the brain of HAE patients.
One of the most important neuropathological alterations associated with HAE is the appearance of multinucleated giant cells or syncytia.1,18–20 Syncytia are a common characteristic of HIV-infected tissues and are present in lymphoid tissues, in the brain, and in the spinal cord.19,21 In the blood and lymph nodes, syncytia arise from the fusion between noninfected and HIV-infected cells through the binding of the HIV-1 envelope (Env) glycoprotein complex (gp120/gp41) to its receptor CD4 and a co-receptor of the chemokine receptor family.22,23 HIV-1 strains that use CXCR4 as co-receptor are particularly efficient in eliciting syncytia.24–26 The CXCR4 chemokine co-receptor is expressed in neurons, astrocytes, microglia, and endothelial cells in the brain.27–30
We have previously characterized the molecular pathway of apoptosis activated in syncytia both in vitro and in lymphoid tissues of HIV-1-infected patients.31–34 Env-elicited syncytia manifest the up-regulation and nuclear translocation of mammalian target of rapamycin (mTOR) and mTOR-mediated phosphorylation of p53 on serine 15 (p53S15P), as well as the transcriptional activation of p53 correlating with its phosphorylation on serine 46 (p53S46P).31,32 p53 then elicits the transcriptional up-regulation of the proapoptotic BH3-only protein Puma, which in turn mediates the insertion of Bax into mitochondrial membranes, thus causing the Bax-dependent mitochondrial release of cytochrome c with subsequent caspase activation and apoptosis.33 In this system, another transcription factor, nuclear factor (NF)-κB, is activated after the phosphorylation of its inhibitor IκB, and cooperates in the process allowing mTOR to gain access to the nucleus (and hence to activate p53).33 Based on these premises, we decided to explore the phenotype of brain syncytia in HAE. Here we report immunohistochemical evidence suggesting that HAE-associated syncytia die by an apoptotic pathway involving NF-κB, mTOR, p53, Puma, as well as another BH3-only protein, transglutaminase type II (TG2).
Materials and Methods
Patients
Autopsy samples of frontal cortex from 43 patients enrolled in prospective studies on HIV-related neuropathology were obtained from the Institute of Pathological Anatomy, Catholic University of Rome, School of Medicine. Seventeen cases were diagnosed as HIV-associated encephalitis (HAE) without superinfection by pathological and histological examination. The remaining samples presented additional opportunistic infections or CNS pathologies unrelated to HIV encephalitis (Table 1). All patients were not treated with highly active anti-retroviral therapy. Brain sections from three control patients were also included in the study. The autoptic samples were formalin-fixed at the time of autopsy and paraffin-embedded.
Table 1.
Characteristics of Enrolled Patients
| All (n = 43) | HAE with syncytia (Syn+) (n = 6) | HAE without syncytia (Syn−) (n = 11) | AIDS-related brain pathologies comprehensive of opportunistic infections and viral encephalitis (n = 26) | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 38.8 | 37 | 35.5 | 40.6 |
| Range | 30–55 | 30–50 | 24–48 | 30–55 |
| Sex | ||||
| Male | 31 | 4 | 9 | 18 |
| Female | 12 | 2 | 2 | 8 |
Macroscopically the most prevalent finding of HAE was pallor of the white matter, more severe in the central and periventricular white matter than in subcortical fibers. The hallmarks of HIV-associated encephalitis were: microglial nodule formation and multinucleated giant cells (syncytia); reactive astrogliosis, indicative of astrocyte activation; myelin pallor or the loss of myelin surrounding neuronal axons, indicating injured oligodendrocytes. Collectively these inflammatory and neuropathological findings based on extensive sampling of cerebral gray and white matter, basal ganglia, thalamus, brain stem, and cerebellum were independently evaluated by two pathologists (L.M.L. and V.A.).
Immunohistochemical Analysis
Paraffin-embedded human frontal cortex sections (5 μm thick) were deparaffinized, rehydrated, and subjected to high-temperature antigen retrieval in 10 mmol/L sodium citrate buffer, pH 6.0. Endogenous peroxidase activity was blocked by 3% H2O2. The primary antibodies used are: mouse RAFT1/FRAP (BD Transduction Laboratories, San Diego, CA) that label the factor mTOR, rabbit p70 S6 kinase (Thr421, Ser 424; Calbiochem, La Jolla, CA) that is the downstream target of mTOR, rabbit phospho-p53 (Ser 46), mouse phospho IκB-α (Ser 32/36; Cell Signaling Technologies, Beverly, MA) that is a marker for NF-κB activation, mouse Puma/Bbc3 (Upstate, Lake Placid, NY), mouse transglutaminase II Ab-1 (CUB 7402; NeoMarkers, Freemont, CA), mouse human immunodeficiency virus p24, mouse neurofilament 200 kd (Novocastra, Newcastle, UK), rabbit glial fibrillary acidic protein (DAKO, Carpinteria, CA).
