Abstract
Transcription activation of some Saccharomyces cerevisiae genes is paralleled by their repositioning to the nuclear periphery, but the mechanism underlying gene anchoring is poorly defined. We show that the nuclear pore complex-associated Mlp1p and the shuttling mRNA export receptor Mex67p contribute to the stable association of the activated GAL10 and HSP104 genes with the nuclear periphery. However, we find no obligatory link between gene positioning and gene expression. Furthermore, gene anchoring correlates with the cotranscriptional recruitment of Mex67p to transcribing genes. Notably, the association of Mex67p with chromatin is not mediated by RNA. Interestingly, a mutant GAL2 gene lacking the coding region is still able to recruit Mex67p upon transcriptional activation and to relocate to the nuclear periphery. Together these data suggest that, at least for GAL2, nascent messenger ribonucleoprotein does not play a major role in gene anchoring and that the early recruitment of Mex67p contributes to gene repositioning by virtue of an RNA-independent process.
A growing number of recent studies point to a functional relationship between gene expression and nuclear organization of chromatin. Indeed, the nucleus is subdivided into spatially defined domains, and the nonrandom distribution of chromosomes within these domains is thought to regulate their transcriptional state (8, 25). Notably, the nuclear periphery has first been viewed as a transcriptionally repressive zone. In Saccharomyces cerevisiae, telomeres and mating-type loci, consisting of silent chromatin, concentrate in nuclear peripheral regions (16), and artificial tethering of nonsilenced DNA to the envelope induces its repression (2). In particular, the nuclear pore complex (NPC) has been implicated in perinuclear gene silencing and maintenance of gene expression states. Indeed, loss of a subset of NPC components, including the nuclear basket-anchored Mlp1p and Mlp2p, results in the activation of subtelomeric reporter genes (10, 12, 20). However, another study showed that artificial tethering of nuclear transport factors to a partially silenced mating-type locus allowed its expression. Thus, recruitment of genes to the nuclear periphery can also have important effects on their activation (24). Consistent with these observations, genome-wide analyses of NPC-bound loci identified preferential association of highly transcribed genes with the nuclear periphery. These studies also showed that transcriptional activation of genes induced by galactose or α-factor is accompanied by their relocation from the nuclear interior to the nuclear periphery (5, 6). INO1 gene activation is also paralleled by its repositioning to the periphery, and this relocation contributes to optimal INO1 gene expression (3).
Importantly, recent studies pointed to a direct physical link between Sus1p, a component of the SAGA histone deacetylase coactivator complex, and the Sac3-Thp1 complex, which is part of the mRNA export machinery associated with pores (31). These data together suggested that transcription regulators could control the recruitment of genes to the nuclear periphery, possibly linking gene repositioning to optimal activation. However, a strict and systematic dependence of gene expression on peripheral positioning has not been demonstrated. More generally, the molecular basis of transcription-induced gene repositioning is poorly understood and whether it is the cause or consequence of transcription activation is still unclear. Several observations indicated a possible role for the nascent messenger ribonucleoprotein (mRNP) in stabilizing the association of a gene with the nuclear periphery. First, mRNP components physically interact with the NPC-associated Mlp1p and Mlp2p proteins (11, 17, 43), and the results of chromatin immunoprecipitation (ChIP) experiments suggest that Mlp1p associates with transcribing genes in an RNA-dependent manner (5). These observations raised the possibility that Mlp proteins contribute to gene anchoring by interacting with nascent transcripts. Second, several mRNA export factors bind mRNA cotranscriptionally (28, 38, 45), consistent with a potential role for growing mRNPs in bridging active genes to the NPC. Moreover, we recently showed that the mRNA export receptor Mex67p, which promotes the translocation of mRNP complexes through the NPC (35), is also recruited cotranscriptionally (19). The association of Mex67p with transcribing genes and its ability to interact with various pore components raised the possibility that mRNP-bound Mex67p helps the anchoring of transcribing loci to the nuclear periphery.
To test the potential roles of Mlp1p and Mex67p in gene anchoring, we compared the localization of inducible genes in wild-type (WT) and Δmlp1 or mex67-5 mutant cells (35). The results indicate that both Mlp1p and Mex67p are required for efficient anchoring of the galactose-inducible GAL10 and stress-inducible HSP104 genes; however, gene anchoring appears to be not essential for the transcription of these two genes. Notably, loss of gene anchoring in the mex67-5 mutant correlates with the inability of the mex67-5 mutant protein to associate with the transcribing genes. Moreover, we find that transcription-induced NPC anchoring of the GAL2 gene does not require the mRNA-coding region, suggesting that nascent mRNP may not be essential for bridging an interaction between an active gene and the NPC. These data and the observation that the cotranscriptional binding of Mex67p is RNA independent suggest that Mex67p may contribute to gene anchoring by interacting with activated chromatin rather than nascent RNA.
MATERIALS AND METHODS
Plasmid constructions.
To insert LacO repeats downstream of the GAL2, GAL10, and HSP104 genes, the 3′ untranslated region (3′UTR) region of each gene was cloned in front of the LacO repeats carried by the integrating plasmid pAFS52 (TRP1) (21) to generate pFS2912, pFS2913, and pFS3013, respectively. For directed insertion, these plasmids were linearized by cleavage of a unique restriction site within the 3′UTR and transformed into relevant strains. Wild-type Mlp1p was expressed from plasmid pFS2614 (pRS316 LEU2 CEN), isolated in a synthetic lethal screen and carrying the MLP1 gene as the only complete open reading frame (D. Zenklusen and F. Stutz, unpublished data).
Yeast strains.
The yeast strains used in this study are listed in Table 1. The mex67-5 strain contains an integrated mutant gene (26). Wild-type and mex67-5 genes were genomically tagged with green fluorescent protein (GFP)-Kanr by homologous recombination (29). GA1320-Δmlp1 and GA1320-mex67-5 strains were obtained by crossing strain GA1320 (LacI-GFP-HIS3 Nup49-GFP) with the mlp1::Kanr (S. Gasser lab) and mex67-5 (26) strains. The GAL10 and HSP104 loci were subsequently tagged with LacO repeats in the GA1320, GA1320-Δmlp1, and GA1320-mex67-5 strains by transformation of linearized pFS2913 and pFS3013, respectively, followed by selection on Trp− plates. Insertions were confirmed by PCR on genomic DNA. The Δgal2 strain was obtained by transformation and homologous recombination of a PCR-generated loxP-URA3 cassette (18) carrying ends complementary to the 5′ and 3′ ends of the GAL2-coding region in the WT GAL2-LacO strain (FSY2817). The forward and reverse primers used were GAL2-loxP-F1 (OFS1071) (5′AACACAAGAT TAACATAATA AAAAAAATAA TTCTTTCATAC AGCTGAAGCT TCGTACGC 3′) and GAL2-loxP-R1 (OFS1072) (5′AAAATTAAGA GAGATGATGG AGCGTCTCAC TTCAAACGCAG CATAGGCCACT AGTGGATCTG3′), respectively. The Δgal2-3′UTR and Δ3′UTR strains were constructed using the same strategy with the forward primers GAL2-loxP-F1 (OFS1071) and GAL2-3′UTR-loxP-F1 (OFS1113) (5′TTACAACATG ACGACAAACC GTGGTACAAG GCCATGCTAG AATAACAGCT GAAGCTTCGT ACGC3′), respectively, in combination with the reverse primer GAL2-3′UTR-loxP-R1 (OFS1104) (5′GTTAGCTCAG GAATTCAACT GGAAGAAAGT CCAGGCAAGT ACCTGACGCA TAGGCCACTA GTGGATCTG3′). The Δprom-gal2 and ΔUAS-3′UTR strains were obtained similarly using the forward primers −200GAL2-loxP-F1 (OFS1103) (5′CAAACATTTC GCAGGCTAAA ATGTGGAGAT AGGATAAGTT TTGTAGCAGC TGAAGCTTCG TACGC3′) and −550GAL2-loxP-F1 (OFS1102) (5′CAAAAGGTAC TCAACGTCAA TTCGGAAAGC TTCCTTCCGG AATGGCCAGC TGAAGCTTCG TACGC3′), with the reverse primer GAL2-loxP-R1 (OFS1072) and GAL2-3′UTR-loxP-R1 (OFS1104), respectively. The URA3 selective marker was subsequently excised by expression of Cre recombinase and selection of colonies on 5-fluoroorotic acid (18). Deletions were confirmed by PCR on genomic DNA.
