Abstract
In plants, the chromatin-associated high mobility group (HMG) proteins occur in two subfamilies termed HMGA and HMGB. The HMGA proteins are characterized by the presence of four AT-hook DNA binding motifs, and the HMGB proteins contain an HMG box DNA binding domain. As architectural factors, the HMG proteins appear to be involved in the regulation of transcription and other DNA-dependent processes. We have examined the subcellular localization of Arabidopsis thaliana HMGA, HMGB1, and HMGB5, revealing that they localize to the cell nucleus. They display a speckled distribution pattern throughout the chromatin of interphase nuclei, whereas none of the proteins associate with condensed mitotic chromosomes. HMGA is targeted to the nucleus by a monopartite nuclear localization signal, while efficient nuclear accumulation of HMGB1/5 requires large portions of the basic N-terminal part of the proteins. The acidic C-terminal domain interferes with nucleolar targeting of HMGB1. Fluorescence recovery after photobleaching experiments revealed that HMGA and HMGB proteins are extremely dynamic in the nucleus, indicating that they bind chromatin only transiently before moving on to the next site, thereby continuously scanning the genome for targets. By contrast, the majority of histone H2B is basically immobile within the nucleus, while linker histone H1.2 is relatively mobile.
INTRODUCTION
In the cell nucleus, organization of the genomic DNA with histones and other proteins into chromatin affects DNA-dependent processes, such as the transcription of genes. The best-studied level of chromatin organization is the packaging of the DNA into nucleosome particles. Posttranslational modification of nucleosomal core histones and nucleosome remodeling mediated by ATP-dependent multiprotein complexes play a critical role in the regulation of gene expression (Reyes et al., 2002; Loidl, 2004; Hsieh and Fischer, 2005). H1 linker histones are the most abundant proteins associated with nucleosomes and with the internucleosomal linker DNA, and they bind to almost all nucleosomes in eukaryotic chromatin (Bustin et al., 2005). The linker histone molecule consists of three domains: a central nonpolar globular domain that is flanked by two unstructured highly basic domains. While the globular domain of H1 binds to nucleosomes around the dyad axis, the C-terminal domain interacts with the linker DNA. The association with H1 can influence the geometry of the linker DNA and generate higher-order structures of the chromatin fiber (Jerzmanowski, 2004).
The high mobility group (HMG) proteins are (after the histones) the second most abundant family of chromosomal proteins occurring in variable amounts, and it has been estimated that they bind to ≤10% of the nucleosomes (Johns, 1982). Their presence in all tissues of eukaryotes favors the possibility that the HMG proteins are required for proper cellular function. Due to their abundance, HMG proteins serve a global structural function in the nucleus, and they act as architectural factors, facilitating various DNA-dependent processes, including transcription (Bustin and Reeves, 1996; Thomas and Travers, 2001; Bianchi and Agresti, 2005). In plants, members of the HMGA and HMGB families have been identified (Grasser, 2003). Depending on the species, plants encode one or two HMGA proteins consisting of an N-terminal domain with similarity to the globular domain of linker histones and a C-terminal domain containing four AT-hook motifs (Klosterman and Hadwiger, 2002; Grasser and Launholt, 2006). Mediated by the AT hooks, HMGA proteins interact with A/T-rich stretches of DNA (Pedersen et al., 1991; Nieto-Sotelo et al., 1994; Webster et al., 1997, 2000; Zhang et al., 2003a, 2003b), four-way junction DNA (Zhang et al., 2003a, 2003b), and nucleosomes (Arwood and Spiker, 1990; Lichota and Grasser, 2001). Plants encode approximately six different HMGB proteins that are characterized by a central HMG box DNA binding domain, which is flanked by a basic N-terminal and an acidic C-terminal domain (Grasser and Launholt, 2006). Mediated by the HMG box domain, the HMGB proteins interact non-sequence specifically with linear DNA (Pedersen et al., 1991; Grasser et al., 1994; Webster et al., 1997, 2000; Wu et al., 2003), but they bind with high affinity certain DNA structures, including four-way junctions and DNA minicircles (Grasser et al., 1994; Stemmer et al., 1997; Ritt et al., 1998b; Webster et al., 2001; Wu et al., 2003; Zhang et al., 2003b), and they interact with nucleosomes (Arwood and Spiker, 1990; Lichota and Grasser, 2001).
Whereas HMGA proteins clearly are nuclear proteins (Amirand et al., 1998), vertebrate HMGB proteins are found primarily in the cell nucleus but to various extents also in the cytosol (Bustin and Neilhart, 1979; Kuehl et al., 1984; Falciola et al., 1997; Bonaldi et al., 2003). It has been suggested that HMGB proteins can shuttle between nucleus and cytosol, and it has been reported that mammalian HMGB1 can be passively released from the nucleus upon cell death and that it can be actively secreted acting as a cytokine (Müller et al., 2004). Since the subcellular localization of plant HMG proteins has not been studied systematically, we have examined here the distribution of Arabidopsis thaliana HMGA and HMGB proteins using immunofluorescence in dividing cells and by analyzing the localization of green fluorescent protein (GFP) fusions. Using fluorescence recovery after photobleaching (FRAP), it was recently found that HMG proteins (in contrast with previous assumptions) are highly mobile in mammalian cell nuclei (Catez et al., 2004; Harrer et al., 2004; Phair et al., 2004b). We have studied by FRAP the mobility of HMGA and HMGB proteins in plant cell nuclei.
RESULTS
Arabidopsis HMGA and HMGB1 Display a Speckled Distribution Pattern in Interphase Chromatin and Are Not Associated with Mitotic Chromosomes
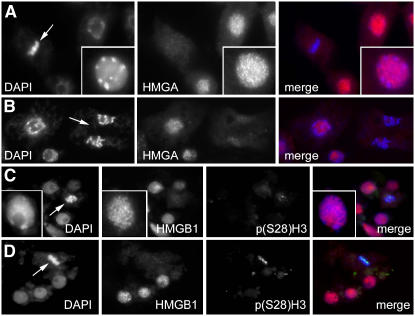
Since the subcellular localization of plant HMGA and HMGB proteins has not been examined in detail, we have studied the distribution of the HMGA and the HMGB1 proteins by indirect immunofluorescence in meristematic root tip cells of Arabidopsis seedlings. For comparison, the chromosomal DNA was visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining, and the HMGA and HMGB1 proteins were detected by immunolabeling using antisera raised against recombinant HMGA and HMGB1. While the preimmune sera did not result in a specific immunostaining (data not shown), the anti-HMGA and anti-HMGB1 sera resulted in labeling of the nuclei of interphase cells. Both HMGA and HMGB1 show a similar speckled distribution pattern in interphase nuclei and can be detected in euchromatin and in the heterochromatic chromocenters (Figure 1). In mitotic cells, the condensed chromosomes revealed no significant immunoreactivity against the HMGA and HMGB1 antisera, and a rather disperse staining of the cytosol is observed (Figure 1). To ascertain whether mitotic cells are equally accessible for antibodies, cells were simultaneously labeled with antibodies recognizing HMGB1 and histone H3 phosphorylated at Ser-28. Cell cycle–specific p(Ser28)H3-immunosignals (Gernand et al., 2003) demonstrated the accessibility of dividing cells (Figures 1C and 1D). Thus, in interphase cells, HMGA and HMGB1 are found throughout the nucleus, whereas in mitotic cells, neither HMGA nor HMGB1 are chromatin associated.
Figure 1.
HMGA and HMGB1 Display a Similar Speckled Distribution Pattern in the Chromatin of Interphase Nuclei, but They Are Not Associated with Metaphase Chromosomes.
Immunolocalization of HMGA ([A] and [B]) and HMGB1 ([C] and [D]) in mitotic interphase chromatin and condensed metaphase/anaphase chromosomes (indicated by arrows) of Arabidopsis root cells. Note that HMGA and HMGB1 immunolabeling occurs only with interphase chromatin but not with condensed mitotic chromosomes. Double immunolabeling for anti-HMGB1 and histone H3 phosphorylated at Ser-28 [p(Ser28)H3] in mitotic cells ([C] and [D]). The nuclear DNA has been counterstained with DAPI, which is depicted in blue in the merged images, while the HMGA and HMGB1 immunofluorescence is in red and the immunofluorescence of p(Ser28)H3 in green. Insets show individual nuclei at higher magnification.
In Vitro DNA Interaction of HMG-GFP Fusion Proteins
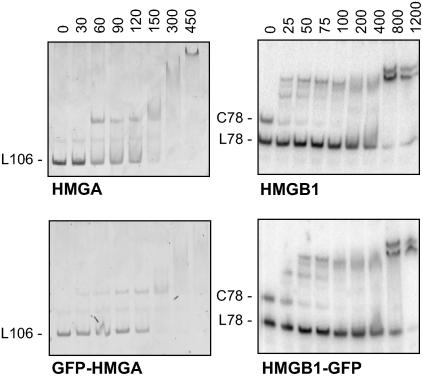
We have studied the subcellular localization and the DNA binding properties of the HMGA and HMGB proteins fused to the GFP. In the case of the HMGB proteins, we selected the HMGB1 and the HMGB5 proteins, which differ significantly in the length of the basic N-terminal and acidic C-terminal domains flanking the central HMG box DNA binding domain (Figure 2). We analyzed the amino acid sequences of HMGA and HMGB1/5 for the presence of subcellular targeting signals using the algorithm Psort (http://psort.nibb.ac.jp/form.html). This revealed predicted nuclear localization signals (NLSs) in the AT-hook region of HMGA and in the HMG box domain of HMGB1 and HMGB5 (Figure 2). Initially, we tested whether the GFP fusion proteins display the DNA binding properties characteristic of HMGA and HMGB proteins (Grasser, 2003). Therefore, the GFP-HMGA and HMGB1-GFP proteins were expressed in Escherichia coli and purified by three-step column chromatography. The DNA binding properties of the GFP fusion proteins were compared with those of the HMG proteins without GFP fusion using electrophoretic mobility shift assays (EMSAs). Increasing amounts of the recombinant proteins were incubated with the DNA substrate, and the formation of protein/DNA complexes was analyzed by native PAGE. Typical of HMGA proteins, both HMGA and GFP-HMGA bind with comparable affinity to an A/T-rich 106-bp DNA fragment of a maize (Zea mays) zein gene promoter (Figure 3). HMGB1 and HMGB1-GFP were incubated with a mixture of a linear and circularized 78-bp DNA fragment. Characteristic of HMGB proteins, HMGB1 and HMGB1-GFP selectively interacted with the circularized form of the DNA fragment, while the linear form of the fragment was bound only at significantly higher protein concentrations. Therefore, the fusion of HMGA and HMGB proteins to GFP apparently does not interfere with the characteristic DNA interactions of the HMG proteins.
