Abstract
Tetraspanins are clustered in specific microdomains (named tetraspanin-enriched microdomains, or TERM) in the plasma membrane and regulate the functions of associated transmembrane receptors, including integrins and receptor tyrosine kinases. We have identified syntenin-1, a PDZ domain-containing protein, as a new component of TERM and show that syntenin-1 specifically interacts with the tetraspanin CD63. Detailed biochemical and heteronuclear magnetic resonance spectroscopy (NMR) studies have demonstrated that the interaction is mediated by the C-terminal cytoplasmic region of the tetraspanin and the PDZ domains of syntenin-1. Upon interaction, NMR chemical shift perturbations were predominantly localized to residues around the binding pocket of PDZ1, indicating a specific mode of recognition of the cytoplasmic tail of CD63. In addition, the C terminus of syntenin-1 has a stabilizing role in the CD63-syntenin-1 association, as deletion of the last 17 amino acids abolished the interaction. The CD63-syntenin-1 complex is abundant on the plasma membrane, and the elevated expression of the wild-type syntenin-1 slows down constitutive internalization of the tetraspanin. Furthermore, internalization of CD63 was completely blocked in cells expressing a syntenin-1 mutant lacking the first 100 amino acids. Previous results have shown that CD63 is internalized via AP-2-dependent mechanisms. Hence, our data indicate that syntenin-1 can counteract the AP-2-dependent internalization and identify this tandem PDZ protein as a new regulator of endocytosis.
Tetraspanins are a large family of four transmembrane domain proteins that are implicated in a variety of biological phenomena including egg-sperm fusion, wound healing, immune responses, tumor cell migration, and invasion (19, 29). At the molecular level, tetraspanins regulate maturation and processing of associated transmembrane proteins (37), as well as their activity on the cell surface (19, 29) and subsequent endocytosis (11, 38). Various tetraspanins are also abundant on late endocytic organelles and could be involved in the intracellular sorting of the associated receptors (18). The biochemical basis for the involvement of tetraspanins in such a wide variety of biological processes is not well understood. One possibility is that tetraspanins control compartmentalization of the associated receptors into specialized microdomains (also called tetraspanin-enriched microdomains, or TERM). While significant progress has been made towards identification of new transmembrane components of TERM and understanding the mechanisms that control their interactions, relatively little is known about the cytoplasmic residents of these microdomains. Earlier experiments established that a class II phosphatidylinositol 4-kinase (PI4-K) and a few isoforms of protein kinase C (PKC) can be coimmunoprecipitated with a number of tetraspanins (7, 42). However, it remains unclear how the presence of PI4-K and PKC in TERM affects the activity of the TERM-associated receptors.
The aim of this study was to identify new cytoplasmic components of TERM. We found that the tetraspanin CD63 directly interacts with syntenin-1, a double PDZ domain-containing protein. The tetraspanin CD63 is a ubiquitously expressed protein which is found in various late endocytic organelles (e.g., lysosomes, platelet dense granules, and Weibel-Palade bodies) and on the plasma membrane. CD63 interacts with other tetraspanin family members and can be coimmunoprecipitated with nontetraspanin TERM components such as integrins (3). Recent data have shown that CD63 is associated with, and plays an important role in, endocytosis of the H,K-ATPase in gastric parietal cells (11). Whether CD63 is involved in the regulation of trafficking routes of other associated proteins remains unknown. The cellular distribution and dynamics of the protein at the plasma membrane are controlled by its C-terminal tyrosine-based sorting motif (G-Y-E-V-M) (34). The direct interaction between this sequence and μ2 and μ3 subunits of the AP-2 and AP-3 complexes, respectively, would link most of the intracellular CD63 trafficking to clathrin-dependent pathways (22, 34). However, recent data also suggested that an AP-2/AP-3-independent pathway(s) may contribute to trafficking of CD63 (22).
Syntenin-1 was originally described as a syndecan-associated protein (17). Later studies have shown that syntenin-1 also interacts with various other transmembrane and cytoplasmic partners through one of its two PDZ domains (36). Notably, despite a clear preference towards a specific PDZ domain, typically PDZ2, interactions with most of its protein partners require the presence of both domains. In addition, both PDZ domains are reportedly required for binding of syntenin-1 to phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], with PDZ1 being most important (43). At the cellular level, syntenin-1 is known to regulate cell migration and adhesion-dependent signaling and promotes cancer metastasis (8, 26). It has been suggested that this function of syntenin-1 may be linked to its ability to regulate trafficking of the associated proteins (13, 45).
In this report, we show that the interaction between the tetraspanin CD63 and syntenin-1 is direct, takes place on the plasma membrane, and also requires both PDZ domains, with PDZ1 playing a pivotal role in complex formation. In addition, we found that the C-terminal 17 amino acids of syntenin-1 are critical for its interaction with CD63 in cells. Finally, we established that the overexpression of syntenin-1 decreases the rate of constitutive internalization of CD63.
MATERIALS AND METHODS
Cell lines and antibodies.
Cos7, 293T, and HeLa cells were grown in Dulbecco's modified Eagle medium (DMEM; Sigma) supplemented with 10% fetal calf serum. CHO-K1 cells were grown in Ham's F-12 media supplemented with 10% fetal calf serum. The mouse anti-CD81 monoclonal antibody (MAb) (M38) was kindly provided by O. Yoshie. The anti-CD63 (6H1) and anti-CD151 (5C11) MAbs were described previously (4, 5). The anti-CD63 MAbs (1B5) were kindly provided by M. Marsh (University College London, London, United Kingdom). The mouse MAbs to human lamp-1 and lamp-2 were from the Development Studies Hybridoma Bank. The anti-rabbit polyclonal Abs to CD151 were provided by L. Ashman (University of Newcastle, Australia). The monoclonal and polyclonal anti-hemagglutinin (HA) tag Abs (F7 and Y-11) were purchased from Autogen Bioclear. The monoclonal Abs against myc tag sequence (9E10) and green fluorescent protein (3E1) were purchased from the Cancer Research UK antibody production facility. The polyclonal and monoclonal anti-myc tag Abs were from Signaling Technology. Rabbit polyclonal Abs against recombinant glutathione S-transferase (GST)-tagged human syntenin-1 were produced by Eurogentec (Liege, Belgium) and affinity purified using coupled recombinant protein.
Plasmids and transfection.
The cDNAs encoding human syntenin-1 (TACIP-18) and the HA-tagged form of syntenin-2 (TACIP) in pcDNA3.1/Zeo(+) were provided by J. Arribas (Vall d'Hebron Research Institute University Hospital, Barcelona, Spain). The cDNA encoding human CD63 in pZeoSV was described previously (5). The plasmid encoding the GST-tagged form of human syntenin-1 was obtained from T. Cierpicki and Z. Derewenda. The plasmid encoding the Flag-tagged NF-2 was kindly provided by M. Crompton (Royal Holloway University of London, United Kingdom). DNA constructs encoding various mutants of syntenin-1 and CD63 were produced by a standard PCR (sequences of the primers are available upon request). Transfection experiments were carried out using Fugene 6 (Roche) according to the manufacturer's instructions. For transfection with small interfering RNA (siRNA), HeLa cells were detached and electroporated with a single pulse at 250 V and 250 μF using a GenePulser Xcell electroporation system (Bio-Rad) and 2.5 μM of siRNA duplex. The efficacy of knocking down was assessed 48 h later by Western blotting as described below. The following siRNA was found to be effective in the knockdown experiments: 5′-GCUAUAGCAUAGCUGCUUATT-3′. Silencer negative control no. 1 siRNA was purchased from Ambion (catalog no. 4611).
Immunoprecipitation and Western blotting.
The proteins were solubilized into the immunoprecipitation buffer containing 0.8% Brij 98-0.2% Triton X-100-phosphate-buffered saline (PBS) (or 0.5% Brij 98-0.5% Triton X-100-PBS), 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin for 4 to 16 h at 4°C. The insoluble material was pelleted at 12,000 rpm for 10 min. The cell lysates were then precleared by incubation for 2 h at 4°C with agarose beads conjugated with goat anti-mouse antibodies (mouse immunoglobulin G [mIgG] beads; Sigma). Immune complexes were collected using appropriate MAbs prebound to the mIgG beads and washed four times with the immunoprecipitation buffer. The anti-green fluorescent protein MAb (3E1) was used as a negative control in all immunoprecipitation experiments. The complexes were eluted from the beads with Laemmli sample buffer. Proteins were resolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to the nitrocellulose membrane, and developed with the appropriate Ab. Protein bands were visualized using horseradish peroxidase-conjugated secondary antibodies (Sigma) and enhanced chemiluminescence reagent (Amersham Pharmacia Biochem).