The incubation with primary antibody was performed for 1 hour at room temperature and followed by a biotinylated goat anti-mouse or goat anti-rabbit IgG as secondary antibody. Then the incubation with a preformed horseradish peroxidase-conjugated streptavidin (Biogenex, San Ramon, CA) was performed. The immunoreaction product was revealed using aminoethylcarbazole or 3–3′ diaminobenzidine as chromogenic substrates and 0.01% H2O2 (Biogenex). As controls, we performed stainings with nonimmune isotype-matched control antibodies and by omitting the primary antibodies. Apoptotic cells were identified by terminal dUTP nick-end labeling (TUNEL) technique (In Situ Cell Death Detection kit; Roche Diagnostics, Penzberg, Germany). Sections were counterstained in Mayer’s acid hemalum and analyzed.
Double-Labeling Protocol by Confocal Microscopy
Paraffin-embedded sections were deparaffinized, rehydrated, and treated with 0.2% Triton X-100 and 1% bovine serum albumin in phosphate-buffered saline. The incubations with primary antibodies were performed for 1 hour at room temperature and followed by the appropriate secondary fluorescent antibodies (Alexa Fluor; Molecular Probes, Eugene, OR) followed by TUNEL labeling fluorescein conjugated. The sections were analyzed with the Leica confocal microscope SP2.
Statistical Analysis
The brain sections of the 17 patients were semiquantitatively evaluated under code by three independent observers using a light microscope without the knowledge of either clinical or histological diagnosis. For each slide, a minimum of 10 fields was examined at ×40 magnification. To describe the histopathological brain alterations a semiquantitative evaluation scale was used based on the percentage of each parameter considered; tissue sections were scored as 0, if the inflammatory/degenerative changes involved an area comprised between 0% and 5% of the total section; 1 if 5 to 25%; 2 if 25 to 75%; and 3 if >75%. To describe the degree of the p24 expression and TUNEL positivity on each specimen, a value comprised between 0 and 3 was assigned based on the percentage of positive cells present on the section as follows: 0: 0 to 5% of stained cells were present; 1: 5 to 25%; 2: 25 to 75%; and 3: 75 to 100%. The Mann-Whitney test for nonparametric data was used to compare medians, and the Pearson’s correlation coefficient was used. Statistical significance was set at P < 0.05. The statistical analysis was performed by SPSS 11.0.1 for Windows (SPSS Science, Chicago, IL).
Results
The Presence of Dying Syncytia as an Index of HAE Severity
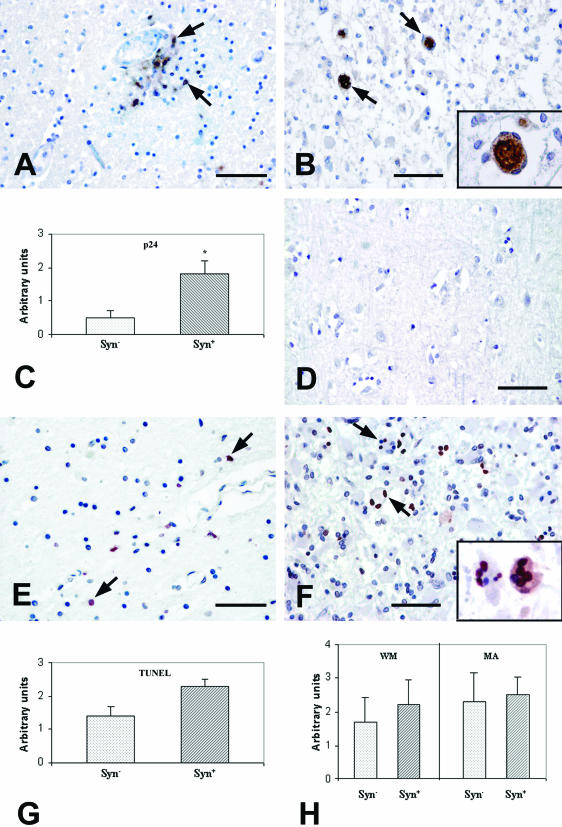
We analyzed autoptic human brain sections from 43 HIV-1-infected patients with different brain pathologies (Table 1) and 3 brains from healthy controls. We subsequently focused our investigation on 17 patients with confirmed HAE, without opportunistic infections or unrelated CNS pathologies. HAE brain sections exhibited perivascular infiltration (Figure 1A) associated with prominent astrocytosis, microglial nodules, and necrotic areas without the presence of syncytia (Syn−, Figure 1A) in 11 of 17 cases; whereas ∼35% of the patient samples with HAE were characterized by the presence of multinucleated giant cells/syncytia (Syn+, Figure 1B). The presence of HIV-1 in HAE brain sections was investigated by immunohistochemical staining with monoclonal