Figure 2.
Domain Structure of HMGA, HMGB1, and HMGB5.
Plant HMGA proteins consist of a C-terminal domain containing four AT-hook motifs and have an additional plant-specific N-terminal domain with similarity to the globular domain of linker histone H1. The GRP core motifs of the four AT hooks of Arabidopsis HMGA (22.0 kD) are highlighted in gray and the N-terminal H1-like domain in black. The Arg residues (Arg-98 and Arg-164) that are part of putative NLSs and that have been mutated here are indicated in bold. Within the amino acid sequences of HMGB1 (20.2 kD) and HMGB5 (14.2 kD), the central HMG box DNA binding domain has been highlighted in black. Note the different length of the basic N-terminal and acidic C-terminal domains of HMGB1 and HMGB5. NLSs predicted by the Psort algorithm (http://psort.nibb.ac.jp/form.html) are indicated by asterisks, and basic residues in the N-terminal region of HMGB1 and HMGB5 (that may contribute to the nuclear targeting) are underlined.
Figure 3.
DNA Binding Properties of HMGA, HMGB1, and the Corresponding GFP Fusion Proteins.
The DNA interaction of HMGA and HMGB proteins examined using EMSA. Increasing amounts (indicated in nM, final concentration) of HMGA and GFP-HMGA have been incubated with an A/T-rich 106-bp DNA fragment (L106) originating from a maize zein gene promoter (left panels). Increasing amounts of HMGB1 and HMGB1-GFP were incubated with a mixture of linear (L78) and circular (C78) 78-bp DNA fragment (right panels). Protein-DNA complexes display a reduced electrophoretic mobility relative to the unbound DNA fragments.
A Basic Monopartite NLS Close to the Third AT Hook Mediates Nuclear Localization of HMGA
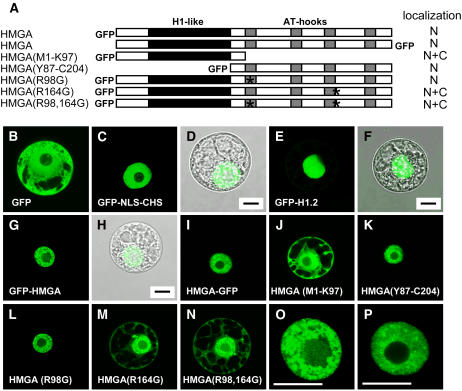
To study the nuclear localization of HMGA in more detail, we constructed plasmids suitable for the expression of GFP fusion proteins in plant protoplasts. In transient transformation assays, full-length and truncated HMGA fusion proteins (Figure 4A) were expressed in tobacco (Nicotiana tabacum) BY-2 suspension cell protoplasts. Transformed protoplasts were analyzed by confocal laser scanning microscopy (CLSM). We used constructs that served as controls in this experiment: GFP alone that is found in both cytosol and nucleus (Figure 4B), GFP fused to chalcone synthase containing a mutated nuclear export signal and the NLS of the SV40 large T antigen as the only functional localization signal (Haasen et al., 1999) (Figures 4C and 4D), and GFP-H1.2 that localizes to the nucleus (Figures 4E and 4F). In case of the nuclear-targeted GFP and linker histone H1.2, colocalization of the nucleus and green fluorescence is demonstrated by overlaying the nuclear GFP fluorescence and the transmission image of the same protoplast (Figures 4C to 4F). The N-terminal and C-terminal GFP fusion proteins of full-length HMGA clearly accumulated in the nucleus (Figures 4G to 4I), and similar to the immunofluorescence experiment (Figure 1), a speckled distribution pattern is observed within the nucleus (Figures 4O and 4P). The individual N-terminal domain of HMGA with sequence similarity to the globular domain of histone H1, however, is found in the cytosol and nucleus (Figure 4J) like GFP alone. By contrast, the individual AT-hook region specifically accumulates in the cell nucleus (Figure 4K). Therefore, the AT-hook region is critical for the nuclear targeting of HMGA. To identify the NLS responsible for the nuclear localization of HMGA, the amino acid sequences of the putative NLSs predicted by Psort (cf. Figure 2) were altered by site-directed mutagenesis. In the context of the full-length protein, the codons for the Arg residues Arg-98 and Arg-164 were either individually or both in combination mutated to code for Gly residues. Whereas mutation of R98 does not interfere with the nuclear localization of HMGA (Figure 4L), mutating R164 individually (Figure 4M) and in combination with R98 (Figure 4N) resulted in a loss of the nuclear accumulation of HMGA. Instead, the mutated proteins are detected both in cytosol and nucleus similar to GFP alone and the individual H1-like domain of HMGA. Thus, the sequence around Arg-164, which overlaps with the third AT hook (cf. Figure 2), is essential for the nuclear targeting of HMGA.
Figure 4.
A Basic Monopartite NLS Close to the Third AT Hook Is Critical for Nuclear Localization of HMGA.
(A) Schematic representation of the analyzed HMGA-GFP fusions. The H1-like domain is highlighted in black, while the AT hooks are highlighted in gray. The position of the GFP relative to HMGA is also indicated for the different constructs. Asterisks indicate the Arg residues (Arg-98 and Arg-164) that have been mutated within the putative NLSs. The predominant subcellular localization of the fusion proteins is indicated (N, nuclear accumulation; N+C, localization in nucleus and cytoplasm) based on inspecting 60 to 80 transformed protoplasts each.
(B) to (P) CLSM images of tobacco BY-2 cell protoplasts transiently transformed with plasmids driving the expression of the GFP fusion constructs indicated in (A). In (D), (F), and (H), overlays of the GFP fluorescence (seen in [C], [E], and [G], respectively) and the transmission image of the same protoplast are shown. Various control construct have been used for the subcellular localization studies: GFP alone ([B]; localizing to the nucleus and cytosol), GFP-NLS-CHS ([C] and [D]; localizing to the nucleus), and GFP-H1.2 ([E] and [F]; localizing to the nucleus). The different HMGA-GFP fusion constructs are shown in (G) to (P). In (O) and (P), the nuclei seen in (G) and (I) are shown in higher magnification. Bars = 10 μm.
Extensive Basic Regions Are Required for Nuclear Accumulation of HMGB1 and HMGB5
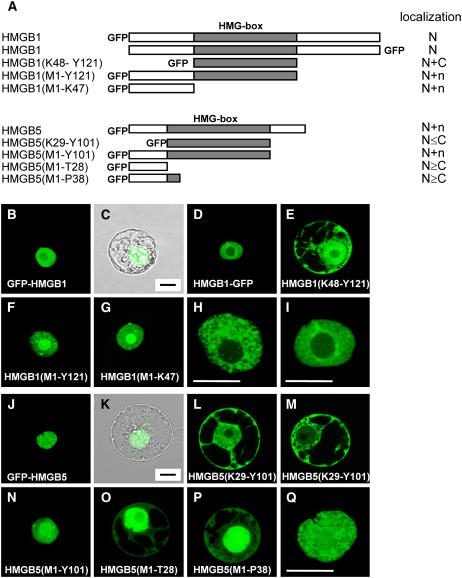
Likewise, we studied the subcellular localization of HMGB1 and HMGB5 in transient transformation assays, and the distribution of GFP fusion proteins (Figure 5A) was analyzed by CLSM. N-terminal and C-terminal GFP fusions of full-length HMGB1 display a prominent nuclear fluorescence (Figures 5B to 5D). The colocalization of the nucleus with GFP fluorescence is evident from the overlay with the transmission image of the same protoplast (Figures 5B and 5C). In line with the immunofluorescence experiments (Figures 1A and 1B), within the nucleus a speckled distribution pattern is seen for HMGB1 (Figures 5H and 5I). Despite the presence of a putative NLS predicted by Psort, the individual HMG box domain of HMGB1 (Figure 5E) was detected in the cytosol and nucleus (similar to GFP alone; Figure 4B). However, a GFP fusion protein comprising the HMG box domain and the N-terminal domain of HMGB1 accumulates in the nucleus (Figure 5F). Similarly, fusion of the individual N-terminal domain to GFP results in nuclear accumulation of the fusion protein (Figure 5H), demonstrating that the N-terminal domain contains the signal(s) for nuclear localization. Compared with full-length HMGB1 (Figures 5B to 5D), both truncated proteins containing the basic N-terminal domain and lacking the C-terminal acidic domain of HMGB1 (Figures 5F and 5G) display a prominent localization also in the nucleolus, suggesting the presence of a signal for nucleolar targeting within the N-terminal domain.
Figure 5.
Different Regions of HMGB1 and HMGB5 Determine Their Subcellular Localization.
(A) Schematic representation of the HMGB1-GFP and HMGB5-GFP fusions used for protoplast transformation. The central HMG box DNA binding domain is highlighted in gray. The position of the GFP relative to the HMGB protein is also indicated for the different constructs. The predominant subcellular localization of the fusion proteins is indicated (N, nuclear accumulation; N+C, localization in the nucleus and cytosol; N+n, nuclear accumulation including localization in the nucleolus; N≤C or N≥C, indicating the tendency of higher fluorescence in the cytosol or in the nucleus, respectively, compared with the other compartment) based on inspecting 60 to 80 transformed protoplasts each.
(B) to (Q) CLSM images of tobacco BY-2 cell protoplasts transiently transformed with plasmids driving the expression of the GFP fusion constructs indicated in (A). In (C) and (K), overlays of the GFP fluorescence (seen in [B] and [J]) and the transmission image of the same protoplast are shown. Nuclei of protoplasts expressing GFP-HMGB1 ([H] and [I]) and GFP-HMGB5 (Q) are shown at higher magnification. Images shown in (O) and (P) are slightly overexposed to clearly show the additional cytosolic localization. Bars = 10 μm.
