Abstract
Cardiac arrhythmias, which occur in a wide variety of conditions where intracellular calcium is increased, have been attributed to the activation of a transient inward current (Iti). Iti is the result of three different [Ca]i-sensitive currents: the Na+–Ca2+ exchange current, a Ca2+-activated chloride current and a Ca2+-activated non-selective cationic current. Using the cell-free configuration of the patch-clamp technique, we have characterized the properties of a Ca2+-activated non-selective cation channel (NSCCa) in freshly dissociated human atrial cardiomyocytes. In excised inside-out patches, the channel presented a linear I–V relationship with a conductance of 19 ± 0.4 pS. It discriminated poorly among monovalent cations (Na+ and K+) and was slightly permeable to Ca2+ ions. The channel's open probability was increased by depolarization and a rise in internal calcium, for which the Kd for [Ca2+]i was 20.8 μm. Channel activity was reduced in the presence of 0.5 mm ATP or 10 μm glibenclamide on the cytoplasmic side to 22.1 ± 16.8 and 28.5 ± 8.6%, respectively, of control. It was also inhibited by 0.1 mm flufenamic acid. The channel shares several properties with TRPM4b and TRPM5, two members of the ‘TRP melastatin’ subfamily. In conclusion, the NSCCa channel is a serious candidate to support the delayed after-depolarizations observed in [Ca2+] overload and thus may be implicated in the genesis of arrhythmias.
Atrial fibrillation (AF) is the most common cardiac arrhythmia in humans. It causes palpitations, decreased cardiac output and heart failure. Furthermore, atrial fibrillation perpetuates itself by inducing progressive electrophysiological and structural changes to the atria (Wijffels et al. 1995). A persistent atrial tachyarrhythmia can overwhelm ion transport processes (pumps, channels and exchangers) and homeostatic mechanisms, resulting in cytosolic calcium overload (Goette et al. 1996). In situations of intracellular calcium ([Ca2+]i) overload, arrhythmias have been attributed to the development of oscillatory depolarizations which follow the normal action potential. High levels of [Ca2+]i may lead to spontaneous calcium release from the sarcoplasmic reticulum. This spontaneous release can be synchronized by membrane voltage changes and occur during diastole, when the activation of calcium-dependent currents initiates premature action potentials (Lakatta & Guarnieri, 1993).
The global calcium-dependent current, called the transient inward current (Iti), has been studied extensively in cardiac Purkinje and ventricular cells during oscillatory depolarizations. It is assumed to reflect at least three components, one supported by Na+–Ca2+ exchange (Karaguezian & Katzung, 1982; Verkerk et al. 2001), a second comprising a [Ca2+]i-activated chloride current (Zygmunt, 1994; Verkerk et al. 2000), and a third component which is carried by non-selective cation channels (Hill et al. 1988; Guinamard et al. 2002b).
In human atrial myocytes, membrane currents carried by the Na+–Ca2+ exchanger have been implicated in delayed after-depolarizations (Benardeau et al. 1996; Li & Nattel, 1996). Koster et al. (1999) reported that a [Ca2+]i-dependent chloride current is not present in human atrial cells but that a non-specific cation current activated by [Ca2+]i may contribute to arrhythmias triggered by calcium overload. Despite this indirect evidence, there are no documented single-channel recordings of Ca2+-activated non-specific channels in human atrial cardiac cells.
Single-channel recording studies have demonstrated that calcium-activated non-selective cation (NSC) channels exist in the plasma membrane of a variety of mammalian cells, both excitable and non-excitable. The main characteristics of these NSC channels are their voltage dependence, a single channel conductance lying between 20 and 35 pS, no discrimination between monovalent cations, low permeability to Ca2+, sensitivity to intracellular calcium ions, inhibition by cytosolic ATP and blockade by flufenamic acid (for review see Teulon, 2000).
The purpose of the present experiments was to determine whether single non-selective cation channels can be identified in inside-out membrane patches from human atrial myocytes and, if so, to establish the biophysical, pharmacological and molecular properties of theses channels.
Preliminary data have been published previously in abstract form (Guinamard et al. 2002a).
Methods
Patients
Right atrial appendages were obtained from 17 patients (65 ± 2.4 years old; 13 males; 4 females) undergoing cardiac surgery (12 aorto-coronary by passes, 5 aortic valve replacements). Sinus rhythm was present in all cases. Myocardial samples were removed during cannulation for cardiopulmonary bypass with extra-corporeal circulation, a routine procedure comprising normal management of the patients. All procedures were carried out in accordance with the Declaration of Helsinki and informed written consent was obtained from the patients.
