Abstract
Cadherin-mediated cell–cell adhesion is dynamically modulated during epithelial–mesenchymal transition triggered by activation of receptor tyrosine kinases (RTK) in epithelial cells. Several cadherin-binding proteins have been identified that control cell–cell adhesion. However, the mechanisms by which intercellular adhesion and cell motility are coregulated are still unknown. Here, we delineate a hitherto uncharted cooperation between RTKs, RhoA GTPase, and p120 catenin in instructing a motile behavior to epithelial cells. We found that expression of an N-terminus–deleted p120 catenin in a variety of epithelial cell types, including primary keratinocytes, effectively competes for endogenous p120 at cadherin binding sites and abrogates EGF-stimulated cell motility as well as HGF-induced cell scattering. The deleted mutant also inhibits the PI3K-dependent RhoA activation ensuing receptor activation. Conversely, we also show that the ectopic expression of full-length p120 in epithelial cells promotes cytoskeletal changes, stimulates cell motility, and activates RhoA. Both motogenic response to p120 and RhoA activation require coactivation of signaling downstream of RTKs as they are suppressed by ablation of the Ras/PI3K pathway. These studies demonstrate that p120 catenin is a necessary target of RTKs in regulating cell motility and help define a novel pathway leading to RhoA activation, which may contribute to the early steps of metastatic invasion.
INTRODUCTION
During development and wound healing, polarized epithelial cells can undergo epithelial-mesenchimal transition (EMT), a morphogenetic program characterized by loss of the epithelial phenotype, disassembly of cadherin-containing adherens junctions (AJs), and enhanced cell motility. EMT is normally instructed by external cues, such as growth factors, through not well-identified pathways and its aberrant regulation is thought to contribute to cancer progression and metastasis (Thiery, 2002).
Cadherins comprise a large family of cell–cell adhesion molecules that are involved in the social behavior of cells (Takeichi, 1990; Yap et al., 1997; Yagi and Takeichi, 2000) and appear well suited to respond to extracellular stimuli. Deregulation of cadherin-mediated cell–cell adhesion can result in EMT and increased cell motility (Birchmeier and Behrens, 1994; Thiery, 2002). The extracellular domain of classical cadherins, such as E-cadherin, mediates calcium-dependent homophilic interactions, whereas the intracellular domain interacts with catenins, which anchor the adhesion complex to the actin cytoskeleton and regulate the strength of cell–cell adhesion (reviewed in Gumbiner, 2000). Both phosphorylation events and activity of Rho GTPases have been implicated in cell–cell adhesion dynamics. Activation of RTKs, including the epidermal growth factor receptor (EGFR) and the hepatocyte growth factor (HGF) receptor (Met), as well as of v-Src tyrosine kinase induces dissociation of epithelial cell clusters and the concomitant tyrosine phosphorylation of cadherin-complex components (Shibamoto et al., 1994; Reynolds et al., 1994; Kinch et al., 1995; Daniel and Reynolds, 1997; Cozzolino et al., 2000). There is much evidence correlating cadherin dysfunction to unscheduled tyrosine phosphorylation of Armadillo catenins; however, the relationship between phosphorylation of catenins and strength of adhesion remains ill defined (Daniel and Reynolds, 1997; Cozzolino et al., 2000).
The small GTPases of the Rho family (including RhoA, Rac, and Cdc42) mediate the formation of specific actin-containing structures and are key determinants of cytoskeletal rearrangements (van Aelst and D'Souza-Schorey, 1997; Hall, 1998). Studies mainly carried out in fibroblasts have shown that the balance between the activities of all three GTPases is responsible for the orchestration of cell polarity and cell locomotion (Nobes and Hall, 1999; Ridley, 2001). More recently, Rho GTPases have also emerged as crucial regulators of epithelial cadherin-based cell–cell adhesion (reviewed in Braga, 2000; Fukata and Kaibuchi, 2001). Two distinct pathways have been delineated with counteracting effects on AJs: RhoA can signal through ROCK to disrupt AJs or through mDia to stabilize components of AJs to cell periphery (Sahai and Marshall, 2002). In polarized epithelial Madin Darby canine kidney (MDCK) cells Tiam1, an activator of Rac, promotes the formation and maintenance of E-cadherin–mediated adhesion on most extracellular matrix substrates, thereby inhibiting the migration of epithelial cells (Sander et al., 1999), thus suggesting that AJ dynamics and cell migration are intimately connected. This appears to be critical during carcinogenesis because a defective cell–cell adhesion has been correlated with the progression of epithelial tumors (Vleminckx et al., 1991; Takeichi, 1993; Birchmeier and Behrens, 1994; Perl et al., 1998).
p120 catenin is a prototypical member of a subfamily of Armadillo repeat domain proteins involved in intercellular adhesion (Anastasiadis and Reynolds, 2000). p120 binds to the membrane-proximal region of cadherin cytoplasmic tail and convincing evidence indicates that it can mediate strong cell–cell adhesion by positive regulation of cadherin clustering (Yap et al., 1998; Thoreson et al., 2000). Importantly, posttranscriptional modifications appear to affect p120 function and “transform” it into a negative regulator of cadherin-mediated adhesiveness (Ozawa and Kemler, 1998; Aono et al., 1999). p120 is phosphorylated on several defined tyrosine residues and on a still imprecisely defined number of serine residues (Mariner et al., 2001; Ozawa and Ohkubo, 2001). Intriguingly, all phosphorylated tyrosine residues and the majority of p120 serine phosphorylation sites are located amino terminal to the Armadillo, suggesting that most regulatory modifications of p120 function are likely to occur within this domain (Aono et al., 1999; Mariner et al., 2001). In support of this possibility, deletion of the amino terminal portion of p120 appears to abrogate a negative regulation of cell–cell adhesion by hyperphosphorylated p120 (Aono et al., 1999). Finally, recent evidence shows that overexpression of p120 in fibroblastic cell lines affects the activation state of Rho GTPases (Anastasiadis et al., 2000; Noren et al., 2000) and that these changes correlate with an enhanced motile phenotype. (Grosheva et al., 2001).
Here, we have addressed the questions of whether in epithelial cells p120 participates in regulating the balance between an adhesive and a motile phenotype and whether it cooperates with signaling pathways originated from motogenic stimuli.
MATERIALS AND METHODS
Materials
EGF was from Upstate Biotechnology (Lake Placid, NY), and human HGF, PD9859 and LY294002 were from Calbiochem (La Jolla, CA). Tissue culture media and sera were from Life Technologies (Grand Island, NY). The following antibodies were used: anti-RhoA and anti-Cdc42 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-E-cadherin, polyclonal anti–α-catenin, and anti-FLAG (Sigma, St. Louis, MO); anti-p120, anti–β-catenin, and anti-Rac (Transduction Labs, Lexington, KY); anti-Akt and anti–phospho-Akt (New England Biolabs, Beverly, MA); and anti-Src 327 monoclonal antibodies (provided by J. Brugge). Anti–cMyc (9E10) mAb was provided by C.J. Marshall. Secondary antibodies (FITC- and TRITC-conjugated donkey anti-rabbit, anti-goat, and anti-mouse antibodies) were from Jackson ImmunoResearch (West Grove, PA). Cytotoxic necrotizing factor 1 (CNF1) was purified as previously described (Falzano et al., 1993). Cell-permeable bacterial Tat-C3 was generated by inserting a C3 cDNA into the pTAT vector (Becker-Hapak et al., 2001) and purified by sonication in 8 M urea followed by passage over a Ni-NTA column (Qiagen, Hilden, Germany), desalted into phosphate-buffered saline (PBS), and then flash frozen in 10% glycerol and stored at –80°C. Purified Tat-C3 protein was added to a final concentration of 50 nM to cells in complete medium.
Cell Culture
Primary keratinocytes were isolated from newborn C57BL/6 mice and cultured in medium with low-calcium concentration (0.05 mM CaCl2; low-calcium medium) and EGF as described previously (Calautti et al., 1998). MDCK epithelial cells, kindly provided by F. Tatò and W. Birchmeier; NIH3T3 cells and 293 human embryonal kidney epithelial cells were propagated in Dulbecco's modified minimal essential medium (DMEM) containing 10% (vol/vol) fetal calf serum. MDCK clones stably expressing FLAG-p120 alleles were established by transfection, selected in hygromycin, and screened by immunofluorescence and Western blot. The cDNAs for FLAG-tagged p120 and ΔN-p120 alleles (Aono et al., 1999) were provided by M. Takeichi (Kyoto, Japan).