The GFP fusion protein comprising full-length HMGB5, which has significantly shorter N- and C-terminal domains than HMGB1, accumulates in the nucleus (Figures 5J and 5K) and displays a speckled distribution pattern (Figure 5Q) similar to HMGB1. The individual HMG box domain of HMGB5 localizes to the cytosol and to varying degrees also to the nucleus (Figures 5L and 5M). Similar to the situation with HMGB1, a GFP fusion protein comprising the HMG box domain and the N-terminal domain to GFP accumulates in the nucleus (Figure 5N). In contrast with the situation with HMGB1, fusion of the individual N-terminal domain of HMGB5 to GFP is not sufficient for nuclear accumulation, as the fusion protein is clearly detected also in the cytosol (Figure 5O). A similar pattern was obtained when the N-terminal domain and the N-terminal part of the HMG box domain was fused to GFP (Figure 5P), although this fusion protein contains the NLS predicted by Psort (Figure 2). Therefore, in the case of HMGB5, both the HMG box domain and the N-terminal domain are required for efficient nuclear accumulation.
HMGA and HMGB Are Highly Dynamic in Cell Nuclei as Determined by FRAP
To study the binding of HMGA and HMGB proteins to chromatin in living cells, we monitored the intranuclear dynamics of HMG-GFP fusion proteins by FRAP. In FRAP experiments, an intense laser pulse is used to irreversibly bleach the emission of light from a fluorescent molecule (e.g., GFP). Within the bleached area, the rate of fluorescence recovery (which is due to influx of unbleached molecules from regions surrounding the bleached area) is monitored by time-lapse microscopy. The rationale for FRAP is that fluorescence recovery kinetics reflect the overall mobility of a protein (Carrero et al., 2004; Sprague and McNally, 2005). Chromatin-associated proteins display a mobility that is reduced compared with proteins (such as GFP) that do not bind to chromatin or nuclear structures. Since the diffusion of proteins is very rapid, even transient chromatin association of a protein severely slows down the recovery rate (Phair et al., 2004a).
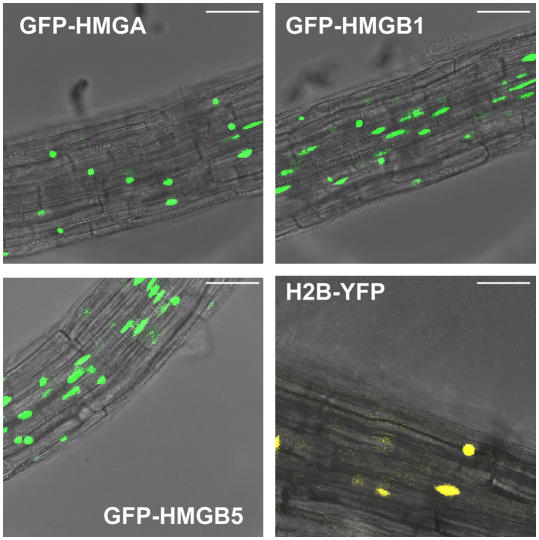
Two experimental systems were used for the FRAP assays to examine the mobility of HMGA, HMGB1, and HMGB5. The HMG-GFP fusions were transiently expressed in tobacco BY-2 cell protoplasts and/or in stably transformed Arabidopsis seedlings. Consistent with the transient transformation experiments (Figures 4 and 5), CLSM revealed that HMGA, HMGB1, and HMGB5 localize to the nuclei of root cells in the transgenic Arabidopsis seedlings expressing the GFP fusion proteins (Figure 6). As reference for the nuclear localization (and nuclear dynamics), plants expressing histone H2B fused to the yellow fluorescent protein (YFP) (Boisnard-Loriq et al., 2001) were analyzed in parallel. Further reference proteins were used in the FRAP experiments, including free GFP representing an inert, nonbinding control protein (Sprague and McNally, 2005), while linker histone H1.2 (and core histone H2B) fused to GFP/YFP served as proteins that (based on experiments using mammalian cells) were expected to display a comparatively low mobility in the nucleus (Lever et al., 2000; Misteli et al., 2000; Kimura and Cook, 2001). We analyzed the mobility of various HMG-GFP fusion proteins in FRAP assays and compared the measured recovery rates with those of the reference proteins. An experiment analyzing the mobility of GFP-H1.2 is used to illustrate the measurements that were performed. After determining the initial fluorescence, a short laser pulse bleached the light emission of the GFP fusion protein within a small area of the nucleus (Figure 7A). Then, the fluorescence recovery was monitored in short time intervals, revealing the recovery time, which is defined as the time after bleaching required for the fluorescence intensity to reach a constant value (Sprague and McNally, 2005). From the recovery curve, the time required to reach half-maximal recovery (t1/2) and the mobile fraction of the protein (Mf) can be determined (Figure 7B). Similarly, we examined the dynamics of full-length and truncated HMGA and HMGB proteins fused to GFP. Since the HMG proteins proved to display a mobility that is remarkably higher than that of the histones, we show the recovery curves in comparison with that of linker histone H1.2 (Figure 8). Fluorescence recovery curves for full-length HMGA, HMGB1, and HMGB5 were recorded both in transiently transformed tobacco protoplasts and in root cells of stably transformed Arabidopsis seedlings. These experiments revealed that data obtained with the two experimental systems are consistent (Figures 8A to 8C), although there is a difference with HMGA. The recovery curves of the reference proteins (Figure 8D, note the different time scale) demonstrate that as expected the fluorescence of GFP (not binding to chromatin) recovers extremely rapidly. As seen with mammalian cells (Kimura and Cook, 2001), the majority of H2B does not recover within the time frame of our experiment. With H1.2, an intermediate recovery rate was observed. Therefore, in contrast with the classical view, linker histone H1.2 displays a remarkable mobility in plant cell nuclei and is not stably bound to chromatin, as previously observed in mammalian cells (Lever et al., 2000; Misteli et al., 2000). However, HMGA and HMGB proteins display a dynamic in Arabidopsis and tobacco cell nuclei that is much higher than that of the linker histone.
Figure 6.
Nuclear Localization of HMGA, HMGB1, HMGB5, and Histone H2B in Stably Transformed Arabidopsis Seedlings.
Detection of nuclear GFP and YFP fluorescence by CLSM of roots of 3-d-old transgenic seedlings, expressing the indicated GFP/YFP constructs. Bars = 40 μm.
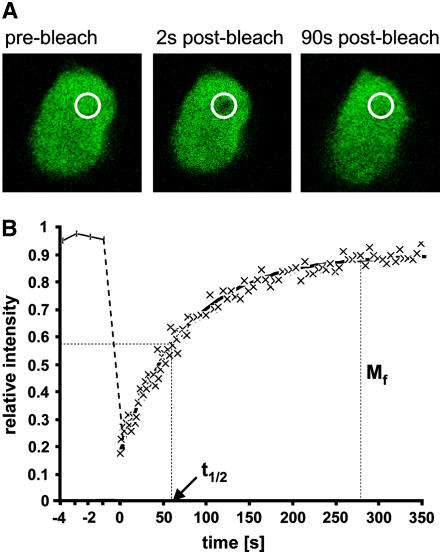
Figure 7.
Measurement of the Mobility of Linker Histone H1.2 in Tobacco Protoplast Nuclei by FRAP.
(A) Images of the GFP fluorescence of the nucleus of a transiently transformed BY-2 cell protoplast expressing the GFP-H1.2 fusion protein. Images have been taken before and 2 and 90 s after bleaching a small area within the nucleus. The bleached area is indicated by a circle.
(B) Quantitative analysis of FRAP experiments summarizing the results from ≥10 individual cells. The time course of the relative fluorescence intensity has been determined within the bleached area. The initial fluorescence intensity measured before bleaching is reduced by ∼80% upon bleaching by a short laser pulse before it recovers over time due to influx of unbleached molecules from the area surrounding the bleached spot. The time required for half-maximal recovery (t1/2) and the mobile fraction (Mf) of the protein can be determined from the recovery data.
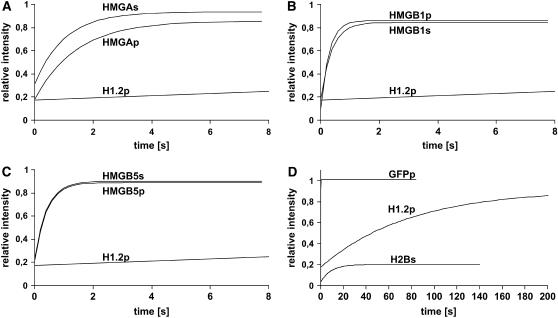
Figure 8.
HMGA and HMGB Proteins Are Highly Mobile in Plant Cell Nuclei.
FRAP curves depicting the time course of fluorescence recovery after bleaching (starting at 0 s). Root cells of stably transformed Arabidopsis seedlings (indicated by s) and transiently transformed BY-2 cell protoplast (indicated by p) expressing HMGA, HMGB1, and HMGB5 proteins and control proteins (H1.2 and H2B) fused to GFP/YFP were analyzed.
(A) to (C) The results obtained with HMGA, HMGB1, and HMGB5 fused to GFP are shown in comparison to the GFP-H1.2 fusion.
(D) The mobility of the control proteins GFP, GFP-H1.2, and H2B-YFP was measured in parallel (note the different time scale in [D] compared with [A] to [C]). GFP that does not interact with chromatin served as an inert nonbinding reference protein (Sprague and McNally, 2005), displaying an extremely rapid fluorescence recovery.
The data obtained from the FRAP experiments and their statistical evaluation are summarized in Table 1. Experimental details, including background values and fluorescence fading, are provided in Supplemental Figures 1 to 4 online as well as control FRAP experiments performed with chemically fixed cells revealing that no redistribution of the GFP fusion proteins occurs after fixation. In the case of HMGA, a half-maximal recovery time (t1/2 ∼0.9 s) was measured, clearly recovering more slowly than free GFP (t1/2 ∼0.1 s) and much more rapidly than H1.2 (t1/2 ∼53 s). The individual N-terminal domain HMGA(M1-K97) with amino acid sequence similarity to the globular domain of linker histone H1 revealed a recovery virtually identical to free GFP, indicating that the N-terminal domain does not interact with chromatin in living cells. The C-terminal domain HMGA(Y87-C204) containing the four AT-hook DNA binding motifs displays a recovery rate that is faster than that of full-length HMGA. For the two HMGB proteins, HMGB1 and HMGB5, we measured a recovery rate that is significantly faster (t1/2 ∼0.25 and ∼0.28 s, respectively) than that of HMGA, indicating that HMGB proteins display an even higher mobility within the nucleus. For both HMGB1 and HMGB5, deletion of the acidic C-terminal domain [HMGB1(M1-Y121) and HMGB5(M1-Y101)] resulted in a decreased mobility as evident from the increase in recovery time. In summary, our data demonstrate that HMGA and HMGB proteins are highly mobile proteins in plant cell nuclei.