antibodies specific for the HIV-1 p24 protein. p24 staining was observed in glial cells, as well as in the multinuclear giant cells (Figure 1, A and B). p24 staining was more pronounced in Syn+ than in Syn− samples (Figure 1C): in fact, a significantly higher presence of p24 was measured (P = 0.015) in Syn+ sections, where the vast majority of syncytia themselves stained positive for p24 (90.25% ± 11.09, number of patients = 6) (Table 2). Moreover, a statistically significant correlation was found between the number of p24-positive cells and the percentage of syncytia in each specimen (R2 = 0.846, P = 0.0094).
Figure 1.
A–D: Immunohistochemical analysis of HIV-1 p24 protein expression in frontal cortex sections from patients with HIV-related encephalitis and controls. A: Section from a patient with HAE without syncytia. p24 staining is present on cells localized preferentially in perivascular area (arrows). B: Section from a patient with HAE characterized by the presence of numerous syncytia and an altered tissue architecture. Syncytia (arrows) stain for p24 inside the cytoplasm, as visible in the higher magnification inset. C: Quantification of p24 protein expression on brain section from patients with HIV-related encephalitis with brain syncytia (Syn+) and without syncytia (Syn−). Statistical analysis underlines a significant difference (*) of p24 staining between the two categories (P = 0.015). D: Frontal cortex section from a control subject negative after staining for HIV-p24. E–H: TUNEL staining of frontal cortex sections from HIV-related encephalitis patients and controls. E: Representative section from a patient with HAE without syncytia. Only a few cells are apoptotic, as indicated by the red nuclear staining (arrows). F: Staining from a patient with brain syncytia. TUNEL-positive syncytia are visible (arrows). Note that in some examples not all of the nuclei of the same syncytium are TUNEL-positive (inset). G: Quantification of TUNEL-positive cells on brain section from Syn+ and Syn− patients. H: Quantification of white matter lesions (WM) and microglia activation (MA) on frontal cortex sections between the two group of patients: with brain syncytia (Syn+) and without this cell type (Syn−). Scale bars, 100 μm.
Table 2.
Histochemical Characteristics of Syncytia in HAE Brain Sections (Number of Patients = 6)
| Characteristics | Frequency of stained syncytia (±SD) |
|---|---|
| HIV-p24 staining | 90.25 ± 11.09 |
| IκB-α (Ser32/36P) | 42.7 ± 25.3 |
| mTOR | 87.77 ± 14.37 |
| p53 (Ser46P) | 72.3 ± 10.2 |
| Puma | 53.16 ± 23.09 |
| TG2 | 64.6 ± 16.8 |
| TUNEL positivity | 48.9 ± 23 |
To study the fate of HAE-associated syncytia, we analyzed our samples by means of the TUNEL technique that detects apoptotic DNA fragmentation (Figure 1, E and F). With respect to Syn− patients, sections from Syn+ patients tended to contain a higher frequency of TUNEL-positive cells. Even though this difference was not significant by statistical analysis (P > 0.05) a high percentage of syncytia (48.9% ± 23, number of patients = 6) contained at least one TUNEL-positive nucleus (Figure 1G). It is noteworthy that some syncytia showed TUNEL positivity affecting only a few nuclei or one single nucleus, indicating that the apoptotic process may occur in an asynchronous manner in different nuclei within the same cytoplasm (Figure 1F).