TABLE 1.
Yeast strains used in this study
| Code | Name | Genotype | Reference or source |
|---|---|---|---|
| W303 background | |||
| W303 | Wild type | MATaade2 his3 leu2trp1ura3 | |
| FSY1982 | mex67-5 | MATaade2 his3 leu2trp1 ura3mex67-5 (integrated mutant) | 26 |
| FSY2395 | MEX67-GFP | MATaade2 his3 leu2trp1 ura3 MEX67-GFP-Kanr | This study |
| FSY2455 | mex67-5-GFP | MATaade2 his3 leu2trp1 ura3mex67-5-GFP-Kanr | This study |
| FSY1651 | HA-Sub2 | MATaade2 his3 leu2trp1 ura3sub2::HIS3 <YCplac111-HA-SUB2> | 45 |
| GA1320 background | |||
| GA1320 | LacI-GFP Nup49-GFP | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP | 21 |
| FSY2811 | WT GAL10-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP GAL10-LacO-TRP1 | This study |
| FSY2812 | WT HSP104-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP HSP104-LacO-TRP1 | This study |
| FSY2813 | Δmlp1GAL10-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP GAL10-LacO-TRP1mlp1::Kanr | This study |
| FSY2814 | Δmlp1HSP104-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP HSP104-LacO-TRP1mlp1::Kanr | This study |
| FSY2815 | mex67-5GAL10-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP GAL10-LacO-TRP1mex67-5 | This study |
| FSY2816 | mex67-5HSP104-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP HSP104-LacO-TRP1mex67-5 | This study |
| FSY2817 | WT GAL2-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP GAL2-LacO-TRP1 | This study |
| FSY2818 | Δgal2-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP Δgal2-LacO-TRP1 | This study |
| FSY3042 | Δ3′UTR-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP Δ3′UTR-LacO-TRP1 | This study |
| FSY3041 | Δgal2-3′UTR-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP Δgal2-3′UTR-LacO-TRP1 | This study |
| FSY2821 | Δprom-gal2-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP Δprom-gal2-LacO-TRP1 | This study |
| FSY2822 | ΔUAS-3′UTR-LacO | MATaade2 his3 leu2trp1 ura3 LacI-GFP-HIS3 Nup49-GFP ΔUAS-3′UTR-LacO-TRP1 | This study |
Live fluorescence microscopy and quantification.
Wild-type, mex67-5, or Δmlp1 strains bearing LacO repeats downstream of GAL10, HSP104, or GAL2 and expressing integrated LacI-GFP repressor and Nup49-GFP fusions were grown and induced as indicated in figure legends. Live microscopy was performed as described previously (41). Briefly, a Zeiss Axioplan microscope was used to capture 21-image stacks of 95-nm step size. In the focal plane in which the GFP spot is brightest, its position was mapped to one of three concentric zones of equal surface by dividing the spot-to-pore distance by the nuclear diameter. A gene was scored as being located at the nuclear periphery when it was present in the most peripheral zone, i.e., when the distance between the spot and the nuclear periphery was <0.184 of 1/2 nuclear diameter. A random distribution of the tagged gene would result in a 33% occurrence in each of the three zones. Values above 33% indicate enrichment in this particular zone. Statistical analysis used a proportion method to compare zone 1 percentages between two different samples. Significance was determined with a 95% confidence interval.
Northern blot analyses.
Total RNA was extracted from the cultures used for gene localization using a hot phenol method, and 10 to 15 μg was fractionated on 1% agarose-formaldehyde gels or 8% polyacrylamide-urea gels. Agarose gels were transferred to Hybond membranes by vacuum blotting, whereas polyacrylamide gels were transferred by semidry blotting as described previously (40). Membranes were hybridized with randomly primed labeled PCR fragments using standard protocols. The GAL1 and HSP104 probes correspond to protein-coding regions. The GAL2 and 3′UTR probes correspond to positions 920 to 1600 of the GAL2 protein-coding region and to positions +123 to +550 of the GAL2 3′UTR, respectively. The signals were quantified with a Bio-Rad Instant Imager and normalized to those obtained with probes specific for 18S rRNA or actin mRNA hybridized to the same membranes.
Chromatin immunoprecipitations.
For ChIP analysis of HSP104, strains were grown in yeast extract-peptone-dextrose (YEPD) rich medium to an optical density at 600 nm (OD600) of 0.8 to 1, divided into three equal cultures for subsequent analysis in triplicate, treated with 10% ethanol for 30 min at 25°C, and cross-linked for 10 min with formaldehyde. For ChIP analysis of GAL10, strains were grown at 25°C in yeast extract-peptone (YEP) plus 2% raffinose to an OD600 of 0.7, divided into three equal cultures, induced with 2% galactose for 2.5 h, mixed with 1 volume of YEP plus 2% galactose at 25°C or 49°C and either kept at 25°C or incubated for 15 min at 37°C, and cross-linked for 10 min at the same temperatures. For ChIP analysis of GAL2, the relevant strains were precultured in YEP plus 2% raffinose as described above, divided into three equal cultures, induced with 2% galactose for 2.5 h, and cross-linked for 10 min. Cross-linking was reduced to 5 min when preparing ChIP extracts for RNase sensitivity tests. In all cases, glass bead extracts were sonicated so as to shear the chromatin down to 300- to 400-bp fragments. Sonicated extracts (1 mg protein in 1-ml final volume) were immunoprecipitated with polyclonal antibodies against Mex67p (gift from C. Dargemont), TATA binding protein (TBP) (gift from M. Collart,) or a monoclonal antibody against RNA polymerase II (PolII) C-terminal domain (8WG16 from Covance). Each immunoprecipitation was performed three times using three independent extracts. Immunoprecipitated DNA was quantified by real-time PCR as described earlier (45) using primer pairs described at http://www.unige.ch/sciences/biologie/bicel/stutz/Dieppois_MCB06_Suppl_Mat.pdf.To calculate the increase in signal in the different gene regions, the absolute values obtained by quantitative PCR were normalized to the values obtained with the nontranscribed intergenic region, arbitrarily set to 1. To evaluate the importance of RNA in ChIP, cross-linked and sonicated extracts, prepared from the galactose-induced HA-Sub2p strain FSY1651, were immunoprecipitated with antibodies against hemagglutinin (HA) (monoclonal 16B12; Covance), RNA PolII, or Mex67p. Immunoprecipitated extracts on beads were washed and resuspended in 1 ml buffer alone or containing 15 μl RNase Cocktail TM (500 U/ml RNase A and 20,000 U/ml RNase T1; Ambion) and incubated for 30 min at room temperature. Beads were washed, and the DNA was purified and quantified as described above.
Western blotting.
Total protein extracts were prepared from aliquots of cultures used for ChIP analysis prior to cross-linking, fractionated on sodium dodecyl sulfate-polyacrylamide gels, and examined by Western blotting with polyclonal antibodies specific for Mex67p (1:10,000) (a gift from C. Dargemont) and TBP (1:2,000) (a gift from M. Collart).
RESULTS
Mlp1p is required for efficient anchoring of the activated GAL10 and HSP104 genes to the NPC.