Cardiomyocyte isolation
Cardiac cells (illustrated in Fig. 1A) were obtained using a procedure modified from Benardeau et al. (1996). Briefly, atrial specimens were collected and finely minced with iridectomy scissors in Krebs buffer containing 30 mm 2,3 butanedione monoxime (BDM) and 0.5 mm EGTA. Enzymatic digestions were then conducted in Krebs buffer supplemented with 0.5% BSA (Invitrogen). A first digestion was performed with 6 IU ml−1 protease type XXIV (Invitrogen) and 165 IU ml−1 collagenase type V (Invitrogen) for 15 min. After a further two to four 10 min steps in the presence of 360 IU ml−1 collagenase type V (Invitrogen), samples were gently triturated using a fire-polished Pasteur pipette in a washing buffer. Isolated cells were filtered to remove undissociated pieces and gradually resuspended in DMEM (Dulbecco's modified Eagle's medium) culture (Biowhittaker, Walkersville, MD, USA) medium supplemented with 1% antibiotics (100 IU ml−1 peniciline-G-Na; 50 IU ml−1 streptomycin sulphate), 1 nm insulin and 10% fetal calf serum. Cells were seeded into 35 mm Petri dishes pretreated with laminin (Invitrogen) and maintained at 37°C in a H2O-saturated 95% air–5% CO2 environment and used for patch-clamp experiments within 24 h.
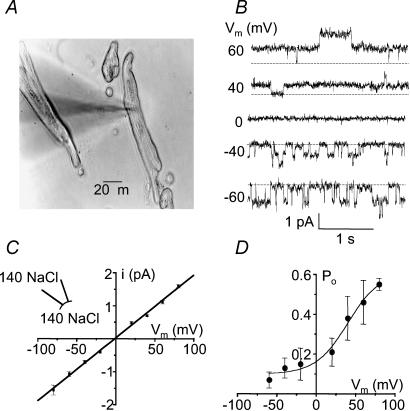
Figure 1. Conductive properties of the non-selective cation channel.
A, phase–contrast micrograph of freshly isolated human atrial cardiomyocytes. Note the shadow cast by the patch pipette used for current recordings. B, single-channel tracings recorded at various voltages from an inside-out patch. Pipette and bath contained 140 NaCl-standard solution (CaCl2: bath 1.8 mm, pipette 1 mm). Vm corresponds to the membrane potential. Dashed lines indicate the current level of closed channels. Two similar channels are present in the patch. C, current–voltage relationship under the same ionic conditions as in B. Data points were fitted by linear regression, yielding the following values: slope conductance (g), 19 ± 0.4 pS; reversal potential (Vrev), −1.7 ± 1.3 (n = 7). D, voltage dependence of open probability (Po). In 4 experiments similar to that shown in B, Po was determined at various voltages using amplitude histograms. Fitting of data with a Boltzmann equation yielded the following values: V0.5= 40 ± 10.7 mV, Pmax= 0.61 ± 0.11, s = 13.5 ± 3.5 mV.
Solutions and chemicals
The Krebs buffer used for cell dissociation procedures contained (mm): 35 NaCl; 7.7 KCl; 16 Na2HPO4; 25 NaHCO3; 1.2 KH2PO4; 10 glucose; 10 Hepes; 134 sucrose. The wash solution contained (mm): 130 NaCl; 4.8 KCl; 1.2 KH2PO4; 5 glucose; 25 Hepes and 5% BSA. Solutions were titrated to pH 7.4 with NaOH.
For electrophysiological recordings, the standard 140 mm NaCl solution for the bath and pipette contained (mm): 140 NaCl; 4.8 KCl; 1.2 MgCl2; 10 glucose; 10 Hepes. Pipette and bath solutions usually contained 1 mm and 1.8 mm CaCl2, respectively. When specified in the text, internal Ca2+ concentrations below 10 μm were determined with a combination of CaCl2 and EGTA (Teulon et al. 1987). Low NaCl solution contained 14 mm NaCl (no KCl included) and was supplemented with 256 mm sucrose to maintain osmolarity. To test monovalent cation selectivity, 140 mm NaCl was replaced by KCl. Ca2+ permeability was determined using a solution containing (mm): 100 CaCl2; 10 glucose; 10 Hepes. External solutions (pipette and bath) were adjusted to pH 7.4. The pH of perfused solutions (inside of the membrane) was adjusted to 7.2. The effect of internal ATP was tested using a solution containing total concentrations of the following constituents (mm): 0.5 ATP, 1 CaCl2 and 1.2 MgCl2, corresponding to 0.016 free-ATP, 0.15 CaATP and 0.32 MgATP, as calculated by the CaBuf program (ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip).
Prior to sealing, cells were incubated for at least 10 min in a solution containing either 140 mm NaCl for control, or with 500 nm phorbol 12-myristate 13-acetate (PMA) dissolved beforehand in DMSO to a final DMSO ratio of 0.1%. At this level the DMSO alone has no effect on channel activity. Glibenclamide was dissolved in DMSO to a final DMSO ratio of 0.01%. Chemical products were from Sigma.