Moreover, it is interesting to note that Syn+ specimens exhibited a more elevated number of white matter lesions than Syn− samples, thus supporting the hypothesis that the presence of syncytia may represent an index of HAE severity (Figure 1H). In fact, the degree of white matter lesions closely correlated with the percentage of syncytia for each specimen (R2 = 0.864, P = 0.0072), whereas there was no correlation between number of syncytia and microglial activation.
Analysis of the Apoptotic Pathway Induced in HIV-Infected Syncytia
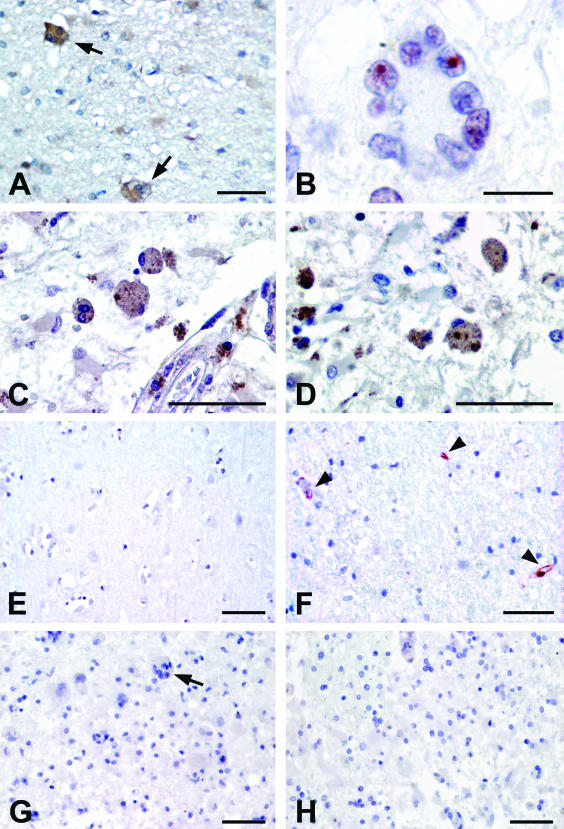
Recent studies underscore the probable importance of p53 for syncytial apoptosis31–33 as well as for the HIV-1-induced neuropathology.17 Garden and colleagues17 described the HAE-associated accumulation of p53 protein in cortical neurons and microglia. In lymphocytes from HIV-1-positive patients, we demonstrated the p53 phosphorylation on Ser 15 and on Ser 46 and that these phosphorylations are mediated by the two kinases mTOR and p38 MAPK, respectively.31,32 Syncytia of HAE patients displayed not only the presence of p38 MAPK,33 but also mTOR (Figure 2A). The effective activation of mTOR in apoptotic syncytia was confirmed by analyzing the nuclear localization of its downstream target, p70 S6 kinase (Figure 2B). The phosphorylation of p53 on Ser 46 (p53S46P) was observed in 72.3% ± 10.2 of brain syncytia (Figure 2C). We have previously demonstrated that NF-κB is required for the mTOR-mediated p53 phosphorylation.33 Accordingly, we detected the activation of NF-κB revealed by the presence of IκB-α phosphorylated on Ser 32 and Ser 36 (IκBS32/36P) in 42.7% ± 25.3 of syncytia, suggesting that NF-κB is indeed released from IκB-mediated inhibition (Figure 2D). These data confirm the involvement of all these factors also in HAE syncytial apoptosis.
Figure 2.
Immunohistochemical analysis of syncytia present on frontal cortex section from HIV-related encephalitis affected patients and controls. A: The section, from a patient with HAE, shows syncytia positively stained for mTOR (arrows). B: Syncytium showing nuclear staining for p70 S6 kinase. C: Syncytia showing p53 phosphorylated on Ser 46. D: Numerous syncytia are labeled for IκB-α phosphorylated on Ser 32/36 that is a marker for NF-κB activation. E: Brain section from control subject submitted to immunostaining for IκB-α phosphorylated on Ser 32/36. No labeling is detectable. F: Brain section from control subject stained with anti-TG2. The immunoreaction product is exclusively localized on endothelial cells (arrowheads) where TG2 is constitutively expressed. G: Frontal cortex section from a patient with HAE submitted to immunostaining by omitting primary antibody and using biotinylated anti-mouse IgG as secondary antibody. No labeling is detectable. The arrow points to a negative syncytium. H: Frontal cortex section from a patient with HAE submitted to immunostaining using isotype-matched mouse IgG as negative control. Scale bars: 100 μm (A, C–H); 25 μm (B).