Adenoviral Infection
Recombinant adenovirus expressing GFP and lacZ (encoding a nuclear-targeted-galactosidase; supplied by M. Crescenzi), p120/FLf, and p120/N346f (Aono et al., 1999; supplied by M. Takeichi), RasN17 (supplied by L. Parada), KI-Src (kinase inactive form of chicken c-Src; supplied by B. Berk), and RacV12 (supplied by G. Tarone) were propagated in 293 cells as described in Latella et al. (2001). Construction of Ad-RacN17 was carried out by inserting a myc-tagged RacN17 mutant (supplied by A. Hall) into the AdEasy-CMV driven vector (Stratagene, La Jolla, CA). Transient transfections of 293 cells were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Viral titer was determined by plaque formation assay using 293 cells and was expressed as plaque-forming units (pfu; Latella et al., 2001). We routinely obtained recombinant adenoviruses with titers in the range of 108-109 pfu/ml.
Infection of MDCK cells was carried out for 1 h; after virus removal, cells were grown for additional 24 h before being subjected to further experimental manipulations. To optimize the protocol for MDCK infection, MDCK cells were infected with Ad-lacZ and Ad-p120, and expression was measured by immunoblotting and immunofluorescence with specific antibodies. There was ∼90% infection at a multiplicity of infection (m.o.i.) of 50 after infection for 48 h. The ratio of expressed p120/ΔN346f to endogenous p120 was 5:1 at an m.o.i. of 50. In all cases, infection of primary keratinocytes was performed for 1 h in low-calcium medium; keratinocytes were then incubated in low-calcium medium and analyzed 36 h postinfection; switch to high-calcium medium (2 mM) was for 12 h. Given the high susceptibility to adenoviral infection exhibited by keratinocytes (Ganly et al., 2000), an m.o.i. of 15–30 was sufficient for a 100% infection of keratinocytes.
Cell Migration Assay
Time-lapse Video Microscopy and Motion Analysis. A Zeiss Axiovert-35 microscope equipped with a JVC digital CCD camera and the IAS2000 software (Deltasistemi, Rome, Italy) was used to take images every 5 min for an observation period of 12–18 h. Applying the “visualize” mode, these series of photographs were displayed as continuous time-lapse movies. Cells were seeded the day before recording into uncoated 25-ml T-flasks. Temperature was adjusted to 37°C with a Peltier apparatus and the medium was buffered with 15 mM HEPES, pH 7.2. A 50× or 80× magnification was applied to investigate a large area to obtain the required number of cases for a representative statistical analysis. To generate migration tracks, the position of the nucleus of individual cells on each image was marked. The migratory speed was calculated based on the sum of distances divided by the time of observation. For each experimental condition, migration of at least 150 cells was analyzed and presented as mean ± SEM. Because descriptive analysis showed that frequency distributions of migration speed values differed from normal distribution, a nonparametric statistical method was used to analyze the data. The significance of differences between populations of data were assessed according to Mann-Whitney rank test with a level of significance of at least p < 0.01.
Immunochemical Procedures
Cell Extraction and Immunoprecipitation For coimmunoprecipitation analysis, cells were solubilized with a 0.5% NP-40 containing CSK extraction buffer (10 mM Pipes buffer, pH 6.8, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 1 mM Na3VO4, 10 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin) for 30 min at 4°C and lysates were clarified by centrifugation at 10,000 × g for 10 min at 4°C. Equal amounts of lysates were incubated at 4°C for 2 h with the appropriate antibodies and the immunocomplexes were collected by binding to either protein A- or protein G-Agarose beads (Roche, Monza, Italy), followed by three washes with 0.5% NP-40 containing extraction buffer. For detection of phospho-Akt cells were lysed on ice in NP-40 buffer (50 mM TrisCl, pH 7.4, 1% NP-40, 15% glycerol, 200 mM NaCl, 5 mM MgCl2) also containing protease inhibitors (10 μg leupeptin/ml, 10 μg aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride, 10 μg pepstatin A/ml). Lysates were cleared by centrifugation, and protein concentrations were determined. Cleared cell lysates were analyzed by immunoblotting with antiphosphorylated Ser 473 or Thr 308 Akt polyclonal antibodies and anti-Akt. For total protein analysis, cell lysates were alcohol-precipitated (ethanol, methanol, acetone, and water, 4:2:2:2) and resuspended in Laemmli sample buffer. The protein concentration of clarified extracts was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Immunoblotting. Total cell extracts or washed immune complexes were boiled for 5 min in Laemmli sample buffer, resolved in SDS-polyacrylamide gels, and electrophoretically transferred to Hybond C-Super nitrocellulose paper (Amersham, Piscataway, NJ). For Western blot analysis, filters were incubated in Tris-buffered saline (TBS) solution containing 0.1% Tween 20 and 5% nonfat milk. Filters were then incubated for 2 h at room temperature with the appropriate antibodies diluted in a TBS also containing 0.1% Tween 20 and 2% nonfat milk. After extensive washing in TBS solution, filters were incubated with either horseradish peroxidase–conjugated anti-mouse or anti-rabbit IgGs (Amersham). The filters were washed as above and developed using the POD chemiluminescence detection system (Roche).
Immunofluorescence. Cells grown on 35-mm dishes were fixed at room temperature for 10 min with 3.7% paraformaldehyde and permeabilized with 0.2% Triton X-100. Cells were stained for α-catenin as previously described (Cozzolino et al., 2000); F-actin was visualized with TRITC-phalloidin. Cells were visualized using a Leitz microscope equipped with 50× oil-immersion objectives. Fluorescence images were recorded on a CCD camera and processed using a Deltasistemi software and Adobe Photoshop.
Rho Family Pull-down Assay
The RhoA-GTP pull-down assay was as in Ren et al. (1999); briefly, cells were plated in 10-cm dishes, serum-starved for 12 h, and lysed in 50 mM TrisCl, pH 7.2, 500 mM NaCl, 1% (v/v) Triton X-100, 5 mM MgCl2, 1 mM dithiothreitol (DTT), and protease inhibitors. One fiftieth of cell lysates was subjected to immunoblotting. Cell lysates were mixed with 10 μg of bacterially expressed GST–Rhotekin (murine amino acids 7–89) bound to glutathione–sepharose and incubated at 4°C with tumbling for 30 min. Beads were collected by centrifugation and washed twice in 50 mM Tris, pH 7.2, 150 mM NaCl, 1% (v/v) Triton X-100, 5 mM MgCl2, and 1 mM DTT before addition of Laemmli buffer and analysis by Western blot with anti-RhoA antibody. The Rac-GTP and Cdc42-GTP pull-down assay was as described in Zondag et al. (2000). Cell lysis and washes were done with 50 mM Tris, pH 7.2, 100 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10% glycerol, 1% NP-40, plus protease inhibitors. Glutathione S-transferase (GST)–PAK (rat PAK amino acids 1–252) was used in place of rhotekin.
RESULTS
p120 Acts in Concert to EGF in Stimulating a Migratory Phenotype in Primary Keratinocytes
Previous studies showed that, when transfected in fibroblasts, p120 influences the actin cytoskeleton and induces a motile behavior (Anastasiadis et al., 2000; Noren et al., 2000; Grosheva et al., 2001). These observations prompt the question of how this experimental paradigm applies to epithelial cells, in which cadherin-based cell–cell adhesion is of paramount importance to tissue formation. To this aim, mouse primary keratinocytes provide a well-defined model for the study of the assembly and regulation of AJs (Vasioukhin et al., 2000). Keratinocytes were infected with recombinant adenoviruses carrying either a full-length p120cnt1 isoform (Ad-p120) or the deletion mutant ΔN346f/p120 (Ad-ΔN-p120), both conveniently Flag-tagged (Aono et al., 1999). The ΔN346f mutant lacks the NH2 terminal 346 aa, but keeps the Armadillo repeat domain intact (Aono et al., 1999) and is identical to the p120ctn4 isoform (Keirsebilck et al., 1998; Aho et al., 2002). Adenoviruses carrying GFP or lacZ were used as controls. By infecting at an m.o.i. of 15–30, we routinely observed expression in 90–100% of the cells in culture with no apparent cytotoxicity.