Immunoprecipitation of the cell surface pool of CD63.
CHO cells were transfected with the plasmids encoding the HA-tagged form of syntenin-1 and human CD63. Forty-eight hours after the transfection, the cells were washed with ice-cold DMEM and incubated for 1 h at 4°C with purified anti-CD63 MAb (6H1) or with the control MAb. Subsequently, the cells were washed three times with ice-cold DMEM and lysed in the immunoprecipitation buffer containing 0.8% Brij 98-0.2% Triton X-100-PBS and the protease and phosphatase inhibitors. Immunoprecipitation and analysis of the CD63-containing complexes were carried out as described above.
Biotinylated peptides, and pull-down, and enzyme-linked immunosorbent assays (ELISA).
N-terminally biotinylated peptides corresponding to the C-termini of tetraspanins were synthesized by AltaBioscience (Birmingham, United Kingdom). The following tetraspanin peptides were used: CD63, VKSIRSGYEVM; CD151, YRSLKLEHY; SAS, RNQKDPRANPSAFL; CD9, AIRRNREMV; and CD82, VHSEDYSKVPKY. For the pull-down experiments, 293T cells, ectopically expressing HA-tagged syntenins, were lysed overnight in the immunoprecipitation buffer (0.5% Brij 98-0.5% Triton X-100-PBS) containing protease and phosphatase inhibitors. Biotinylated peptides were conjugated to avidin-agarose (Sigma) for 2 h at 4°C in the washing buffer (0.5% Brij 98-0.5% Triton X-100-PBS) and subsequently incubated with the cell lysates for 5 to 16 h at 4°C. After three washes, pulled-down proteins were eluted from agarose into 1× Laemmli buffer, boiled, and resolved in SDS-PAGE. The proteins were then transferred to the nitrocellulose membrane and probed with the anti-HA tag rabbit polyclonal antibodies. The GST-tagged recombinant syntenin-1 proteins for ELISA were produced in the BL21 strain of Escherichia coli by using a standard protocol. The proteins (0.5 μg) were immobilized on 96-well plates overnight in 50 μl of coating buffer (0.1 M Na2HPO4, adjusted to pH 9 with 0.1 M NaH2PO4) at 4°C. Plates were then washed gently twice in PBS-Tween (0.05%). The wells were then incubated in blocking buffer (2% bovine serum albumin in PBS-Tween [0.05%]) for 1 h at 37°C and subsequently washed once with PBS-Tween (0.05%). Biotinylated peptides at a range of concentrations (made up in 50 μl of blocking buffer) were added to the wells and incubated for a minimum of 2 h at 37°C. After the incubation period, the plates were washed four times in PBS-Tween (0.05%), followed by addition of streptavidin-horseradish peroxidase (1:2,000 in blocking buffer) and subsequent incubation for 1 h at room temperature. Wells were washed eight times in PBS-Tween (0.05%) and 100 μl of 3,3′,5,5′ tetramethylbenzidine (Tebu-bio Laboratories) was added. The reaction was allowed to proceed for 20 min and then stopped by the addition of 100 μl 1 M HCl. Levels of binding were analyzed by measuring absorbance at A450 using a plate reader.
NMR spectroscopy and C-terminal peptide ligand titration.
For the heteronuclear magnetic resonance spectroscopy (NMR) analysis of syntenin-1 and CD63 interaction, uniformly 15N-labeled syntenin-1 PDZ tandem domain (113 to 273), referred to as “syntenin-1 PDZ12,” was expressed as a GST fusion in Escherichia coli BL21 (DE3) at 25°C in M9 minimal medium using 15NH4Cl as the sole nitrogen source. The purification of GST-fused protein was performed as reported previously (24). The NMR samples contained 200 μM protein, 150 mM NaCl, 500 μM TCEP [Tris(2-carboxyethyl)phosphine], and 50 μM AEBSF in 50 mM Tris buffer (pH 7.5). All NMR spectra were recorded at 30°C on a Varian Inova 800-MHz spectrometer equipped with a room temperature 5-mm 1H/13C/15N z-axis pulse field gradient probe, with data processed using the Azara package and analyzed using Ansig (28). Earlier chemical shift assignments (9) were confirmed by tracing sequential nuclear Overhauser effect connectivities across the protein backbone from a three-dimensional heteronuclear single quantum correlation (HSQC)-nuclear Overhauser effect spectroscopy spectrum recorded with a mixing time of 150 ms using a 400 μM sample of 15N-labeled syntenin-1 PDZ12 protein. Synthesized C-terminal CD63 peptide (RVKSIRSGYEVM) was purchased from Sigma-Genosys and contained an extra Arg residue at the N terminus, to improve peptide solubility. Titrations of syntenin-1 PDZ12 proteins with C-terminal CD63 peptide were conducted by recording a series of 1H,15N HSQC spectra of 15N-labeled protein (200 μM), with increasing molar concentrations of the peptide ligand, up to a protein-to-peptide ratio of 1:8. Some amide proton resonances were obscured at pH 7.5 due to rapid exchange with solvent, and hence the peptide titration was also performed at pH 6.5, with chemical shifts being assigned to the nearest neighbors. A nonlinear regression fit of the chemical changes as a function of ligand concentration was used to calculate the dissociation constant for the syntenin-1 PDZ12-CD63C complex (2). The combined backbone 1H and 15N chemical shift changes, Δδ, were calculated according to equation 1, where ΔN is the 15N chemical shift difference in Hz, and ΔH is the 1H chemical shift difference in Hz, between free and peptide (eightfold excess) titrated protein:
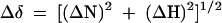 |
(1) |
Immunofluorescence staining.
Cells were grown on glass coverslips in complete media for 24 to 36 h. Spread cells were fixed with 2% paraformaldehyde-PBS for 10 to 15 min. The staining with primary and fluorochrome-conjugated secondary Abs was carried out as previously described (6). The staining was analyzed using a Nikon Eclipse E600 microscope. Images were acquired using a Leica DC200 digital camera and subsequently processed using the DC200 image processing program.
Antibody uptake/pulse-chase.
HeLa cells grown on the glass coverslips were transfected with the plasmids encoding various HA-tagged syntenin-1 constructs. After 48 h posttransfection, cells were incubated with 10 μg/ml MAb 6H1 in serum-free DMEM (supplemented with 10 mM HEPES) for 1 h at 4°C. Cells were rinsed three times with ice-cold DMEM (zero time point) and then transferred to prewarmed complete growth media at 37°C for different lengths of time (30 min to 4 h). At each time point, a set of coverslips was taken out of the incubator and cells were immediately fixed for 20 min with 2% paraformaldehyde at room temperature. Cells were subsequently permeabilized in 0.1% Triton X-100. Cells were then rinsed in PBS and blocked for 1 h with blocking buffer (20% heat-inactivated normal goat serum-2% human serum-PBS). After blocking, cells were incubated with Alexa-conjugated goat anti-mouse Ab (1:1,000 in blocking buffer) for 1 h at room temperature. Finally, cells were rinsed in PBS and water. Coverslips were subsequently processed for analysis as described above. The internalization was scored as blocked/impaired when MAb clearly outlined cell perimeter. At least three independent experiments were performed for an individual construct with at least 70 to 80 syntenin-1-positive cells scored in each of the experiments.
RESULTS
Syntenin-1 is associated with tetraspanin-enriched microdomains: interaction of syntenin-1 with CD63.
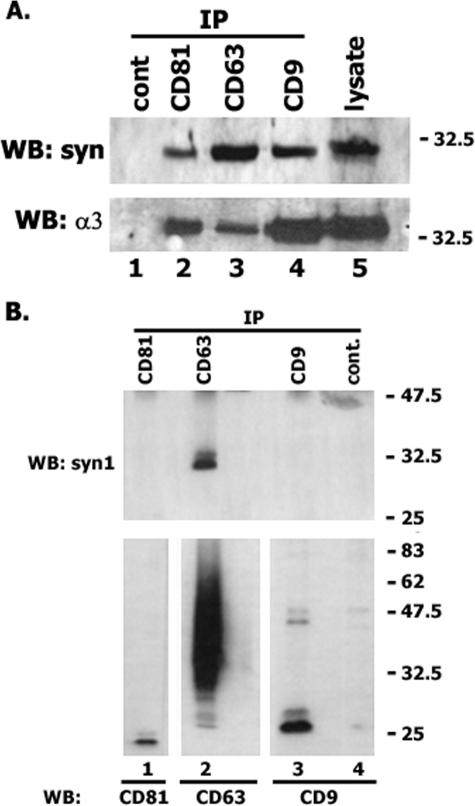
It has previously been shown that tetraspanins are abundant on exosomes, 50- to 90-nm vesicles secreted by various cell types (12, 33, 39). We hypothesized that some of the cytoplasmic proteins that are recruited to exosomes can interact with tetraspanins directly. Indeed, we found that syntenin-1 can be coimmunoprecipitated with various tetraspanins (e.g., CD9, CD63, and CD81) from the lysates of MDA-MB-231 cells (Fig. 1A). The association with syntenin-1 was specific, since tetraspanins did not interact with annexin II or fatty acid synthase, two proteins that are also abundant on exosomes (results are not shown). To establish whether the interactions between the tetraspanins and syntenin-1 are direct, we initially performed immunoprecipitation experiments under conditions that disrupt the association of tetraspanins with most of their transmembrane partners (i.e., in the presence of 0.5% Brij 98-0.5% Triton X-100). In these experiments, only CD63 remained associated with syntenin-1 (Fig. 1B). These data suggest that CD63 directly interacts with syntenin-1, while the association of syntenin-1 with CD9 and CD81 may be mediated by other proteins.