In Figure 2, E and F, we report the brain sections from HIV-negative patients showing no expression of IκB-α phosphorylated on Ser 32 and Ser 36 (Figure 2E) and the detection of TG2 restricted to endothelial cells (Figure 2F, arrowheads), in which the enzyme is constitutively expressed. The specificity of the used staining procedures was confirmed by performing the methodological controls such as the omission of primary antibodies (Figure 2G) and the utilization of nonimmune isotype-matched control antibodies (Figure 2H) on HIV-positive patients.
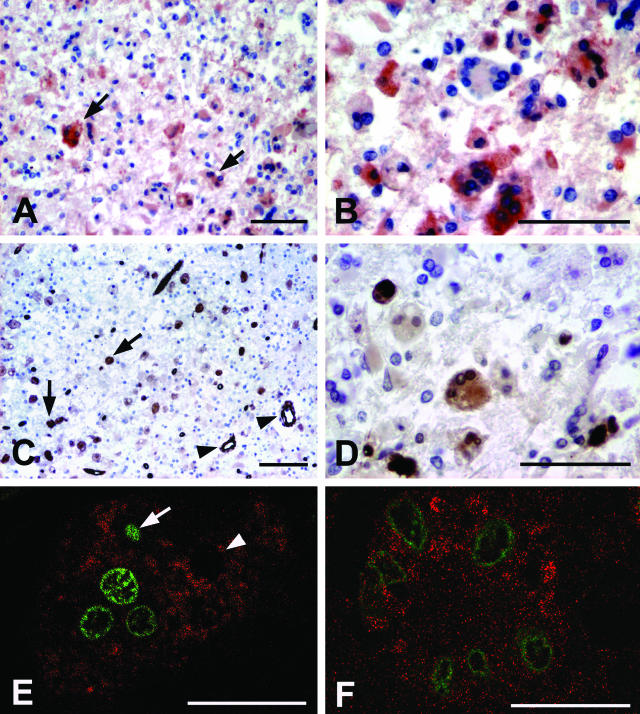
Next, we determined the expression of one particular p53 target, the BH3-only protein Puma, which has been found to be essential for the death of HIV-1 Env-elicited syncytia.33 Enhanced Puma expression was consistently observed in HAE brain sections (Figure 3, A and B) suggesting that Puma could be a mediator of p53 signaling involved in the neuropathology. Syncytia exhibited a variable degree of Puma expression in their cytoplasm (Figure 3B); at least 53.16% ± 23.09 multinucleated cells were judged to contain more Puma than adjacent nonsyncytial cells. Further support to the role of Puma in p53-dependent killing of multinucleated cells was obtained by detecting its up-regulation in syncytia showing DNA fragmentation (Figure 3E).
Figure 3.
Immunohistochemistry of syncytia present on frontal cortex sections from patients with HIV-related encephalitis. Analysis of p53-dependent effectors of the apoptotic pathway. A: Brain section from a patient, displaying altered tissue architecture, with high expression of Puma protein in syncytia (arrows). Also other histiomacrophagic cells are labeled. B: Higher magnification displays a variable degree of Puma expression in different syncytia. C: TG2 labeling. Numerous syncytia are labeled (arrows). Endothelial cells constitutively express TG2 (arrowheads). D: Higher magnification displays syncytia exhibiting cytoplasmic expression of TG2. E: Confocal microscopy image of a syncytium showing cytoplasmatic Puma expression (labeled with Alexa 546, red) and nuclear TUNEL reaction product (fluorescein, green). The syncytium nuclei show different stage of apoptosis; in fact, condensed nucleus (arrow) is present in the same syncytium with TUNEL-negative (arrowhead) and TUNEL-positive nuclei. F: Confocal microscopy image of a syncytium stained with anti-TG2 (labeled with Alexa 546, red) and nuclear TUNEL reaction product (fluorescein, green). Scale bars: 100 μm (A–D); 25 μm (E, F).