Measurements
Single-channel currents were recorded under voltage clamp with a RK400 (Biologic, Claix, France) patch-clamp amplifier from patches of isolated cardiomyocytes, using the inside-out variant of the patch-clamp technique (Hamill et al. 1981). The bath reference was 0.5 m KCl (in a 4% agar bridge) connected to an Ag–AgCl pellet. Liquid junction potentials arising from changes in bath solutions at the inner surface of the membrane patch were determined using a pipette containing 2.7 m KCl, which allowed the zero-current voltage deflection to be monitored. The measured values were similar to those calculated by the JPCalc program (Barry, 1994) with Clampex software (version 8.1; Axon Instruments, Foster City, USA). Applied potentials (Vm = Vbath − Vpipette) were corrected accordingly. Currents due to the migration of cations from the inner to the outer surface of the membrane were positive and were registered as upward deflections in single-channel current tracings. All experiments were conduct at room temperature (20–25°C).
Data analysis
Signals for analysis were stored on digital audio tapes and then played back through a filter (Bessel model 902LPF, Frequency Devices, Inc., Haverhill, MA, USA) and digitized at 1 kHz using a Digidata 1200A analog–digital interface and Fetchex software (version 6.02; Axon Instruments). Currents were analysed with Bio-patch software (version 3.30; Biologic), generating amplitude histograms for the construction of I–V curves and for the estimation of open probability (Po). Relative permeabilities and associated reversal potentials for current flow (Vrev) were estimated by fitting experimental measurements to the Goldman–Hodgkin–Katz equation:
where g = PNa[Na]oF2/RT, and where V, PNa, F, R and T have their usual meanings. Ps represents the permeability of the channel to the ion S; subscripts ‘i’ and ‘o’ refer to the internal and outer sides, respectively. The relative permeability of the non-selective cation channel to K+ was deduced from Vrev values after linear fitting of the data points since no current rectification was apparent when K+ was substituted for the bath Na+. Po as a function of voltage was fitted using a Boltzmann function:
where Pmin and Pmax represent the minimum and maximum open probabilities, V0.5 is the potential of half-maximal activation and s is the slope parameter. Experimental results are reported as mean ±s.e.m. A paired Student's t test was applied when Po was determined under different conditions on the same cell. An analysis of variance (ANOVA) was used to compare conductances and reversal potentials under different conditions. Statistical significance was accepted for values of P < 0.05.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from human atrial samples by the Chomczynski method, using the RNABle reagent (Eurobio). cDNA was synthesized using the Pd(N)6 random hexamer primers (Amersham). 10 μl RNA, 2.4 μg random primers, 11.3 mm DTT (Gibco) and 1.1 mm dNTPs (Invitrogen) were mixed in a total volume of 22 μl and incubated for 2 min at 65°C. Then 30 U RNAguard (Amersham) and 400 U reverse transcriptase M-MLV (Invitrogen) were added to the mixture and incubated for 1 h at 37°C, in a final volume of 50 μl. Primers for the amplification of the TRPM4b N-terminal region and of the pore domain region consisted of the following sequences: N-terminal sense TCTCTATTCCGGACCACACC, N-terminal antisense ACACACCACGGAGAAGCC, pore domain sense TTGGCATACTGGGAGACGCA, pore domain antisense GGCCCAAGATCGTCATCGT. The PCR amplification was carried out with 0.5–1 μg cDNA, 0.25 mm dNTPs (Invitrogen), 0.2 μm each primer, 2 mm MgCl2, 1.25 U Taq polymerase (Gibco) and 2.5 μl TAQ buffer (Gibco) in a total volume of 25 μl. Thermal cycles performed in a PTC-100 (M.J. Research, Inc.) were: 95°C for 3 min, followed by 30 cycles at 62°C for 30 s, 72°C for 30 s and 95°C for 30 s. Amplification was stopped by a step at 62°C for 2 min and a final one at 72°C for 10 min. Amplified products were electrophoresed on 1% agarose gels and stained with ethidium bromide. They were then sequenced using the ABI Prism Big Dye terminator method (Applied biosystems, Perkin-Elmer).
Results
A calcium-activated non-selective cation current has been characterized in atrial cardiomyocytes (Koster et al. 1999) but as yet nothing is known about its single channel properties. In parallel, we have previously described the single channel properties of a calcium-activated non-selective cation channel (NSCCa) in ventricular cardiomyocytes in culture (Guinamard et al. 2002b). To investigate whether the same channel could participate in the macroscopic current described in atrial myocytes, single channel currents were recorded from isolated cardiomyocytes. Channel activity was recorded using the inside-out patch configuration with 140 mm NaCl standard solution on both sides of the membrane ([CaCl2]i= 1.8 mm; [CaCl2]o= 1 mm). Figure 1B illustrates channel activity under these conditions as a function of the membrane holding potential. For symmetrical ionic solutions, the corresponding current–voltage relationship (Fig. 1C) was linear with a slope conductance of 19 ± 0.4 pS (n = 7) and a reversal potential at −1.7 ± 1.3 mV. The open probability values determined for various membrane potentials clearly indicate that channel activity increases with depolarization (Fig. 1D). The data were fitted to a Boltzmann function with values of 0.61 ± 0.11 for Pmax, 13.5 ± 3.5 mV for s and +40 ± 10.7 mV for V0.5 (n = 4).