FIG. 1.
Syntenin-1 is associated with the tetraspanin-enriched microdomains. MDA-MB-231 cells were lysed in the buffer containing either 0.8% Brij-0.2% Triton X-100 (A) or 0.5% Brij-0.5% Triton X-100 (B). Protein complexes were immunoprecipitated with the anti-CD9 MAb Syb1 (lane 2), anti-CD63 MAb 6H1 (lane 3), or anti-CD81 MAb M38 (lane 4). An irrelevant MAb (3E1) was used as a negative control (lane 1). The protein lysate (lane 5) was used as a positive control. Immunocomplexes were separated in 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were developed with the polyclonal Abs to syntenin-1, the α3 integrin subunit, or MAbs to CD9 (C9-BB), CD81 (JS-64), or CD63 (1B5). WB, Western blot.
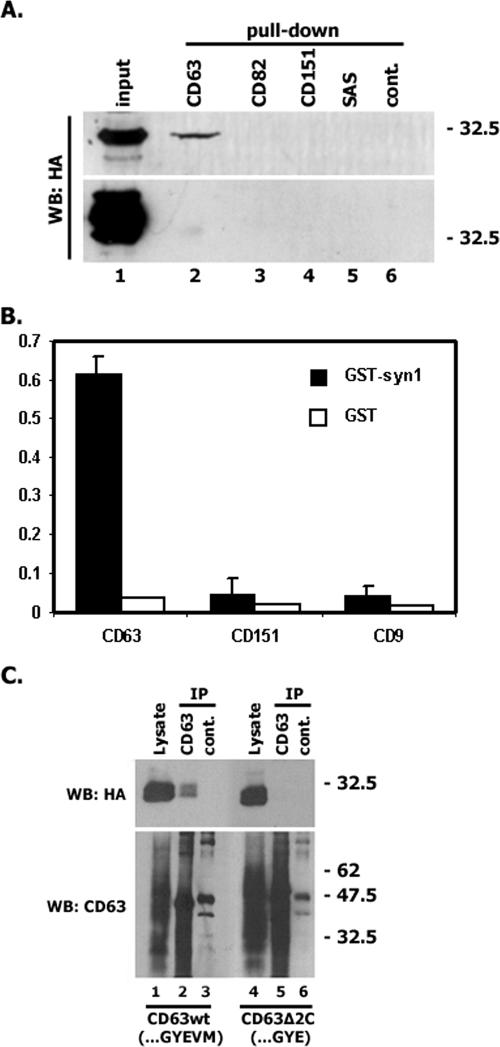
Syntenin-1 is known to interact with various transmembrane proteins via their C-terminal cytoplasmic domains (36). A series of experiments were carried out to examine whether the C-terminal cytoplasmic portion of CD63 is involved in the interaction with syntenin-1. Firstly, we found that the immobilized CD63 C-terminal peptide (CD63-C), but not the corresponding regions of some other tetraspanins (e.g., CD151, CD9, SAS, and CD82), can retain syntenin-1 from cell lysates (Fig. 2A). The specificity of this interaction was further confirmed by the fact that CD63-C did not pull down syntenin-2, a closely related PDZ domain-containing protein (Fig. 2A). Secondly, the CD63-C peptide directly binds to recombinant GST-syntenin-1 in ELISA experiments (Fig. 2B). In a series of control ELISA experiments, we found that the CD63-C peptide did not bind to recombinant GST, nor did the CD9-C or CD151-C peptides interact with GST-syntenin-1 (Fig. 2B). Finally, we found that the deletion of the last two amino acids in CD63 completely abolished the association of the full-length protein with syntenin-1 (Fig. 2C). Thus, we concluded that the interaction between CD63 and syntenin-1 is direct and mediated by the C-terminal cytoplasmic tail of the tetraspanin.
FIG. 2.
The C-terminal cytoplasmic region mediates the interaction of CD63 with syntenin-1. (A) 293T cells were transiently transfected with the plasmids encoding syntenin-1-HA (or syntenin-2-HA) and 48 h later lysed in 0.8% Brij-0.2% Triton X-100. Retention of syntenins by immobilized tetraspanin peptides was analyzed by Western blotting (WB). (B) Interaction of biotinylated peptides (0.25 μg) with the immobilized GST-syntenin-1 (or GST) was analyzed by ELISA as described in Materials and Methods. (C) CHO cells were transiently transfected with the plasmids encoding syntenin-1-HA and human CD63 (CD63h) constructs. After 48 h, cells were lysed in 0.8% Brij-0.2% Triton X-100. Protein complexes were immunoprecipitated (IP) with the anti-CD63 MAb (6H1) or an irrelevant MAb (3E1). The protein lysates (lanes 1 and 4) were used as positive controls. Immunocomplexes were separated in 12% SDS-PAGE and transferred on nitrocellulose membranes. Membranes were probed with the anti-HA polyclonal Abs or anti-CD63 MAb (1B5). wt, wild type.
The contribution of PDZ domains in the interaction of syntenin-1 with CD63.
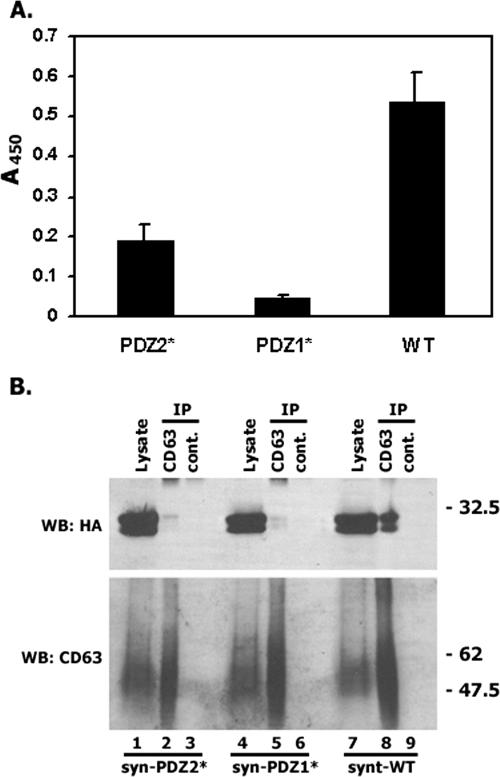
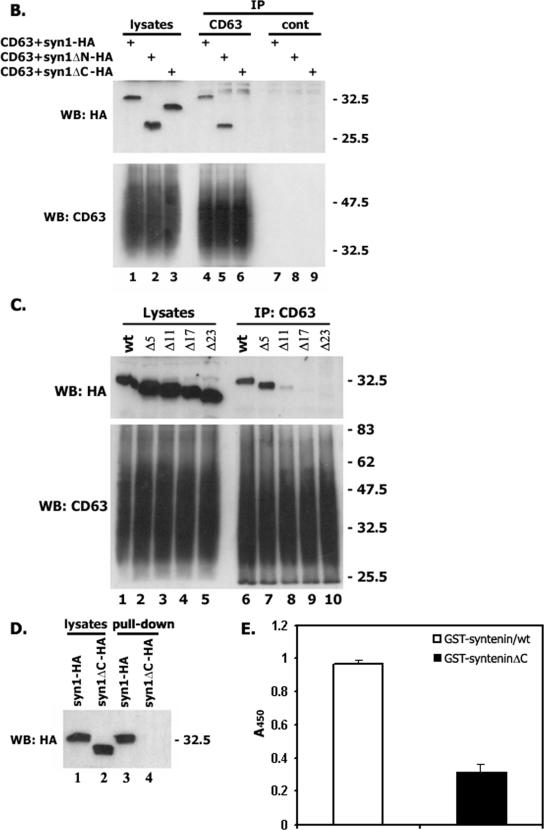
Previous studies have shown that the presence of both PDZ domains is critical for the interaction of syntenin-1 with its various transmembrane partners (36). Indeed, the Gly126→Asp and Gly210→Glu mutations in the interacting loops of PDZ1 and PDZ2, respectively, completely abolished these interactions (27). The interaction of the mutants with CD63-C peptide was examined by ELISA and in coimmunoprecipitation experiments with the native CD63. As shown in Fig. 3A, the mutations in both PDZ domains markedly diminished interaction of CD63-C peptide with syntenin-1. Interestingly, the mutation in PDZ1 had a more dramatic effect on this interaction. These data were in agreement with the results of the coimmunoprecipitation experiments, in which we found that mutations in either of the PDZ domains dramatically decreased the amount of syntenin-1 associated with native CD63 (Fig. 3B).