The expression of TG2, another proapoptotic BH3-only protein35 involved in viral-induced pathologies36,37 and in the regulation of apoptosis of HIV-infected cells,38–40 was also analyzed in the same samples (Figure 3, C and D). As expected, high levels of TG2 were found in the cytoplasm of HAE syncytia (64.6% ± 16.8 positive) (Table 2 and Figure 3, C and D) and, by confocal microscopy studies, we observed its localization exactly in the syncytia showing DNA fragmentation (Figure 3F). TG2 was also detected in endothelial cells, which constitutively express the enzyme (Figure 2F).41 Interestingly, ∼30% of TUNEL-negative syncytia expressed mTOR and p53S46P (but not Puma or TG2) corroborating a hypothetical sequence of events in which the activation of p53 (as indicated by mTOR induction and p53 phosphorylation) precedes the transactivation of Puma and TG2, both of which act as terminal effectors.
Analysis of the Apoptotic Pathway Induced in Neurons in HIV-Infected Brains
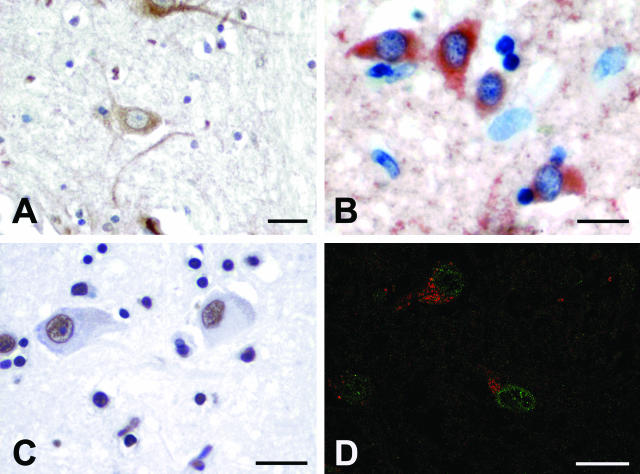
Several studies indicate that HAE is associated with neuronal apoptosis.8–11 As observed for syncytia, a fraction of neurons manifested the up-regulation of mTOR (44.8% ± 19.7) and Puma induction (56.8% ± 7.5) (Figure 4, A and B). Again, the same cells, which expressed Puma, showed extensive DNA fragmentation as detected by TUNEL staining (53.4% ± 17.8) (Figure 4, C and D). As recently reported, neuronal cells showing Puma-expression are primed toward apoptosis.42 By contrast, IκB-α phosphorylation and TG2 induction were not observed in the neuronal populations, thus suggesting that neuronal and syncytial cell death follows partially overlapping, yet distinct apoptotic pathways.
Figure 4.
Immunohistochemical analysis and TUNEL staining on neurons from patients with HIV-related encephalitis. A: The microphotograph shows neurons stained for mTOR. The brown reaction product is uniformly distributed on the cytoplasm. B: Expression of Puma protein on frontal cortex neurons. The cytoplasm of numerous neurons is positively stained with anti-Puma antibody. C: Neurons showing DNA fragmentation detectable by brown precipitate in the nucleus. D: Confocal microscopy image showing Puma expression (labeled with Alexa 546, red) and TUNEL reactivity (fluorescein, green) in neurons. Scale bars, 50 μm.
Discussion
The introduction of highly active anti-retroviral therapy has modified the observed disease progression in HIV-induced encephalitis and AIDS-related CNS opportunistic infections. After the introduction of protease inhibitors, a decline in both HIV encephalitis and CNS opportunistic infections was observed.1,7,24,40 However, with the increasing resistance of HIV strains to anti-retrovirals, there has been a rebound in the frequency of HIV encephalitis and HIV leukoencephalopathy. The brain is considered as a potential HIV reservoir; in fact the virus can persist even after long periods of treatment with highly active anti-retroviral therapy.1,7,24,40,43
The mechanistic dissection of HAE should be envisaged to elaborate novel therapeutic strategies. To this aim, we have analyzed HIV-induced apoptotic pathway(s) in brain sections from HAE-affected patients. We described a substantial difference between HAE cases showing the presence or the absence of syncytia. On the basis of our results, syncytia can be considered as an index of HAE severity because their presence correlates with an elevated number of HIV-1-infected cells (as detected by p24 staining). In addition, a pronounced apoptosis (as detected by TUNEL staining) and histopathological brain alterations (white matter lesions and microglia activation) are also detected in the same patients.