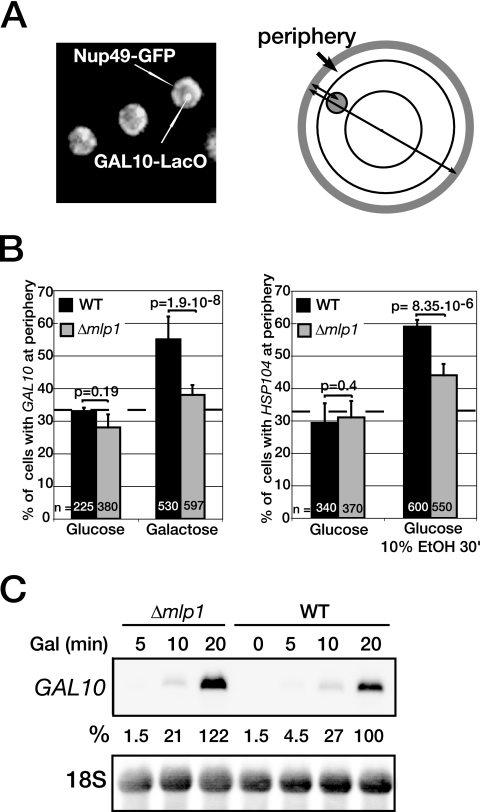
The NPC-associated Mlp1p and related Mlp2p could be involved in stable association of active genes with the NPC, as they were proposed to contact chromatin and/or nascent transcripts, as well as to influence the expression of genes located at the nuclear periphery (5, 6, 9, 17, 43). To test whether Mlp1p contributes to gene anchoring, two inducible genes, GAL10 and HSP104 were localized in wild-type and Δmlp1 cells. GAL10 is part of a cluster of galactose-inducible genes that relocate to the nuclear periphery upon transcriptional activation (6), and HSP104 transcription can be activated by heat stress or ethanol at 25°C (32). To locate the genes with respect to the nuclear periphery, LacO repeats were inserted 600 to 700 bp downstream of the GAL10 and HSP104 loci in wild-type or Δmlp1 strains that also expressed chromosomally encoded GFP-LacI repressor and GFP-Nup49 (Fig. 1A) (21). The GAL10-LacO strains were grown in glucose- or galactose-containing medium at 25°C followed by gene localization in living cells by fluorescence microscopy. Consistent with earlier observations (6), transcription activation of GAL10 in wild-type cells promoted the repositioning of the GAL10 locus, as the percentage of cells with the tagged gene at the periphery increased from 30% in glucose to 55% in galactose (Fig. 1B, left panel). Similarly, transcription activation of HSP104 with ethanol in HSP104-LacO wild-type cells resulted in the repositioning of the locus to the nuclear periphery (from 30% to 60%) (Fig. 1B, right panel). Ethanol is unlikely to trigger general chromatin reorganization, as it had no effect on the localization of the GAL10 locus (data not shown). Interestingly, for both conditions, the absence of Mlp1p resulted in a significant loss of peripheral localization for these two genes (from 55% to 38% for GAL10 and from 60% to 44% for HSP104) (Fig. 1B, left and right panels). Northern blot analyses showed that neither GAL10 nor HSP104 mRNA levels were substantially affected in the absence of Mlp1p (Fig. 1C and data not shown). These results indicate first that Mlp1p contributes to transcription-induced GAL10 and HSP104 gene anchoring and second that GAL10 and HSP104 gene expression does not require stable association with the NPC.
FIG. 1.
Mlp1p is required for efficient transcription-induced GAL10 and HSP104 gene relocation. (A) Yeast cells with GAL10 tagged with LacO repeats and expressing LacI-GFP and Nup49-GFP. Binding of LacI-GFP to LacO gives rise to the intense bright spot, whereas Nup49-GFP marks the nuclear periphery. A spot was scored as peripheral when located in the most peripheral 33% cross-sectional area of the nucleus (see Materials and Methods). (B) Localization of GAL10 or HSP104 tagged with LacO repeats in the wild-type (black bars) or Δmlp1 (gray bars) cells. To ensure isogenicity, WT and Δmlp1 strains were obtained by transforming the Δmlp1 strain with a plasmid expressing Mlp1p or an empty vector, respectively. GAL10-LacO cells were grown at 25°C in selective medium containing 2% raffinose to an OD600 of 0.5 and shifted to 2% glucose or 2% galactose for 2.5 h. HSP104-LacO cells were grown at 25°C in YEPD to an OD600 of 0.5 and examined before or after a 30-min treatment with 10% ethanol (10% EtOH 30′). A population of live cells was photographed with a fluorescence microscope. In each cell, the position of the GAL10 or HSP104 gene spot was defined with respect to the nuclear periphery. Graph bars represent the percentage of cells with the indicated tagged gene positioned at the periphery. Error bars were derived from three independent experiments; n is the total number of cells counted for each strain and condition. The broken line at 33% marks a random distribution; based on a proportional test, two distributions were considered as significantly different when P < 0.05. (C) Northern blot analysis of GAL10 mRNA in Δmlp1 and WT cells after induction with galactose (Gal) at 25°C for the indicated times. The GAL10 mRNA signal was normalized to 18S rRNA used as internal control and expressed as a percentage of the WT level, after 20 min in galactose at 25°C.
Mex67p is required for transcription-induced anchoring of GAL genes.
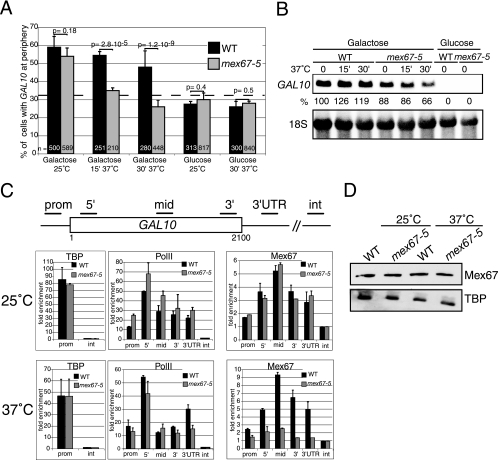
A similar approach was taken to investigate a contribution of the mRNA export receptor Mex67p in peripheral gene anchoring by comparing the wild-type strain and the mex67-5 strain. The mex67-5 strain is a temperature-sensitive mutant with a single amino acid substitution in the NTF2-like domain interacting with Mtr2p. At 37°C, the mex67-5 mutant protein dissociates from the nuclear periphery, and the cells exhibit a very rapid poly(A)+ RNA export defect (35). GAL10-LacO-tagged wild-type and mex67-5 strains were grown in glucose- or galactose-containing medium at 25°C and either kept at 25°C or shifted to 37°C for 15 or 30 min prior to fluorescence microscopy analysis (Fig. 2A). In both wild-type and mex67-5 cells at 25°C, the percentage of cells with GAL10 at the periphery increased from 25% in glucose to more than 55% in galactose, indicating efficient transcription-induced GAL10 gene anchoring at the permissive temperature. In contrast, a 15-min shift to 37°C strongly decreased peripheral GAL10 localization in mex67-5 cells compared to the wild type. After 30 min at 37°C, only 25% of mex67-5 cells were scored positive, a value similar to that in glucose, indicating complete loss of GAL10 gene association with the nuclear periphery. Comparable results were obtained with a LacO-tagged GAL2 gene located on a different chromosome (see below; also data not shown).
FIG. 2.
Mex67p is required for transcription-induced GAL10 gene relocation. (A) The GAL10 gene tagged with LacO was localized in wild-type (black bars) and mex67-5 (gray bars) cells also expressing LacI-GFP and Nup49-GFP. Cells were grown from an OD600 of 0.1 to 0.5 at 25°C in synthetic complete (SC) medium containing 2% raffinose and shifted to 2% glucose or 2% galactose for 2 h. Cells were then either kept at 25°C or shifted to 37°C for 15 min (15′) or 30 min (30′). GAL10 gene localization was defined as described in the legend to Fig. 1. (B) Northern blot analysis of GAL10 mRNA in wild-type and mex67-5 cells grown as described above for panel A and shifted to 37°C for the indicated times. The GAL10 mRNA signal was normalized to 18S rRNA and expressed as a percentage of the wild-type value before the shift to 37°C. (C) Association of TATA binding protein, RNA polymerase II, and Mex67p with galactose-induced GAL10 in wild-type and mex67-5 cells at 25°C and 37°C. Cross-linked and sonicated extracts were immunoprecipitated with antibodies against TBP, PolII, or Mex67p. Coprecipitating DNA was amplified by real-time PCR with primers specific for the GAL10 promoter (prom), 5′, middle (mid), 3′, 3′UTR, and a nontranscribed intergenic region (int), as indicated. The relative enrichment of the GAL10 gene segments in each ChIP was expressed as the increase with respect to the nontranscribed intergenic region value, arbitrarily set to 1. Values correspond to the means of three independent experiments. Bars correspond to standard deviations. (D) Western blot analysis of Mex67p levels in wild-type and mex67-5 extracts from cells used for ChIP. TBP was used as an internal loading control.
To examine the relationship between gene localization and expression, GAL10 transcripts were analyzed by Northern blotting. mRNA levels were somewhat lower in mex67-5 cells than in wild-type cells at 25°C and decreased slightly (from 88% to 66% of the wild-type level) in the mutant shifted to 37°C for 30 min (Fig. 2B). ChIP experiments revealed comparable amounts of TBP associated with the GAL10 promoter in wild-type and mex67-5 cells at 25°C or 37°C, and the association of RNA PolII with the coding region was slightly reduced in mex67-5 cells after 30 min at 37°C (Fig. 2C). The lower GAL10 mRNA levels in mex67-5 cells at 37°C could be due, at least in part, to reduced transcription but also to mRNA instability as a result of the mRNA export block (see Discussion). In any case, the loss of GAL10 from the periphery was more pronounced and occurred faster than the decrease in mRNA levels. These observations indicate that association with the nuclear periphery is not absolutely required for GAL10 gene transcription. They also suggest that mRNA production is not sufficient for gene anchoring and that Mex67p is required for stable association with the nuclear periphery.