FIG. 3.
Both PDZ domains contribute to binding of CD63 with syntenin-1. (A) The interaction of biotinylated CD63 peptide (0.25 μg) with the immobilized GST-tagged syntenins was analyzed by ELISA as described in Materials and Methods. WT, wild type. (B) CHO cells were transiently transfected with the plasmids encoding human CD63 and syntenin-1-HA (or PDZ domain mutants of syntenin-1). The association between the proteins was analyzed as described in the legend to Fig. 2C. syn-PDZ1* and syn-PDZ2* indicate the Gly126→Asp and Gly210→Glu mutations in the interacting loops of PDZ1 and PDZ2, respectively.
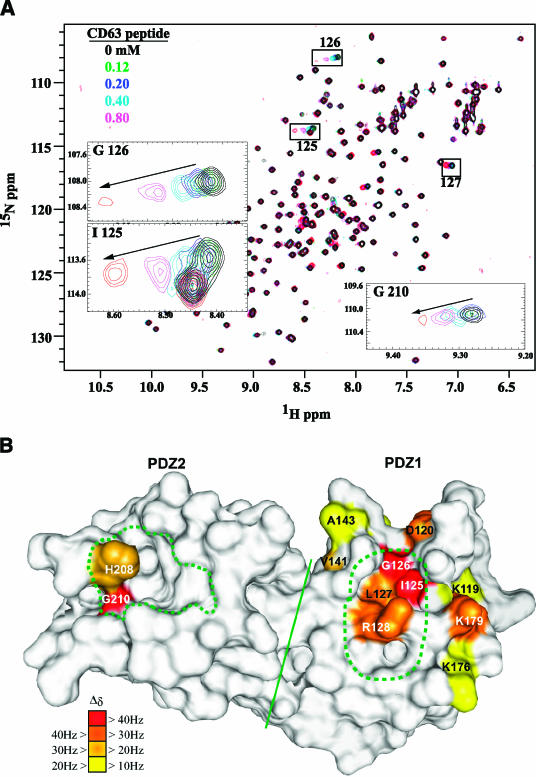
These experiments suggested either that both PDZ domains can bind CD63-C (although with a preference for PDZ1) or that the tetraspanin tail is bound by one PDZ domain, with mutation of the other having an allosteric effect on the interaction. To examine the interaction in more detail, we carried out NMR titration analysis of 15N-labeled syntenin-1 PDZ12 with unlabeled CD63 C-terminal peptide. The most significant chemical shift changes were induced in the PDZ1 domain upon CD63-C binding (Fig. 4A), with only minimal changes occurring in the PDZ2 domain. Even when the peptide was added to an eightfold excess, it was insufficient to saturate the binding of CD63-C to syntenin-1 PDZ12, indicating a weak affinity for the peptide. The chemical shift perturbations were used to map the binding sites, revealing a contact surface predominantly in the PDZ1 domain binding pocket (Fig. 4B). Chemical shifts of residues Gly126 and Ile125 in the carboxylate binding loop of PDZ1 domain were perturbed even with stoichiometric amounts of CD63-C peptide (Fig. 4A, insets). Chemical shift perturbations were also observed for residues Arg119, Asp120, Val141, Ala143, Arg176, Leu127, Arg128, and Arg179 in the vicinity of the carboxylate binding loop of PDZ1 (Fig. 4B). In contrast, only minor chemical shift changes were found in the PDZ2 domain, being limited to residues Gly210 and His208 in the carboxylate binding loop (Fig. 4B). Data from the NMR titration experiment were also used to estimate the dissociation constants for the binding of CD63-C to PDZ domains of syntenin-1. As inferred from the HSQC spectra, CD63-C peptide binds to PDZ1 domain with a Kd of 3.9 ± 1.2 mM (results not shown). In contrast, the affinity of the PDZ2 domain was too weak to measure precisely. Thus, the NMR results suggest that CD63 can bind both PDZ domains of syntenin-1 with clear preference for PDZ1, thus corroborating our results from ELISA and coimmunoprecipitation (Fig. 3A).
FIG. 4.
CD63 interacts specifically with the PDZ1 domain of syntenin-1. (A) Superposition of six two-dimensional 1H-15N-HSQC spectra of uniformly 15N-labeled syntenin-1 PDZ12 (200 μΜ), which are color coded, according to the concentration of titrated CD63 C-terminal peptide. Shown in the insets are traces for residues exhibiting chemical shift changes greater than 40 Hz. (B) The locations of PDZ12 residues involved in binding to the C-terminal peptide of CD63, based on chemical shift perturbation, are indicated on the surface of syntenin-1 PDZ12 monomer (1N99.pdb). The residues displaying combined chemical shift changes (Δδ) greater than the mean value plus one (10 Hz) are color coded as indicated. The canonical peptide binding pocket in PDZ2 (traced from 1W9E.pdb) and a corresponding putative pocket in PDZ1 are represented within green dotted lines. The bulk of the two domains are separated by a solid line.
CD63 is associated with syntenin-1 on the plasma membrane: the role of the C terminus of syntenin-1.
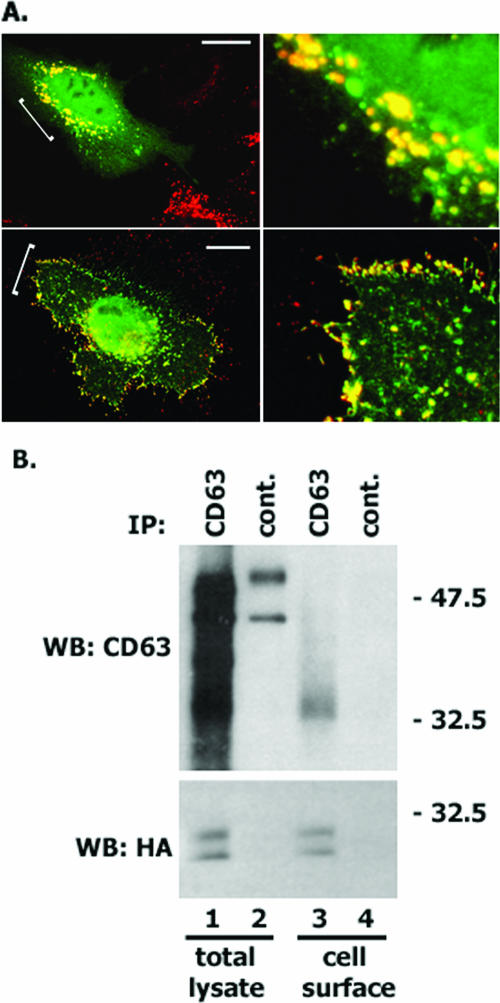
Syntenin-1 is found in various membranous compartments in cells, including early and recycling endosomes, and also on the plasma membrane (14, 45). Double immunofluorescence experiments showed that in permeabilized HeLa cells, ectopically expressed syntenin-1 is associated with vesicular organelles, some of which were also positive for CD63 (Fig. 5A, upper panels). Although CD63 is expressed on the surface of HeLa cells (as detected by flow cytometry), no surface staining was observed in these experiments. However, when we preincubated cells with the anti-CD63 MAb at 4°C prior to fixation and permeabilization, both CD63 and syntenin-1 were readily detectable and colocalized at the plasma membrane (Fig. 5A, lower panels). This apparent “discrepancy” could be explained by a relatively high sensitivity of the surface pools of CD63 and syntenin-1 to detergent extraction after fixation with paraformaldehyde.
FIG. 5.