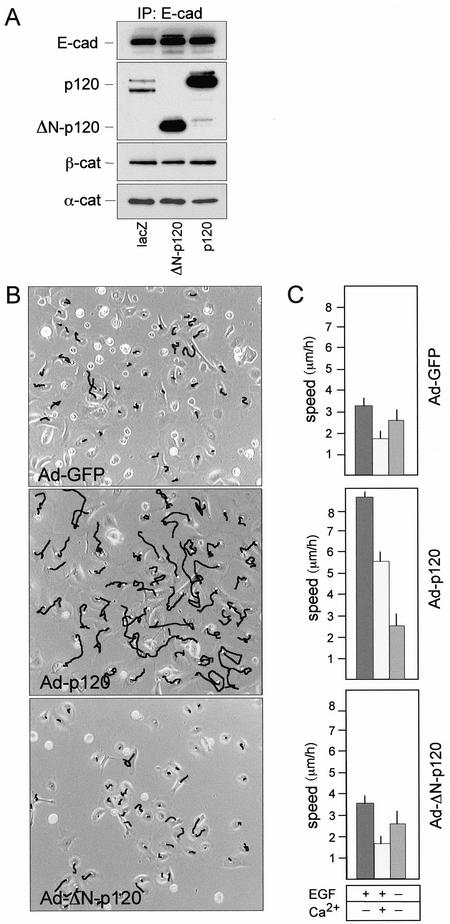
For direct biochemical determination of components of cadherin-complexes, infected cells were processed for immunoprecipitation with E-cadherin antibodies followed by sequential immunoblotting with antibodies against p120, β-catenin, and α-catenin (Figure 1A). Both forms of p120 bound efficiently to cadherins without interfering significantly with the binding of β- and α-catenins. ΔN-p120 expression did not affect levels of endogenous p120 isoforms but efficiently competed with endogenous p120 for binding to cadherins (Figure 1A), demonstrating that the N-terminus deleted form is not impaired in its affinity for cadherins. Intriguingly, the cadherin-bound pools of ectopic p120 protein species were much more conspicuous than the endogenous one (Figure 1A), suggesting that in these cells the overexpressed proteins can saturate preexisting cadherin binding sites or somewhat induce a state of higher affinity for E-cadherin.
Figure 1.
p120 catenin induces cell migration in primary keratinocytes. (A) Primary mouse keratinocytes were either infected with Ad-lacZ or infected with the Ad-p120 or Ad-ΔN-p120 viruses at a similar m.o.i. (30 pfu/cell). Total cell extracts were prepared 36 h after infection and immunoprecipitated with anti–E-cadherin antibody. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with monoclonal antibodies to E-cadherin, p120, β-catenin, and α-catenin, as indicated. Note that in immunocomplexes from cells infected with Ad-lacZ two bands, corresponding to endogenous p120 isoforms, are clearly resolved. (B) Representative frames from cell locomotion analysis of keratinocytes either infected with Ad-GFP or infected with the Ad-p120 or Ad-ΔN-p120 viruses. Mouse primary keratinocytes cultures were plated in low-calcium medium and infected at a similar m.o.i. (30 pfu/cell). After 36 h, cultures were analyzed by time-lapse video microscopy; migratory paths of cells filmed for 4 h are shown. (C) Quantitative analysis (see MATERIALS AND METHODS) of cell migratory activities in the absence or the presence of 10 ng/ml EGF and calcium ions is reported in right panels. Cultures were shifted to a high-calcium medium or to a medium depleted of EGF 24 h postinfection and migration measured over a 12–16-h treatment. The data are representative of five separate experiments. Migration of at least 150 cells was analyzed and presented as mean ± SEM
To determine cell migration we used time-lapse video microscopy and quantitative analysis the migratory paths of individual cells (Figure 1B). Examination of cell migratory activity (Figure 1C) showed that primary keratinocyte in low-calcium media moved randomly with an average velocity of 3.2 ± 0.3 μm/h. On differentiation in the presence of calcium the velocity of random migration dropped to 1.8 ± 0.3 μm/h (p < 0.001; Figure 1C). Keratinocytes expressing full-length p120 exhibited a large increase in random migration both in the absence and in the presence of calcium ions (p < 0.001; Figure 1C). Basal cell locomotion was moderately growth-factor–dependent, as it decreased to 2.5 ± 0.5 μm/h when cultures were deprived of EGF (p < 0.01; Figure 1C). Strikingly, the increase in cell motility induced by p120 was fully inhibited upon removal of EGF (Figure 1C). Migration of ΔN-p120 cells was indistinguishable from control cells under all conditions (Figure 1C).
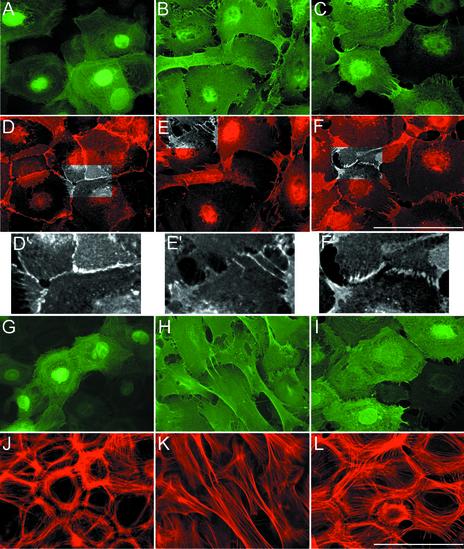
A polarized epithelial morphology developed upon switch to high-calcium media and became evident after 12–16 h (Figure 2). The keratinocytes, originally more fibroblast-like in shape, adopted a polygonal shape, assembled the actin cytoskeleton in circumferential rings and sparse bundles of stress fibers and organized well-sealed adherens junctions as evidenced by staining for α-catenin and F-actin shown in Figure 2, D, and D′, and J. p120-keratinocytes did not form epithelial sheets even after prolonged incubation in high-calcium media. In these cells, α-catenin and p120 staining was rarely observed at membrane borders and did not evidence any discernible AJ; rather, intercellular contacts were substituted by loosely interdigitating membrane blebs and filopodia (Figure 2, B, E, and E′). Overall, the actin cytoskeleton seen in p120-keratinocytes, consistent essentially of an extensive array of longitudinal actin bundles, was reminiscent of that of motile cells (Figure 2K). Expression of ΔN-p120 in keratinocytes in high-calcium media permitted the formation of epithelial sheets and the organization of the actin cytoskeleton was largely comparable to that of GFP-expressing cells (Figure 2L). Nevertheless, they showed adhesive defects since gap regions were visible between adjacent cells and intercellular junctions in most infected cells were formed by double rows of α-catenin-positive puncta (adhesion zippers; Vasioukhin et al., 2000), which apparently could not progress into well-organized, sealed single rows of puncta (Figure 2, F and F′). These defective contacts also appear to contain little ΔN-p120, whose peripheral localization (see Figure 1A) is dulled by its high levels of expression (Figure 2I). The phenotype imposed by ΔN-p120 on primary keratinocytes is that expected if p120 regulates clustering of cadherins (Anastasiadis and Reynolds, 2000; Gumbiner, 2000) and Δ 78-p120 acts as a dominant-negative of p120 at AJs by competing with endogenous p120 for binding to cadherins.
Figure 2.
The forced expression of p120 results in distinct cytoskeletal changes in primary keratinocytes. Mouse primary keratinocytes were either infected with Ad-GFP (A, D, G, and J) or infected with the Ad-p120 (B, E, H, and K) or Ad-Δ 78-p120 (C, F, I, and L) as described in Figure 1 and 24 h postinfection switched to 2 mM calcium containing medium to stimulate AJ formation. After 12 more hours, cultures were fixed and stained with anti-Flag (B, C, H, and I) to visualize expression of Δ 78-p120; GFP was detected by epifluorescence (A and G). Cultures were also stained with anti–α-catenin (D, E, and F) to visualize cell–cell junctions or with TRITC-phalloidin (J, K, and L) to decorate F-actin. Insets in D′, E′, and F′ are higher magnifications of areas highlighted in gray scale to show AJs. Note that in E′ well-sealed α-catenin junctions (D′) have been substituted by areas of intercellular separations or loosely interdigitating membrane blebs; in F′ areas of intercellular contact show aberrant junctions, reminiscent of unsealed adhesion zippers. The data are representative of five separate experiments. Scale bar, 20 μm.
Expression of Δ 78-p120 in MDCK Cells Prevents HGF-induced Motility and Scattering
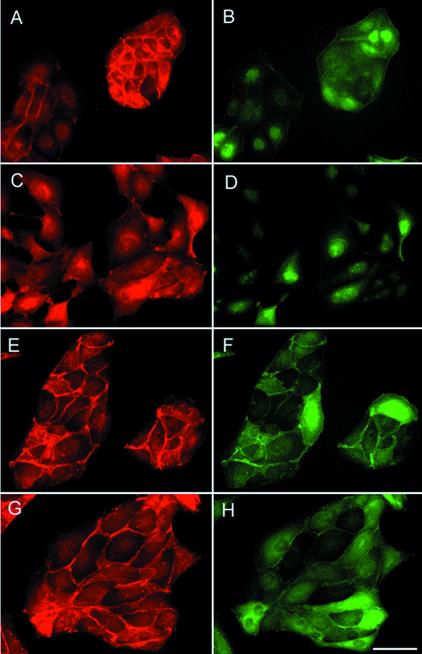
The scattering induced by HGF in MDCK cells is a well-established experimental paradigm to study the mechanisms underlying EMT (Thiery, 2002; Trusolino and Comoglio, 2002). Moreover, some of the signaling pathways used by HGF/Met to induce scattering have been delineated in these cells (Ponzetto et al., 1994; Ridley et al., 1995; Royal et al., 2000; Zondag et al., 2000). To confirm the growth factor dependence of p120-induced cell migration observed in keratinocytes (Figure 1C), and to dissect the biochemical steps downstream of p120, we introduced p120 and Δ 78-p120 into MDCK cells by either adenoviral infection or transfection and isolation of cell clones. Infection of MDCK cells with the adenoviruses described above resulted in >90% of infection, as assessed by immunofluorescence from GFP (Figure 3, B and D) and with anti-Flag (Figure 3, F and H). The amount of exogenous p120 protein was measured by immunoblotting of total lysates with anti-p120 antibodies and averaged to ∼5-fold over the endogenous one (our unpublished results). Subconfluent cultures of control GFP-MDCK cells exhibited a typical cobble-stone morphology and formed round colonies. In these cells α-catenin (Figure 3A) and E-cadherin (Figure 4A) were localized at adherens junctions displaying a characteristic honeycomb pattern. F-actin localized mainly at the periphery of the cells in circumferential rings (Figure 4B). MDCK cells expressing Δ 78-p120 appeared well spread onto the substrate but the overall morphology was similar to control cells; immunofluorescence analysis identified E-cadherin (Figure 4C) and α-catenin at cell–cell contact sites, where it largely colocalized with Δ 78-p120 protein (Figure 3, E–H). AJs in Δ 78-p120-MDCK cells exhibited discrete morphological defects, but this trait was not further investigated.