Preincubation of cells for at least 10 min before patching with the protein kinase C (PKC) activator PMA (0.5 μm) increased the channel activity. While activity was detected in only one inside-out patch over 15 trials without PMA preincubation, channel activity increased significantly following the preincubation step (46.3%versus 6.7% in control; n = 95; P = 0.003). This observation demonstrates that PMA potentiates human atrial channel activity, as has been previously described for an NSCCa in ventricular cells (Guinamard et al. 2002b, 2004). Thus, while the mechanism underlying this activation was not further characterized, all subsequent experiments were performed following PMA preincubation.
Selectivity
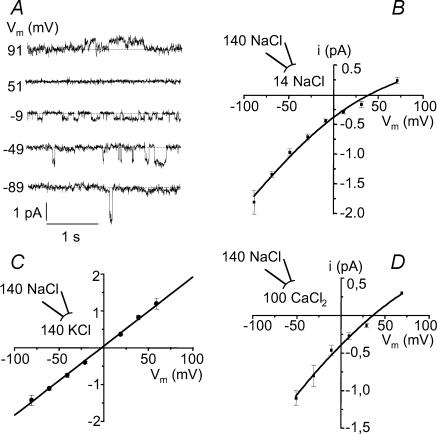
The ionic selectivity of the channel was studied in inside-out patches by changing the ionic composition on the cytoplasmic side. As show in Fig. 2A and B, reducing the NaCl concentration from 140 to 14 mm on the internal side of the membrane caused a pronounced inward rectification and shifted the reversal potential to more positive voltages. Fitting of the Goldman–Hodgkin–Katz (GHK) equation to the data yielded mean conductance and reversal potential values of 17.4 ± 0.5 pS and +38.5 ± 4.2 mV (n = 7), respectively. The corresponding permeability ratio PCl/PNa was 0.1 ± 0.04. The selectivity among monovalent cations was assessed by replacing NaCl (140 mm) in the bath solution by KCl (140 mm). The channel conductance (18.7 ± 0.1 pS) and Vrev (−0.7 ± 1.7 mV; n = 6) were not significantly affected (Fig. 2C). The corresponding permeability ratio PK/PNa was 1.17 ± 0.08. To test the calcium permeability of the NSC channel, the cytoplasmic side was bathed in a solution containing 100 mm CaCl2. Under these conditions, the channel displayed a conductance of 17.8 ± 3.5 pS with a reversal potential at +39.5 ± 4.1 mV (n = 4), corresponding to a PCa/PNa of 0.13 ± 0.02 (Fig. 2D). The results indicate that the channel primarily conducts monovalent Na+ as well as K+ ions, and that it is slightly permeable to calcium.
Figure 2. Channel selectivity.
A, single channel current tracings recorded at various voltages from an inside-out patch. Pipette: 140 mm NaCl, bath 14 mm NaCl. B, current–voltage relationship under the same conditions as in A averaged from 7 patches. Data were fitted by the GHK equation, giving values of Vrev=+38.5 ± 4.2 mV and PCl/PNa= 0.1 ± 0.04. C, current–voltage relationship in the presence of 145 mm KCl in the bath (pipette: 140 mm NaCl) (n = 6). Data points were fitted by a linear regression (g = 18.7 ± 0.1 pS; Vrev=−0.7 ± 1.7 mV, PK/PNa= 1.17 ± 0.08). D, current–voltage relationship averaged from 4 inside-out patches with pipette 140 mm NaCl and bath 100 mm CaCl2. Data points were fitted by the GHK equation (g = 17.8 ± 3.5 pS; Vrev=+39.5 ± 4.1 mV, PCa/Na= 0.13 ± 0.02).
Effect of internal calcium and internal ATP
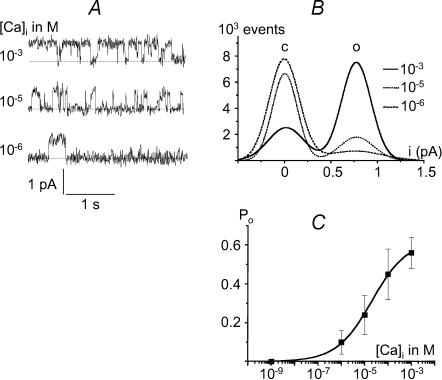
As reported for experiments on several tissues (Teulon, 2000), the non-selective cation channel in human atrial myocytes is also dependent upon the cytosolic calcium concentration. Figure 3A clearly shows that reducing the calcium concentration from 1 mm to 1 μm on the cytoplasmic face of an excised inside-out patch decreased channel activity. Corresponding amplitude histogram distributions confirmed this effect (Fig. 3B). The open probability of active channels determined at +40 mV is plotted in Fig. 3C as a function of the Ca2+ concentration in the bath, rising from 1 nm to 1 mm (n = 4). A similar sensitivity to calcium was observed at −40 mV (data not shown). Channel openings were seen at [Ca2+]i≥ 1 μm. Fitting of the Hill equation to the experimental data points yielded an apparent dissociation constant (Kd) of 20.8 μm and a Hill coefficient of 0.8.