To further examine the role of Mex67p in gene anchoring, ChIP was used to compare the association of Mex67p with the GAL10 gene in wild-type and mex67-5 mutant cells. Wild-type Mex67p was detected in association with GAL10 when cells were grown in galactose but not in glucose, confirming that cotranscriptional binding of Mex67p correlates with active transcription (Fig. 2C) (19). Mex67p was detected at very low levels at the promoter, clearly increased at the 5′ end, reached a maximum at the middle of the gene, and declined towards the 3′end and 3′UTR. This profile indicates that Mex67p recruitment starts at an early phase of transcription. The mex67-5 mutant protein showed a similar binding profile at 25°C. At 37°C, however, while association of wild-type Mex67p with GAL10 was even more efficient, the recruitment of mex67-5 was strongly inhibited. Western blotting of total cell extracts confirmed that wild-type and mex67-5 proteins were present in similar amounts (Fig. 2D). These data suggest that the binding of Mex67p to GAL10 contributes to the stable association of this transcribing gene with the nuclear periphery.
Mex67p is required for transcription-induced HSP104 gene repositioning.
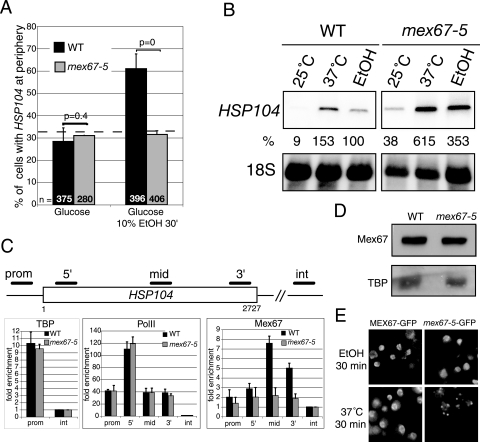
To address the role of Mex67p in gene anchoring under different inducing conditions, HSP104-LacO was localized in wild-type and mex67-5 cells before or after a 30-min treatment with 10% ethanol at 25°C (Fig. 3A). As shown above (Fig. 1B), the number of cells with HSP104 at the nuclear periphery increased from 25% to nearly 60% after ethanol treatment in the wild-type cells. In contrast, HSP104 relocation was completely abolished in mex67-5 cells. To verify that ethanol has no indirect effects on gene positioning, the localization of the LacO-tagged PHO84 gene was examined in these cells under different conditions described at http://www.unige.ch/sciences/biologie/bicel/stutz/Dieppois_MCB06_Suppl_Mat.pdf.The PHO84 gene is located at 23 kb from the telomere of chromosome XIII. As a consequence of telomere anchoring, the LacO-tagged PHO84 gene is detected at the nuclear periphery in 60% of wild-type cells independent of its transcriptional state. Importantly, shifting the LacO-tagged mex67-5 strain to 37°C or exposing the cells to 10% ethanol for 30 min did not dissociate the PHO84 locus from the periphery. Therefore, ethanol treatment of mex67-5 cells does not induce general chromatin rearrangements.
FIG. 3.
Mex67p is required for transcription-induced HSP104 gene relocation. (A) The HSP104 gene tagged with LacO was localized in wild-type (black bars) and mex67-5 (gray bars) cells expressing LacI-GFP and Nup49-GFP. Cells were grown in YEPD at 25°C and scored before and after a 30-min treatment with 10% ethanol (10% EtOH 30′) as described in the legend to Fig. 1. (B) Northern blot of HSP104 mRNAs in wild-type and mex67-5 strains grown at 25°C, shifted to 37°C, or treated with 10% ethanol for 30 min. HSP104 mRNA levels were normalized to 18S rRNA values and expressed as a percentage of the value for the wild type incubated with ethanol for 30 min at 25°C. (C) ChIP analysis of TBP, RNA PolII, and Mex67p association with HSP104. Extracts were prepared from wild-type or mex67-5 cells induced with 10% ethanol for 30 min at 25°C and immunoprecipitated with antibodies against TBP, RNA PolII, or Mex67p as indicated. Coprecipitating DNA was amplified with primers specific for HSP104 promoter (prom), 5′, middle (mid), 3′, and a nontranscribed intergenic region (int). The relative enrichment of the HSP104 gene segments was calculated as described in the legend to Fig. 2C. Values correspond to the means of three independent experiments. (D) Western blot analysis of Mex67p and TBP levels in wild-type and mex67-5 extracts from cells used for ChIP. (E) Genomically tagged MEX67-GFP and mex67-5-GFP strains were grown in YEPD at 25°C, incubated with 10% ethanol, or shifted to 37°C for 30 min and immediately examined with a fluorescence microscope.
Northern blot analysis confirmed that HSP104 transcripts were induced in wild-type cells after a 30-min ethanol treatment at 25°C and reached even higher levels in mex67-5 cells. For comparison, heat stress induced accumulation of even more HSP104 mRNAs in both wild-type and mex67-5 cells (Fig. 3B) (23). ChIP analysis of wild-type and mex67-5 extracts revealed similar amounts of TBP and RNA PolII associated with the HSP104 promoter and coding regions upon ethanol induction at 25°C (Fig. 3C), indicating comparable transcription rates in the two strains. The higher HSP104 mRNA levels in the mutant may therefore result from different mRNA turnover rates. These observations indicate that efficient HSP104 gene transcription does not require peripheral localization.
The role of Mex67p in HSP104 gene anchoring was further examined by comparing the association of wild-type and mutant mex67-5 with the HSP104 gene by ChIP. These experiments showed that wild-type Mex67p was efficiently recruited to HSP104 after ethanol induction at 25°C, starting at the 5′ end, reaching a maximum in the middle of the gene, and decreasing towards the 3′ end (Fig. 3C). In contrast, mex67-5 was barely detectable, despite comparable Mex67p and mex67-5 protein levels in these cells (Fig. 3D). Notably, ethanol stress at 25°C does not affect mex67-5-GFP localization, in contrast to heat stress, which rapidly dissociates mex67-5-GFP from the nuclear periphery (Fig. 3E) (35). Ethanol stress therefore affects more severely the binding of mex67-5 to the HSP104 gene than to the NPC. Thus, the lack of mex67-5 cotranscriptional recruitment in ethanol is not due to the dissociation of the mutant protein from the NPC but to its inability to associate with the transcribing gene. As for GAL10, these observations suggest that Mex67p binding to HSP104 contributes to the stable association of this transcribing gene with the nuclear periphery.
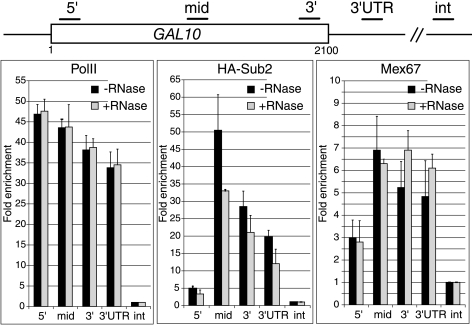
Cotranscriptional recruitment of Mex67p is RNA independent.
To determine whether the recruitment of Mex67p to transcribing genes is mediated by nascent mRNP, the association of Mex67p with GAL10 was examined before or after RNase treatment (Fig. 4). RNA PolII and Sub2p were used as negative and positive controls, as the cotranscriptional recruitment of these proteins is RNA independent and dependent, respectively (1). Extracts prepared from a galactose-induced strain expressing HA-Sub2p were immunoprecipitated with antibodies against RNA PolII, HA, or Mex67p. Consistent with earlier studies (1), quantification of coprecipitating DNA showed that the association of RNA PolII along the GAL10 gene was not affected by RNase treatment, whereas the binding of Sub2p was substantially reduced. Importantly, the association of Mex67p was insensitive to RNase, indicating that the early recruitment of Mex67p to transcribing genes is primarily mediated by adaptors associated with the transcription machinery.
FIG. 4.