Figure 3.
An N-terminus–deleted allele of p120 catenin inhibits HGF-induced cell scattering in MDCK cells. MDCK cells were either infected with Ad-GFP as control (A–D) or Ad-Δ 78-p120 (E–H) at a similar m.o.i. (50). Twenty-four hours later cultures were left untreated (A, B, E, and F) or treated with 10 ng/ml HGF (C, D, G, and H) for 12 h. After fixation, cells were stained with anti–α-catenin (A, C, E, and G) to visualize adherens junctions or with anti-Flag (F and H) to visualize expression of Δ 78-p120; GFP was detected by epifluorescence (B and D). The data are representative of four separate experiments. Scale bar, 20 μm.
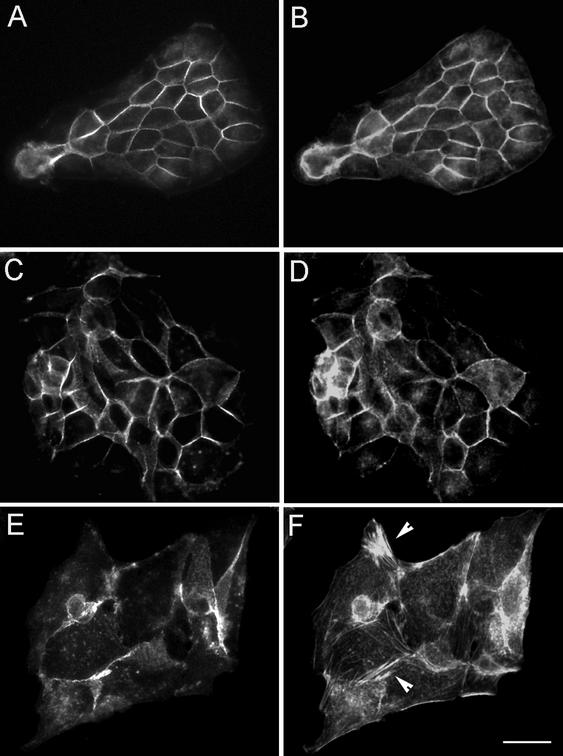
Figure 4.
p120 promotes the loss of polarized localization of E-cadherin and F-actin in MDCK cells. MDCK cells were either infected with Ad-GFP (A and B) or infected with the Ad-Δ 78-p120 (C and D) or Ad-p120 (E and F) as described in Figure 3. Forty-eight hours postinfection, cells were fixed and either stained with anti–E-cadherin (A, C, and E) to visualize cell–cell junctions or with TRITC-phalloidin (B, D, and F) to decorate F-actin. Note in F the de novo formation of intracellular clusters of F-actin filaments (arrowhead). Scale bar, 20 μm.
Control MDCK cells lost their organization as compact cell clusters within 4–12 h of treatment with HGF and adopted a highly motile behavior. Scattering was accompanied by a redistribution of junctional markers that acquired an intracellular localization (Figure 3C). In contrast, HGF-scattering of Δ 78-p120 MDCK cells was markedly prevented (Figure 3G) and cells retained their junctional relationships as visualized by α-catenin antibody labeling (Figure 3G) and E-cadherin (Figure 4C).
Ad-p120–infected cells (Figure 4, E and F) and p120-expressing clones (our unpublished results) lost a polygonal morphology, appeared flattened, and displayed irregular shapes; this was accompanied by loss of peripheral localization of E-cadherin (Figure 4E), dissolution of the actin circumferential rings and de novo formation of thin F-actin fibers and asters of F-actin scattered in the cytoplasm (Figure 4F). Only very rarely did they assume the dendritic phenotype described in acutely transfected fibroblasts (Anastasiadis et al., 2000; Grosheva et al., 2001; Aho et al., 2002).
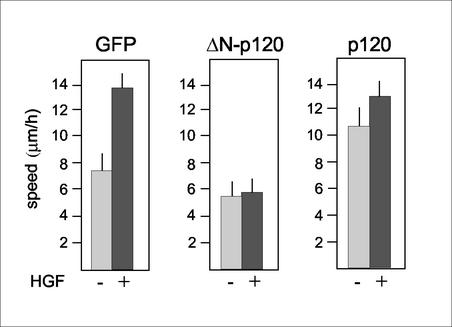
In cell motility assays, Ad-GFP-MDCK cells exhibited a basal rate of 7.3 ± 0.8 μm/h that nearly doubled upon exposure to HGF for 8–12 h (p < 0.0001; Figure 5). Again, competition of endogenous p120 by Δ 78-p120 was accompanied by suppression of HGF-induced cell migration (p < 0.001; Figure 5). A severe reduction in HGF-stimulated cell scattering was also measured in MDCK clones derived from Δ 78-p120–transfected cells (our unpublished results). Importantly, the inhibitory effect of Δ 78-p120 was not confined to the HGF/MDCK cell model as it inhibited EGF-stimulated cell motility in EGFR-expressing cells, including A431 and NIH3T3-EGFR cell lines (our unpublished results). As described in keratinocytes, the basal rate of migration was increased by overexpression of full-length p120 in MDCK cells (p < 0.0001) and was further increased in the presence of HGF (Figure 5). It is worth noting that p120 was unable to stimulate motility in MDCK cells under serum-free conditions, further suggesting that its effect is not autonomous and requires cooperation with extracellular cues (our unpublished results). Altogether, these data demonstrate that p120 is required for disruption of epithelial integrity and cell migration induced by HGF and assign this function to the N-terminus region of the p120 protein.
Figure 5.
Effect of p120 and Δ 78-p120 expression on the motile phenotype of MDCK cells. MDCK cells infected with the indicated recombinant adenoviruses as described in Figure 2 were analyzed by time-lapse video microscopy. A quantitative analysis of cell migratory activities in the absence or presence of 10 ng/ml HGF is shown. Note that basal cell motility is increased in full-length p120-infected cells, whereas it is diminished in Δ 78-p120–expressing cells.
