Abstract
Increasing intake of folic acid would be a relatively cheap and simple way of reducing heart disease, if it works. Can we draw a definitive conclusion from the current evidence?
Debate remains over whether raised serum homocysteine concentrations cause ischaemic heart disease and stroke and whether folic acid, which lowers homocysteine, will help reduce the risk of these disorders. Different groups of researchers have used the same evidence to reach opposite conclusions.1 2 We examine why the differences have occurred and draw conclusions using evidence from the various types of study used to investigate the relation.
Cohort studies
Meta-analyses of cohort studies show significant positive associations between serum homocysteine concentrations and ischaemic heart disease events (fatal and non-fatal myocardial infarction and sudden cardiac death) and stroke. A 3 µmol/l decrease in serum homocysteine (achievable with 0.8 mg/day folic acid) lowers the risk of myocardial infarction by 15% and stroke by 24%.1 3 These estimates were adjusted for confounding by other cardiovascular risk factors.
Evidence from patients with homocystinuria
Lowering homocysteine concentrations has been shown to have a large effect on cardiovascular disease risk in patients with homocystinuria. Untreated people who are homozygous for this rare genetic disorder have homocysteine concentrations about five times above the average for unaffected people and about a 50% chance of a vascular event by age 30.4 In two studies, homozygous patients taking treatment to reduce homocysteine concentrations had only two vascular events when 59 would have been expected from previous observations in untreated patients.5 6 Although these were not randomised trials, selection bias is unlikely to explain so large a difference. The absence of a threshold in the dose-response relation between homocysteine and cardiovascular disease over a wide range3 7 suggests that lowering moderately raised homocysteine concentrations would also have a preventive effect.
Evidence from genetic polymorphism studies
Moderately raised homocysteine concentrations occur as a result of a mutation in the methylenetetrahydrofolate reductase gene (MTHFR). A base pair substitution of cytosine for thymidine reduces the activity of the enzyme. People who are homozygous for the mutant gene (TT) have homocysteine concentrations about 25% higher than people who are homozygous for the normal gene (CC),1 although the effect varies between populations because it is dependent on environmental factors such as dietary folate.8 The mutation is common (about 10% of people are TT)1 so it has been possible to study the risk of ischaemic heart disease and stroke in people with and without the mutation. These genetic studies avoid the confounding that could affect cohort studies; people with and without the mutation would not be expected to differ in other cardiovascular risk factors, and direct observation indicates that they do not.1 8 The studies are, in effect, natural randomised experiments, capable of testing whether moderately raised homocysteine causes ischaemic heart disease and stroke.
Two meta-analyses published in 2002 showed that TT homozygotes (with higher homocysteine concentrations) had about a 20% excess risk of ischaemic heart disease (P<0.001).1 8 Both groups interpreted their results as indicating causality, as did the authors of another meta-analysis, showing about a 25% excess risk of stroke.9 In 2005 Lewis and colleagues published an updated meta-analysis (80 studies) reporting a 14% higher risk of ischaemic heart disease in people who were TT compared with those who were CC (P<0.001) for an observed 2.2 µmol/l homocysteine difference between the two groups.2 This is equivalent to a 16% lower risk for a 3 µmol/l decrease in homocysteine, the same as our 2002 result.1 However, the authors concluded that their meta-analysis did not indicate causality because they suspected publication bias (smaller studies more likely to be reported if they had positive results than negative results) and because there was heterogeneity (greater variation between study results than would be expected through chance alone).
Publication bias
Lewis and colleagues suspected publication bias because of a positive Egger's test result (P=0.03). This is a test of significance of the slope of a regression of the size of an effect against the size of a study.10 An excess of small positive studies in relation to small negative ones (publication bias) can create a significant downward slope (a positive result), but this is not the only way a significant slope could arise. A few large studies that have odds ratios near 1.0 could have the same effect, even in the absence of publication bias. For example, studies in places with higher dietary folate would be expected to have odds ratios near 1.0 (because the effect of the mutation on increasing homocysteine will be reduced) and a few large studies of this nature could produce a significant slope in the absence of publication bias.
In any event, publication bias is not sufficient to explain the overall difference in risk observed between TT and CC homozygotes because the number of unpublished studies that would need to be invoked is implausibly large. In the 2005 meta-analysis, 11 of the 80 studies had significantly positive results and only one had significantly negative results. If there were no association between homocysteine and ischaemic heart disease, the probability of observing a significantly positive or negative association by chance (at the 5% level of significance) is 1 in 20, or 1 in 40 for positive results only. For publication bias to have accounted for the result, the 11 significantly positive studies would have to come from a pool of 440 studies (11×40). This would mean that only 18% (80/440) of the studies were published. Similarly, in the meta-analysis of stroke,9 five of the 30 studies had significantly positive results and none negative results; for publication bias to account for this result would require a pool of 200 (5×40) studies, of which only 30 (15%) were published. Although some unpublished studies may be expected, it is unlikely that so large a proportion of researchers would fail to publish their results.
