Abstract
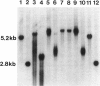
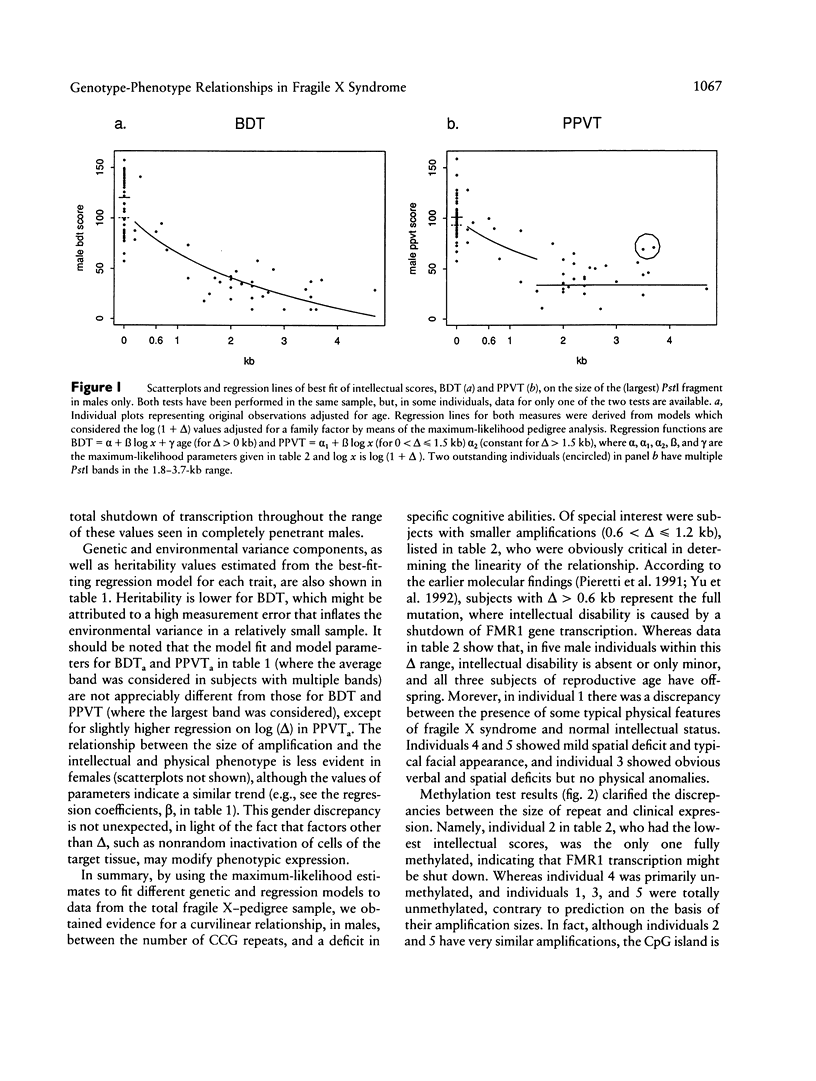
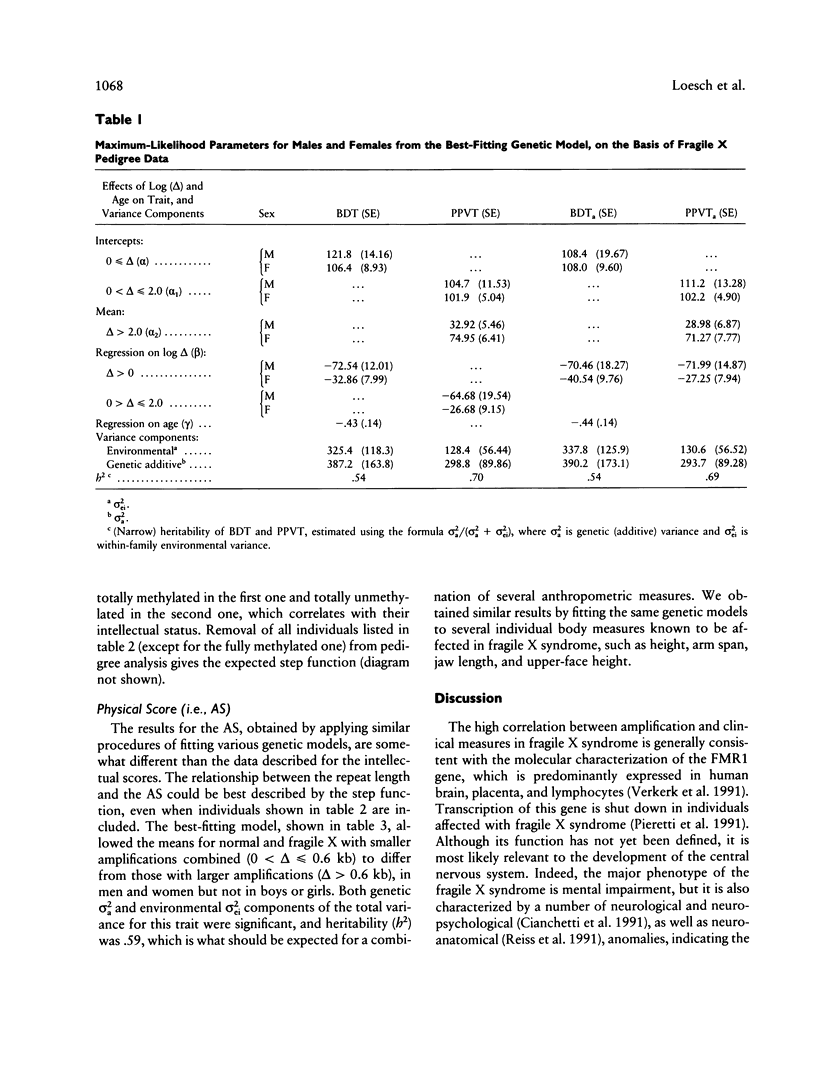
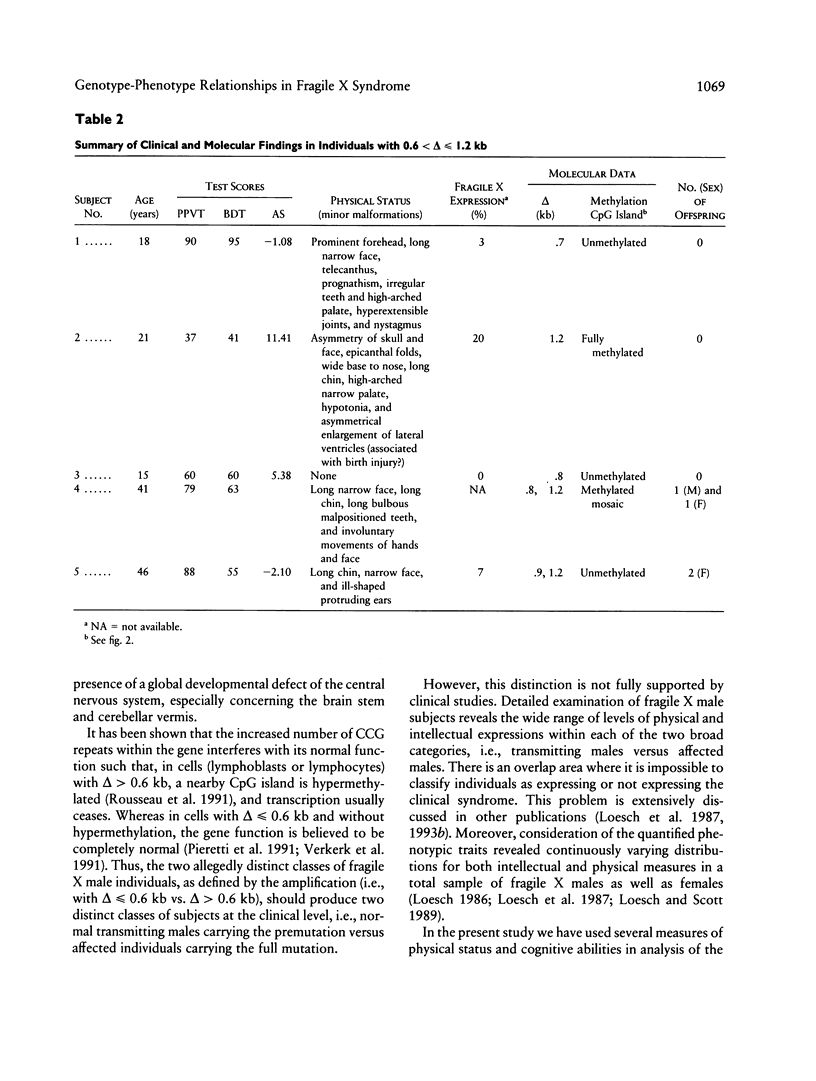
Relationships between the measures of intellectual and physical status in the fragile X syndrome and the size of amplification of the fragile X-specific fragment, equivalent to the number of CCG repeats within the FMR1 locus, were studied by a maximum-likelihood scoring technique for analysis of pedigree data. This allows for estimation of random effects (genetic and environmental variance) concurrently with other (fixed) effects in a quantitative trait. FMR1 expression is usually shut down in males penetrant for the fragile X syndrome who have hypermethylated CCG amplifications of > or = 0.6 kb. The assumption of the step versus curvilinear function representing this relationship was tested by the likelihood-ratio criterion. The maximum-likelihood parameters were based on the most appropriate model for each measure. The results were indicative of the presence of a curvilinear relationship between the amplification size and the two intellectual scores, the Peabody Picture Vocabulary Test and Block Design Test, measuring verbal and spatial abilities, respectively. Reasons for the unexpected curvilinear regression between the amplification size and intellectual scores were explained further by methylation analysis of fragile X males with amplifications of 0.6 < delta < or = 1.2 kb who appeared to be responsible for the curvilinearity of the relationship. Four of these showed unmethylated status of the