Figure 3. Effect of internal calcium on channel activity.
A, single channel currents recorded from an inside-out patch showing that channel activity increases with rising [Ca2+]i (Vm=+40 mV; pipette and bath contained 140 mm NaCl). B, corresponding amplitude histograms. ‘c’ and ‘o’ indicate the current level for the closed and open states of the channel, respectively. C, values of Po (mean ±s.e.m.) at various [Ca2+]i (Vm=+40 mV; n = 4).
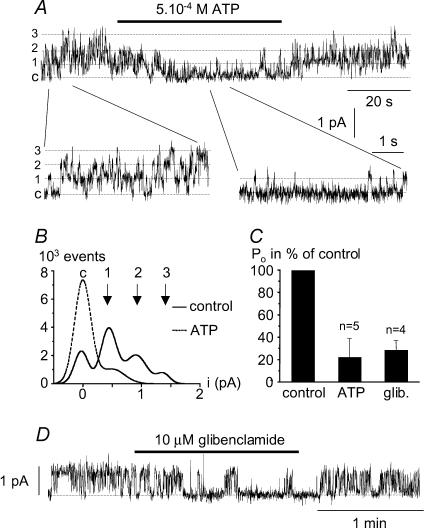
Non-selective cation channels have also been shown to be sensitive to cytosolic adenine nucleotides (Teulon, 2000). This effect on the channel of internal ATP was assessed using the inside-out configuration of the patch-clamp technique. Figure 4A–C shows that addition of 5 × 10−4m ATP (corresponding to a [ATP]free of 16 μm; see Methods) to the cytoplasmic surface of inside-out excised patches decreased Po to 22.1 ± 16.8% of control (Vm=+40 mV; n = 5). This effect, confirmed by the corresponding amplitude histograms (Fig. 4B), was completely reversible.
Figure 4. Effect of internal ATP and glibenclamide on channel activity.
A, single channel currents recorded from an inside-out patch showing the reversible inhibition of channel activity by 0.5 mm internal ATP corresponding to 16 μm free-ATP (Vm=+30 mV). Three identical channels were present in the patch membrane, labelled ‘c’ for the closed state, and 1, 2 and 3 indicating the current opening level for the three channels. Current variations at the single channel level can be seen more easily at the bottom of the panel. B, corresponding amplitude histograms for control conditions and with ATP in the bath. Current amplitude corresponding to the opening of one channel is 0.5 pA, as expected at this voltage. C, open probability of the channel as a percentage of control in the presence of 0.5 mm ATP or 10 μm glibenclamide averaged for 5 and 4 experiments, respectively. D, single channel currents recorded from an inside-out patch showing the reversible inhibition of channel activity by 10 μm glibenclamide (Vm=+40 mV)
Pharmacology
Analogous to previous reports concerning the non-selective cation channel identified in native reactive astrocytes (Chen et al. 2003) and on IKATP channels in insulin-secreting cells (Schmid-Antomarchi et al. 1987), the ATP effect described above could suggest sulphonylurea sensitivity. The inhibition of channel activity in atrial cells by the sulphonylurea glibenclamide, a hypoglycaemic agent, was thus assessed. Figure 4C and D illustrates the reversible action of 10 μm of this compound on channel activity recorded from inside-out patches held at +40 mV. When added to the cytoplasmic side of the patch, the drug reduced channel activity to 28.5 ± 8.6% of that of control (Vm=+40 mV; n = 4).
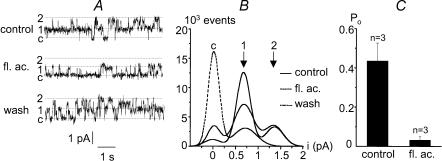
Flufenamic acid, a non-steroidal anti-inflammatory drug has also been reported to block NSC channels (Gogelein et al. 1990). Figure 5A and B illustrates the reversible action of this compound on channel activity recorded from an inside-out patch held at +40 mV. The perfusion of 0.1 mm flufenamic acid in the bath clearly reduced channel activity, with Po decreasing from 0.44 ± 0.09 to 0.03 ± 0.02 (Vm=+40 mV; n = 3) (Fig. 5C).
Figure 5. Inhibition of channel activity by flufenamic acid.