Table 1.
Mobility of GFP/YFP Fusion Proteins as Determined by FRAP
| Fusion Protein | Samplea | Mf (%)b | t1/2 (s)cd | t Test on t1/2e |
|---|---|---|---|---|
| HMGA* | p | 83.3 ± 1.6 | 0.894 ± 0.082 | — |
| HMGA | s | 84.5 ± 3.0 | 0.778 ± 0.061 | <0.01 |
| HMGA(M1-K97) | p | 80.0 ± 3.7 | ∼0.1 | <1×10−9 |
| HMGA(Y87-C202) | p | 90.5 ± 1.4 | 0.756 ± 0.092 | <0.001 |
| HMGB1* | p | 84.7 ± 1.8 | 0.254 ± 0.008 | — |
| HMGB1 | s | 88.4 ± 1.9 | 0.255 ± 0.045 | 0.41 |
| HMGB1(M1-Y121) | p | 80.9 ± 2.4 | 0.290 ± 0.036 | <0.01 |
| HMGB5* | p | 86.1 ± 1.6 | 0.284 ± 0.014 | — |
| HMGB5 | s | 91.0 ± 2.6 | 0.285 ± 0.018 | 0.41 |
| HMGB5(M1-Y101) | p | 87.3 ± 2.2 | 0.351 ± 0.006 | <0.01 |
| H1.2 | p | 88.4 ± 2.8 | 53.00 ± 3.70 | — |
| H2B | s | 17.1 ± 2.0 | 5.96 ± 0.24 | — |
| GFP | p | 104 ± 3.3 | ∼0.1 | — |
Protein mobility was measured in transiently transformed BY-2 cell protoplasts (p) and/or root cells of transgenic Arabidopsis seedlings (s).
The mobile fraction of the GFP/YFP fusion protein.
The time required to obtain 50% FRAP. Due to the extremely rapid redistribution of GFP and HMGA(M1-K97), the values could not be determined precisely for these proteins.
Regarding H2B the value has been determined for the mobile fraction, whereas the majority of H2B is immobile within the time frame of the experiment.
Evaluation of the statistical significance of observed differences relative to the first experiment (indicated by an asterisk) in each of the sets (HMGA, HMGB1, and HMGB5) as determined by Student's t test.
DISCUSSION
Plant HMG proteins are usually isolated from nuclear extracts, but their subcellular localization has not been analyzed in detail (Grasser, 2003). Here, we have examined the localization of Arabidopsis HMGA and two typical HMGB proteins, HMGB1 and HMGB5. Indirect immunofluorescence microscopy and CLSM of cells expressing HMG-GFP fusions demonstrated that HMGA, HMGB1, and HMGB5 clearly localize to the cell nucleus. Mammalian HMGA proteins are also nuclear proteins (Amirand et al., 1998), while mammalian HMGB1 and HMGB2 are found in the nucleus but also in the cytosol (Bustin and Neilhart, 1979; Kuehl et al., 1984; Falciola et al., 1997; Bonaldi et al., 2003). In mammalian cells, the subcellular distribution of HMGB1 is regulated by the acetylation of Lys residues (Bonaldi et al., 2003). This mechanism appears not to be conserved in plants, since maize HMGB proteins are phosphorylated by protein kinase CK2 at Ser residues within their acidic C-terminal domain, but there is no evidence for acetylation (Stemmer et al., 2002). Within interphase nuclei, the Arabidopsis HMG proteins display a speckled distribution pattern that is seen similarly in tobacco BY-2 cell protoplasts and in root cells of Arabidopsis seedlings. In mitotic cells, HMGA and HMGB1 are not associated with condensed chromosomes, and a disperse immunostaining of the cytosol is observed. Mammalian HMGA is found on metaphase chromosomes (Disney et al., 1989), while there are conflicting results regarding the association of mammalian HMGB1 with condensed mitotic chromosomes (Falciola et al., 1997; Pallier et al., 2003).
To identify the regions of HMGA required for directing it to the nucleus, deletion analyses and point mutations of HMGA fusion proteins with GFP were performed. These experiments clearly identify a single NLS that is necessary and sufficient for nuclear targeting of HMGA. Whereas the N-terminal H1-like domain does not contain nuclear targeting information, the C-terminal domain of HMGA does. Point mutation of specific Arg residues (Arg-98 and Arg-164) within putative NLSs in the C-terminal domain of HMGA identified the second predicted NLS (around Arg-164) as the only functional one, although both motifs are similar and close to AT hooks (Figure 2). The predicted NLS (PKR164PRT) contains only three basic residues, although a contribution of two close Arg residues cannot be ruled out. The motif resembles a monopartite NLS of the SV40 type; however, it is Arg rich, whereas typical monopartite NLSs contain predominantly Lys residues (Görlich and Kutay, 1999). The presence of one monopartite NLS suggests nuclear import of HMGA by the importin α/β heterodimer (Merkle, 2003). Nuclear import via the importin α/β pathway has been reported for the structurally unrelated mammalian HMGN proteins (Hock et al., 1998).
In HMGB1, no short basic NLS was identified, and the entire basic N-terminal domain of HMGB1 is necessary and sufficient for nuclear accumulation. Despite the presence of a predicted NLS, the individual HMG box domain was not targeted efficiently to the nucleus and revealed a localization that was similar to GFP alone. Nuclear targeting of HMGB5 depends on signals arranged differently compared with those in HMGA and HMGB1. Here, relative to HMGB1, an even larger portion of the protein is required for efficient nuclear accumulation, since both the basic N-terminal domain and the HMG box domain are necessary for proper nuclear targeting of HMGB5. Since there is a basic cluster at the N-terminal end of the HMG box domain, this motif may comprise the NLS. However, HMGB5(M1-P38) that contains this motif was not directed completely to the nucleus, suggesting that additional amino acid residues C-terminal of the motif contribute to the nuclear targeting. In line with these findings, mammalian HMGB1 and HMGB2 are targeted to the nucleus by several basic regions scattered over the protein. These amino acid motifs contribute to the nuclear localization in the context of the whole protein but not as isolated peptides (Shirakawa et al., 1997; Bonaldi et al., 2003). The finding that a rather long basic region in HMGB1 and HMGB5 is necessary and sufficient for nuclear targeting suggests nuclear import via the heterodimeric importin β/RanBP7 import receptor that has been reported to be functional for histone H1 (Jäkel et al., 1999). Naturally, the importin α/β pathway cannot be ruled out. The localization of GFP fusions of rather unusual Arabidopsis HMGB proteins was studied by fluorescence microscopy. These experiments revealed that the HMGB6 protein and the HMGB-type protein encoded by the Arabidopsis Genome Initiative locus At2g34450 are nuclear proteins, whereas the protein encoded by At5g23405 primarily is found in the cytosol (Grasser et al., 2004, 2006). Interestingly, the nuclear import mechanism of HMGB proteins appears to differ from that of transcription factors containing HMG box DNA binding domains. In these transcription factors, including SRY, LEF-1, TCF-1, and Ste11p, basic motifs within the HMG box domain are sufficient for nuclear accumulation (Poulat et al., 1995; Prieve et al., 1998; Qin et al., 2003), whereas the individual HMG box domains of mammalian (Shirakawa et al., 1997) and plant HMGB proteins (this work) do not localize to the nucleus. The acidic C-terminal domain modulates the subnuclear localization of HMGB1. Fusion proteins that contained the basic N-terminal domain but lacked the acidic C-terminal domain displayed nuclear accumulation and a prominent localization in the nucleolus that was not seen to the same extent with the full-length protein or with the individual HMG box domain. This suggests the presence of a nucleolus targeting signal within the N-terminal domain. However, the acidic C-terminal domain obviously plays an important role in subnuclear targeting of HMGB proteins, as prominent nucleolar targeting is only revealed in the absence of the acidic tail. The acidic C-terminal domain of plant HMGB proteins can interact intramolecularly with the basic N-terminal domain, which in case of the maize HMGB1 protein is regulated by phosphorylation of residues within the acidic tail (Stemmer et al., 2002; Thomsen et al., 2004). Thus, in full-length HMGB1, the acidic tail may interfere with the accessibility of the nucleolus targeting signal within the basic N-terminal domain.
Recent studies on nuclear architecture have revealed that the dynamic properties of proteins play an essential role in various processes occurring in the cell nucleus, including transcription. These results have implications on the view of how transcription is regulated in the context of chromatin components (Hager et al., 2002; Gorski and Misteli, 2005). We have expressed various GFP fusion proteins in plant cells to study by FRAP the nuclear mobility of HMGA and HMGB proteins in comparison to core histone H2B and linker histone H1.2 as well as free GFP. The analyzed proteins display the following relative recovery rates in plant cell nuclei: free GFP < HMGB1/5 < HMGA ≪ H1.2 ≪ H2B. Since the FRAP recovery rates are only weakly dependent on protein mass, and all proteins that have been fused to GFP in this study are in the range of 10 to 30 kD, the different recovery rates determined for the chromosomal proteins (relative to free GFP) cannot arise from size-dependent molecular sieving effects (Sprague and McNally, 2005). In the case of chromosomal proteins, the FRAP recovery is slower than that of the nonbinding GFP alone due to interaction with DNA and/or other chromatin proteins, and the diffusional mobility of the proteins is much faster than the rate-limiting binding events (Carrero et al., 2004; Phair et al., 2004a; Sprague and McNally, 2005). Therefore, linker histone H1.2 and in particular HMGA and HMGB1/5 interact with plant chromatin only transiently and constantly exchange binding sites within the nuclear microenvironment.