The cotranscriptional recruitment of Mex67p is RNA independent. Strain FSY1651 expressing HA-tagged Sub2p was induced for 2 hours with 2% galactose. Cross-linked and sonicated extracts were immunoprecipitated with antibodies against HA, RNA PolII, or Mex67p. Coprecipitating DNA was purified from beads before or after RNase treatment and quantified by real-time PCR with primers specific for GAL10 as described in the legend to Fig. 2C. Values correspond to the means of three independent immunoprecipitation experiments.
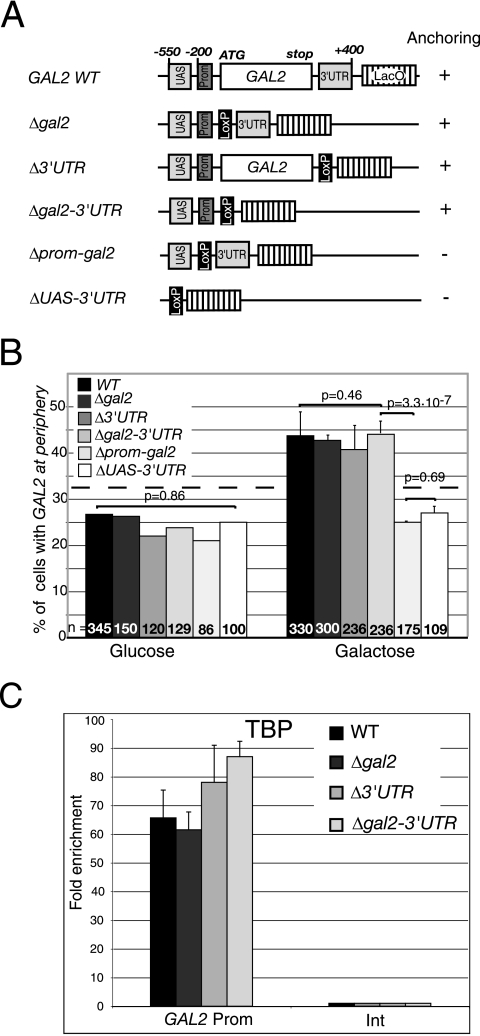
GAL2 gene anchoring does not require the mRNA-coding region.
The RNase-insensitive early recruitment of Mex67p suggested that Mex67p might contribute to gene anchoring in a process independent of nascent mRNP. To test the importance of the mRNA-coding region or other cis-acting sequences in gene anchoring more directly, repositioning of the galactose-inducible GAL2 gene was examined in strains containing the wild-type gene or various deletions of this gene. Similar experiments could not be performed with GAL10 or HSP104, as GAL10 is part of a cluster of galactose-inducible genes, and microarray analyses report that HSP104 lies in a region containing several ethanol-inducible genes (13). In contrast, GAL2 is the only galactose-inducible gene within more than 100 kb on the left arm of chromosome XII (13). Cre-LoxP recombination (18) was used to generate five strains with increasing GAL2 deletions on the chromosome (Fig. 5A). The first mutant strain lacks the GAL2 protein-coding region (Δgal2), the second lacks only the 3′UTR (Δ3′UTR), the third lacks the mRNA-coding and 3′UTR sequences (Δgal2-3′UTR), the fourth lacks the promoter region encompassing the TATA box and the protein-coding region (Δprom-gal2), and the fifth lacks the whole GAL2 gene unit, including the upstream activation sequence (UAS), promoter region, protein-coding, and 3′UTR sequences (ΔUAS-3′UTR) (22). The GAL2 locus was tagged with LacO repeats in wild-type and mutant strains, and its position was examined in cells grown in glucose or galactose (Fig. 5B). In the wild-type GAL2 strain, the number of cells with the tagged gene at the periphery increased from 25% in glucose to nearly 45% in galactose. In contrast, no galactose-induced repositioning was observed in the ΔUAS-3′UTR mutant, confirming that the relocation observed in wild-type cells is exclusively due to GAL2 gene activation. Surprisingly, deletion of the protein-coding region (Δgal2) or 3′UTR (Δ3′UTR) or both (Δgal2-3′UTR) did not substantially affect GAL2 gene anchoring (locus repositioning from 25% in glucose to more than 40% in galactose), indicating that the protein-coding and 3′UTR regions are not essential for GAL2 gene anchoring. In contrast, repositioning was completely lost in the Δprom-gal2 cells. These experiments indicate that the promoter region containing the TATA box is required and that the UAS alone is not sufficient for the association of GAL2 with the nuclear periphery.
FIG. 5.
The promoter, but not the protein-coding region or 3′UTR, is required for transcription-induced GAL2 gene anchoring. (A) Diagram of GAL2 genomic deletions. The positions −200 and −550 are relative to GAL2 ATG. The UAS lies between −350 and −550, the TATA box lies between −100 and −200, and transcription initiation was mapped at −97 (22). The deleted 3′UTR lies between the GAL2 stop codon and position +400 relative to the stop codon. The LoxP sequence is 106 bp long and results from the Cre-LoxP recombination procedure. (B) Localization of the GAL2 locus tagged with LacO repeats in wild-type, Δgal2, Δ3′UTR, Δgal2-3′UTR, Δprom-gal2, and ΔUAS-3′UTR cells grown in YEP medium containing 2% glucose or 2% galactose to an OD600 of 0.5. (C) ChIP analysis of TBP binding at the GAL2 promoter (position −181 to −106 encompassing the TATA box) in the wild type and the indicated mutant strains induced for 2 h with 2% galactose. The relative enrichment of the GAL2 promoter segment in each ChIP was expressed as the enrichment with respect to the nontranscribed intergenic region (Int) value, set to 1. Values are the means of three independent experiments.
To determine whether the repositioning of truncated GAL2 genes correlates with transcription activation, ChIP was used to compare TBP binding to the GAL2 promoter in the wild-type locus and in the Δgal2, Δ3′UTR, and Δgal2-3′UTR mutant loci following galactose induction (Fig. 5C). Interestingly, TBP is recruited to similar levels to the GAL2 promoter region encompassing the TATA box in all four strains, indicating that GAL2 transcription activation is not affected in the absence of the mRNA-coding regions. These results together with the gene localizations suggest that the mRNA-coding region is not essential for GAL2 gene anchoring, and that transcription activation and TBP recruitment are both necessary and sufficient for transcription-induced GAL2 gene repositioning.
Stable mRNP biogenesis is not required for early recruitment of Mex67p.
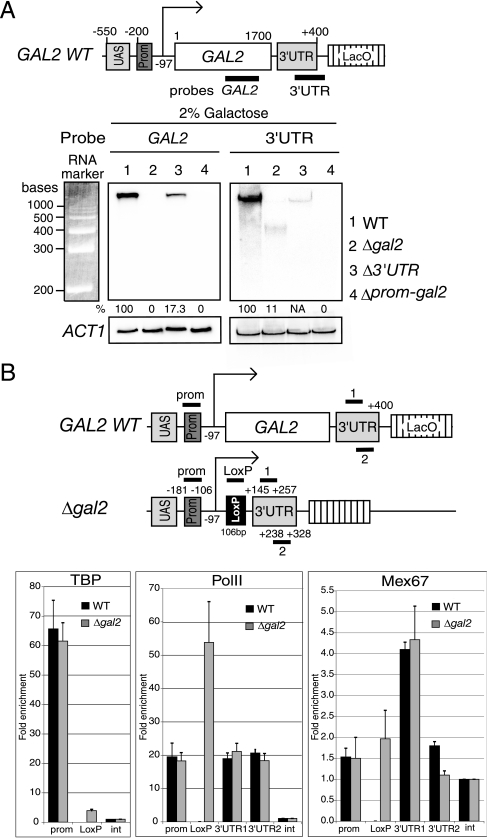
To define the nature and amounts of transcripts encoded by the GAL2 locus in the absence of the protein-coding region or the 3′UTR, total RNA from galactose-induced wild-type, Δgal2, Δ3′UTR, and Δprom-gal2 strains was analyzed by Northern blotting with probes spanning either the protein-coding region (probe GAL2) or the 3′UTR (probe 3′UTR) (Fig. 6A). The GAL2 probe detects GAL2 transcripts encoded in the wild type and the mutant lacking the 3′UTR, whereas the 3′UTR probe detects transcripts encoded in the wild type and the Δgal2 mutant. Neither probe should generate a signal in the mutant lacking the promoter and coding region. The GAL2 probe detected no transcript in the Δgal2 strain and showed that in the absence of the 3′UTR, GAL2 transcripts were expressed at roughly 20% of wild-type GAL2 mRNA levels (Fig. 6A, left panel), indicating that correct 3′-end formation contributes to optimal GAL2 mRNA levels. Notably, the 3′UTR probe detected a low-abundance 450-base-long transcript in strain Δgal2 (Fig. 6A, right panel, lane 2). Since the 5′ end of the GAL2 mRNA has been mapped to position −97 upstream of the ATG codon, and the LoxP-derived sequence is 106 bp (18, 22), the 3′end of this short 450-base transcript is predicted to lie around position +250 of the 3′UTR. Northern blot quantification as well as reverse transcription combined with real-time PCR (see http://www.unige.ch/sciences/biologie/bicel/stutz/Dieppois_MCB06_Suppl_Mat.pdf)indicate that this small transcript accumulates to roughly 10% of wild-type GAL2 mRNA levels. The low level of expression of this short transcript despite wild-type levels of TBP recruitment at the promoter (Fig. 5C) suggests that this small RNA is very unstable.