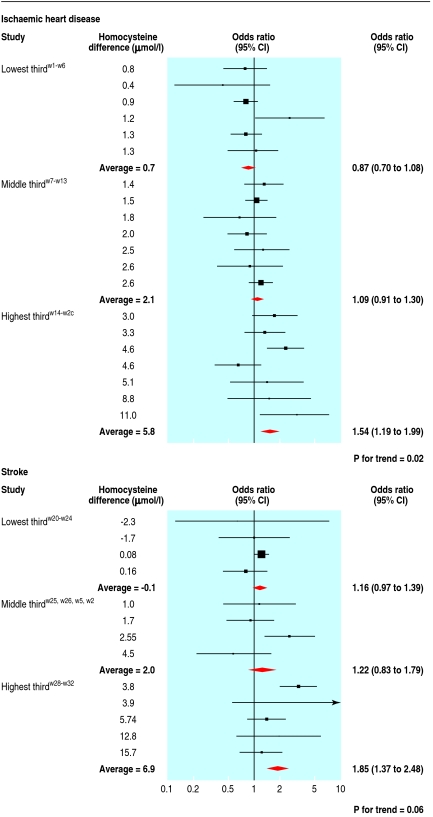
Additional evidence against the overall result being due to publication bias comes from the observation that studies in populations that had similar homocysteine concentrations in the TT and CC groups tended to find no difference in risk of ischaemic heart disease, whereas those in populations in which homocysteine was higher in the TT group than the CC group tended to find higher risk. Most of the genetic studies did not measure homocysteine, but the 19 studies from the 2005 ischaemic heart disease meta-analysisw1-19 and the 13 from the stroke meta-analysisw20-w32 that reported homocysteine concentrations show a dose-response relation (fig 1). The risk of ischaemic heart disease (P=0.02) and stroke (P=0.06) rose across tertile groups with increasing difference in homocysteine between the TT and CC groups. For publication bias to account for this observation would require a systematic failure to publish negative studies with large homocysteine differences and positive studies with small homocysteine differences, and both tendencies would have to occur in small rather than large studies. This is unlikely and can reasonably be excluded as an explanation.
Fig 1 Dose-response relation between odds ratio of ischaemic heart disease and stroke and difference in homocysteine concentrations between TT and CC homozygotes
Heterogeneity
Heterogeneity between the results from different studies is not surprising. Some populations (for example, those with a higher dietary folate intake) overcome the homocysteine raising effect of the TT genotype,8 so the TT genotype will increase risk of ischaemic heart disease less in studies of these populations than in others. Heterogeneity in this context is expected and was observed.1 2 8 9 Moreover, the heterogeneity is useful in that the dose-response relation between difference in homocysteine concentrations and ischaemic heart disease (fig 1) can be used as a test of causality and this favours a causal effect.
The heterogeneity does not mean that any preventive effect of folic acid would be limited to populations in which homocysteine concentrations differ between TT and CC homozygotes.2 Folic acid lowers homocysteine concentrations in people of both genotypes, its effect increasing up to 0.8 mg/day. In practical terms, folic acid supplementation will be expected to have a variable effect in preventing cardiovascular disease, with greater prevention in populations with relatively low folate intakes and less prevention in populations with higher folate intakes.
Randomised controlled trials
Randomised trials, although valuable, are not the only source of evidence on efficacy of interventions. In some situations they are not needed—for example, in establishing that stopping smoking prevents ischaemic heart disease and lung cancer. It is sometimes said that associations in epidemiological studies—for example, between antioxidant vitamins and ischaemic heart disease events11 12—have incorrectly been thought to be causal when subsequent randomised trials showed otherwise.13 However, confounding by socioeconomic status was acknowledged as a reasonable explanation for the observed association with antioxidant vitamins.11 12 Trials were needed because of legitimate doubt. In the case of homocysteine and ischaemic heart disease, the position is different; the evidence from cohort studies is supported independently by the genetic evidence from MTHFR polymorphism studies, which are not subject to such confounding, and the observations from patients with homocystinuria show that the risk is reversible.