According to the FRAP analysis (Table 1), HMGA is highly mobile within the cell nucleus, and it displays a somewhat higher mobility in tobacco BY-2 cell protoplasts than in Arabidopsis root cells, whereas no difference between the two cell types is seen with HMGB1 and HMGB5. The nuclear mobility of HMGA may be modulated by cell type– or species-specific effects. Based on FRAP data, a remarkable dynamic has been also reported for mammalian HMGA (Harrer et al., 2004). The N-terminal H1-like domain (which occurs exclusively in plant HMGA proteins) does not interact with chromatin in the nucleus and displays a FRAP recovery like free GFP. Another truncated Arabidopsis HMGA protein comprising the C-terminal domain, including the four AT-hook DNA binding motifs, recovers faster than full-length HMGA, indicating that in the context of the full-length protein, the H1-like domain contributes to the interaction with chromatin. These results are in line with DNA binding experiments demonstrating that the H1-like domain of rice (Oryza sativa) and wheat (Triticum aestivum) HMGA does not bind DNA in vitro, but in full-length HMGA, it enhances the DNA binding mediated by the AT-hook region (Nieto-Sotelo et al., 1994; Zhang et al., 2003a). The recovery of the HMGB1 and HMGB5 proteins in the FRAP experiments is even more rapid than that of HMGA, demonstrating that HMGB proteins are extremely dynamic within plant cell nuclei. The high mobility of HMGB1 and HMGB5 is consistent with the nuclear dynamics observed for mammalian HMGB1 (Catez et al., 2004; Phair et al., 2004b), although plant HMGB proteins (compared with mammalian HMGB1 that contains two HMG box domains) bind significantly more tightly to linear DNA (Ritt et al., 1998b). Interestingly, with both Arabidopsis HMGB proteins, deletion of the acidic C-terminal domain resulted in a slower recovery, suggesting a tighter binding to chromatin of the C-terminally truncated proteins relative to full-length HMGB1 and HMGB5. This is in contrast with the assumption that the acidic tail contributes to the chromatin binding of HMGB proteins by interacting with histones (Travers, 2003), but it is consistent with DNA binding experiments, demonstrating that deletion of the acidic tail of maize and rice HMGB1 markedly enhances the affinity for DNA (Ritt et al., 1998a; Wu et al., 2003). Therefore, the reduced mobility of C-terminally truncated HMGB1 and HMGB5 suggests that the interaction with chromatin in vivo is mainly determined by the DNA binding properties of the proteins.
HMG proteins are widely distributed in chromatin and contribute to the regulation of DNA-dependent processes by globally modulating chromatin structure and as architectural factors in cooperation with specific regulators facilitating the assembly of higher-order nucleoprotein complexes (Bustin and Reeves, 1996; Bianchi and Agresti, 2005; Grasser and Launholt, 2006). Because members of the chromatin-associated linker histone and HMG protein families display overlapping modes of interaction with DNA and chromatin, it has been postulated that these structurally distinct protein families cooperate in regulating gene expression by modulating chromatin structure (Nightingale et al., 1996; Ragab and Travers, 2003). Nucleosomal arrays bound by linker histones are generally assumed to adopt a rather compact chromatin structure, restricting the access of regulatory factors and nucleosome modifying complexes, while the interaction with HMG proteins decreases the compactness of the chromatin fiber favoring access of regulatory factors (Bustin and Reeves, 1996; Thomas, 1999; Travers, 2003; Bianchi and Agresti, 2005; Bustin et al., 2005). Therefore, the replacement of linker histones by HMG proteins has been suggested as a mechanism involved in transcriptional activation (Zlatanova and van Holde, 1998; Zlatanova et al., 2000). The high mobility of linker histones and in particular of the HMGA and HMGB proteins within the cell nucleus makes this model very attractive. Dynamic and competitive interactions of linker histones and HMG proteins may constantly modulate the structure of the chromatin fiber and the accessibility of regulatory DNA sites, such as promoter regions (Bustin et al., 2005). An example of histone H1-HMGB protein exchange in transcriptional regulation in vivo is the transcriptional activation induced by nuclear receptors that involves replacement of H1 by HMGB1 at a specific nucleosome containing a receptor response element (Ju et al., 2006). Consistently, maize HMGA could relieve in vitro the inhibitory effect exerted by linker histone H1 on transcription driven by an A/T-rich zein gene promoter (Zhao and Grafi, 2000). Moreover, linker histone-mediated inhibition and HMG protein–mediated stimulation of ATP-dependent chromatin remodeling (Bonaldi et al., 2002; Horn et al., 2002) may contribute to establishing different states of local chromatin structure at transcriptional control regions in vivo.
In collaboration with transcription factors, HMGA and HMGB proteins as architectural factors facilitate the assembly of regulatory complexes at promoter/enhancer regions (Bianchi and Agresti, 2005; Grasser and Launholt, 2006). Since HMG proteins bind DNA with little or no sequence specificity (Webster et al., 1997), the question regarding the recruitment of the HMG proteins to their sites of action in the genome was puzzling. HMG proteins can be recruited by specific protein interaction with transcription factors or by structural trapping at certain (protein-induced) DNA structures (Bianchi and Agresti, 2005; Grasser and Launholt, 2006). It has been suggested early on that the recruitment of HMG proteins and other non-sequence-specific architectural factors involves random association and dissociation with DNA, occasionally leading to productive complexes (Segall et al., 1994). The remarkable dynamics of HMGA and HMGB proteins in combination with their abundance allows efficient searching of the genome for DNA binding sites and protein partners by three-dimensional scanning. Many chromatin-associated proteins bind chromatin only transiently (residence time of a few seconds or less) before moving on to the next site, but due to the efficiency of the diffusion process, the vast majority of any given protein is still bound to chromatin at any given time (Hager et al., 2002; Gorski et al., 2006). The dynamic property of chromosomal proteins, rapidly exchanging between binding sites by a three-dimensional hopping mechanism, ensures their availability throughout the genome and contributes to the overall plasticity of chromatin assisting the rapid induction/repression of gene transcription (Hager et al., 2002; Gorski and Misteli, 2005). Intriguingly, mammalian HMGB1 and its interaction partner glucocorticoid receptor increase each other's residence time within chromatin, forming a rather stable complex at promoter response elements (Agresti et al., 2005). Kinetic cooperativity of this type between transcription factors and assistant chromatin proteins may emerge as a general theme in transcriptional regulation.
Originally, the HMG proteins were operationally termed high mobility group proteins because of their unusual mobility in gel electrophoresis (Goodwin et al., 1973), but now, in view of their extraordinary dynamics in the cell nucleus, the name chosen more than 30 years ago is also biologically relevant.
METHODS
Plasmid Constructions
Except where stated otherwise, the inserts of the plasmids were produced by PCR using Pfu DNA polymerase (MBI Fermentas) and an Arabidopsis thaliana cDNA library (derived from the green parts of seedlings of the ecotype Columbia) as a template. Purified PCR fragments were digested using terminal restriction enzyme recognition sites introduced through the PCR primers as specified below and cloned by standard methods into the different plasmids digested with the corresponding restriction enzymes. All plasmid constructions were checked by DNA sequencing.
For protein production in Eschericha coli, the coding sequences were cloned in frame with the 6xHis-tag of pQE9 expression plasmids. The coding sequence of HMGA was amplified by PCR using primers P1 (5′-AATTGGATCCATGGCCTTCGATCTCCACCAT-3′) and P2 (5′-AATTAAGCTTTCAGCACCCAACCGGAGCAA-3′), digested with BamHI-HindIII, and inserted into BamHI-HindIII–digested plasmid pQE9 (Qiagen), giving pQE9-HMGA. The GFP coding sequence fused in frame to the HMGA coding sequence was amplified by PCR using primers P3 (5′-AATTCCCGGGTATGAGTAAAGGAGAAGAACT-3′) and P4 (5′-AATTAAGCTTATCAGCACCCACCAACCGGA-3′) and plasmid p5′GFP-HMGA as template, digested with SmaI-HindIII, and inserted into SmaI-HindIII–digested plasmid pQE9, giving pQE9-GFP-HMGA. The HMGB1 coding sequence fused in frame to the GFP coding sequence was amplified by PCR using primers P5 (5′-AATTTGTCGACATGAAAACAGCAAAGGGG-3′) and P6 (5′-AATTAAGCTTAAGCGGGAGCTTTGTATAGT-3′) and plasmid p3′GFP-HMGB1 as template, digested with SalI-HindIII, and inserted into SalI-HindIII–digested plasmid pQE9, giving pQE9-HMGB1-GFP.
For the construction of HMG protein–GFP fusions suitable for transient protoplast transformation, the sequences encoding full-length or truncated HMG proteins were cloned in frame 5′ or 3′ of the GFP coding sequence. The HMGA coding sequence was obtained by treating pQE9-HMGA consecutively with HindIII, Klenow enzyme, and EcoRI. The DNA fragment was inserted into EcoRI-SmaI–digested plasmid p5′GFP (Haasen et al., 1999), giving p5′GFP-HMGA. The HMGA coding sequence was amplified by PCR using primers P7 (5′-AATTTCTAGAATGGCCTTCGATCTCCACC-3′) and P8 (5′-AATTGGATCCAAGCACCCAACCGGAGCAACC-3′), digested with XbaI-BamHI, and inserted into the XbaI-BamHI–digested plasmid p3′GFP (Haasen et al., 1999), giving p3′GFP-HMGA. The sequence encoding HMGA(M1-K97) was amplified by PCR using the primers P9 (5′-AATTCCCGGGAATGGCCTTCGATCTCCACCA-3′) and P10 (5′-AATTGGATCCTCACTTAGGAGGAGCATCT-3′), digested with SmaI-BamHI, and inserted into the SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGA(M1-K97). The sequence encoding HMGA(Y87-C204) was amplified by PCR using the primers P11 (5′-AATTCCCGGGATATATGAAACCAGATCCAGA-3′) and P12 (5′-AATTGGATCCTCAGCACCCAACCGGAG-3′), digested with SmaI-BamHI, and inserted into the SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP- HMGA(Y87-C204). In plasmid p5′GFP-HMGA, a point mutation (R98G) was introduced by PCR using the QuikChange site-directed mutagenesis kit according to the instructions of the manufacturer (Stratagene) and primers P13 (5′-CAGATGCTCCTCCTAAGGGTGGTCGTGGCCGTCCTCCG-3′) and P14 (5′-CGGAGGACGGCCACGACCACCCTTAGGAGGAGCATCTG-3′), giving p5′GFP-HMGA(R98G). Similarly, the point mutation (R164G) was introduced in p5′GFP-HMGA using primers P15 (5′-GAGGACGACCACCGAAGGGACCGAGAACAGATTC-3′) and P16 (5′-GAATCTGTTCTCGGTCCCTTCGGTGGTCGTCCTC-3′), giving p5′GFP-HMGA(R164G). The point mutation (R164G) was introduced in p5′GFP-HMGA(R98G) using the primers P15 and P16, giving the double mutant p5′GFP-HMGA(R98G,R164G).