p120 Activates RhoA in a Ras-dependent Manner
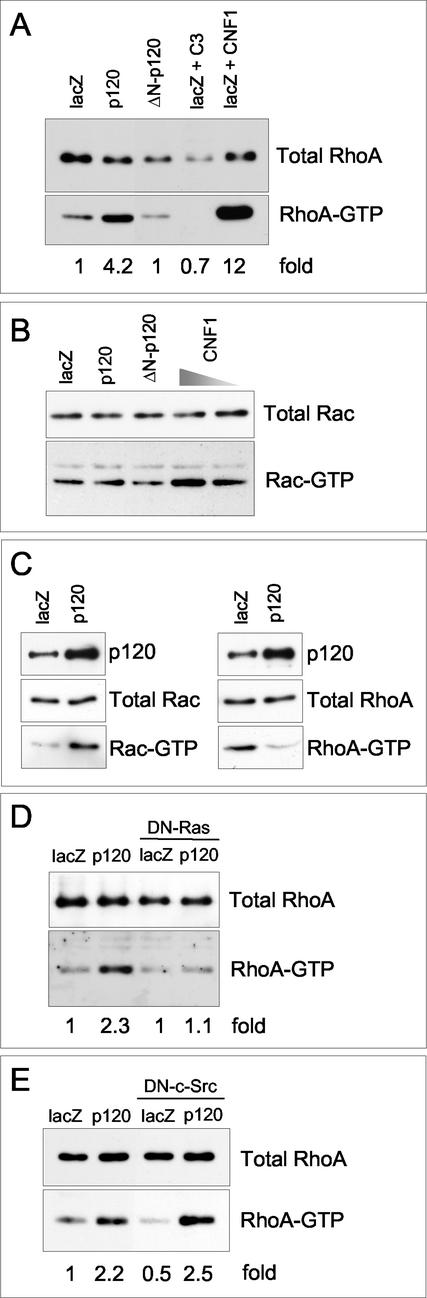
Previous studies have indicated that Rho A is actively involved in cell migration (reviewed in Ridley, 2001) and in AJ assembly (Fukata and Kaibuchi, 2001; Sahai and Marshall, 2002). Interestingly, Rho proteins have been shown to be modulated by p120 when overexpressed in fibroblasts (Anastasiadis et al., 2000; Noren et al., 2000; Grosheva et al., 2001). To determine whether endogenous RhoA GTPase activity is regulated upon forced expression of p120 and Δ 78-p120 in epithelial cells, we first measured RhoA-GTP in keratinocytes under basal conditions by pull-down assay using the Rho-binding domain of rhotekin as bait (Ren et al., 1999). In these cells, RhoA activity was constitutively increased by full-length p120 but not by Δ 78-p120 (Figure 6A). The sensitivity of the assay was validated by pretreatment of Ad-lacZ-keratinocytes with either a cell-permeable Tat-C3 transferase toxin, which selectively inactivates RhoA or with low concentrations of cytotoxic necrotizing factor 1 (CNF1), which mainly activate RhoA (Falzano et al., 1998; Figure 6, A and B). The forced expression of p120 and Δ 78-p120 had no discernible effect on basal activation of Rac (Figure 6B) and Cdc42 GTPases, when analyzed with the Rac/Cdc42-binding domain of Pak1 (Sander et al., 1999). Comparison of the levels of activated Rho proteins in NIH-3T3 murine fibroblasts infected with the same protocols revealed strong augmentation of Rac1 activity and a downregulation of basal RhoA activity (Figure 6C). Infection with Ad-Δ 78-p120 caused no discernible differences in Rho proteins activity (our unpublished results). Thus, p120 ability of influencing the activity of Rho GTPases appears to be cell context dependent.
Figure 6.
p120 catenin induces RhoA activation in epithelial cells. Levels of active GTP-bound RhoA (A) or GTP-bound Rac (B) were measured in primary mouse keratinocytes infected with Ad-p120 (m.o.i., 50), Ad-Δ 78-p120 (m.o.i., 50) or with Ad-lacZ (m.o.i., 50) as a control. Ad-lacZ-keratinocytes were either left untreated or were treated with Tat-C3 transferase (50 nM) and CNF1 for 8 h before pull-down assays. CNF1 was used at 0.1 nM concentrations to activate specifically RhoA and at 10-fold higher concentrations to also stimulate Rac. Activity of Rho family GTPases was assayed as described in MATERIALS AND METHODS using GST fusion proteins derived from either Rhotekin RBD or PAK RBD. Pull-downs were immunoblotted with mAbs recognizing specifically the various family members. Total cell lysates were also blotted to compare levels of endogenous Rho GTPases in infected cells. The numbers represent fold stimulation above normalized activity in control lacZ-expressing cells (referred to as 1); densitometric scanning of the films was used to determine relative levels of RhoA activation vs. protein amounts. (C) Levels of RhoA-GTP and Rac-GTP were assayed in NIH3T3 fibroblasts infected with Ad-p120 or with Ad-lacZ and processed as in panels A and B. An anti-p120 mAb was used to monitor endogenous and ectopic p120 protein species in total lysates. (D and E) Levels of RhoA-GTP were also measured in keratinocytes infected with Ad-lacZ, Ad-p120 alone or in combination with similar m.o.i. (50/50) of either Ad-RasN17 (DN-Ras) or Ad-KI-Src (DN-c-Src) and processed as described above. The data are representative of three separate experiments.
To investigate the function of RhoA signaling in p120-promoted cell remodelling and migration, we treated keratinocytes with Tat-C3 and CNF1 to either inhibit or activate RhoA. In C3-treated cells, inhibition of RhoA was accompanied by the disruption of junctional localization of α-catenin, a dramatic reduction of F-actin and a severe inhibition of both basal and p120-induced cell motility (our unpublished results). Keratinocytes treated with CNF1 at concentrations (0.1 nM) that only affected levels of RhoA-GTP showed an overall increase of F-actin and an augmented cell motility rate (9.0 ± 1.1 μm/h).
Next, given the observed growth factor dependence of p120-stimulated cell migration and previous studies showing that activation of RhoA by RTKs is Ras dependent (reviewed in Bar-Sagi and Hall, 2000), we asked whether in order to activate RhoA p120 was cooperating with pathways downstream of RTKs. To this aim keratinocytes were coinfected with Ad-p120 and either a recombinant adenovirus encoding a dominant-negative RasN17 allele (Figure 6D) or an adenovirus encoding a kinase-inactive c-Src mutant (Figure 6E), which had been shown to inhibit c-Src functions (Okuda et al., 1999). Pull-down experiments carried out on these cells showed that although basal levels of RhoA-GTP remained unaffected, RasN17 completely inhibited RhoA activation by p120 and KI-c-Src had no effect (Figure 6, D–E). Cell migration assays conducted on these cultures revealed that both RasN17 and KI-c-Src fully inhibited p120-induced cell motility (our unpublished results), suggesting that although Ras is required for both RhoA activation and induction of cell migration, c-Src is only required downstream of RhoA for motility.
Δ 78-p120 Abrogates Activation of RhoA by HGF
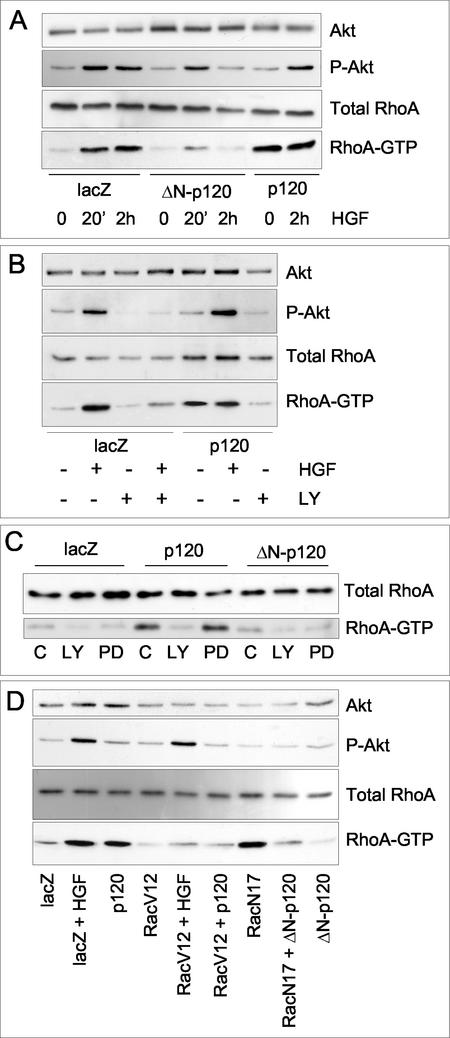
Growth factors like HGF, EGF, and PDGF have been shown to stimulate Rac (Ridley et al., 1995; Scita et al., 1999; Royal et al., 2000; Zondag et al., 2000) and RhoA activities (Zondag et al., 2000) both in fibroblasts and epithelial cells. Accordingly, HGF stimulation of control MDCK cells resulted in a rapid and transient increase (between 2 and 10 min after addition of the growth factor) of Rac-GTP (our unpublished results). At variance, a pronounced and long-lasting increase in RhoA-GTP by HGF in control MDCK cells was measurable after 10 min and persisted for at least 4 h (Figure 7A), thus coinciding with the early morphological changes and ensuing scattering elicited by HGF. As in keratinocytes, basal RhoA activity, but not Rac, was increased in Ad-p120-MDCK cells (Figure 7A) and in MDCK clones stably expressing p120 (our unpublished results). Strikingly, the sustained activation of RhoA by HGF (Figure 7A), but not the transient activation of Rac (our unpublished results), was inhibited in Δ 78-p120-MDCK cells, indicating that, in epithelial cells, Δ 78-p120 uncouples HGF/Met from one of its effector pathways, critical to induction of cell scattering.
Figure 7.