amplified bands in lymphocytes, which were presumably transcriptionally active. Removal of the aberrant individuals led to the anticipated step function between amplification and intellectual scores. For the combined anthropometric score, as well as for several single physical measures, the step function was the most appropriate model regardless of the inclusion or omission of the aberrant individuals in the pedigree sample.
Full text
PDF


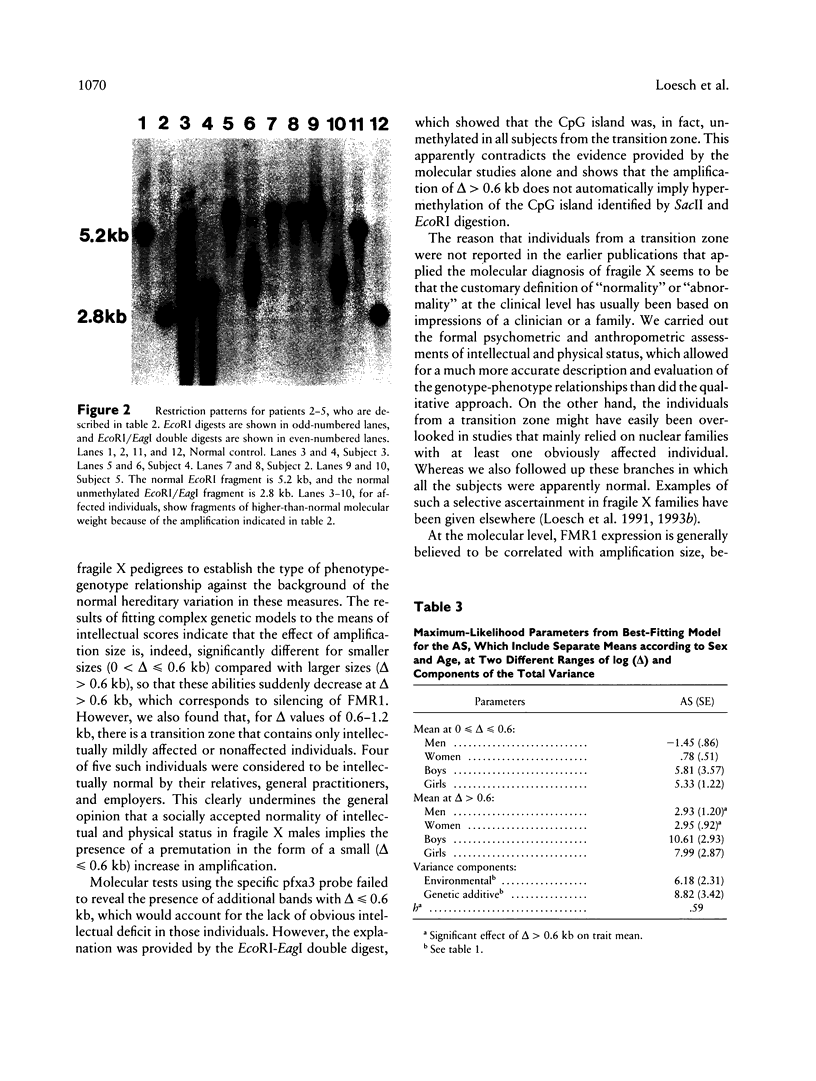



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler M. G., Najjar J. L. Do some patients with fragile X syndrome have precocious puberty? Am J Med Genet. 1988 Dec;31(4):779–781. doi: 10.1002/ajmg.1320310408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchetti C., Sannio-Fancello G., Fratta A. L., Manconi F., Orano A., Pischedda M. P., Pruna D., Spinicci G., Archidiacono N., Filippi G. Neuropsychological, psychiatric, and physical manifestations in 149 members from 18 fragile X families. Am J Med Genet. 1991 Aug 1;40(2):234–243. doi: 10.1002/ajmg.1320400222. [DOI] [PubMed] [Google Scholar]
- Crowe S. F., Hay D. A. Neuropsychological dimensions of the fragile X syndrome: support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia. 1990;28(1):9–16. doi: 10.1016/0028-3932(90)90082-y. [DOI] [PubMed] [Google Scholar]
- D'Amato R. C., Gray J. W., Dean R. S. Construct validity of the PPVT with neuropsychological, intellectual, and achievement measures. J Clin Psychol. 1988 Nov;44(6):934–939. doi: 10.1002/1097-4679(198811)44:6<934::aid-jclp2270440614>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Gedeon A. K., Baker E., Robinson H., Partington M. W., Gross B., Manca A., Korn B., Poustka A., Yu S., Sutherland G. R. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. doi: 10.1038/ng0892-341. [DOI] [PubMed] [Google Scholar]