FIG. 6.
The Δgal2 mutant gene encodes a short unstable transcript but recruits wild-type levels of RNA PolII and Mex67p. (A) Northern blot analysis of total RNA from the wild type (lane 1) and indicated mutant strains (lanes 2 to 4) with probes diagrammed at the top and spanning the GAL2 protein-coding region (GAL2 probe [left panel]) or the GAL2 3′UTR region (3′UTR probe [right panel]). For quantification, the GAL2 RNA signals were normalized to endogenous actin mRNA levels. The 3′UTR probe weakly hybridized to the GAL2 mRNA produced in the Δ3′UTR strain, as both the probe and the transcript produced by this mutant extend beyond the 3′UTR-deleted region. This band was not quantified, as it is only partially complementary to the probe. NA, not applicable. (B) ChIP analyses of TBP, RNA PolII and Mex67p on wild-type GAL2 and the mutant Δgal2 gene. Extracts from galactose-induced cultures were immunoprecipitated with antibodies against TBP, PolII, and Mex67p. Coprecipitating DNA segments (diagrammed as short bars on top) were quantified by real-time PCR with primers specific for the GAL2 promoter (positions −181 to −106), LoxP (106 bp derived from Cre-Lox recombination), 3′UTR1 (positions +145 to +257) and 3′UTR2 (positions +238 to +328). The 3′UTR was numbered positively starting from the GAL2 stop codon, and 3′ end formation is predicted to occur around +250 (A). The relative enrichment of DNA segments in each ChIP was expressed as the enrichment with respect to the nontranscribed intergenic (int) value, set to 1. Values are means of three independent experiments.
The efficient repositioning of the Δgal2 locus despite the production of very limited amounts of RNA suggests that mRNP does not play a major role in transcription-induced gene repositioning. Furthermore, the RNase-insensitive recruitment of Mex67p (Fig. 4) raises the possibility that Mex67p contributes to gene anchoring in the absence of stable mRNP formation. To define whether Mex67p is recruited to the Δgal2 gene, ChIP was used to compare the recruitment of TBP, RNA PolII, and Mex67p on the wild-type and Δgal2 genes after galactose induction (Fig. 6B). Four primer pairs were used to examine the association of these proteins with chromatin: one corresponds to the GAL2 promoter region, another to the LoxP sequence present only in the Δgal2 mutant, and two others are specific for the 3′untranslated region and amplify gene segments from +145 to +257 (3′UTR1) and +238 to +328 (3′UTR2) (Fig. 6B, top). As shown in Fig. 5C, similar levels of TBP were recruited to the GAL2 promoter in the two strains. TBP was barely detectable at the LoxP site located less than 200 bp downstream from the promoter region in Δgal2. The very low level of TBP at this site demonstrates the high resolution of these ChIP analyses and is consistent with efficient shearing of chromatin in the extracts. RNA PolII levels were comparable within the promoter and 3′UTR regions of the two strains. The RNA PolII signal was higher at the LoxP site in the Δgal2 strain, consistent with the increased levels of RNA polymerase at the 5′ end of GAL10 (Fig. 2C). Finally, Mex67p was detected at very low levels at the GAL2 promoter in both strains, increased at the LoxP site, and reached a fourfold enrichment in the 3′UTR1 region of both strains, before dropping in the 3′UTR2 region. Notably, the size of the short transcript produced in Δgal2 cells predicts that 3′-end formation occurs somewhere between the 3′UTR1 and 3′UTR2 regions (Fig. 6A). These results suggest that 3′-end processing may coincide with the loss of Mex67p from the chromatin. More importantly, the association of Mex67p with the Δgal2 locus strengthens the view that early recruitment of Mex67p depends on the transcription machinery rather than mRNP formation and that Mex67 may act as a trans-acting factor in NPC gene anchoring by virtue of an RNA-independent process.
DISCUSSION
Live imaging of GFP-tagged yeast cells has revealed that genomic loci constantly move within the nucleus and frequently ricochet off the nuclear membrane (24, 30). Transcription-induced gene anchoring is likely to result from the stabilization of these transient contacts by specific interactions between the activated gene and NPC components. The aim of this study was to test the potential role of the nascent mRNP complex in GAL2, GAL10, and HSP104 gene anchoring by examining gene repositioning in both cis- and trans-acting mutants.
GAL2 gene anchoring does not require the mRNA-coding region.
Our analyses of GAL2 gene anchoring in strains lacking various portions of the GAL2 gene show that the protein-coding and 3′UTR regions are dispensable, while the UAS and promoter region encompassing the TATA box are necessary for GAL2 association with the nuclear periphery (Fig. 5A and B). The results of ChIP experiments confirmed that similar amounts of TBP and RNA PolII associate with the GAL2 promoter of wild-type, Δgal2, Δ3′UTR, and Δgal2-3′UTR cells, indicating that transcription activation and initiation occur efficiently in the absence of the protein-coding and 3′UTR regions (Fig. 5C and 6B; also data not shown). Despite efficient TBP recruitment, GAL2 transcripts encoded by the Δ3′UTR mutant were poorly expressed (20% of wild-type levels), probably due to message instability as a result of improper 3′-end processing. Notably, the short 450-base transcript encoded by the Δgal2 mutant was present at even lower levels (10% of wild-type levels). Thus, the poor expression of this short RNA together with the efficient transcription-induced repositioning of the Δgal2 gene suggests that RNA is unlikely to act as a major determinant in GAL2 gene anchoring. However, the possibility that the GAL2 mRNA encoded in the wild-type strain contributes to the maintenance of the induced gene at the periphery cannot be excluded. This possibility could be tested by comparing the dissociation kinetics of wild-type GAL2, Δgal2, or Δ3′UTR loci from the nuclear envelope after transcriptional shut-off.
So far, our data on GAL2 support the view that transcription activation but not mRNA production plays a major role in NPC anchoring of this gene. Accordingly, physical interactions have been identified between Sus1p, a component of the SAGA coactivator complex recruited upstream of galactose-inducible genes, and components of the mRNA export machinery associated with pores (31). Moreover, a recent study showed that the promoter regions of numerous active genes, including the GAL genes, physically interact with the NPC component Nup2p. Notably, these interactions are independent of transcription, suggesting that early activating events might be sufficient for connecting a gene to the NPC (33, 34). The results of our gene localization experiments on GAL2 deletion strains are consistent with the results of these biochemical studies, but this may not be the case for all genes. Indeed, a recent study shows that efficient repositioning of the HXK1 gene requires the 3′UTR (42). One possibility is that gene-to-pore interactions involve a number of partially redundant interactions, which may occur simultaneously or sequentially during the tethering process. Thus, the contribution of the mRNP to gene anchoring may depend on the strength of other, i.e., transcription linked, interactions.
How do Mex67p and Mlp1p contribute to gene anchoring?