Codistribution of CD63 with syntenin-1. CD63 is associated with the cell surface pool of syntenin-1. (A) CD63 and syntenin-1 are colocalized at the plasma membrane and vesicular organelles. Upper panels. HeLa cells were transiently transfected with the plasmid encoding syntenin-HA and 48 h later processed for immunofluorescence staining. The MAbs 6H1 (IgG1) and F7 (IgG2a) were used to examine the distribution of CD63 and HA-tagged syntenin-1. Staining was visualized using Alexa 594-conjugated goat anti-mouse IgG1 Ab and Alexa 488-conjugated goat anti-mouse IgG2a. The scale bar represents 10 μΜ. The right panel represents a magnified image of the area marked with a bracket on the left image. Note, some of the syntenin-1-positive vesicular organelles are devoid of CD63. Lower panels. Transfected HeLa cells were processed for immunofluorescence staining as described above except that they were preincubated with the anti-CD63 MAb for 1 h at 4°C prior to fixation and permeabilization. (B) CHO cells were transiently transfected with the plasmids encoding syntenin-HA and human CD63 and 48 h later were preincubated with the anti-CD63 MAb for 1 h at 4°C. After removal of unbound MAb, the association between CD63 and syntenin-1 was analyzed as described in the legend to Fig. 2C.
Next, we examined whether CD63 and syntenin-1 are associated with each other at the cell surface. As illustrated in Fig. 5B (upper panel), only a minor proportion (<10%) of the total amount of CD63 can be immunoprecipitated by anti-CD63 MAb prebound to the cells. In spite of this, ∼60% of the total pool of cellular syntenin-1 is associated with the surface-exposed CD63 (Fig. 5B, lower panel). These data suggest that the CD63-syntenin-1 complex is abundant at the cell surface.
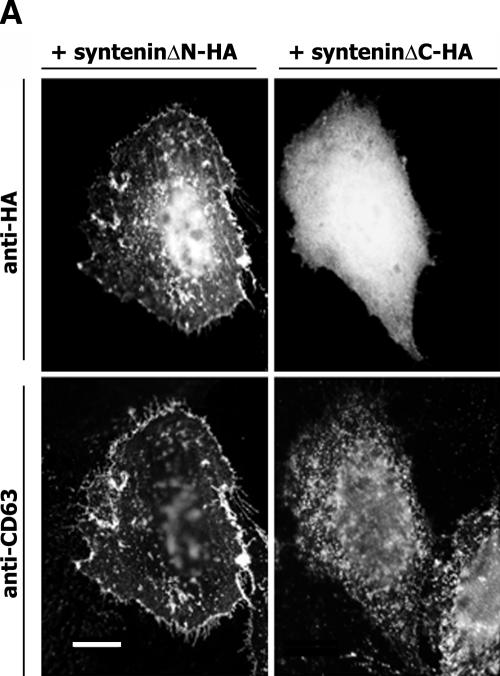
It has previously been reported that deletion of the first 101 amino acids shifts equilibrium of the cellular distribution of syntenin-1 towards the plasma membrane (44). Hence, one might expect that deletion of the sequence N-terminal to PDZ1 would enhance the interaction between syntenin-1 and CD63. However, the immunoprecipitation experiments showed that the enhanced recruitment of the syntenin-1ΔN mutant (with 100 N-terminal amino acids deleted) to the plasma membrane did not result in the stoichiometric increase of its association with CD63 (Fig. 6B). On the other hand, we observed that the deletion of the last 23 amino acids following PDZ2 (syntenin-1ΔC mutant) obliterated its association with CD63 (Fig. 6B). Further analysis revealed that the region between the residues 281 and 293 is important for the stability of the CD63-syntenin-1 complex (Fig. 6C). Notably, the pattern of distribution of the syntenin-1ΔC mutant in cells was markedly different compared to that of syntenin-1 or syntenin-1ΔN, with less of the protein associated with the internal and plasma membranes (Fig. 6A). To examine whether the negative effect of the ΔC deletion on this association is due to the redistribution of the syntenin-1ΔC mutant in cells, we carried out pull-down experiments using CD63-C peptide. As illustrated in Fig. 6D, the syntenin-1ΔC mutant could not be retained by the immobilized peptide. Finally, ELISA experiments also showed that the direct binding of CD63-C peptide to purified syntenin-1ΔC is significantly weaker than that of the wild-type protein (Fig. 6E). These data strongly suggest that the C terminus is directly involved in stabilizing the association of syntenin-1 with CD63.
FIG.6.
The C-terminal part of syntenin-1 stabilizes its association with CD63. (A) Distribution of deletion mutants of syntenin-1. HeLa cells were transiently transfected with the plasmids encoding syntenin-1ΔC-HA or syntenin-1ΔN-HA and 48 h later processed for immunofluorescence staining. The MAbs 6H1 (IgG1) and F7 (IgG2a) were used to examine the distribution of CD63 and HA-tagged syntenin-1. Staining was visualized using Alexa 594-conjugated goat anti-mouse IgG1 Ab and Alexa 488-conjugated goat anti-mouse IgG2a. The scale bar represents 10 μM. (B and C) Cos7 cells were transiently transfected with the plasmids encoding various HA-tagged syntenin-1 constructs and human CD63. After 48 h, cells were lysed in 0.5% Brij-0.5% Triton X-100. Protein complexes were immunoprecipitated (IP) with the anti-CD63 MAb (6H1) or an irrelevant MAb (3E1). The protein lysates (lanes 1 to 3 in panel B and lanes 1 to 5 in panel C) were used as positive controls. Immunocomplexes were separated in 11 SDS-PAGE and transferred on nitrocellulose membranes. Membranes were probed with the anti-HA polyclonal Abs or anti-CD63 MAb (1B5). (D) 293T cells were transiently transfected with the plasmids encoding syntenin-1-HA and syntenin-1ΔC-HA and 48 h later lysed in 0.5% Brij-0.5% Triton X-100. Retention of syntenins by immobilized CD63-C peptides was analyzed by Western blotting (WB) with the anti-HA polyclonal Abs. (E). Interaction of biotinylated CD63-C peptide (0.25 μg) with the immobilized GST-tagged syntenins was analyzed by ELISA as described in Materials and Methods. wt, wild type.
Overexpression of merlin does not affect interaction of syntenin-1 with CD63.
Previous studies have suggested that NF-2/merlin/schwannomin can also interact with syntenin-1 via the PDZ1 domain (21, 24). Thus, it was conceivable that elevated expression of merlin may substitute for CD63 in the complex with syntenin-1. A possible regulatory role of merlin was examined in transient transfection experiments. Figure 7 shows that increased amounts of merlin in Cos7 cells had no effect on the association of syntenin-1 with CD63. These results indicated that in cells the CD63-syntenin-1 and NF-2-syntenin-1 complexes coexist independently of one another.
FIG. 7.
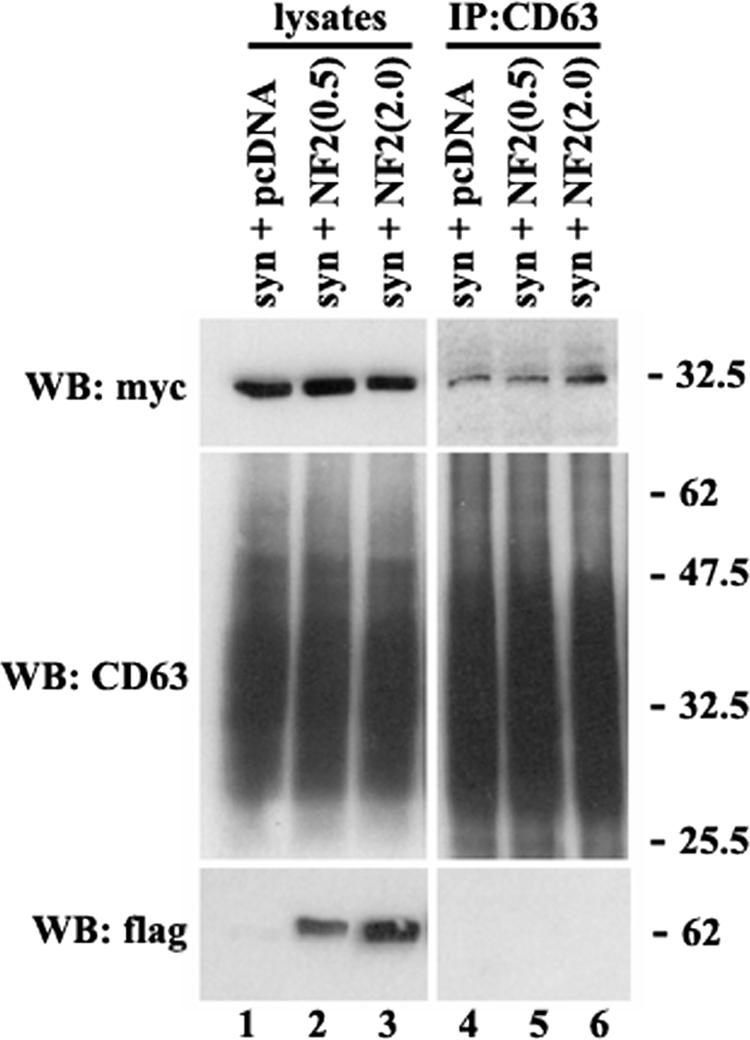
Elevated expression of NF-2/merlin does not interfere with the assembly of the CD63-syntenin-1 complex. Cos7 cells were transiently transfected with the plasmid encoding syntenin-1-myc (0.5 μg) and indicated amounts of the plasmid encoding Flag-tagged human NF-2/merlin. After 48 h, cells were lysed in 0.5% Brij-0.5% Triton X-100. Protein complexes were immunoprecipitated (IP) with the anti-CD63 MAb (6H1). The protein lysates (lanes 1 to 3) were used as positive controls. Immunocomplexes were separated in 11 to 12% SDS-PAGE and transferred on nitrocellulose membranes. Membranes were probed with the anti-myc and anti-Flag polyclonal Abs or anti-CD63 MAb (1B5). WB, Western blot.