- Hopper J. L., Mathews J. D. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982 Oct;46(Pt 4):373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kemper M. B., Hagerman R. J., Ahmad R. S., Mariner R. Cognitive profiles and the spectrum of clinical manifestations in heterozygous fra (X) females. Am J Med Genet. 1986 Jan-Feb;23(1-2):139–156. doi: 10.1002/ajmg.1320230109. [DOI] [PubMed] [Google Scholar]
- Lange K., Weeks D., Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5(6):471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- Lange K., Westlake J., Spence M. A. Extensions to pedigree analysis. III. Variance components by the scoring method. Ann Hum Genet. 1976 May;39(4):485–491. doi: 10.1111/j.1469-1809.1976.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z. Dermatoglyphic findings in fragile X syndrome: a causal hypothesis points to X-Y interchange. Ann Hum Genet. 1986 Oct;50(Pt 4):385–398. doi: 10.1111/j.1469-1809.1986.tb01759.x. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Hay D. A., Leversha M. Problems in ascertainment of transmitting males in Martin-Bell syndrome. Am J Med Genet. 1991 Dec 15;41(4):410–416. doi: 10.1002/ajmg.1320410405. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Hay D. A., Sutherland G. R., Halliday J., Judge C., Webb G. C. Phenotypic variation in male-transmitted fragile X: genetic inferences. Am J Med Genet. 