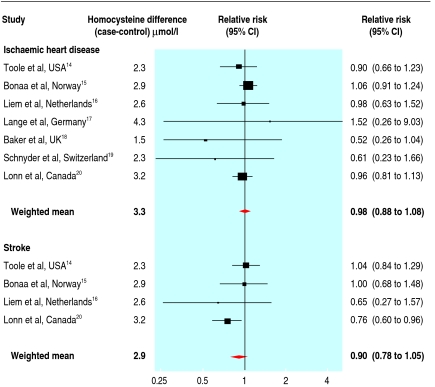
Randomised trials of the effect of reducing homocysteine concentrations on myocardial infarction and stroke are in progress, and some have been reported. Folic acid is expected to reduce cardiovascular disease events by only about 10-15% (compared, for example, to about an 80% reduction in neural tube defects from taking 5 mg folic acid daily). The modest effect and the relatively small number of events recorded in the published randomised trials (about 2000 compared to 32 000 in the meta-analyses of genetic studies) mean that the trials lack statistical power. Despite this, the reports from individual studies tend to inappropriately interpret non-significant effects as evidence of no effect. This is shown in figure 2 by the wide confidence intervals around the estimates from each trial in a meta-analysis of published trials of homocysteine reduction on disease events.14 15 16 17 18 19 20 Even with all the reported trial results together there is a lack of statistical power; the results are consistent with a 12% reduction in ischaemic heart disease and a 22% reduction in stroke (from the lower 95% confidence limits on the summary relative risk estimates) but also consistent with no reduction. If the only evidence available were the trial results, we would still be in the dark.
Fig 2 Meta-analyses of the randomised trials of lowering homocysteine concentrations on ischaemic heart disease and stroke events. In some cases the relative risk estimates in the figure are not identical to those published in the original papers because they are based on coronary deaths and non-fatal myocardial infarction only, without including endpoints such as angioplasty and coronary artery bypass surgery
In addition, some of the trials were short term (less than two years), and it is uncertain how long it would take for any risk reversal to emerge. With reductions in serum cholesterol concentrations it takes two years for the near maximal effect to become apparent.21 In the recent heart outcomes prevention evaluation (HOPE-2) trial, which showed a significant reduction overall in the risk of stroke, the published survival curve shows no reduction in risk of cardiovascular disease in the first two years but a modest risk reduction in the third and fourth years.20 We believe it is misleading to conclude that the results from trials such as HOPE-2 are negative.
An analogy exists with medical judgments made after the early randomised trials of treatment to reduce serum cholesterol concentrations. The early trials achieved only modest reductions in serum cholesterol and their duration was short; the two years necessary for the near maximal reduction in ischaemic heart disease events was not appreciated. Consequently, the modest risk reductions observed were not significant and were widely interpreted as negative. Cholesterol reduction was claimed to be harmful, and in 1992 a moratorium on the use of all cholesterol lowering drugs was suggested.22
Examining all the evidence together
The table shows the summary results from the meta-analyses of the cohort studies,1 the genetic polymorphism studies,2 9 and the randomised trials,14 15 16 17 18 19 20 the first two adjusted to the average homocysteine decrease of 3.3 µmol/l for ischaemic heart disease and 2.9 µmol/l for stroke, observed in the randomised trials. The cohort and genetic studies give similar results even though they do not share the same sources of error. The dose-response relation in the genetic studies is particularly relevant in suggesting a causal effect. The summary estimate from the trials is consistent with a short term protective effect of 12% on ischaemic heart disease events and 22% on stroke, or a larger long term effect.
Table.
Combining the evidence from the three types of meta-analyses (cohort, MTHFR and randomised trials) for IHD and stroke
| IHD | Stroke | ||||
|---|---|---|---|---|---|
| No. of studies | Relative risk*(95% CI) | No. of studies | Relative risk**(95% CI) | ||
| Observational data | |||||
| Cohort studies | 16 | 0.83 (0.78 to 0.89) | 11 | 0.79 (0.71 to 0.86) | |
| MTHFR studies | 80 | 0.79 (0.67 to 0.93) | 30 | 0.67 (0.56 to 0.82) | |
| Experimental data | |||||
| Randomised trials | 7 | 0.98 (0.88 to 1.08) | 4 | 0.90 (0.78 to 1.05) | |
*Relative risk for observational data expressed as odds ratio for 3.3 µmol/L homocysteine reduction (average effect in the trials)
**Relative risk for observational data expressed as odds ratio for a 2.9 µmol/L homocysteine reduction (average effect of in the trials)
The conclusion that homocysteine is a cause of cardiovascular disease explains the observations from all the different types of study, even if the results from one type of study are, on their own, insufficient to reach that conclusion. No single alternative explanation can account for all the observations. Since folic acid reduces homocysteine concentrations, to an extent dependent on background folate levels, it follows that increasing folic acid consumption will reduce the risk of heart attack and stroke by an amount related to the homocysteine reduction achieved. We therefore take the view that the evidence is now sufficient to justify action on lowering homocysteine concentrations, although the position should be reviewed as evidence from ongoing clinical trials emerges.