Overexpression of syntenin-1 decreases the rate of internalization of CD63.
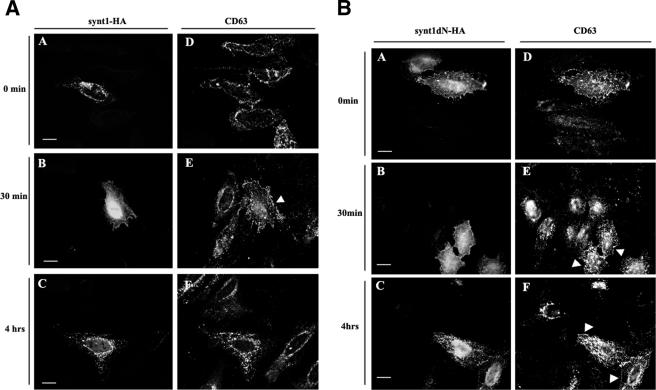
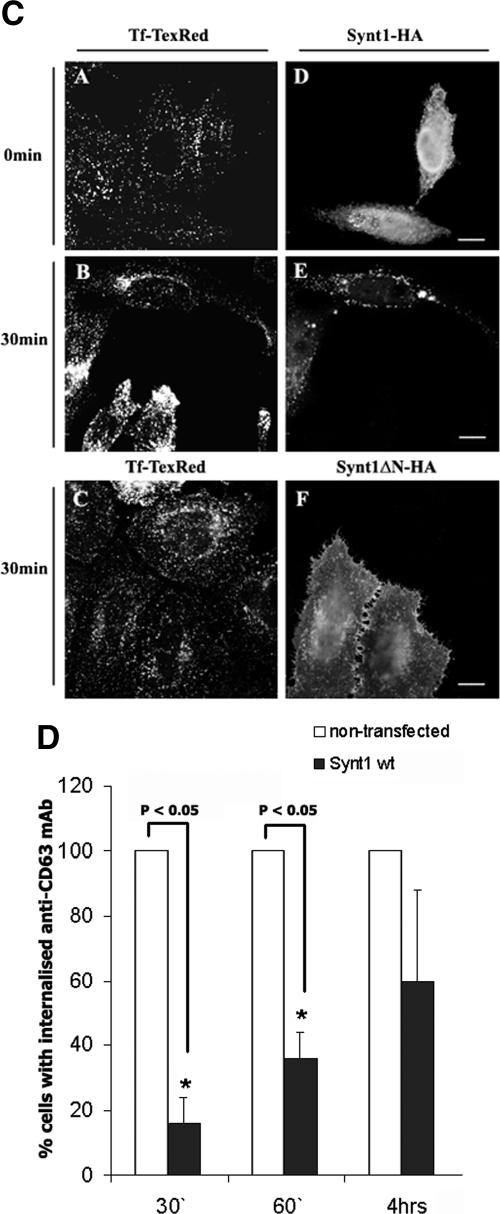
It is thought that CD63 undergoes rapid constitutive internalization from the plasma membrane by mechanisms involving the AP-2 complex (22). The μ2 chain of AP-2 directly interacts with the tyrosine-based sorting motif in the C-terminal cytoplasmic domain of CD63 (G-Y-E-V-M) (34) and directs the protein to clathrin-dependent endocytosis (22). We hypothesized that syntenin-1, which binds to the same region of CD63, may interfere with the assembly of the CD63-AP-2 complex and thereby inhibit endocytosis of the protein from the plasma membrane. Indeed, the rate of MAb-induced internalization of CD63 was reduced in cells expressing high levels of the exogenously introduced wild-type syntenin-1 (Fig. 8A and D). Specifically, 30 min after the temperature shift, the intracellular staining of the anti-CD63 MAb was observed in only ∼16% of the syntenin-1-expressing cells compared to 100% in the nontransfected cells. As shown in Fig. 8, the differences were also apparent at the later time points: ∼37% and ∼60% of the anti-CD63 MAbs were internalized after cells were incubated at 37°C for one and four hours, respectively. The inhibitory effect of syntenin-1 on CD63 expression was even more pronounced in cells expressing the N-terminal deletion mutant of this protein. We did not observe any internalization of the anti-CD63 MAb even after 4 h of incubation at 37°C (Fig. 8B and D). In control experiments, we found that neither wild-type syntenin-1 nor the syntenin-1ΔN mutant changed the kinetics of internalization of labeled transferrin (Fig. 8C). Taken together, these results show that syntenin-1 plays an important role in the regulation of constitutive endocytosis of CD63.
FIG.8.
Syntenin-1 regulates internalization of CD63. HeLa cells were transiently transfected with the plasmids encoding HA-tagged variants of syntenin-1 (A to C). Forty-eight hours later, cells were surface labeled with the anti-CD63 MAb (A and B) or Texas Red-labeled transferrin (Tf-TexRed) (C) for 1 h at 4°C and then placed to 37°C for indicated time intervals. Cells were subsequently processed for the immunofluorescence staining as described in the legend for Fig. 5A, upper panels. Arrowheads point to cells expressing syntenin-1 constructs. Note the distinct peripheral staining with anti-CD63 MAb. The scale bar represents 10 μM. (D) Quantitative analysis of three independent internalization experiments with 70 to 80 transfected cells counted in each experiment. Shown are the results of the experiments with cells expressing wild-type (wt) syntenin-1. Expression of the syntenin-1ΔN mutant completely blocked internalization of the anti-CD63 MAb. Error bars represent standard errors of the means.
DISCUSSION
A considerable body of recent work has firmly established that TERM contribute to regulation of the activities of various transmembrane proteins (19, 29). While tetraspanin-dependent lateral clustering of associated proteins may be considered as one of the possible mechanisms underlying the modulatory activity of tetraspanins, TERM-associated cytoplasmic proteins (e.g., PKC and PI4-K) may also contribute to this phenomenon. In this report, we identified syntenin-1 as a new cytoplasmic component of TERM and showed that CD63 may function as a specific anchor that recruits syntenin-1 to TERM. Given the ability of syntenin-1 to interact with a number of cytoplasmic signaling proteins such as Rab GTPases and Ulk1 (40), the CD63-syntenin-1 complex may represent a novel horizontal pathway of regulation within TERM.
The two PDZ domains of syntenin-1 are known to bind to a variety of target proteins by employing their degenerate specificities and cooperative interactions (8, 16, 23, 24). For the first time we have identified a protein that preferentially targets the PDZ1 domain of syntenin-1 within the PDZ tandem. Although it was recently reported that the C-terminal peptide of NF-2/merlin can bind preferentially to the isolated recombinant PDZ1 domain (but not PDZ2) in biochemical assays (24), the structural basis of this interaction has not been studied. We noticed that the C-terminal NF-2 peptide (VAFFEEL) used in this study has a pronounced negative potential, and may, therefore, bind to the positively charged PDZ1 of syntenin-1 in an electrostatic manner. A canonical mode of peptide binding, observed in a number of PDZ domain-containing proteins, assumes that the bound peptides adopt a regular secondary structure in the form of an additional antiparallel strand between the helix α2 and the adjoining strand β2 of the PDZ domains (10). However, recent reports describe noncanonical modes of peptide binding to PDZ domains of NHERF, X11/Mint, and syntenin-1 (16, 25, 31) where peptide binding occurs without the formation of a regular β strand. In the case of syntenin-1, the noncanonical mode of binding with a synthetic peptide is facilitated by structural differences within PDZ1. These differences include an unusually narrow peptide binding groove compared to that of PDZ2 (Fig. 4B) and a hydrogen bond between a side chain of His175 with the backbone amide of Leu129 (16), both of which prevent a peptide from pairing in a canonical β-strand conformation.