1987 Jun;27(2):401–417. doi: 10.1002/ajmg.1320270219. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R. M., Chin W. F. Effect of fragile X on physical and intellectual traits estimated by pedigree analysis. Am J Med Genet. 1993 Jun 1;46(4):415–422. doi: 10.1002/ajmg.1320460414. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R. M. Fixed and random effects in the variation of the finger ridge count: a study of fragile-X families. Am J Hum Genet. 1992 May;50(5):1067–1076. [PMC free article] [PubMed] [Google Scholar]
- Loesch D. Z., Lafranchi M., Scott D. Anthropometry in Martin-Bell syndrome. Am J Med Genet. 1988 May-Jun;30(1-2):149–164. doi: 10.1002/ajmg.1320300113. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Scott D. Application of the anthropometric discriminant functions in estimation of carrier probabilities in Martin-Bell syndrome. Clin Genet. 1989 Sep;36(3):145–151. doi: 10.1111/j.1399-0004.1989.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Sheffield L. J., Hay D. A. Between-generation differences in ascertainment and penetrance: relevance to genetic hypotheses in fragile X. Hum Genet. 1993 Jun;91(5):469–474. doi: 10.1007/BF00217774. [DOI] [PubMed] [Google Scholar]
- Mulley J. C., Yu S., Gedeon A. K., Donnelly A., Turner G., Loesch D., Chapman C. J., Gardner R. J., Richards R. I., Sutherland G. R. Experience with direct molecular diagnosis of fragile X. J Med Genet. 1992 Jun;29(6):368–374. doi: 10.1136/jmg.29.6.