To identify trans-acting factors implicated in gene anchoring and following up on the initial hypothesis that anchoring may be mediated by factors interacting with nascent mRNPs, we found that both Mex67p and Mlp1p contribute to GAL10 and HSP104 gene anchoring (Fig. 1B, 2A, and 3A). Indeed, the activated GAL10 gene rapidly dissociated from the periphery in mex67-5 cells shifted to 37°C. Similarly, ethanol-induced HSP104 gene anchoring observed in wild-type cells at 25°C was abolished in mex67-5 cells at this temperature. Importantly, the loss of gene anchoring was paralleled by the loss of cotranscriptional binding of the mex67-5 protein to ethanol-induced HSP104, a condition under which the mex67-5 protein remains at the nuclear periphery (Fig. 3C and E). These observations indicate that maintenance of the mex67-5 protein at the periphery is not sufficient for gene anchoring and that productive interaction of Mex67p with the gene is required. Although we cannot exclude the possibility that gene movement is restricted in a mex67-5 mutant heated to 37°C or exposed to ethanol or distinguish whether Mex67p cotranscriptional recruitment is the cause or consequence of peripheral gene association, the results of both the GAL10 and HSP104 experiments support the view that Mex67p contributes to NPC anchoring by physical association with activated genes.
Our ChIP analyses show that association of Mex67p with GAL10 and HSP104 is clearly detectable at the 5′ end, reaches a maximum in the middle part, and decreases at the 3′ end of these genes (Fig. 2C and 3C). Importantly, the interaction of Mex67p with the GAL10 gene is not sensitive to RNase treatment, indicating that the early recruitment of Mex67p to transcribing genes is not mediated by RNA (Fig. 4). Thus, Mex67p is likely to be recruited via adaptors associated with the transcription machinery. Despite the good resolution of our ChIP experiments, the Mex67p signal detected at the promoter is too weak to conclude that Mex67p already binds in this region. The ChIP profile more likely indicates that Mex67p becomes associated with activated genes at a very early step of transcription, possibly within the transition from initiation to elongation.
Notably, Mex67p is efficiently recruited to the Δgal2 gene, which does not produce a stable mRNP (Fig. 6A and B). This observation further supports the view that the early recruitment of Mex67p is not mediated by RNA, but by an adaptor(s) associated with the transcription machinery. Mex67p is probably transferred to the mRNA at a later step, an event not easily detected by ChIP. Notably, analysis of Mex67p recruitment on the wild-type GAL2 and Δgal2 genes indicated a drop in Mex67p binding around position +250 within the 3′UTR. Interestingly, this site corresponds to the region within which the 450-base transcript encoded by the Δgal2 gene is predicted to end (Fig. 6A and B). This observation suggests that transfer of Mex67p from chromatin to mRNA may coincide with 3′-end processing. This view is consistent with an earlier study proposing that binding of Mex67p to mRNA is coupled to 3′-end processing and release of the mRNP from the transcribing gene (15).
The finding that early recruitment of Mex67p is RNA independent raises the question of the nature of the adaptor(s) mediating the association of Mex67p to active genes. One candidate is Npl3p, an hnRNP protein interacting with Mex67p and recruited to active genes by the RNA PolII complex at an early step of transcription (15, 28). Another is the hnRNP-like protein Yra1p, which interacts with the N-terminal domain of Mex67p (37, 39, 44). The association of Yra1p with transcribing genes is largely RNase insensitive, suggesting that this mRNA export adaptor is first recruited via interaction with the transcription machinery and subsequently transferred to mRNA (1, 28, 45). Thus, Mex67p could be recruited via interaction of its N-terminal domain with Yra1p. However, the results of our recent studies indicate that the early recruitment of Mex67p largely depends on its C-terminal UBA domain (19). Interestingly, Hpr1p, a component of the THO complex implicated in transcription elongation and mRNA export (7, 38, 45), directly interacts with the Mex67p UBA domain, and this interaction facilitates the recruitment of Mex67p to the GAL10 gene (19). Future studies will address whether early Mex67p recruitment is mediated via multiple, possibly sequential, adaptors associated with the transcription initiation and elongation machineries.
This work also shows that the NPC-attached Mlp1 protein contributes to efficient transcription-induced GAL10 and HSP104 gene anchoring (Fig. 1B). Genome-wide mapping of Mlp1p-bound DNA sequences suggested that interaction of Mlp1p with chromatin occurs according to different modes. Whereas the association of Mlp1p with induced genes is RNA dependent and biased towards the 3′ end, the binding to subtelomeric regions is largely RNA independent (5). Mlp1p has also been found in association with components of the SAGA coactivator and mediator complexes implicated in transcription activation (14). Thus, Mlp proteins may contribute to stable GAL10 and HSP104 gene tethering at an early step by interaction with chromatin-associated transcription regulators, or at a later step by interacting with nascent transcripts, or both. In contrast to our observations, a recent report identified no effect of Δmlp1 on gene anchoring (4). It is presently unclear whether this discrepancy results from strain background or experimental differences. Importantly, this recent study identified additional factors implicated in gene anchoring, including Ada2p and Sus1p, two components of the SAGA coactivator complex, as well as Nup1 and Sac3, two factors belonging to the mRNA export machinery associated with pores (4). Whether these factors, as well as Mex67p and Mlp1p, act in the same pathway and in a defined chronological order during the anchoring process are questions for the future. It is possible that both early transcription-linked and later potentially mRNP-dependent tethers contribute to gene anchoring. However, the relative importance of individual tethers may vary from gene to gene.
Relationship between gene expression and anchoring.
Although peripheral localization has been proposed to optimize the expression of some inducible genes (3, 42), our analyses indicate that gene anchoring may not be a general requirement for gene expression. Indeed, whereas HSP104 gene anchoring was strongly inhibited in the mex67-5 mutant (Fig. 3A), HSP104 mRNA levels were even higher in mex67-5 cells than in the wild-type cells (Fig. 3B). ChIP analyses of TBP and RNA PolII indicated similar transcription rates for HSP104 in wild-type and mex67-5 cells exposed to ethanol, indicating that dissociation of HSP104 from the periphery affects mRNA turnover rather than transcription rates (Fig. 3C). In contrast, a shift of mex67-5 cells to 37°C led to a slight decrease in GAL10 mRNA levels and RNA PolII recruitment to this gene (Fig. 2B and C). Whether this decrease is due to indirect effects of mex67-5 on transcription and/or a decrease in mRNA stability as a result of the mRNA export block is unclear. However, the slow and modest effect on mRNA levels compared to the rapid and strong effect on GAL10 gene localization suggests that NPC anchoring does not influence the expression of this gene. This conclusion is consistent with the report by Cabal et al. (4) showing that the changes in GAL locus positioning, observed in strains lacking the SAGA components Ada2p or Sus1p or the pore-associated mRNA export factors Nup1 or Sac3, do not affect GAL1 mRNA transcription levels. Our analyses with Δmlp1 cells led to similar conclusions. As this mutant presents no mRNA export defect (27, 36), the relationship between gene localization and expression could be examined without potential indirect effects of an export block. Neither GAL10 nor HSP104 levels were affected in Δmlp1 cells despite a clear effect on gene repositioning (Fig. 1B and C; also data not shown). At this time, it is unclear whether the increased expression of INOI and HXKI, observed when these genes were positioned at the periphery, reflects a natural regulatory pathway or results from their artificial tethering to the nuclear envelope (3, 42).
The functional significance of GAL10 and HSP104 gene anchoring is an open question, as gene repositioning appears to be the consequence rather than the cause of their transcriptional activity. Peripheral localization may be more important for the efficient expression of other types of genes under different physiological or inducing conditions. Another likely possibility is that gene anchoring contributes to gene expression efficiency by facilitating mRNA export through nuclear pores. Since Mlp proteins have been implicated in the nuclear retention of unprocessed pre-mRNAs (11), an interesting view is that gene anchoring also contributes to this late step of mRNA surveillance at the nuclear periphery.
Acknowledgments
We are grateful to Susan Gasser, Catherine Dargemont, and Martine Collart for plasmids, strains, and antibodies. We also thank Sylvie Dersi and Céline Fickentscher for technical assistance and Thierry Laroche and Christophe Bauer for help in microscopy. We thank Michael Rosbash for discussions and Martine Collart, Catherine Dargemont, and Jurgi Camblong for critical comments on the manuscript.
This work was supported by the Swiss National Science Foundation (SNF grant 102235 to F.S.) and support from the SNF program “Frontiers in Genetics” to F.S.