HGF-dependent activation of RhoA and PI3K/Akt pathway in MDCK cells expressing p120 and Δ 78-p120 alleles. MDCK cells were infected with Ad-p120 or Δ 78-p120 and with Ad-lacZ as a control as described in Figure 4. Thirty-six hours postinfection cultures were assayed for activation of RhoA upon exposure to 10 ng/ml HGF for 20 min and 2 h (A). RhoA-GTP was specifically precipitated using GST-Rhotekin RBD-conjugated glutathione-Sepharose beads and detected by immunoblotting with a specific antibody. (B and C) RhoA activation by HGF (10 ng/ml, 2 h) (B) and p120 (C) is PI3K dependent. Whole-cell lysates from MDCK cultures infected as in A were separated by SDS-PAGE and specific antibodies were used to detect total and phosphorylated Akt in the presence of 20 μM LY294002, 50 μM PD9859 (a MEK inhibitor), or the equivalent volume of dimethyl sulfoxide carrier (C) added for 2 h before harvesting. RhoA-GTP was measured as in A. (D) RhoA activation by HGF and p120 is modulated by Rac activity. MDCK cells were infected with Ad-p120, Ad-Δ 78-p120, Ad-RacV12 and Ad-RacN17 either alone (50 pfu/cell) or in combination (50 pfu/cell each). Activation of the Akt/PI3K pathway and of RhoA were measured as in D, in the absence or the presence of 10 ng/ml HGF for 2 h. The data are representative of three separate experiments.
Both HGF and p120 Require Integrity of the Ras/PI3K Pathway for Accumulation of RhoA-GTP and Stimulation of Cell Motility
It was shown previously that HGF-induced dissociation of MDCK epithelial cells is dependent on phosphatidylinositol 3-kinase (PI3K) activity (Potempa and Ridley, 1998). It was also suggested that whereas HGF-induced Ras activation increases Rac-GTP in a PI3K-dependent manner (Ridley et al., 1995; Scita et al., 1999; Royal et al., 2000; Zondag et al., 2000), RhoA might be activated through a PI3K-independent pathway (Zondag et al., 2000). In contrast to the latter report, we found that both RhoA (Figure 7B) and Rac activation by HGF were strictly dependent on PI3K activity, being inhibited by treatment of the cultures with the PI3K inhibitor LY294002 (2 h, 20 μM; Figure 7B). The same inhibition of RhoA activation by HGF was obtained using wortmannin (1 h, 1 μM), an independent PI3K inhibitor (Royal and Park, 1995; our unpublished results). These findings suggest that in our cell derivatives HGF/Met activates RhoA and Rac by means of the same signaling pathway. Similarly, RhoA activation by p120 was also abrogated by LY294002 and wortmannin, but not by PD9859 (2 h, 50 μM), a specific MEK inhibitor (Figure 7, B and C, and our unpublished results). Thus, the integrity of the PI3K pathway, but not of the MEK pathway, appears to play a critical role in activation of Rho GTPases by p120. Indeed, the cell motility stimulated by p120 in MDCK cells was also fully inhibited by LY294002 (8 h, 10 μM; our unpublished results).
Furthermore, we observed a time-dependent inhibitory action by Δ 78-p120 on the kinetics of PI3K/Akt activation after treatment of control MDCK cells with HGF. Although modest at 20 min, there was full inhibition of the accumulation of phospho-Akt species at 120 min (Figure 7A). These findings can be rationalized by postulating a positive feedback loop involving PI3K and Rho GTPases (Bakin et al., 2000; Weiner et al., 2002). Interestingly, no apparent inhibition of basal phospho-Akt levels by Δ 78 p120 or full-length p120 was observed (Figure 7, A and D), suggesting that this regulatory cascade is only operative in specific signaling contexts (e.g., HGF signaling program).
p120 Can Affect the Balance between Rac and RhoA Activity
We next examined whether Rac activity could affect levels of RhoA-GTP. Sustained activation or downregulation of Rac was obtained by infection of MDCK cells with recombinant adenovirus respectively encoding constitutively active RacV12 (Ad-RacV12; 50 m.o.i.) and dominant-negative RacN17 (Ad-RacN17; 50 m.o.i.) alleles (Figure 7D). In these cells, high levels of activated Rac resulted in downregulation of HGF- and p120-stimulated RhoA activity (Figure 7D), similarly to what observed in Tiam1-expressing cells (Zondag et al., 2000). Interestingly, RacV12 also inhibited cell scattering by HGF and p120-induced cell migratory activity (our unpublished results), suggesting that active Rac can modify a signaling mechanism shared by both inducers. Conversely, cells expressing RacN17 displayed fairly high levels of basal RhoA-GTP (Figure 7D), a modulation that, however, could be reversed by coinfection with Ad-Δ 78-p120 (Figure 7D). From these data we conclude that Rac can negatively regulate RhoA activation; because this epistatic interaction appears to be suppressed by Δ 78-p120, we also conclude that 1) Δ 78-p120 acts downstream of PI3K/Akt; 2) endogenous p120 may be involved in this regulation, and 3) lack of this negative regulation may be cause of the increased RhoA activity observed in cells over expressing full-length p120.
DISCUSSION
It was previously proposed that p120 catenin by regulating cadherin clustering is a key determinant of strong cell–cell adhesion (Yap et al., 1998; Thoreson et al., 2000; reviewed in Anastasiadis and Reynolds, 2000). It was further suggested that in fibroblasts p120 could affect other cell functions such as organization of the actin cytoskeleton and cell motility by virtue of its influence on the activity of Rho GTPases (Noren et al., 2000; Anastasiadis and Reynolds, 2001; Grosheva et al., 2001). In this study, the observations of prototypic epithelial cells expressing full-length and N-terminus deleted alleles of p120, while supporting the proposed roles for p120, provide new relevant elements for the understanding of epithelial dispersion orchestrated by growth factors. This advance should allow future studies to focus on key questions such as: How does Rho GTPases activation in epithelia be spatially and temporally coupled to apparently antithetic processes such as stability of AJs and cell motility? What are the steps linking p120 modifications mediated by RTKs to Rho proteins activation?
Our main result is that the expression of the Δ 78-p120 mutant, which binds cadherins but lacks the N-terminus regulatory region, inhibits the response of epithelial cells to motogenic stimuli. In addition, Δ 78-p120 partially suppresses cell scattering and restores adhesiveness in ts-v-Src–transformed cells when cultured at the permissive temperature (Ozawa and Ohkubo, 2001; our unpublished observations). Conversely, overexpression of full-length p120 in epithelial cells remodels profoundly the cell phenotype and the actin cytoskeleton and promotes a more motile behavior. Of importance, however, the finding that expression of p120 is unable to facilitate migration of mouse primary keratinocytes when cultured in the absence of EGF clearly indicates that p120 acts in concert to growth factor–activated pathways to regulate the motogenic response. Thus, our investigation of p120 function in epithelial cells points to a crucial role of this catenin as a mediator of growth factor–stimulated cell migration and assigns this function to the N-terminus region of the protein.
Current thinking holds that growth factor–activated motogenic responses involve cytoskeleton rearrangements through subtle and temporal changes of the activities of Rho GTPases, and considerable evidence points to the balance between Rac and RhoA activities as a major determinant of epithelial-mesenchymal transition (Sander et al., 1999; Royal et al., 2000; Zondag et al., 2000). Accordingly, HGF-stimulated motility of MDCK cells is mediated by a transient, PI3K-dependent increase of Rac activity and a sustained activation of RhoA. Whether Rac and RhoA are independently regulated by HGF remains unclear, but evidence has been reported suggesting that activation of RhoA occurs concomitantly to the downregulation of Rac (Zondag et al., 2000). We found that in MDCK cells HGF activates both Rac and RhoA in a PI3K-dependent manner. Moreover, inhibition of Rac in MDCK cells by expression of RacN17 is sufficient to up-regulate RhoA-GTP and, conversely, RacV12 inhibits HGF- and p120-stimulated RhoA, suggesting a Rac-dependent regulation of RhoA.
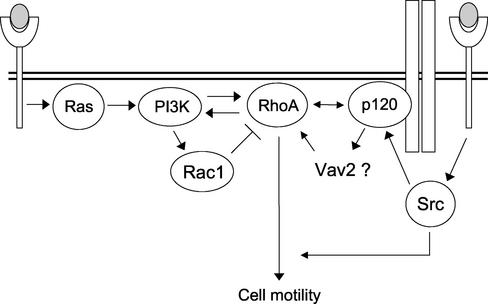
A biochemical analysis of GTPase activation in Δ 78-p120–expressing MDCK cells revealed that in these cells the HGF-induced pathway leading to RhoA activation is fully inhibited. On the contrary, overexpressed p120 promotes activation of RhoA without direct stimulation of the PI3K/Akt pathway. Importantly, activation of RhoA by p120 requires integrity of the signaling flow through PI3K. We conclude that 1) p120 provides signals that cooperate necessarily with major HGF-triggered pathways leading to RhoA activation and 2) the N-terminal domain of p120 is required for RhoA activation. We also infer that the endogenous p120 competed off cadherins by Δ 78-p120 is unable to influence the activated state of Rho GTPases. Thus, the stimulation of RhoA and cell migration observed in epithelial cells overexpressing p120 are not autonomous functions of p120 but probably arise as a consequence of its positive cooperation with incoming signals provided by serum growth factors. In light of these findings we propose a model that provides a potential mechanism for p120-dependent activation of Rho GTPases in response to a motile stimulus by growth factors (Figure 8). It posits that HGF/Met- and p120-derived signals interact downstream of PI3K in order to activate RhoA and ensuing cell motility; how this functional interaction is achieved is not yet clear, but we note that Drosophila p120 has been recently shown to bind Rho (Magie et al., 2002; see also Anastasiadis et al., 2000) and Vav2, a GEF for Rac and RhoA, has been shown to interact with p120 (Noren et al., 2000). The possibility of p120 acting upstream of PI3K, as suggested by the observation that activation of Akt by HGF is significantly reduced in ΔN-p120–expressing cells, should also be considered. However, there is evidence that advocates against it: 1) overexpression of p120 does not affect the PI3K/Akt pathway (Figure 7); 2) expression of Δ 78-p120 is able to inhibit Rac-dependent RhoA activation, suggesting that it influences GEF activity; 3) accumulation of RhoA-GTP induced by p120 is PI3K dependent. Therefore, the observed inhibition of phospho-Akt by Δ 78-p120 could be a consequence of Δ 78-p120–promoted RhoA inhibition via a positive feedback loop involving PI3K and Rho GTPases (Bakin et al., 2000; Weiner et al., 2002).