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey M. E., Winter R. M., Davies K. E. A premutation that generates a defect at crossing over explains the inheritance of fragile X mental retardation. Am J Med Genet. 1985 Aug;21(4):709–717. doi: 10.1002/ajmg.1320210413. [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Reiss A. L., Freund L., Tseng J. E., Joshi P. K. Neuroanatomy in fragile X females: the posterior fossa. Am J Hum Genet. 1991 Aug;49(2):279–288. [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R., Gedeon A., Kornman L., Donnelly A., Byard R. W., Mulley J. C., Kremer E., Lynch M., Pritchard M., Yu S. Prenatal diagnosis of fragile X syndrome by direct detection of the unstable DNA sequence. N Engl J Med. 1991 Dec 12;325(24):1720–1722. doi: 10.1056/NEJM199112123252407. [DOI] [PubMed] [Google Scholar]
- Theobald T. M., Hay D. A., Judge C. Individual variation and specific cognitive deficits in the fra(X) syndrome. Am J Med Genet. 1987 Sep;28(1):1–11. doi: 10.1002/ajmg.1320280102. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kotzot D., Hirst M. C., Manca A., Korn B., Schmidt A., Barbi G., Rott H. D., Poustka A., Davies K. E. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Mulley J., Loesch D., Turner G., Donnelly A., Gedeon A., Hillen D., Kremer E., Lynch M., Pritchard M. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet. 1992 May;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Pritchard M., Kremer E., Lynch M., Nancarrow J., Baker E., Holman K., Mulley J. C., Warren S. T., Schlessinger D. Fragile X genotype characterized by an unstable region of DNA. Science. 1991 May 24;252(5009):1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]