Summary points
Debate continues over whether raised serum homocysteine is a cause of ischaemic heart disease and stroke
Cohort and genetic polymorphism studies show a quantitatively similar association between decreased serum homocysteine concentrations and risk of heart disease and stroke, even though they are subject to different sources of error
Among the genetic polymorphism studies, those with the greatest difference in homocysteine concentration had the greatest difference in cardiovascular disease risk
Randomised trials are consistent with a short term protective effect but lack the statistical power to be conclusive
Taken together the evidence supports a modest protective effect of folic acid
Supplementary Material
Contributors and sources: All authors are involved in research into the causes and prevention of cardiovascular disease and have published and presented work on homocysteine, folic acid and cardiovascular disease. All the authors were involved in the ideas behind the paper and participated in the interpretation of the results and preparation of the submitted version. DSW is guarantor.
Competing interests: ML, NJW, and DSW have interests in a combined pill (polypill) to simultaneously reduce four cardiovascular risk factors including homocysteine. NJW and ML have patents (granted and pending) on the formulation of the polypill.
References
- 1.Wald DS, Law M, Morris J. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002;325:1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis SJ, Ebrahim S, Davey-Smith G. Meta-analysis of the MTHFR C to T polymorphism and coronary heart disease; does the totality of evidence support a causal role for homocysteine and the preventive potential of folate? BMJ 2005;331:1053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homocysteine Studies Collaboration. Homocysteine and the risk of ischemic heart disease and stroke. JAMA 2002;288:2015-22. [DOI] [PubMed] [Google Scholar]
- 4.Mudd SH, Skovby F, Levy H, Pettigrew KD, Wilcken B, Pyeritz RE, et al. The natural history of homocystinuria due to cystathionine β synthase deficiency. Am J Hum Genet 1985;37:1-31. [PMC free article] [PubMed] [Google Scholar]
- 5.Kluijtmans LAJ, Boers GHD, Kraus JP, van den Heuvel LP, Cruysberg JR, Trijbels FJ, et al. The molecular basis of cystathionine β synthase deficiency in Dutch patients with homocystinuria: effect of SBS genotype on biochemical and clinical phenotype and on response to treatment. Am J Hum Genet 1999;65:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap S, Naughten E. Homocysteinemia due to cystathionine β synthase deficiency in Ireland: 25 years experience of newborn screened and treated population with reference to a clinical outcome and biochemical control. J Inherit Metab Dis 1998;21:738-47. [DOI] [PubMed] [Google Scholar]
- 7.Wald DS, Law M, Morris JK. The dose-response relation between serum homocysteine and cardiovascular disease: implications for treatment and screening. Eur J Cardiovasc Prev Rehabil 2004;11:250-3. [DOI] [PubMed] [Google Scholar]
- 8.Klerke M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, et al. MTHFR 677 C to T polymorphism and risk of coronary heart disease. JAMA 2002;288:2023-31. [DOI] [PubMed] [Google Scholar]
- 9.Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet 2005;365:224-32. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Ascherio A, Giaovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328:1450-6. [DOI] [PubMed] [Google Scholar]
- 12.Stampfer MJ, Hennekens CH, Manson JT, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary heart disease in women. N Engl J Med 1993;328:1444-9. [DOI] [PubMed] [Google Scholar]
- 13.Heart Protection Study Collaborative Group. MRC/BHF heart protection study of antioxidant vitamin supplementation in 20 536 high risk individuals; a randomised placebo controlled study. Lancet 2002;360:23-33.12114037 [Google Scholar]
- 14.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death. JAMA 2004;291:565-75. [DOI] [PubMed] [Google Scholar]
- 15.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006;354:1578-88. [DOI] [PubMed] [Google Scholar]
- 16.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005;91:1213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med 2004;350:2673-81. [DOI] [PubMed] [Google Scholar]
- 18.Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial [abstract]. Circulation 2002;106(suppl II):2-741.12093758 [Google Scholar]
- 19.Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med 2001;345:1593-600. [DOI] [PubMed] [Google Scholar]
- 20.Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 2006;354:1567-77. [DOI] [PubMed] [Google Scholar]
- 21.Law MR, Wald NJ, Rudnicka A. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey Smith G, Pekkanen J. Should there be a moratorium on the use of cholesterol lowering drugs? BMJ 1992;304:431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.