The HMGB1 coding sequence was obtained by treating the plasmid pQE9cm-HMGB1 (Stemmer et al., 1997) consecutively with HindIII, Klenow enzyme, and EcoRI. The DNA fragment was inserted into EcoRI-SmaI–digested plasmid p5′GFP, giving p5′GFP-HMGB1. The HMGB1 coding sequence was amplified by PCR using primers P17 (5′-AATCGGATCCATGAAAACAGCAAAGGGGAA-3′) and P18 (5′-AATTGGATCCAAGTCTTCTTCCTCGTCGTC-3′), digested with BamHI, and inserted into the BamHI-digested plasmid p3′GFP, giving p3′GFP-HMGB1. The sequence encoding HMGB1(K48-Y121) was amplified by PCR using primers P19 (5′-AATTCCCGGGAAAGGACCCAAACAAACCAAA-3′) and P20 (5′-AACCGGATCCTTAGTATGCATCCATTTGCTTC-3′), digested with SmaI-BamHI, and inserted into SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB1(K48-Y121). The sequence encoding HMGB1(M1-Y121) was amplified by PCR using primers P21 (5′-AATTCCCGGGAATGAAAACAGCAAAGG-3′) and P20, digested with SmaI-BamHI, and inserted into Sma-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB1(M1-Y121). The sequence encoding HMGB1(M1-K47) was amplified by PCR using primers P21 and P22 (5′-AACCGGATCCTTATTTAGCCTTCTTCTCTT-3′), digested with SmaI-BamHI, and inserted into SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB1(M1-K47).
The HMGB5 coding sequence was obtained by treating the plasmid pQE9cm-HMGB5 (Stemmer et al., 1997) consecutively with HindIII, Klenow enzyme, and EcoRI. The DNA fragment was inserted into EcoRI-SmaI–digested plasmid p5′GFP, giving p5′GFP-HMGB5. The sequence encoding HMGB5(K28-Y101) was amplified by PCR using primers P23 (5′-AATTCCCGGGAAAGGATCCAAACAGGCCTAA-3′) and P24 (5′-AATTGGATCCCTACTGTTGCATAGTCACAGC-3′), digested with SmaI-BamHI, and inserted into SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB5(K29-Y101). The sequence encoding HMGB5(M1-Y101) was amplified by PCR using primers P25 (5′-AATTCCCGGGAATGAAAGATAACCAAACGG-3′) and P24, digested with SmaI-BamHI, and inserted into SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB5(M1-Y101). The sequence encoding HMGB5(M1-T28) was amplified by PCR using primers P25 and P26 (5′-AATTGGATCCCTACTTTGTTTTCTTTCCAA-3′), digested with SmaI-BamHI, and inserted into SmaI-BamHI–digested plasmid p5′GFP, giving p5′GFP-HMGB5(M1-T28). The sequence encoding HMGB5(M1-P38) was amplified by PCR using primers P25 and P27 (5′-AATTGAGCTCCTATGGTGGTTTCTTAGGCCTG-3′), digested with SmaI-SacI, and inserted into SmaI-SacI–digested plasmid p5′GFP, giving p5′GFP-HMGB5(M1-P38).
The linker histone H1.2 coding sequence (At2g30620) was amplified by PCR using primers P28 (5′-AATGAATTCATGTCTATAGAGGAAGAAAACGTTCCAACGACTG-3′) and P29 (5′-AATGGATCCTCACTTCTTAGCCTTCCTAGTCGAAGCTCTCTT-3′), digested with EcoRI-BamHI, and inserted into EcoRI-BamHI–digested plasmid p5′GFP, giving p5′GFP-H1.2.
For construction of HMG protein–GFP fusions suitable for Agrobacterium tumefaciens–mediated stable plant transformation, the fused coding sequences were introduced into a derivative of the binary plant transformation vector pGreen (Hellens et al., 2000). The cauliflower mosaic virus 35S promoter/terminator expression cassette was isolated by digesting the plasmid p35S-2 with EcoRV and inserted into the EcoRV-SmaI–digested plasmid pGII0179, giving pGII0179-35S. The coding sequence of the GFP-HMGA fusion was amplified by PCR using primers P3 and P30 (5′-AATTCCCGGGTCAGCACCCAACCGGAGCAA-3′) and p5′GFP-HMGA as template. The DNA fragment was inserted into the SmaI-digested plant transformation vector pGII0179-35S, giving pGII0179-35S-GFP-HMGA. The coding sequence of the GFP-HMGB1 fusion was amplified by PCR using primers P3 and P31 (5′-AATTCCCGGGTTAGTCTTCTTCCTCGTCGTCA-3′) and p5′GFP-HMGB1 as template. The DNA fragment was inserted into the SmaI-digested plasmid pGII01709-35S, giving pGII0179-35S-GFP-HMGB1. The coding sequence of the GFP-HMGB5 fusion was amplified by PCR using primers P3 and P32 (5′-AATTCCCGGGCTAATCATCAGCAGCTTTCTC-3′) and p5′GFP-HMGB5 as template. The DNA fragment was inserted into the SmaI-digested plasmid pGII01709-35S, giving pGII0179-35S-GFP-HMGB5.
Production and Purification of Recombinant Proteins
The plasmids pQE9cm-HMGB1 (Stemmer et al., 1997), pQE9-HMGA, pQE9-GFP-HMGA, and pQE9-HMGB1-GFP were used to transform the E. coli strain M15 (Qiagen) for protein expression. The recombinant 6xHis-tagged proteins were purified by three-step fast protein liquid chromatography column chromatography (Ni-NTA agarose, S Sepharose Fast Flow; ResourseQ) as previously described (Grasser et al., 1996). Purified proteins were checked by SDS-PAGE and matrix-assisted laser-desorption ionization time of flight mass spectrometry.
EMSA
Binding of HMGA and GFP-HMGA to a 106-bp fragment of a maize (Zea mays) zein gene promoter was examined by EMSA as previously described (Grasser et al., 1994). The DNA was visualized by staining with SYBR Gold (Molecular Probes) and detected by scanning the gels using the Typhoon 8600 phosphor imager (Amersham Biosciences). Binding of HMGB1 and HMGB1-GFP to the radiolabeled linear and circularized 78-bp fragment derived from pBluescript SK− (Stratagene) was analyzed by EMSA as previously described (Grasser et al., 1994, 1996). The DNA was visualized by scanning the dried gels using the Typhoon 8600 phosphor imager (Amersham Biosciences).
Indirect Immunofluorescence
Antisera against purified recombinant HMGA and HMGB1 proteins were produced and tested as previously described (Duroux et al., 2004). Preparation of interphase and mitotic cells from root tips of Arabidopsis seedlings was performed following the method previously described (Gernand et al., 2003). To avoid nonspecific antibody binding, slides were blocked for 30 min in 4% (w/v) BSA and 0.1% Triton X-100 in PBS at room temperature prior to two washes in PBS for 5 min each, and incubated with the primary antibodies in a humid chamber. The HMGA- and HMGB1-specific rabbit antibodies were diluted 1:500, and a rat monoclonal antibody against H3 phosphorylated at Ser-28 [p(Ser28)H3] (Goto et al., 1999) was diluted 1:400 in PBS with 1% BSA. After a 12-h incubation at 4°C and washing for 15 min in PBS, the slides were incubated with rhodamine-conjugated anti-rabbit IgG (Dianova) and FITC-conjugated anti-rat IgG (Dianova) diluted 1:200 in PBS and 3% BSA for 1 h at 37°C. After a 12-h incubation at 4°C and washing for 15 min in PBS, the slides were incubated with rhodamine-conjugated anti-rabbit IgG (Dianova) diluted 1:100 in PBS and 3% BSA for 1 h at 37°C. After final washes in PBS, the preparations were mounted in antifade containing DAPI as counterstain. Imaging of immunofluorescence was recorded with an Olympus BX61 microscope equipped with an ORCA-ER CCD camera (Hamamatsu). All images were collected in gray scale and pseudocolored with Adobe Photoshop.
Agrobacterium-Mediated Arabidopsis Transformation
The pGII0179 plasmids and the plasmid pSOUP (Hellens et al., 2000) were used to cotransform Agrobacterium strain pGV3101 by electroporation. Transformed cells were selected on Luria-Bertani agar plates containing 25 mg/L kanamycin, 6 mg/L tetracycline, and 50 mg/L gentamycine. Arabidopsis plants of the ecotype Columbia-0 were transformed using the floral dip method (Clough and Bent, 1998). Seeds were selected on Murashige and Skoog agar plates, pH 5.8 (Murashige and Skoog, 1962), containing 30 mg/L hygromycin. Plants growing on the selective medium were transferred to soil and propagated at 16 h light/8 h darkness at a light intensity of 8000 lux. Genomic DNA isolated from the leaves of progeny was tested by PCR (with primers specific for the cauliflower mosaic virus 35S promoter and terminator and for the different HMG coding sequences) for the presence of the transgene.
Transient Protoplast Transformation Assays with GFP Fusion Constructs
Protoplasts of dark-grown tobacco (Nicotiana tabacum) BY-2 cells were transiently transformed using polyethylene glycol–mediated transformation as previously described (Merkle et al., 1996). Excitation of GFP was performed with a standard UV light source and fluorescein isothiocyanate filters. For CLSM, samples were directly examined under oil with a ×63 objective and a DM RBE TCS4D microscope (Leica) equipped with an argon-krypton laser (excitation at 488 nm, beam splitter at 510 nm, filter at 515 nm) using Leica Scanware. Analysis of the localization of the GFP fusion proteins was performed in three independent experiments representing ∼60 to 80 transformed protoplasts.