As far as the cell death mechanisms involved in the demise of brain cells are concerned, we revealed the expression on brain syncytia of the tumor suppressor transcription factor p53 phosphorylated on Ser 46. p53 phosphorylation is known to increase the transactivating function of p5344 and thus may contribute to apoptosis induction.45,46 We previously showed that phosphorylated p53 plays a key role in the death of syncytia elicited by HIV infection in vitro.31 In keeping with these findings, an increased p53 protein expression in its proapoptotic form has been revealed in syncytia of HAE patients, indicating that p53-dependent death signaling is required for HIV-induced degeneration of multinucleated cells. We previously reported the involvement of mTOR in the phosphorylation of p53 in lymphoid tissues of HIV-infected patients.31,32 Here, we show that mTOR is not only highly activated in brain syncytia of HIV-infected patients, thus suggesting its general participation in the lethal p53-driven signaling cascade involved in HAE. We detected the presence of the BH3-only protein Puma in brain syncytia, especially in TUNEL-positive syncytia.
Another proapoptotic protein, TG2, was also found in the cytoplasm of syncytia, especially at an advanced stage of the process, when syncytia were TUNEL-positive. TG2 has been shown to play an important role in neurodegenerative pathologies, for instance in Huntington’s disease.47 Recently, TG2 has been shown to act as a multifunctional BH3-only protein at the mitochondrial level.35
Altogether, the results obtained in this study are compatible with the notion that the formation and death of syncytia correlates with or contributes to the neuropathology of HAE. Once formed, presumably as the result of a fusion process involving at least one HIV-infected (p24+) cell, syncytia activate a lethal signal transduction cascade whose early events (mTOR up-regulation and IκB-α phosphorylation) manifest before cells become TUNEL-positive, whereas late events (Puma and TG2 induction) coincide with cellular demise.
Some of the factors involved in syncytial cell death are overexpressed also in neurons of HAE patients. In fact, we detected the neuronal up-regulation of mTOR and Puma, thus suggesting their participation to the neuronal death pathway. In keeping with this assumption, the overexpression of Puma during neuronal cell death has been recently described.42
However, dying cortical neurons found in HAE patients were TG2-negative, suggesting that neuronal and syncytial death obeys different rules. In accord with this speculation, neurons also lacked signs of NF-κB activation, which were detected in HAE syncytia. These findings suggest that neuronal and syncytial cell death follows partially overlapping, yet distinct apoptotic pathways. This knowledge may lay the ground for future studies and intervention on HAE-related cell death.
Acknowledgments
We thank Dr. Loredana Polsinelli and Sig. E. Stigliano for technical assistance and Dr. Patrizia Lorenzini for statistical advice.
Footnotes
Address reprint requests to Mauro Piacentini, National Institute for Infectious Diseases, IRCCS ≪ Lazzaro Spallanzani≫ Via Portuense 292, 00149 Rome, Italy. E-mail: mauro.piacentini@uniroma2.it.
Supported by the European Commission (QLK3-CT-2002-01956, Sidaction, ANRS (to G.K. and M.P.), Istituto Superiore di Sanità (no. 40F.60), Ricerca Corrente e Finalizzata “Ministero della Salute,” and MIUR (COFIN and FIRB-2001).
References
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Major EO. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/s1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Graham DI, Lantos PL. New York: Oxford University Press, Inc.; Greenfield’s Neuropathology. 1997:pp 15–25. [Google Scholar]
- Cornford ME, Holden JK, Boyd MC, Berry K, Vinters HV. Neuropathology of the acquired immune deficiency syndrome (AIDS): report of 39 autopsies from Vancouver, Br Columbia. Can J Neurol Sci. 1992;19:442–452. [PubMed] [Google Scholar]
- Kibayashi K, Mastri AR, Hirsch CS. Neuropathology of human immunodeficiency virus infection at different disease stages. Hum Pathol. 1996;27:637–642. doi: 10.1016/s0046-8177(96)90391-3. [DOI] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001;312:67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Rotiroti D, Nappi G, Bagetta G. Neurobiological mediators of neuronal apoptosis in experimental neuro-AIDS. Toxicol Lett. 2003;139:199–206. doi: 10.1016/s0378-4274(02)00434-4. [DOI] [PubMed] [Google Scholar]