A, single channel currents recorded from an inside-out patch illustrating the reversible blocking effect of flufenamic acid (fl. ac., 0.1 mm) (Vm=+40 mV). ‘c’ indicates the closed channel current level while 1 and 2 indicate the current levels corresponding to the open states of one or two identical channels. B, corresponding amplitude histograms indicating a current level of 0.7 pA for the opening of one channel, as expected at this voltage. C, open probability in the absence (control) or presence of 0.1 mm flufenamic acid (Vm=+40 mV; n = 3)
TRPM4b expression in atrial cells
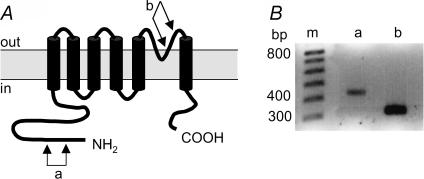
The cardiac non-selective cation channel described here shares biophysical properties with the newly cloned TRPM4b channel (Launay et al. 2002; Nilius et al. 2003). To verify its expression in the human atrium, RT-PCR was used to probe for TRPM4b. Two fragments were studied, one of which includes amino acids coding for the expected pore region (7 samples), and the other coding for the N-terminal amino acid tail that is absent on the first TRPM4 form that was cloned (7 samples) (Xu et al. 2001; Launay et al. 2002). In all cases, the presence of PCR products was detected. Figure 6 shows an example of amplification for each fragment. Amplified products were sequenced and were similar (100%) to the human TRPM4b-mRNA sequence (Launay et al. 2002), thus showing that TRPM4b is expressed in human atrial cells.
Figure 6. TRPM4b mRNA expression on human atrium.
A, the predicted topology of TRPM4b. The channel is composed of six membrane-spanning segments (M1 to M6) and a pore domain between M5 and M6. For PCR reactions, two pairs of primers were used. One in the segment encoding for a part of the N-terminal domain (a) and the other for a part of the pore domain (b). B, total RNA was extracted from human atrial samples and was reverse-transcribed. cDNA was amplified by PCR for 30 cycles. The two pairs of primers (a and b) were used separately and amplified products were obtained at the expected size (399 and 300 bp, respectively). ‘m’ is the 100 bp DNA size marker.
Discussion
Using a cell-free configuration of the patch-clamp technique, we have obtained evidence that a calcium-activated cation channel exists in human atrial cardiomyocytes. In a manner similar to the NSCCa channel characterized in dedifferentiated adult rat ventricular cells (Guinamard et al. 2002b), the channel is activated by micromolar [Ca2+]i and its open probability increases with depolarization. NSCCa channels are characterized by a single channel conductance around 20 pS, an absence of discrimination between monovalent cations, inhibition by cytosolic ATP and blockade by flufenamic acid. In agreement with previous reports for other NSCCa channel types (for review see Teulon, 2000), the channel is not completely impermeable to calcium ions. The permeability ratio (PCa/PNa), estimated in the presence of high intracellular calcium concentration (100 mm), was about 0.13. This value is similar to those reported for rat ventricular cells (Guinamard et al. 2002b), rat distal colon cells (Siemer & Gogelein, 1992) and guinea-pig outer hair cells (Van den Abbeele et al. 1994), whicht are 0.09, 0.14 and 0.1, respectively.
The physiological role for this channel in cardiac function remains unclear, but it may be involved in an arrhythmogenic mechanism in situations of intracellular calcium overload or cytoplasmic ATP depletion. As described for the heart in general (Fozzard, 1992; for review) and for human atrial cells in particular (Koster et al. 1999), a calcium-dependent non-specific cation current may be involved in the transient inward current (Iti), which depolarizes the cell membrane, generating delayed after-depolarizations. Indeed, since this NSC channel was activated by micromolar [Ca2+]i (Fig. 3C), which is believed to prevail during Iti (Eisner & Valdeolmillos, 1986), activation of this channel should occur during the transient inward current. Moreover, the intracellular calcium overload observed during atrial arrhythmias could be mediated partly by the non-selective cationic channel itself. Consequently, the channel may participate in the initiation of atrial fibrillation and flutter under ischaemic conditions. Furthermore, it is plausible that the current might also be activated during cardiac glycoside intoxication and under ischaemic conditions where the level of intracellular calcium is drastically enhanced. It has been reported that oxygen-derived free radicals can induce a calcium-activated non-selective cationic current in ventricular cells of the guinea-pig (Jabr & Cole, 1993, 1995). These NSC currents may also be associated with pro-arrhythmic signalling during post-ischaemic reperfusion.
In the present study, channel activity was rarely observed in isolated human atrial myocytes maintained under normal conditions. As previously reported for cultured rat cardiomyocytes (Guinamard et al. 2002b, 2004), pre-stimulation with phorbol ester facilitated detection of the channel in human atrial cells by raising their level of activity, suggesting that PKC might be an activator of this channel. The increased channel activity observed for the inside-out configuration after PMA treatment indicates that PKC phosphorylation increases the number of functional channels. Therefore, NSC channels could facilitate the appearance of arrhythmias upon stimulation of hormonal receptors that are coupled to phospholipase C (PLC) signalling pathways, such as angiotensin II, α1-adrenergic or purinergic receptors.