FRAP Analyses of GFP Fusion Proteins in Protoplasts and Roots
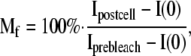
The dynamic behavior of various HMG-GFP and free GFP, H1.2-GFP, and H2B-YFP fusion proteins transiently expressed in tobacco BY-2 cell protoplasts and/or stably expressed in Arabidopsis seedlings was examined by FRAP. For live-cell imaging in Arabidopsis roots, 3-d-old seedlings were used, while protoplasts were examined ∼24 h after transformation. The analyses were performed using a TCS SP2 confocal laser scanning microscope (Leica) equipped with an argon-krypton laser with fluorescein isothiocyanate filter setting (excitation at 488 nm and spectrometric filter setting of emission filter between 520 and 580 nm) and a HCX PL APO ×63 1.2-W CORR lens. Four single prebleach scans were performed at 15% laser power attenuation, 256 × 256-pixel image size, XYT-mode, and single line scan. For bleaching, a single 1-s bleach pulse at 100% laser power attenuation was used without imaging. The bleach area has a radius of ∼0.5 μm, corresponding to ∼1% of the total nuclear area. The fluorescence recovery period was monitored by collecting images at different times after bleaching. For HMG-GFP proteins, images were recorded at maximum speed in 0.2-s intervals (60 images) and in 1-s intervals (40 images). For H1.2-GFP, images were recorded in 2-s intervals (40 images) and 5-s intervals (60 images). For H2B-YFP, images were recorded using excitation at 514 nm without single line scan and 40% laser power attenuation. After bleaching, images were recorded in 0.4-s intervals (60 images) and 2-s intervals (40 images). FRAP recovery curves were generated taking background and fading into account (Kappel and Eils, 2004). The data were normalized to the relative intensity in the bleached area measured before the bleach (prebleach), setting this value to 1 unit. The mobile fraction (Mf) was estimated from these curves using Equation 1 (Kappel and Eils, 2004):
 |
(1) |
where Ipostcell is post-bleach intensity within whole cell at equilibrium after bleaching, Iprebleach is prebleach intensity in bleached area (region of interest), and I(0) is intensity in region of interest at 0 s after bleaching.
The data at each time point (t) were fitted to Equation 2 using the goal seek function of the Microsoft Excel to reduce the standard error to a minimum, thereby getting values for the constants a, b, and τ in Equation 2 (Kappel and Eils, 2004):
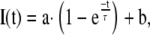 |
(2) |
where τ is correlated to the recovery half-time as in Equation 3 (Kappel and Eils, 2004):
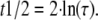 |
(3) |
Quantitative values represent averages of at least 10 analyzed cells from two independent experiments. As control, roots fixed in 1% paraformaldehyde, pH 7.0, were analyzed using the same settings as described above. Student's t test was used to determine the statistical significance of the results.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes in this article are as follows: HMGA (At1g14900), HMGB1 (At3g51880), and HMGB5 (At4g35570).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Quantitative FRAP Analysis of HMGA-GFP Fusions.
Supplemental Figure 2. Quantitative FRAP Analysis of HMGB1-GFP Fusions.
Supplemental Figure 3. Quantitative FRAP Analysis of HMGB5-GFP Fusions.
Supplemental Figure 4. Quantitative FRAP Analysis of Control Proteins.
Supplementary Material
Acknowledgments
We thank Frédéric Berger for kindly providing the Arabidopsis line expressing H2B-YFP. This work was supported by grants from the Danish Research Council. D.L. is a Danish Biotech Research Academy fellow.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Klaus D. Grasser (kdg@bio.aau.dk).
Online version contains Web-only data.
References
- Agresti, A., Scaffidi, P., Riva, A., Caiolfa, V.R., and Bianchi, M.E. (2005). GR and HMGB1 interact only within chromatin and influence each other's residence time. Mol. Cell 18 109–121. [DOI] [PubMed] [Google Scholar]
- Amirand, C., Viari, A., Ballini, J.-P., Rezaei, H., Beaujean, N., Jullien, D., Käs, E., and Debey, P. (1998). Three distinct sub-nuclear populations of HMG-I protein of different properties revealed by co-localization image analysis. J. Cell Sci. 111 3551–3561. [DOI] [PubMed] [Google Scholar]
- Arwood, L.J., and Spiker, S. (1990). Binding of wheat and chicken high mobility group chromosomal proteins to DNA and to wheat and chicken mononucleosomes. J. Biol. Chem. 265 9771–9777. [PubMed] [Google Scholar]
- Bianchi, M.E., and Agresti, A. (2005). HMG proteins: Dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 15 496–506. [DOI] [PubMed] [Google Scholar]
- Boisnard-Loriq, C., Colon-Carmona, A., Bauch, M., Hodge, S., Doerner, P., Bancharel, E., Dumas, C., Haseloff, J., and Berger, F. (2001). Dynamic analyses of the expression of the HISTONE∷YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldi, T., Längst, G., Strohner, R., Becker, P.B., and Bianchi, M.E. (2002). The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. EMBO J. 21 6865–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldi, T., Talamo, F., Scaffidi, P., Ferrera, D., Porto, A., Bachi, A., Rubartelli, A., Agresti, A., and Bianchi, M.E. (2003). Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22 5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin, M., Catez, F., and Lim, J.-H. (2005). The dynamics of histone H1 function in chromatin. Mol. Cell 17 617–620. [DOI] [PubMed] [Google Scholar]
- Bustin, M., and Neilhart, N.K. (1979). Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell 16 181–189. [DOI] [PubMed] [Google Scholar]
- Bustin, M., and Reeves, R. (1996). High-mobility-group chromosomal proteins: Architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 54 35–100. [DOI] [PubMed] [Google Scholar]
- Carrero, G., Crawford, E., Th'ng, J., de Vries, G., and Hendzel, M.J. (2004). Quanitification of protein-protein and protein-DNA interactions in vivo, using fluorescence recovery after photobleaching. Methods Enzymol. 375 415–442. [DOI] [PubMed] [Google Scholar]
- Catez, F., Yang, H., Tracey, K.J., Reeves, R., Misteli, T., and Bustin, M. (2004). Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol. Cell. Biol. 24 4321–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Disney, J.E., Johnson, K.R., Magnuson, N.S., Sylvester, S.R., and Reeves, R. (1989). High-mobility group protein HMG-I localizes to G/Q- and C-bands of human and mouse chromosomes. J. Cell Biol. 109 1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duroux, M., Houben, A., Ruzicka, K., Friml, J., and Grasser, K.D. (2004). The chromatin remodelling complex FACT associates with actively transcribed regions of the Arabidopsis genome. Plant J. 40 660–671. [DOI] [PubMed] [Google Scholar]
- Falciola, L., Spada, F., Calogero, S., Längst, G., Voit, R., Grummt, I., and Bianchi, M.E. (1997). High mobility group 1 protein is not stably associated with the chromosomes of somatic cells. J. Cell Biol. 137 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand, D., Demidov, D., and Houben, A. (2003). The temporal and spatial pattern of histone H3 phosphorylation at serine 28 and serine 10 is similar in plants but differs between mono- and polycentric chromosomes. Cytogenet. Genome Res. 101 172–176. [DOI] [PubMed] [Google Scholar]
- Goodwin, G.H., Sanders, C., and Johns, E.W. (1973). A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 38 14–19. [DOI] [PubMed] [Google Scholar]
- Görlich, D., and Kutay, U. (1999). Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15 607–660. [DOI] [PubMed] [Google Scholar]
- Gorski, S., and Misteli, T. (2005). Systems biology in the cell nucleus. J. Cell Sci. 118 4083–4092. [DOI] [PubMed] [Google Scholar]
- Gorski, S.A., Dundr, M., and Misteli, T. (2006). The road much traveled: Trafficking in the cell nucleus. Curr. Opin. Cell Biol. 18 284–290. [DOI] [PubMed] [Google Scholar]
- Goto, H., Tomono, Y., Ajiro, K., Kosako, H., Fujita, M., Sakurai, M., Okawa, K., Iwamatsu, A., Okigaki, T., Takahashi, T., and Inagaki, M. (1999). Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J. Biol. Chem. 274 25543–25549. [DOI] [PubMed] [Google Scholar]
- Grasser, K.D. (2003). Chromatin-associated HMGA and HMGB proteins: Versatile co-regulators of DNA-dependent processes. Plant Mol. Biol. 53 281–295. [DOI] [PubMed] [Google Scholar]
- Grasser, K.D., Grill, S., Duroux, M., Launholt, D., Thomsen, M.S., Nielsen, B.V., Nielsen, H.K., and Merkle, T. (2004). HMGB6 from Arabidopsis thaliana specifies a novel type of plant chromosomal HMGB protein. Biochemistry 43 1309–1314. [DOI] [PubMed] [Google Scholar]
- Grasser, K.D., Grimm, R., and Ritt, C. (1996). Maize chromosomal HMGc: Two closely related structure-specific DNA-binding proteins specify a second type of plant HMG-box protein. J. Biol. Chem. 271 32900–32906. [DOI] [PubMed] [Google Scholar]
- Grasser, K.D., Krech, A.B., and Feix, G. (1994). The maize chromosomal HMGa protein recognises structural features of DNA and increases DNA flexibility. Plant J. 6 351–358. [DOI] [PubMed] [Google Scholar]
- Grasser, K.D., and Launholt, D. (2006). Chromatin-associated architectural HMGA and HMGB proteins assist transcription factor function. In Regulation of Transcription in Plants, K.D. Grasser, ed. (Oxford, UK: Blackwell Publishing), pp. 54–78.
- Grasser, M., Lentz, A., Lichota, J., Merkle, T., and Grasser, K.D. (2006). The Arabidopsis genome encodes structurally and functionally diverse HMGB-type proteins. J. Mol. Biol. 358 654–664. [DOI] [PubMed] [Google Scholar]
- Haasen, D., Köhler, C., Neuhaus, G., and Merkle, T. (1999). Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant J. 20 695–706. [DOI] [PubMed] [Google Scholar]
- Hager, G.L., Elbi, C., and Becker, M. (2002). Protein dynamics in the nuclear compartment. Curr. Opin. Genet. Dev. 12 137–141. [DOI] [PubMed] [Google Scholar]
- Harrer, M., Lührs, H., Bustin, M., Scheer, U., and Hock, R. (2004). Dynamic interaction of HMGA1a proteins with chromatin. J. Cell Sci. 117 3459–3471. [DOI] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42 819–832. [DOI] [PubMed] [Google Scholar]
- Hock, R., Scheer, U., and Bustin, M. (1998). Chromosomal proteins HMG-14 and HMG-17 are released from mitotic chromosomes and imported into the nucleus by active transport. J. Cell Biol. 143 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, P.J., Carruthers, L.M., Logie, C., Hill, D.A., Solomon, M.J., Wade, P.A., Imbalzano, A.N., Hansen, J.C., and Peterson, C.L. (2002). Phosphorylation of linker histones regulates ATP-dependent chromatin remodeling enzymes. Nat. Struct. Biol. 9 263–267. [DOI] [PubMed] [Google Scholar]
- Hsieh, T.-F., and Fischer, R.L. (2005). Biology of chromatin dynamics. Annu. Rev. Plant Biol. 56 327–351. [DOI] [PubMed] [Google Scholar]
- Jäkel, S., Albig, W., Kutay, U., Bischoff, F.R., Schwamborn, K., Doenecke, D., and Görlich, D. (1999). The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 18 2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzmanowski, A. (2004). The linker histones. In Chromatin Structure and Dynamics: State-of-the-Art, J. Zlatanova and S.H. Leuba, eds (Amsterdam/New York: Elsevier), pp. 75–102.