In the context of the syntenin-1 structures (16), our NMR experiments with the CD63 C-terminal peptide are consistent with a noncanonical mode of binding to the syntenin-1 PDZ1 domain. That is, the chemical shift perturbations were localized to the residues in the vicinity of the carboxylate binding loop, with no significant perturbations in residues corresponding to those of canonical peptide binding grooves (Leu129, Lys130, His175, and His171 on β2 and α2). Notably, we also observed slight chemical shift changes in the PDZ2 domain upon titration with the CD63-C peptide. Furthermore, G210D mutation in PDZ2 had a negative effect on the interaction between CD63 and syntenin-1 (Fig. 3). Importantly, NMR analysis of the G210D mutant of syntenin-1 PDZ12 indicates that there are no major conformational changes in the structure of the protein, with the chemical shift perturbations being primarily confined to the vicinity of the mutation. However, we also observed subtle chemical shift changes in the carboxylate binding groove of PDZ1 domain (results not shown). Taken together, these data suggest that there may be a weak allosteric interaction between the PDZ domains of syntenin-1.
While the engagement of PDZ domains is essential for most interactions involving syntenin-1, our data illustrate for the first time the importance of the C-terminal 12 amino acids in the PDZ domain-dependent interactions. There are two possibilities that may explain how the region outside the PDZ domains can control the interaction involving syntenin-1. Firstly, we found that the amount of syntenin-1ΔC at the plasma membrane is markedly decreased compared to that of the wild-type protein. Thus, the impaired recruitment of the mutant to the plasma membrane could shift a stoichiometric balance between CD63 and syntenin-1 and prevent interaction between the proteins in cells. Secondly, our pull-down and ELISA experiments clearly indicate that deletion of the C-terminal region also affects a direct contact between the proteins. These data suggest that the 281-to-293 region is involved in allosteric regulation of the association between syntenin-1 and CD63. For example, it may have a direct stabilizing effect on binding of the cytoplasmic tail of CD63 to PDZ1 (see above).
While the interactions of class I ligands (Ser/Thr-X-Φ, where Φ represents a C-terminal hydrophobic residue) with PDZ domains can be influenced by ligand phosphorylation (1, 41), the regulatory mechanisms that control the associations involving class II (Φ-X-Φ) and noncanonical ligands remain unknown. One possibility would be that those interactions are controlled by a direct competition between various ligands for a given PDZ domain-containing protein. This mode of regulation would rely on the events that modulate the local concentrations of the alternative ligands. Previous studies have suggested that the C terminus of merlin can also bind the syntenin-1 PDZ1 domain with relatively high affinity (21, 24), and weak interactions with syndecan and neurexin have also been described (16). Thus, it is conceivable that fluctuations in the expression of alternative ligands may influence the association of CD63 with syntenin-1. However, our results indicate that elevated expression of merlin in cells does not influence the formation of the CD63-syntenin-1 complex. These data suggest that either these complexes are spatially separated in cells or the affinity of CD63 tail to syntenin-1 is higher than that of NF-2/merlin. It was also reported that PDZ1 of syntenin-1 binds PI(4,5)P2 (43). Although the structural basis of this interaction is unclear, mutational analysis suggests involvement of residues Lys119, Ser171, Asp172, and Lys173 (43). Our NMR results of the CD63-C interaction show a slight chemical shift change only for Lys119, thus suggesting that the proposed PI(4,5)P2 interaction would not exclude CD63 binding. Accordingly, we found overexpression of various isoforms of PIP5-K [enzymes responsible for conversion of PI(4)P into PI(4,5)P2] does not have an effect on the interaction of CD63 with syntenin-1 (results are not shown).
The tyrosine-based sorting signal at the C terminus of CD63, G-Y-E-V-M, interacts with μ2 and μ3 subunits of the AP-2 and AP-3 adaptor complexes (34). Crystal structures of μ2 with the peptides containing the Y-X-X-Φ sorting signal reveal that both tyrosine and the bulky hydrophobic amino acid at theY + 3 position are engaged in binding to the surface of two parallel β-sheet strands of the adaptor subunit (32, 35). In contrast, both classical and noncanonical modes of interaction of PDZ domains with their ligands require positioning of the last two amino acids of the peptide into the interactive groove (16). Hence, it is highly likely that the interactions of CD63 with syntenin-1 and μ subunits of AP complexes are mutually exclusive. Consequently, one would expect syntenin-1 to influence the AP-2/AP-3-dependent trafficking of CD63. Although modulation of syntenin-1 (i.e., overexpression or knocking down with siRNA) does not have an obvious effect on the steady-state distribution of CD63 in HeLa cells (results are not shown), the rate of internalization of CD63 was decreased in cells expressing high levels of syntenin-1 (Fig. 8). Importantly, this effect is dependent on the interaction of syntenin-1 with CD63. Our data thus highlight a hitherto unknown function of syntenin-1 as a regulator of constitutive endocytosis. Given that three other transmembrane partners of syntenin-1, including ephrin-B2 (30), CD6 (15), and mGluR7a (20), have the canonical Y-X-X-Φ sorting signal juxtaposed to or overlapping with the PDZ binding motif, one would expect that endocytosis/trafficking of these proteins is also influenced by syntenin-1 interactions.
Although the most plausible model suggests that syntenin-1 would function as a “molecular plug” that physically precludes the binding of AP-2 and AP-3 complexes to the cytoplasmic tail of CD63, one cannot exclude that the effect of syntenin-1 on endocytosis is due to modification rather than suppression of the internalization pathway. In fact, a decrease in the internalization rate (but not a complete block of the process) may indicate that syntenin-1 diverts endocytosis from the fast, AP-2-dependent to a slow, AP-2-independent route. This pathway(s) may involve the N-terminal 100 amino acids of syntenin-1, deletion of which completely blocked antibody-induced internalization of CD63 but had no effect on the formation of the CD63-syntenin-1 complex. Notably, the existence of an AP-independent sorting pathway for the proteins containing the G-Y-X-X-Φ motif has been predicted in a recent work by Janvier and Bonifacino (22).
In summary, this report describes syntenin-1 as a new component of tetraspanin microdomains and identifies CD63 as one of its specific targets. Importantly, we demonstrated the functional relevance of this association. As growing evidence strongly suggests that tetraspanins are involved in regulating the membrane dynamics of their transmembrane partners, the association with syntenin-1 may represent a key determinant of this activity.
Acknowledgments
We are very grateful to all our colleagues for their generous gifts of the reagents that were used in this study. We acknowledge HWB-NMR, University of Birmingham, for NMR spectrometer access, and we thank Felican Dancea for useful discussions. The backbone 1H and 15N assignments of the syntenin-1 PDZ12 domain were kindly provided by Tomasz Cierpicki (University of Virginia School of Medicine).
This work was supported by CR UK grants C1322/A2945 and C1322/A5705 (to F.B.), the BBSRC grant 2003:574 (to N.A.H. and F.B.), and grants from the BBSRC (10714) and Wellcome Trust (071684) (to M.O.).
Footnotes
Published ahead of print on 14 August 2006.