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotoxicol Res. 2004;5:605–615. doi: 10.1007/BF03033180. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lifson J, Coutre S, Huang E, Engleman E. Role of envelope glycoprotein carbohydrate in human immunodeficiency virus (HIV) infectivity and virus-induced cell fusion. J Exp Med. 1986;164:2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J, Goh WC, Rosen C, Campbell K, Haseltine WA. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Martin J, LaBranche CC, Gonzalez-Scarano F. Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J Virol. 2001;75:3568–3580. doi: 10.1128/JVI.75.8.3568-3580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ER, Zijenah LS, Mutetwa S, Kantor R, Kittinunvorakoon C, Katzenstein DA. High frequency of syncytium-inducing and CXCR4-tropic viruses among human immunodeficiency virus type 1 subtype C-infected patients receiving antiretroviral treatment. J Virol. 2003;77:7682–7688. doi: 10.1128/JVI.77.13.7682-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonis VR, de Souza MS, Darden JM, Chantakulkij S, Chuenchitra T, Nitayaphan S, Brown AE, Robb ML, Birx DL. Human immunodeficiency virus type 1 primary isolate neutralization resistance is associated with the syncytium-inducing phenotype and lower CD4 cell counts in subtype CRF01_AE-infected patients. J Virol. 2003;77:8570–8576. doi: 10.1128/JVI.77.15.8570-8576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Ferri KF, Blanco J, Roumier T, Larochette N, Barretina J, Amendola A, Nardacci R, Metivier D, Este JA, Piacentini M, Kroemer G. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med. 2001;194:1097–1110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, Leduc P, Ingher DE, Druillennec S, Roques B, Leibovitch SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M, Kroemer G. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 2002;21:4070–4080. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini JL, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, Lazar V, Ciccosanti F, Nardacci R, Penninger J, Piacentini M, Kroemer G. NF-{kappa}B and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med. 2005;20:279–289. doi: 10.1084/jem.20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolfo C, Mormone E, Matarrese P, Ciccosanti F, Farrace MG, Garofano E, Piredda L, Fimia GM, Malorni W, Piacentini M. “Tissue” transglutaminase is a multifunctional BH3-only protein. J Biol Chem. 2004;279:54783–54792. doi: 10.1074/jbc.M410938200. [DOI] [PubMed] [Google Scholar]
- Nardacci R, Ciccosanti F, Falasca L, Lo Iacono O, Amendola A, Antonucci G, Piacentini M. Tissue transglutaminase in HCV infection. Cell Death Differ. 2003;10(Suppl 1):S79–S80. doi: 10.1038/sj.cdd.4401112. [DOI] [PubMed] [Google Scholar]
- Nardacci R, Lo Iacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxi A, Fimia GM, Iadevaia V, Melino G, Ruco L, Tocci G, Ippolito G, Piacentini M. Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol. 2003;162:1293–1303. doi: 10.1016/S0002-9440(10)63925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola A, Rodolfo C, Di Caro A, Ciccosanti F, Falasca L, Piacentini M. “Tissue” transglutaminase expression in HIV-infected cells: an enzyme with an antiviral effect? Ann NY Acad Sci. 2001;946:108–120. [PubMed] [Google Scholar]
- Amendola A, Fesus L, Piacentini M, Szondy Z. “Tissue” transglutaminase in AIDS. J Immunol Methods. 2002;265:145–159. doi: 10.1016/s0022-1759(02)00077-7. [DOI] [PubMed] [Google Scholar]
- Antinori A, Cingolani A, Giancola ML, Forbici F, De Luca A, Perno CF. Clinical implications for HIV-1 drug resistance in the neurological compartment. Scand J Infect Dis Suppl. 2003;35(Suppl 106):S41–S44. doi: 10.1080/03008870310009650. [DOI] [PubMed] [Google Scholar]
- Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- Cregan SP, Arbour NA, Maclaurin JG, Callaghan SM, Fortin A, Cheung EC, Guberman DS, Park DS, Slack RS. P53 activation domain 1 is essential for Puma upregulation and p53-mediated neuronal cell death. J Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr Opin Neurol. 2003;16:403–409. doi: 10.1097/01.wco.0000073943.19076.98. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 1999;18:7002–7010. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH. Activation of the p53 tumor suppressor protein. Biochim Biophys Acta. 2002;1602:47–59. doi: 10.1016/s0304-419x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- Mastroberardino PG, Iannicola C, Nardacci R, Bernassola F, De Laurenzi V, Melino G, Moreno S, Pavone F, Oliverio S, Fesus L, Piacentini M. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington’s disease. Cell Death Differ. 2002;9:873–880. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]