Figure 8.
Schematic model for the roles of p120 catenin in the signaling pathways leading to growth factor–induced RhoA stimulation and cell migration. Under unstimulated conditions Ras/PI3K and the small GTPases RhoA and Rac are present in the cell in an inactive state, and p120 catenin is mainly bound to cadherin complexes. On engagement of RTKs (Met) by growth factors (HGF) and ensuing activation of Ras, signals are propagated downstream in order to activate Rac1 transiently and RhoA steadily. The integrity of the PI3K pathway, but not of the MEK pathway, appears to play a critical role in activation of Rho GT-Pases. Long-term activation of RhoA, which is required for epithelial-mesenchimal transition, on the one hand appears to be negatively controlled by Rac and on the other hand is achieved through the collaboration of a Ras-independent pathway originated at the cell surface and involving the integrity of p120 catenin. All these pathways likely converge either on a direct interaction of p120 with RhoA or on yet undefined regulators of the GEF (e.g., Vav2) or GAP families. It is postulated that in order to coordinately modulate cell motility and weaken cell–cell adhesion, RTK activation somewhat impinges on p120 catenin function, by posttranslational modifications at the N-terminus or by affecting its localization. One likely candidate is c-Src tyrosine kinase that lies downstream of RTKs and may control cell motility acting both upstream, by affecting p120 phosphorylation state, and downstream of RhoA.
In contrast with what found in epithelial cells, we have measured activation of Rac and inhibition of RhoA in NIH3T3 cells infected with Ad-p120. The latter finding is coherent with previous studies reporting that when transfected in fibroblastic cells p120 activates Rac and Cdc42 (Noren et al., 2000; Grosheva et al., 2001) and inhibits basal and LPA-stimulated RhoA activity (Anastasiadis et al., 2000; Noren et al., 2000). Thus, it appears that cell context can direct the activity of p120 in modulating the functional state of Rho GTPases. A possible basis of this differential behavior may reside in epithelial cells being engaged in extensive cell–cell contacts. Here, the high density of α-catenin at AJs and/or the recruitment of overexpressed p120 to cadherin complexes could influence RhoA compartmentalization, for instance by direct binding (Magie et al., 2002), and thus make it accessible to specific GEFs and its downstream effectors. Relevant to this issue is the comforting observation that in MDCK cells both overexpressed p120 and HGF adopt a PI3K-dependent sustained activation of RhoA to control cell migration.
Our observations beyond further elucidating the role of p120 in control of epithelial integrity impinge on several critical issues. A first issue concerns the physiological role of p120 catenin at AJs, which remains controversial (Anastasiadis and Reynolds, 2000; Gumbiner, 2000). A number of experiments on cultured cells suggest that the formation of adherens junctions depends on and is preceded by the proper organization of a submembranous actin cytoskeleton (Vasioukhin et al., 2000). The immature junctions formed upon overexpression of Δ 78-p120 suggest an intriguing correlation between maturation of adhesion zippers, which are an intermediate step in the formation of AJs (Vasioukhin et al., 2000), strength of adhesion and integrity of p120. Overexpressed Δ 78-p120, by competing off endogenous p120, could act as a dominant-negative of p120 at AJs and cause loose adhesion. Therefore, once membrane contact is made and adhesion zippers are formed, one of the functions of p120 at cadherin complexes could be to signal or to influence the cytoskeleton in order to reorganize it locally enabling closure of the zippers and ensuing stabilization of adherens junctions. This interpretation indicates a function for p120 at AJs in the absence of motogenic signals and is consistent with the proposed role of p120 in regulating cadherin clustering (Gumbiner, 2000; Anastasiadis and Reynolds, 2000).
A second issue is provoked by an apparent paradox: on the one hand Δ 78-p120 weakens cadherin-mediated cell–cell adhesion in keratinocytes; on the other hand it hampers epithelial dispersal and cell scattering that follow activation of RTKs. A plausible explanation reconciling these two apparently opposite effects sees endogenous full-length p120 switching between two functional states depending on the nature and strength of incoming signaling events (discussed in Anastasiadis and Reynolds, 2001). In one state, p120 promotes cadherin clustering and strong adhesion, whereas in the other, for instance in response to growth factor–activated signals, it actively mediates dismantling of junctions and cell motility. Irrespective of the mechanism responsible for the switch, our data would argue that both states require integrity of p120 molecule as they provide direct evidence linking the amino terminus to both functions. p120 is a privileged substrate of cellular and oncogenic Src tyrosine kinases (Daniel and Reynolds, 1997; Colautti et al., 1998; Cozzolino et al., 2000). Other posttranslational modifications of p120 by yet unidentified oncogenic functions are supposed to affect the functional state of p120 (Aono et al., 1999). It has been shown that once located at the cell periphery c-Src acts in concert with the Rho GTPases to regulate cell polarity and motility of fibroblasts (Timpson et al., 2001). Clearly, Src can affect RhoA activation both upstream, by modifying the activity or the localization of p120 catenin at AJs, and down-stream, by remodelling integrin-mediated adhesion (Arthur et al., 2000) or modulating the function of RhoA effectors such as mDia (Tominaga et al., 2000; Figure 8). In the light of the recently delineated pathways downstream of RhoA in epithelial cells (Sahai and Marshall, 2002), whereby signaling through mDia results in the stabilization of AJs, whereas signaling through ROCK results in destabilization of AJs, one could speculate that depending on the biochemical background (for instance an active Src) the modulation of RhoA activity by p120 can be oriented toward either ROCK or mDia, thus justifying the described positive or negative effects on cadherin function (Aono et al., 1999; Thoreson et al., 2000).
Finally, whereas the available evidence is compatible with the contention that the pool of p120 exerting its negative function is the cadherin-bound one (Aono et al., 1999; Anastasiadis and Reynolds, 2001), the question remains as to whether this same pool of p120 is also responsible for modulation of RhoA activity and cell motility in collaboration with incoming motogenic stimuli. We have not addressed this issue directly but note that Δ 78-p120 competes efficiently with endogenous p120 protein species at cadherin complexes, thus enabling the generation of a “cytoplasmic” pool of p120. In these cells, “cytoplasmic” p120 apparently neither stimulates cell motility nor activates RhoA. Moreover, p120 can only be tyrosine phosphorylated by Src when cadherin-bound (Ozawa and Ohkubo, 2001) and, conversely, phosphorylated p120 species are found enriched in cadherin complexes (Kinch et al., 1995; Cozzolino et al., 2000). Clearly, resolution of this issue is important for the understanding of the spatial and temporal control of small GTPases in AJ assembly and during cell migration, which is critical in normal development and invasive growth.
Acknowledgments
Stefano Alemà and Anna Maria Salvatore dedicate this article to the memory of Franco Tatò. We thank Masatoshi Takeichi (Kyoto University, Kyoto), Al Reynolds (Vanderbilt University, Memphis), John Collard (Netherland Cancer Institute, Amsterdam), Martin Schwartz (Scripps Research Institute, La Jolla), Marco Crescenzi (ISS, Rome), Alan Hall (MRC LMCB, London), Louis Parada (Southwestern University, Dallas), Bradford C. Berk (CRC, Rochester) for plasmids and reagents, Marco Crescenzi for help and advice, and Loriana Castellani for critical reading of the manuscript. L.S. was the recipient of a long-term EMBO fellowship. This work was supported by grants from Telethon Italy, Associazione Italiana Ricerca sul Cancro (AIRC), and Consiglio Nazionale delle Ricerche (Progetto Strategico Postgenomica).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-08-0469. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0469.