- Johns, E. W., ed (1982). The HMG Chromosomal Proteins. (London: Academic Press).
- Ju, B.-G., Lunyak, V.V., Perissi, V., Garcia-Bassets, I., Rose, D.W., Glass, C.K., and Rosenfeld, M.G. (2006). A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312 1798–1802. [DOI] [PubMed] [Google Scholar]
- Kappel, C., and Eils, R. (2004). Fluorescence recovery after photobleaching with the Leica TCS SP2. Confocal Appl. Lett. 18 1–12. [Google Scholar]
- Kimura, H., and Cook, P.R. (2001). Kinetics of core histones in living human cells: Little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 153 1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterman, S.J., and Hadwiger, L.A. (2002). Plant HMG proteins bearing the AT-hook motif. Plant Sci. 162 855–866. [Google Scholar]
- Kuehl, L., Salmond, B., and Tran, L. (1984). Concentrations of high-mobility-group proteins in the nucleus and cytoplasm of several rat tissues. J. Cell Biol. 99 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever, M.A., Th'ng, J.P., Sun, X., and Hendzel, M.J. (2000). Rapid exchange of histone 1.1 on chromatin in living human cells. Nature 408 873–876. [DOI] [PubMed] [Google Scholar]
- Lichota, J., and Grasser, K.D. (2001). Differential chromatin association and nucleosome binding of the maize HMGA, HMGB, and SSRP1 proteins. Biochemistry 40 7860–7867. [DOI] [PubMed] [Google Scholar]
- Loidl, P. (2004). A plant dialect of histone language. Trends Plant Sci. 9 84–90. [DOI] [PubMed] [Google Scholar]
- Merkle, T. (2003). Nucleo-cytoplasmic partitioning of proteins in plants: Implications for the regulation of environmental and developmental signalling. Curr. Genet. 44 231–260. [DOI] [PubMed] [Google Scholar]
- Merkle, T., Leclerc, D., Marshallsay, C., and Nagy, F. (1996). A plant in vitro system for the nuclear import of proteins. Plant J. 10 1177–1186. [DOI] [PubMed] [Google Scholar]
- Misteli, T., Gunjan, A., Hock, R., Bustin, M., and Brown, D.T. (2000). Dynamic binding of histone H1 to chromatin in living cells. Nature 408 877–881. [DOI] [PubMed] [Google Scholar]
- Müller, S., Ronfani, L., and Bianchi, M.E. (2004). Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokinin function. J. Intern. Med. 255 332–343. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 15 473–497. [Google Scholar]
- Nieto-Sotelo, J., Ichida, A., and Quail, P.H. (1994). PF1: An A-T hook containing DNA binding protein from rice that interacts with a functionally defined d(AT)-rich element in the oat phytochrome A3 gene promoter. Plant Cell 6 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale, K., Dimitrov, S., Reeves, R., and Wolffe, A.P. (1996). Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organising chromatin. EMBO J. 15 548–561. [PMC free article] [PubMed] [Google Scholar]
- Pallier, C., Scaffidi, P., Chopineau-Proust, S., Agresti, A., Nordmann, P., Bianchi, M.E., and Marechal, V. (2003). Association of chromatin proteins high mobility group box (HMGB) 1 and HMGB2 with mitotic chromosomes. Mol. Biol. Cell 14 3414–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, T.J., Arwood, L.J., Spiker, S., Guiltinan, M.J., and Thompson, W.F. (1991). High mobility group proteins bind to AT-rich tracts flanking plant genes. Plant Mol. Biol. 16 95–104. [DOI] [PubMed] [Google Scholar]
- Phair, R.D., Gorski, S.A., and Misteli, T. (2004. a). Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Methods Enzymol. 375 393–413. [DOI] [PubMed] [Google Scholar]
- Phair, R.D., Scaffidi, P., Elbi, C., Vecerová, J., Dey, A., Ozato, K., Brown, D.T., Hager, G., Bustin, M., and Misteli, T. (2004. b). Global nature of dynamic protein-chromatin interactions in vivo: Three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol. Cell. Biol. 24 6393–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulat, F., Girad, F., Chevron, M.-P., Gozé, C., Rebillard, X., Calas, B., Lamb, N., and Berta, P. (1995). Nuclear localization of the testis determining gene product SRY. J. Cell Biol. 128 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieve, M.G., Guttridge, K.L., Munguia, J., and Waterman, M.L. (1998). Differential importin-α recognition and nuclear transport by nuclear localization signals within the high-mobility-group DNA-binding domains of lymphoid enhancer factor 1 and T-cell factor 1. Mol. Cell. Biol. 18 4819–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, J., Kang, W., Leung, B., and McLeod, M. (2003). Ste11p, a high-mobility-group box DNA-binding protein, undergoes pheromone- and nutrient-regulated nuclear-cytoplasmic shuttling. Mol. Cell. Biol. 23 3253–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab, A., and Travers, A. (2003). HMG-D and histone H1 alter the local accessibility of nucleosomal DNA. Nucleic Acids Res. 31 7083–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, J.C., Henning, L., and Gruissem, W. (2002). Chromatin-remodeling and memory factors. New regulators of plant development. Plant Physiol. 130 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritt, C., Grimm, R., Fernández, S., Alonso, J.C., and Grasser, K.D. (1998. a). Basic and acidic regions flanking the HMG domain of maize HMGa modulate the interactions with DNA and the self-association of the protein. Biochemistry 37 2673–2681. [DOI] [PubMed] [Google Scholar]
- Ritt, C., Grimm, R., Fernández, S., Alonso, J.C., and Grasser, K.D. (1998. b). Four differently chromatin-associated maize HMG domain proteins modulate DNA structure and act as architectural elements in nucleoprotein complexes. Plant J. 14 623–631. [DOI] [PubMed] [Google Scholar]
- Segall, A.M., Goodman, S.D., and Nash, H.A. (1994). Architectural elements in nucleoprotein complexes: Interchangeability of specific and non-specific DNA binding proteins. EMBO J. 13 4536–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa, H., Tanigawa, T., Sugiyama, S., Kobayashi, M., Terashima, T., Yoshida, K., Arai, T., and Yoshida, M. (1997). Nuclear accumulation of HMGB2 protein is mediated by basic regions interspaced with a long DNA-binding sequence, and retention within the nucleus requires the acidic carboxyl terminus. Biochemistry 36 5992–5999. [DOI] [PubMed] [Google Scholar]
- Sprague, B.L., and McNally, J.G. (2005). FRAP analysis of binding: Proper and fitting. Trends Cell Biol. 15 84–91. [DOI] [PubMed] [Google Scholar]
- Stemmer, C., Ritt, C., Igloi, G.L., Grimm, R., and Grasser, K.D. (1997). Variability in Arabidopsis thaliana chromosomal high-mobility-group-1-like proteins. Eur. J. Biochem. 250 646–652. [DOI] [PubMed] [Google Scholar]
- Stemmer, C., Schwander, A., Bauw, G., Fojan, P., and Grasser, K.D. (2002). Protein kinase CK2 differentially phosphorylates maize chromosomal high mobility group B (HMGB) proteins modulating their stability and DNA interactions. J. Biol. Chem. 277 1092–1098. [DOI] [PubMed] [Google Scholar]
- Thomas, J.O. (1999). Histone H1: Location and role. Curr. Opin. Cell Biol. 11 312–317. [DOI] [PubMed] [Google Scholar]
- Thomas, J.O., and Travers, A.A. (2001). HMG1 and 2, and related architectural DNA-binding proteins. Trends Biochem. Sci. 26 167–174. [DOI] [PubMed] [Google Scholar]
- Thomsen, M.S., Franßen, L., Launholt, D., Fojan, P., and Grasser, K.D. (2004). Interactions of the basic N-terminal and the acidic C-terminal domains of the maize chromosomal HMGB1 protein. Biochemistry 43 8029–8037. [DOI] [PubMed] [Google Scholar]
- Travers, A.A. (2003). Priming the nucleosome - A role for HMGB proteins? EMBO Rep. 4 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, C.I., Cooper, M.A., Packman, L.C., Williams, D.H., and Gray, J.C. (2000). Kinetic analysis of high-mobility-group proteins HMG-1 and HMG-I/Y binding to cholesterol-tagged DNA on a supported lipid monolayer. Nucleic Acids Res. 28 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, C.I., Packman, L.C., and Gray, J.C. (2001). HMG-1 enhances HMGI/Y binding to an A/T-rich enhancer element from the pea plastocyanin gene. Eur. J. Biochem. 268 3154–3162. [DOI] [PubMed] [Google Scholar]
- Webster, C.I., Packman, L.C., Pwee, K.-H., and Gray, J.C. (1997). High mobility group proteins HMG-1 and HMGI/Y bind to a positive regulatory region of the pea plastocyanin gene. Plant J. 11 703–715. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Zhang, W., Pwee, K.-H., and Kumar, P.P. (2003). Rice HMGB1 protein recognizes DNA structures and bends DNA efficiently. Arch. Biochem. Biophys. 411 105–111. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Wu, Q., Pwee, K.-H., Jois, S.D.S., and Kini, R.M. (2003. a). Characterization of the interaction of wheat HMGa with linear and four-way junction DNA. Biochemistry 42 6596–6607. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Wu, Q., Pwee, K.-H., and Kini, R.M. (2003. b). Interaction of wheat high-mobility-group proteins with four-way-junction DNA and characterization of the structure and expression of HMGA gene. Arch. Biochem. Biophys. 409 357–366. [DOI] [PubMed] [Google Scholar]
- Zhao, J., and Grafi, G. (2000). The high mobility group I/Y protein is hypophosphorylated in endoreduplicating maize endosperm cells and is involved in alleviating histone H1-mediated transcriptional repression. J. Biol. Chem. 275 27494–27499. [DOI] [PubMed] [Google Scholar]
- Zlatanova, J., Caiafa, P., and van Holde, K. (2000). Linker histone binding and displacement: Versatile mechanism for transcriptional regulation. FASEB J. 14 1697–1704. [DOI] [PubMed] [Google Scholar]
- Zlatanova, J., and van Holde, K. (1998). Linker histones versus HMG1/2: A struggle for dominance? Bioessays 20 584–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.