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Abruzzi, K. C., S. Lacadie, and M. Rosbash. 2004. Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. EMBO J. 23:2620-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrulis, E. D., A. M. Neiman, D. C. Zappulla, and R. Sternglanz. 1998. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 394:592-595. [DOI] [PubMed] [Google Scholar]
- 3.Brickner, J. H., and P. Walter. 2004. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabal, G. G., A. Genovesio, S. Rodriguez-Navarro, C. Zimmer, O. Gadal, A. Lesne, H. Buc, F. Feuerbach-Fournier, J. C. Olivo-Marin, E. C. Hurt, and U. Nehrbass. 2006. SAGA interacting factors confine subdiffusion of transcribed genes to the nuclear envelope. Nature 441:770-773. [DOI] [PubMed] [Google Scholar]
- 5.Casolari, J. M., C. R. Brown, D. A. Drubin, O. J. Rando, and P. A. Silver. 2005. Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev. 19:1188-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casolari, J. M., C. R. Brown, S. Komili, J. West, H. Hieronymus, and P. A. Silver. 2004. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117:427-439. [DOI] [PubMed] [Google Scholar]
- 7.Chavez, S., T. Beilharz, A. G. Rondon, H. Erdjument-Bromage, P. Tempst, J. Q. Svejstrup, T. Lithgow, and A. Aguilera. 2000. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 19:5824-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockell, M., and S. M. Gasser. 1999. Nuclear compartments and gene regulation. Curr. Opin. Genet. Dev. 9:199-205. [DOI] [PubMed] [Google Scholar]
- 9.Dilworth, D. J., A. J. Tackett, R. S. Rogers, E. C. Yi, R. H. Christmas, J. J. Smith, A. F. Siegel, B. T. Chait, R. W. Wozniak, and J. D. Aitchison. 2005. The mobile nucleoporin Nup2p and chromatin-bound Prp20p function in endogenous NPC-mediated transcriptional control. J. Cell Biol. 171:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuerbach, F., V. Galy, E. Trelles-Sticken, M. Fromont-Racine, A. Jacquier, E. Gilson, J. C. Olivo-Marin, H. Scherthan, and U. Nehrbass. 2002. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat. Cell Biol. 4:214-221. [DOI] [PubMed] [Google Scholar]
- 11.Galy, V., O. Gadal, M. Fromont-Racine, A. Romano, A. Jacquier, and U. Nehrbass. 2004. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 116:63-73. [DOI] [PubMed] [Google Scholar]
- 12.Galy, V., J. C. Olivo-Marin, H. Scherthan, V. Doye, N. Rascalou, and U. Nehrbass. 2000. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature 403:108-112. [DOI] [PubMed] [Google Scholar]
- 13.Gasch, A. P., and M. Werner-Washburne. 2002. The genomics of yeast responses to environmental stress and starvation. Funct. Integr. Genomics 2:181-192. [DOI] [PubMed] [Google Scholar]
- 14.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, W., and C. Guthrie. 2004. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell 13:201-212. [DOI] [PubMed] [Google Scholar]
- 16.Gotta, M., T. Laroche, A. Formenton, L. Maillet, H. Scherthan, and S. M. Gasser. 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134:1349-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green, D. M., C. P. Johnson, H. Hagan, and A. H. Corbett. 2003. The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export. Proc. Natl. Acad. Sci. USA 100:1010-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueldener, U., J. Heinisch, G. J. Koehler, D. Voss, and J. H. Hegemann. 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwizdek, C., I. Iglesias, M. S. Rodriguez, B. Ossareh-Nazari, M. Hobeika, G. Divita, F. Stutz, and C. Dargemont. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 20.Hediger, F., and S. M. Gasser. 2002. Nuclear organization and silencing: putting things in their place. Nat. Cell Biol. 4:E53-E55. [DOI] [PubMed] [Google Scholar]
- 21.Heun, P., T. Laroche, K. Shimada, P. Furrer, and S. M. Gasser. 2001. Chromosome dynamics in the yeast interphase nucleus. Science 294:2181-2186. [DOI] [PubMed] [Google Scholar]
- 22.Huibregtse, J. M., P. D. Good, G. T. Marczynski, J. A. Jaehning, and D. R. Engelke. 1993. Gal4 protein binding is required but not sufficient for derepression and induction of GAL2 expression. J. Biol. Chem. 268:22219-22222. [PubMed] [Google Scholar]
- 23.Hurt, E., K. Strasser, A. Segref, S. Bailer, N. Schlaich, C. Presutti, D. Tollervey, and R. Jansen. 2000. Mex67p mediates nuclear export of a variety of RNA polymerase II transcripts. J. Biol. Chem. 275:8361-8368. [DOI] [PubMed] [Google Scholar]
- 24.Ishii, K., G. Arib, C. Lin, G. Van Houwe, and U. K. Laemmli. 2002. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109:551-562. [DOI] [PubMed] [Google Scholar]
- 25.Isogai, Y., and R. Tjian. 2003. Targeting genes and transcription factors to segregated nuclear compartments. Curr. Opin. Cell Biol. 15:296-303. [DOI] [PubMed] [Google Scholar]
- 26.Jimeno, S., A. G. Rondon, R. Luna, and A. Aguilera. 2002. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 21:3526-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosova, B., N. Pante, C. Rollenhagen, A. Podtelejnikov, M. Mann, U. Aebi, and E. Hurt. 2000. Mlp2p, a component of nuclear pore attached intranuclear filaments, associates with nic96p. J. Biol. Chem. 275:343-350. [DOI] [PubMed] [Google Scholar]
- 28.Lei, E. P., H. Krebber, and P. A. Silver. 2001. Messenger RNAs are recruited for nuclear export during transcription. Genes Dev. 15:1771-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 30.Marshall, W. F., A. Straight, J. F. Marko, J. Swedlow, A. Dernburg, A. Belmont, A. W. Murray, D. A. Agard, and J. W. Sedat. 1997. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr. Biol. 7:930-939. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Navarro, S., T. Fischer, M. J. Luo, O. Antunez, J. E. Perez-Ortin, R. Reed, and E. Hurt. 2004. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116:75-86. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez, Y., J. Taulien, K. A. Borkovich, and S. Lindquist. 1992. Hsp104 is required for tolerance to many forms of stress. EMBO J. 11:2357-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid, M., G. Arib, C. Laemmli, J. Nishikawa, T. Durussel, and U. K. Laemmli. 2006. Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol. Cell 21:379-391. [DOI] [PubMed] [Google Scholar]
- 34.Schmid, M., T. Durussel, and U. K. Laemmli. 2004. ChIC and ChEC: genomic mapping of chromatin proteins. Mol. Cell 16:147-157. [DOI] [PubMed] [Google Scholar]
- 35.Segref, A., K. Sharma, V. Doye, A. Hellwig, J. Huber, R. Luhrmann, and E. Hurt. 1997. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 16:3256-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strambio-de-Castillia, C., G. Blobel, and M. P. Rout. 1999. Proteins connecting the nuclear pore complex with the nuclear interior. J. Cell Biol. 144:839-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strasser, K., and E. Hurt. 2000. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 19:410-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strasser, K., S. Masuda, P. Mason, J. Pfannstiel, M. Oppizzi, S. Rodriguez-Navarro, A. G. Rondon, A. Aguilera, K. Struhl, R. Reed, and E. Hurt. 2002. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417:304-308. [DOI] [PubMed] [Google Scholar]
- 39.Stutz, F., A. Bachi, T. Doerks, I. C. Braun, B. Seraphin, M. Wilm, P. Bork, and E. Izaurralde. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6:638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stutz, F., E. Gouilloud, and S. G. Clarkson. 1989. Oocyte and somatic tyrosine tRNA genes in Xenopus laevis. Genes Dev. 3:1190-1198. [DOI] [PubMed] [Google Scholar]
- 41.Taddei, A., M. R. Gartenberg, F. R. Neumann, F. Hediger, and S. M. Gasser. 2005. Multiple pathways tether telomeres and silent chromatin at the nuclear periphery: functional implications for sir-mediated repression. Novartis Found. Symp. 264:140-156, 156-165, 227-230. [PubMed] [Google Scholar]
- 42.Taddei, A., G. Van Houwe, F. Hediger, V. Kalck, F. Cubizolles, H. Schober, and S. M. Gasser. 2006. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441:774-778. [DOI] [PubMed] [Google Scholar]
- 43.Vinciguerra, P., N. Iglesias, J. Camblong, D. Zenklusen, and F. Stutz. 2005. Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. EMBO J. 24:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenklusen, D., P. Vinciguerra, Y. Strahm, and F. Stutz. 2001. The yeast hnRNP-like proteins Yra1p and Yra2p participate in mRNA export through interaction with Mex67p. Mol. Cell. Biol. 21:4219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zenklusen, D., P. Vinciguerra, J. C. Wyss, and F. Stutz. 2002. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol. Cell. Biol. 22:8241-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]