The recent discovery of a novel subfamily of cation channels, which belong to the ‘transient receptor potential’ (TRP) protein family, revives interest in the subject of non-selective channels. On the basis of structural information, the TRP-related channels have been divided into three main subfamilies: TRPC for canonical TRP channels, TRPV for the vanilloid group, and TRPM homologous to the archetypal melastatin. The functions of the various TRP channels differ markedly, even within the same subfamily (for review see Vennekens et al. 2002).
Interestingly, the cardiac NSCCa channel has biophysical properties that are similar to those of the second form of TRPM4 (so-called TRPM4b). This TRP channel was recently cloned and characterized by Launay et al. (2002). When TRPM4b was expressed in HEK-293 cells, it produced a non-selective cation channel of 25–23 pS unitary conductance that was directly activated by [Ca2+]i, with an apparent Kd ranging between 0.4 (Launay et al. 2002) and 9.3 μm (Nilius et al. 2004). This difference in calcium sensitivity may reflect species differences and/or differences in experimental conditions. As observed for the NSCCa channel, TRPM4b was equally selective for Na+ and K+ (Launay et al. 2002) and was blocked by intracellular free ATP in the 10 μm range (Nilius et al. 2004). In addition, as has been reported for TRPM4b (Nilius et al. 2003), the NSCCa channel exhibits a Boltzmann-type activation; the fraction of open channels increased at positive potentials and was low at negative potentials. The slope parameter s of the activation curve estimated in the present experiment is close to that reported by Nilius et al. (2003) for TRPM4b (13.5 mV versus 9.7 mV). Moreover, while the TRPM4b channel is expressed widely in a variety of mammalian cells in both excitable and non-excitable tissues, it appears to have a particularly pronounced expression in heart tissue (Launay et al. 2002; Nilius et al. 2003). In the present study, an RT-PCR signal corresponding to TRPM4b-transcript was detected in human atrial cells. This is a more precise localization of the channel in cardiac tissue, where it coincides with the appearance of an NSCCa channel.
Within the TRPM subfamily, TRPM5 displays characteristics of a calcium-activated, non-selective channel with a unitary conductance of 25 pS. This new TRPM channel is monovalent cation-specific (Na+, K+), directly activated by [Ca2+]i at concentrations of 0.3–1 μm and exhibits a voltage dependence (Hofmann et al. 2003). Taken together, the data indicate that the TRPM4b and TRPM5 share many of the hallmarks of NSCCa. There are no other channels described with these features. Therefore, these two TRP channels are presently the only molecular candidates that could account for NSCCa.
Despite the indirect evidence, further experiments are needed to establish the exact relationship between the NSCCa channel characterized in human atrial cells and the second form of TRPM4b and/or TRPM5. For example, the sensitivity of both TRPM4b and 5 to sulphonylurea agents has not yet been investigated. Similarly to the cystic fibrosis transmembrane conductance regulator (CFTR) and ATP-sensitive K+ channel (KATP) (Schmid-Antomarchi et al. 1987; Anderson et al. 1992), the NSCCa exhibits sensitivity to glibenclamide. This effect of this sulphonylurea derivative suggests the presence of a glibenclamide binding site, directly on the channel and/or on a sulphonylurea receptor. Indeed, as has been recently described for a non-selective cation channel identified in native reactive astrocytes (Chen et al. 2003), the possibility cannot be excluded that the NSC channel is functionally coupled to sulphonylurea receptors in human atrial cells. On the other hand, we cannot rule out the possibility that the glibenclamide blocks the NSCCa current by directly acting on the channel protein. These issues require further investigation.
In conclusion, a Ca2+-activated non-selective cation channel present in the human atria may play a primary role in the genesis of arrhythmias. An understanding of the regulatory processes that govern this channel may well lead to improved drug targeting and therapies.
Acknowledgments
We would like to thank Chantal Jougla, Christophe Magaud and Françoise Mazin for technical help, and Dr David McKenzie and Michael Paterson for critically reading the manuscript.