References
- Aho S., Levansuo L., Montonen O., Kari C., Rodeck U., and Uitto J. (2002). Specific sequences in p120ctn determine subcellular distribution of its multiple isoforms involved in cellular adhesion of normal and malignant epithelial cells. J. Cell Sci. 115, 1391–1402. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., and Reynolds, A.B. (2000). The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113, 1319–1334. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637–644. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., and Reynolds, A.B. (2001). Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13, 604–610. [DOI] [PubMed] [Google Scholar]
- Aono, S., Nakagawa, S., Reynolds, A.B., and Takeichi, M. (1999). p120ctn acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J. Cell Biol. 145, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W.T., Petch, L.A., and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722. [DOI] [PubMed] [Google Scholar]
- Bakin, A.V., Tomlinson, A.K., Bhowmick, N.A., Moses, H.L., and Arteaga, C.L. (2000). Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi, D., and Hall, A. (2000). Ras, and Rho GTPases. a family reunion. Cell 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Becker-Hapak, M., McAllister, S.S., and Dowdy, S.F. (2001). TAT-mediated protein transduction into mammalian cells. Methods 24, 247–256. [DOI] [PubMed] [Google Scholar]
- Birchmeier, W., and Behrens, J. (1994). Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 1198, 11–26. [DOI] [PubMed] [Google Scholar]
- Braga, V. (2000). Epithelial cell shape: cadherins and small GTPases. Exp. Cell Res. 261, 83–90. [DOI] [PubMed] [Google Scholar]
- Calautti, E., Cabodi, S., Stein, P.L., Hatzfeld, M., Kedersha, N., and Dotto, P. (1998). Tyrosine phosphorylation and Src family kinases control keratinocyte cell-cell adhesion. J. Cell Biol. 141, 1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino, M., Giovannone, B., Serafino, A., Knudsen, K., Levi, A., Alemà, S., and Salvatore, A. M (2000). Activation of TrkA tyrosine kinase in embryonal carcinoma cells promotes cell compaction, independently of tyrosine phosphorylation of catenins. J. Cell Sci. 113, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Daniel, J.M., and Reynolds, A.B. (1997). Tyrosine phosphorylation and cadherin/catenin function. BioEssays 19, 883–891. [DOI] [PubMed] [Google Scholar]
- Falzano, L., Fiorentini, C., Donelli, G., Michel, E., Kochks, C., Cossart, P., Cabaniè, L., Oswald, E., and Boquet, P. (1998). Induction of phagocytic behavior in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9, 1247–1254. [DOI] [PubMed] [Google Scholar]
- Fukata, M., and Kaibuchi, K. (2001). Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell. Biol. 2, 887–897. [DOI] [PubMed] [Google Scholar]
- Ganly, I., Mautner, V., and Balmain, A. (2000). Productive replication of human adenoviruses in mouse epidermal cells. J. Virol. 74, 2895–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosheva, I., Shtutman, M., Elbaum, M., and Bershadsky, A.D. (2001). p120 catenin affects cell motility via modulation of activity of Rho-family GTPases. a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114, 695–707. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (2000). Regulation of cadherin adhesive activity. J. Cell Biol. 148, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Keirsebilck, A., Bonne, S., Staes, K., van Hengel, J., Nollet, F., Reynolds, A., and van Roy, F. (1998). Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics 50, 129–146. [DOI] [PubMed] [Google Scholar]
- Kinch, M.S., Clark, G.F., Der, C.J., and Burridge, K. (1995). Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J. Cell Biol. 130, 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latella, L., Sacco, A., Pajalunga, D., Tiainen, M., Macera, D., D'Angelo, M., Felici, A., Sacchi, A., and Crescenzi, M. (2001). Reconstitution of cyclin D1-associated kinase activity drives terminally differentiated cells into the cell cycle. Mol. Cell. Biol. 21, 5631–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magie, C.R., Pinto-Santini, D., and Parkhurst, S.M. (2002). Rho1 interacts with p120ctn, and α-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 129, 3771–3782. [DOI] [PubMed] [Google Scholar]
- Mariner, D.J., Anastasiadis, P., Keilhack, H., Bohmer, F.D., Wang, J., and Reynolds, A.B. (2001). Identification of Src phosphorylation sites in the catenin p120ctn. J. Biol. Chem. 276, 28006–28013. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., Liu, B.P., Burridge, K., and Kreft, B. (2000). p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, M., Takahashi, M., Suero, J., Murry, C.E., Traub, O., Kawakatsu, H., and Berk, B.C. (1999). Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J. Biol. Chem. 274, 26803–26809. [DOI] [PubMed] [Google Scholar]
- Ozawa, M., and Ohkubo, T. (2001). Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J. Cell Sci. 114, 503–512. [DOI] [PubMed] [Google Scholar]
- Ozawa, M., and Kemler, R. (1998). The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J. Cell Biol. 142, 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl, A.K., Wilgenbus, P., Dahl, U., Semb, H., and Christofori, G. (1998). A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193. [DOI] [PubMed] [Google Scholar]
- Ponzetto, C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G., and Comoglio, P.M. (1994). A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261–271. [DOI] [PubMed] [Google Scholar]
- Potempa, S., and Ridley, A.J. (1998). Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for Hepatocyte Growth Factor/Scatter Factor-induced adherens junction disassembly. Mol. Biol. Cell 9, 2185–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, A.B., Daniel, J., McCrea, P., Wheelock, M.M., Wu, J., and Zhang, Z. (1994). Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 14, 8333–8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J. (2001). Rho GTPases, and cell migration. J. Cell Sci. 114, 2713–2722. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Comoglio, P.M., and Hall, A. (1995). Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal, I., and Park, M. (1995). Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J. Biol. Chem. 270, 27780–27787. [DOI] [PubMed] [Google Scholar]
- Royal, I., Lamarche-Vane, N., Lamorte, L., Kaibuchi, K., and Park, M. (2000). Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 11, 1709–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai, E., and Marshall, C.J. (2002). ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 4, 408–415. [DOI] [PubMed] [Google Scholar]
- Sander, E.E., ten Klooster, J.P., van Delft, S., van der Kammen, R.A., and Collard, J.G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita, G., Nordstrom, J., Carbone, R., Tenca, P., Giardina, G., Gutkind, S., Bjarnegard, M., Betsholtz, C., and Di Fiore, P.P. (1999). EPS8 and E3B1 transduce signals from Ras to Rac. Nature 401, 290–293. [DOI] [PubMed] [Google Scholar]
- Shibamoto, S., Hayakawa, M., Takeuchi, K., Hori, T., Oku, N., Miyazawa, K., Kitamura, N., Takeichi, M., and Ito, F. (1994). Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes. Commun. 1, 295–305. [DOI] [PubMed] [Google Scholar]
- Takeichi, M. (1990). Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59, 237–252. [DOI] [PubMed] [Google Scholar]
- Takeichi, M. (1993). Cadherins in cancer: implication for invasion and metastasis. Curr. Opin. Cell Biol. 5, 803–811. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P. (2002). Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2, 442–454. [DOI] [PubMed] [Google Scholar]
- Thoreson, M.A., Anastasiadis, P.Z., Daniel, J.M., Ireton, R.C., Wheelock, M.J., Johnson, K.R., Hummingbird, D.K., and Reynolds, A.B. (2000). Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson, P., Jones, G.E., Frame, M.C., and Brunton, V.G. (2001). Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11, 1836–1846. [DOI] [PubMed] [Google Scholar]
- Tominaga, T., Sahai, E., Chardin, P., McCormick, F., Courtneidge, S.A., and Alberts, A.S. (2000). Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell 5, 13–25. [DOI] [PubMed] [Google Scholar]
- Trusolino, L., and Comoglio, P.M. (2002). Scatter-factor and semaphorin receptors: cell signaling for invasive growth. Nat. Rev. Cancer 2, 289–300. [DOI] [PubMed] [Google Scholar]
- van Aelst, L., and D'Souza-Schorey, C. (1997). Rho GTPases and signaling networks. Genes Dev. 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., Bauer, C., Yin, M., and Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209–219. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., Vakaet, L., Jr., Mareel, M., Fiers, W., and van Roy, F. (1991). Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119. [DOI] [PubMed] [Google Scholar]
- Weiner, O.D., Neilsen, P.O., Prestwich, G.D., Kirschner, M.W., Cantley, L.C., and Bourne, H.R. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi, T., and Takeichi, M. (2000). Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14, 1169–1180. [PubMed] [Google Scholar]
- Yap, A.S., Brieher, W.M., and Gumbiner, B.M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119–146. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., Niessen, C.M., and Gumbiner, B.M. (1998). The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 141, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag, G.C., Evers, E.E., ten Klooster, J.P., Janssen, L., van der Kammen, R.A., and Collard, J.G. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity, and epithelial-mesenchymal transition. J. Cell Biol. 149, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]