REFERENCES
- 1.Adey, N. B., L. Huang, P. A. Ormonde, M. L. Baumgard, R. Pero, D. V. Byreddy, S. V. Tavtigian, and P. L. Bartel. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 60:35-37. [PubMed] [Google Scholar]
- 2.Amezcua, C. A., S. M. Harper, J. Rutter, and K. H. Gardner. 2002. Structure and interactions of PAS kinase N-terminal PAS domain: model for intramolecular kinase regulation. Structure 10:1349-1361. [DOI] [PubMed] [Google Scholar]
- 3.Berditchevski, F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 115:4143-4151. [DOI] [PubMed] [Google Scholar]
- 4.Berditchevski, F., G. Bazzoni, and M. E. Hemler. 1995. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 270:17784-17790. [DOI] [PubMed] [Google Scholar]
- 5.Berditchevski, F., S. Chang, J. Bodorova, and M. E. Hemler. 1997. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272:29174-29180. [DOI] [PubMed] [Google Scholar]
- 6.Berditchevski, F., and E. Odintsova. 1999. Characterization of integrin/tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J. Cell Biol. 146:477-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berditchevski, F., K. F. Tolias, K. Wong, C. L. Carpenter, and M. E. Hemler. 1997. Novel link between integrins, TM4SF proteins (CD63, CD81) and phosphatidylinositol 4-kinase. J. Biol. Chem. 272:2595-2598. [DOI] [PubMed] [Google Scholar]
- 8.Boukerche, H., Z. Z. Su, L. Emdad, P. Baril, B. Balme, L. Thomas, A. Randolph, K. Valerie, D. Sarkar, and P. B. Fisher. 2005. mda-9/syntenin: a positive regulator of melanoma metastasis. Cancer Res. 65:10901-10911. [DOI] [PubMed] [Google Scholar]
- 9.Cierpicki, T., J. H. Bushweller, and Z. S. Derewenda. 2005. Probing the supramodular architecture of a multidomain protein: the structure of syntenin in solution. Structure 13:319-327. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, D. A., A. Lee, J. Lewis, E. Kim, M. Sheng, and R. MacKinnon. 1996. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell 85:1067-1076. [DOI] [PubMed] [Google Scholar]
- 11.Duffield, A., E. J. Kamsteeg, A. N. Brown, P. Pagel, and M. J. Caplan. 2003. The tetraspanin CD63 enhances the internalization of the H,K-ATPase beta-subunit. Proc. Natl. Acad. Sci. USA 100:15560-15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escola, J.-M., M. J. Kleijmeer, W. Stoorvogel, J. Griffith, O. Yoshie, and H. J. Geuze. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273:20121-20127. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Larrea, J., A. Merlos-Suarez, J. M. Urena, J. Baselga, and J. Arribas. 1999. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-alpha to the cell surface. Mol. Cell 3:423-433. [DOI] [PubMed] [Google Scholar]
- 14.Fialka, I., P. Steinlein, H. Ahorn, G. Bock, P. D. Burbelo, M. Haberfellner, F. Lottspeich, K. Paiha, C. Pasquali, and L. A. Huber. 1999. Identification of syntenin as a protein of the apical early endocytic compartment in Madin-Darby canine kidney cells. J. Biol. Chem. 274:26233-26239. [DOI] [PubMed] [Google Scholar]
- 15.Gimferrer, I., A. Ibanez, M. Farnos, M. R. Sarrias, R. Fenutria, S. Rosello, P. Zimmermann, G. David, J. Vives, C. Serra-Pages, and F. Lozano. 2005. The lymphocyte receptor CD6 interacts with syntenin-1, a scaffolding protein containing PDZ domains. J. Immunol. 175:1406-1414. [DOI] [PubMed] [Google Scholar]
- 16.Grembecka, J., T. Cierpicki, Y. Devedjiev, U. Derewenda, B. S. Kang, J. H. Bushweller, and Z. S. Derewenda. 2006. The binding of the PDZ tandem of syntenin to target proteins. Biochemistry 45:3674-3683. [DOI] [PubMed] [Google Scholar]
- 17.Grootjans, J. J., P. Zimmermann, G. Reekmans, A. Smets, G. Degeest, J. Durr, and G. David. 1997. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA 94:13683-13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemler, M. E. 2003. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19:397-422. [DOI] [PubMed] [Google Scholar]
- 19.Hemler, M. E. 2005. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6:801-811. [DOI] [PubMed] [Google Scholar]
- 20.Hirbec, H., O. Perestenko, A. Nishimune, G. Meyer, S. Nakanishi, J. M. Henley, and K. K. Dev. 2002. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J. Biol. Chem. 277:15221-15224. [DOI] [PubMed] [Google Scholar]
- 21.Jannatipour, M., P. Dion, S. Khan, H. Jindal, X. Fan, J. Laganiere, A. H. Chishti, and G. A. Rouleau. 2001. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J. Biol. Chem. 276:33093-33100. [DOI] [PubMed] [Google Scholar]
- 22.Janvier, K., and J. S. Bonifacino. 2005. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell. 16:4231-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, B. S., D. R. Cooper, Y. Devedjiev, U. Derewenda, and Z. S. Derewenda. 2003. Molecular roots of degenerate specificity in syntenin's PDZ2 domain: reassessment of the PDZ recognition paradigm. Structure 11:845-853. [DOI] [PubMed] [Google Scholar]
- 24.Kang, B. S., D. R. Cooper, F. Jelen, Y. Devedjiev, U. Derewenda, Z. Dauter, J. Otlewski, and Z. S. Derewenda. 2003. PDZ tandem of human syntenin: crystal structure and functional properties. Structure 11:459-468. [DOI] [PubMed] [Google Scholar]
- 25.Karthikeyan, S., T. Leung, G. Birrane, G. Webster, and J. A. Ladias. 2001. Crystal structure of the PDZ1 domain of human Na(+)/H(+) exchanger regulatory factor provides insights into the mechanism of carboxyl-terminal leucine recognition by class I PDZ domains. J. Mol. Biol. 308:963-973. [DOI] [PubMed] [Google Scholar]
- 26.Koo, T. H., J. J. Lee, E. M. Kim, K. W. Kim, H. D. Kim, and J. H. Lee. 2002. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene 21:4080-4088. [DOI] [PubMed] [Google Scholar]
- 27.Koroll, M., F. G. Rathjen, and H. Volkmer. 2001. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J. Biol. Chem. 276:10646-10654. [DOI] [PubMed] [Google Scholar]
- 28.Kraulis, P. J., P. J. Domaille, S. L. Campbell-Burk, T. Van Aken, and E. D. Laue. 1994. Solution structure and dynamics of ras p21.GDP determined by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 33:3515-3531. [DOI] [PubMed] [Google Scholar]
- 29.Levy, S., and T. Shoham. 2005. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 5:136-148. [DOI] [PubMed] [Google Scholar]
- 30.Lin, D., G. D. Gish, Z. Songyang, and T. Pawson. 1999. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J. Biol. Chem. 274:3726-3733. [DOI] [PubMed] [Google Scholar]
- 31.Long, J. F., W. Feng, R. Wang, L. N. Chan, F. C. Ip, J. Xia, N. Y. Ip, and M. Zhang. 2005. Autoinhibition of X11/Mint scaffold proteins revealed by the closed conformation of the PDZ tandem. Nat. Struct. Mol. Biol. 12:722-728. [DOI] [PubMed] [Google Scholar]
- 32.Owen, D. J., and P. R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelchen-Matthews, A., G. Raposo, and M. Marsh. 2004. Endosomes, exosomes and Trojan viruses. Trends Microbiol. 12:310-316. [DOI] [PubMed] [Google Scholar]
- 34.Rous, B. A., B. J. Reaves, G. Ihrke, J. A. Briggs, S. R. Gray, D. J. Stephens, G. Banting, and J. P. Luzio. 2002. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol. Biol. Cell 13:1071-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royle, S. J., O. S. Qureshi, L. K. Bobanovic, P. R. Evans, D. J. Owen, and R. D. Murrell-Lagnado. 2005. Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J. Cell Sci. 118:3073-3080. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar, D., H. Boukerche, Z. Z. Su, and P. B. Fisher. 2004. mda-9/syntenin: recent insights into a novel cell signaling and metastasis-associated gene. Pharmacol. Ther. 104:101-115. [DOI] [PubMed] [Google Scholar]
- 37.Shoham, T., R. Rajapaksa, C. Boucheix, E. Rubinstein, J. C. Poe, T. F. Tedder, and S. Levy. 2003. The tetraspanin CD81 regulates the expression of CD19 during B cell development in a postendoplasmic reticulum compartment. J. Immunol. 171:4062-4072. [DOI] [PubMed] [Google Scholar]
- 38.Takino, T., H. Miyamori, N. Kawaguchi, T. Uekita, M. Seiki, and H. Sato. 2003. Tetraspanin CD63 promotes targeting and lysosomal proteolysis of membrane-type 1 matrix metalloproteinase. Biochem. Biophys. Res. Commun. 304:160-166. [DOI] [PubMed] [Google Scholar]
- 39.Thery, C., M. Boussac, P. Veron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166:7309-7318. [DOI] [PubMed] [Google Scholar]
- 40.Tomoda, T., J. H. Kim, C. Zhan, and M. E. Hatten. 2004. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 18:541-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez, F., S. R. Grossman, Y. Takahashi, M. V. Rokas, N. Nakamura, and W. R. Sellers. 2001. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 276:48627-48630. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, X. A., A. L. Bontrager, and M. E. Hemler. 2001. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J. Biol. Chem. 276:25005-25013. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann, P., K. Meerschaert, G. Reekmans, I. Leenaerts, J. V. Small, J. Vandekerckhove, G. David, and J. Gettemans. 2002. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol. Cell 9:1215-1225. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann, P., D. Tomatis, M. Rosas, J. Grootjans, I. Leenaerts, G. Degeest, G. Reekmans, C. Coomans, and G. David. 2001. Characterization of syntenin, a syndecan-binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol. Biol. Cell 12:339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann, P., Z. Zhang, G. Degeest, E. Mortier, I. Leenaerts, C. Coomans, J. Schulz, F. N′Kuli, P. J. Courtoy, and G. David. 2005. Syndecan recyling [sic] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 9:377-388. [DOI] [PubMed] [Google Scholar]