References
- Anderson MP, Sheppard D, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airways and intestinal epithelia. Am J Physiol. 1992;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Meth. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Benardeau A, Hatem SN, Rucker-Martin C, Le Grand B, Mace L, Dervanian P, et al. Contribution of Na+/Ca2+ exchange to action potential of human atrial myocytes. Am J Physiol. 1996;271:H1151–H1161. doi: 10.1152/ajpheart.1996.271.3.H1151. [DOI] [PubMed] [Google Scholar]
- Chen M, Dong Y, Simard JM. Functional coupling between Sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neuroscience. 2003;23:8568–8577. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Valdeolmillos M. A study of intracellular calcium oscillations in sheep cardiac Purkinje fibres measured at the single cell level. J Physiol. 1986;372:539–556. doi: 10.1113/jphysiol.1986.sp016024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol. 1992;87(2):105–113. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- Goette A, Honeycutt C, Langberg JJ. Electrical remodelling in atrial fibrillation. Time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- Gogelein H, Dahlem D, Englert H, Lang HJ. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channel in the rat exocrine pancreas. FEBS Lett. 1990;268:79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Chatelier A, Corbi P, Rahmati M, Lenfant J, Bois P. A Ca2+-activated nonselective cation channel expressed in human atrial cardiomyocytes. J Physiol. 2002a;544:S136. [Google Scholar]
- Guinamard R, Chatelier A, Lenfant J, Bois P. Activation of the Ca2+-activated nonselective cation channel by Diacylglycerol analogues in rat cardiomyocytes. J Cardiovasc Electrophysiol. 2004;15:342–348. doi: 10.1046/j.1540-8167.2004.03477.x. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Rahmati M, Lenfant J, Bois P. Characterization of a Ca2+-activated nonselective cation channel during dedifferentiation of cultured rat ventricular cardiomyocytes. J Memb Biol. 2002b;188:127–135. doi: 10.1007/s00232-001-0180-4. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill JA, Coronado R, Strauss HC. Reconstitution and characterization of a calcium-activated channel from heart. Circ Res. 1988;62:411–415. doi: 10.1161/01.res.62.2.411. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Current Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Cole WC. Alterations in electrical activity and membrane currents induced by intracellular oxygen-derived free radical stress in guinea pig ventricular myocytes. Circ Res. 1993;72:1229–1244. doi: 10.1161/01.res.72.6.1229. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Cole WC. Oxygen-derived free radical stress activates nonselective cation current in guinea pig ventricular myocytes. Role of sulfhydryl groups. Circ Res. 1995;76:812–824. doi: 10.1161/01.res.76.5.812. [DOI] [PubMed] [Google Scholar]
- Karaguezian HS, Katzung BG. Voltage-clamp studies of transient inward current and mechanical oscillations induced by ouabain in ferret papillary muscle. J Physiol. 1982;327:255–271. doi: 10.1113/jphysiol.1982.sp014230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster OF, Szigeti GP, Beuckelmann DJ. Characterization of a [Ca2+]i-dependent current in human atrial and ventricular cardiomyocytes in the absence of Na+ and K+ Cardiovasc Res. 1999;41:175–185. doi: 10.1016/s0008-6363(98)00202-8. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation? J Card Elec. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Li GR, Nattel S. Demonstration of a inward Na+/Ca2+ exchange current in adult human atrial myocytes. Ann NY Acad Sci. 1996;779:525–528. doi: 10.1111/j.1749-6632.1996.tb44827.x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch. 2004;448:70–75. doi: 10.1007/s00424-003-1221-x. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987;262:15840–15844. [PubMed] [Google Scholar]
- Siemer C, Gogelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflugers Arch. 1992;420:319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- Teulon J. Ca2+-activated nonselective cation channels. In: Endo M, Kurachi Y, Mishina M, editors. Pharmacology of Ionic Channel Function: Activators and Inhibitor. Berlin: Springer-Verlag; 2000. pp. 625–649. [Google Scholar]
- Teulon J, Paulais M, Bouthier M. A Ca2+-activated cation-selective channel in the basolateral membrane of the cortical thick ascending limb of Henle's loop of the mouse. Biochim Biophys Acta. 1987;905:125–132. doi: 10.1016/0005-2736(87)90016-2. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele T, Tran Ba Huy P, Teulon J. A calcium-activated nonselective cationic channel in the basolateral membrane of outer hair cells of the guinea-pig cochlea. Pflugers Arch. 1994;427:56–63. doi: 10.1007/BF00585942. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Verkerk A, Veldkamp MW, Baartscheer A, Schumacher CA, Klopping C, van Ginneken AC, et al. Ionic mechanisms of delayed afterdepolarizations in ventricular cells isolated from human end-stage failing hearts. Circulation. 2001;104:2728–2733. doi: 10.1161/hc4701.099577. [DOI] [PubMed] [Google Scholar]
- Verkerk AO, Veldkamp MW, Bouman LN, van Ginneken AC. Calcium-activated Cl− current contributes to delayed afterdepolarizations in single Purkinje and ventricular myocytes. Circulation. 2000;101:2639–2644. doi: 10.1161/01.cir.101.22.2639. [DOI] [PubMed] [Google Scholar]
- Wijffels M, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt AC. Intracellular calcium activates a chloride current in canine ventricular myocytes. Am J Physiol. 1994;267:H1984–H1195. doi: 10.1152/ajpheart.1994.267.5.H1984. [DOI] [PubMed] [Google Scholar]