Abstract
The Saccharomyces cerevisiae REV3/7-encoded polymerase ζ and Rev1 are central to the replicative bypass of DNA lesions, a process called translesion synthesis (TLS). While yeast polymerase ζ extends from distorted DNA structures, Rev1 predominantly incorporates C residues from across a template G and a variety of DNA lesions. Intriguingly, Rev1 catalytic activity does not appear to be required for TLS. Instead, yeast Rev1 is thought to participate in TLS by facilitating protein-protein interactions via an N-terminal BRCT motif. In addition, higher eukaryotic homologs of Rev1 possess a C terminus that interacts with other TLS polymerases. Due to a lack of sequence similarity, the yeast Rev1 C-terminal region, located after the polymerase domain, had initially been thought not to play a role in TLS. Here, we report that elevated levels of the yeast Rev1 C terminus confer a strong dominant-negative effect on viability and induced mutagenesis after DNA damage, highlighting the crucial role that the C terminus plays in DNA damage tolerance. We show that this phenotype requires REV7 and, using immunoprecipitations from crude extracts, demonstrate that, in addition to the polymerase-associated domain, the extreme Rev1 C terminus and the BRCT region of Rev1 mediate interactions with Rev7.
In all eukaryotes examined to date, Rev1 and the REV3/REV7-encoded polymerase zeta (Polζ) are absolutely required for the mutagenic bypass of DNA lesions, a process called translesion DNA synthesis (TLS). Even on undamaged DNA, most TLS occurs at a low fidelity, relative to replicative DNA synthesis, and represents a major source of DNA damage-induced base substitutions and frameshift mutations (7, 36).
In the yeast Saccharomyces cerevisiae, the majority of mutagenic DNA lesion bypass is carried out by Polζ, a member of the B family of DNA polymerases, and the Y-family polymerase Rev1 (21). Both Polζ and Rev1 are part of the “error-prone” branch of the RAD6 epistasis pathway (20). Inactivating mutations in the rev genes render cells sensitive to DNA-damaging agents and confer a “reversionless” phenotype after DNA damage (23, 25). Together, Polζ and Rev1 functions are required for the generation of 95% to 98% of UV radiation (UV)-induced base pair substitutions (22, 24).
Though the yeast Polζ can, in some cases, insert nucleotides across damaged bases, it possesses a unique facility to extend DNA synthesis from terminally mismatched primers and, in general, from distorted DNA structures (15, 45). The Polζ enzyme consists of a catalytic subunit, Rev3, and an accessory subunit, Rev7, that enhances the polymerase activity of Rev3 by about 200-fold (33). Polζ replicates past abasic sites in the DNA template, an activity that is greatly stimulated by Rev1 in vitro (32).
The Rev1 polymerase is endowed with the unique ability to insert predominantly dCMP both across an undamaged template G and abasic sites as well as a variety of other DNA lesions (11, 21, 32). In this capacity, Rev1 uses a novel mechanism of DNA synthesis whereby the incoming dCTP pairs with an arginine rather than the template base, and the template G is evicted from the DNA helix (30). Intriguingly, the catalytic activity of Rev1 appears not to be essential for the bypass of DNA damage, as mutations inactivating this activity leave Rev1 still competent to participate in translesion synthesis, (12; L. Waters, S. D'Souza and G. C. Walker, unpublished observations), albeit with an altered mutation spectrum (35).
In contrast to the polymerase domain, the N-terminal BRCT motif of yeast Rev1 is required for mutagenesis. A G193R point mutation in the Rev1 BRCT motif (the rev1-1 allele) abrogates the ability of Rev1 to function in induced mutagenesis and confers a moderate sensitivity to DNA damaging agents (19, 25). BRCT domains (BRCA1 C terminus) are ubiquitous motifs that facilitate physical interactions among proteins involved in the cellular response to DNA damage (4, 44). Interestingly, the Rev1-1 protein retains its catalytic dCMP transferase activity in vitro (31). Thus, the TLS function of yeast Rev1 is thought to require protein-protein interactions, rather than its polymerase activity, and has been proposed to nucleate a protein complex at the site of a stalled replication fork, possibly to facilitate a switch between the replicative DNA polymerase and other TLS polymerases (12). However, the recent observation that the yeast Rev1 protein levels fluctuate dramatically in the yeast cell cycle and peak during the G2/M phase suggests that Rev1 functions mainly in the G2/M phase rather than during the S phase of the cell cycle when replication occurs (46).
In addition to its BRCT and polymerase domains, Rev1 possesses a C-terminal region which, in higher eukaryotes, has been shown to interact with a variety of translesion DNA polymerases, including Polκ, Polλ, Polη, and Polι and the Polζ accessory subunit, Rev7 (9, 29, 34, 40, 41). Unlike human and mouse Rev1, relatively little is known about the C terminus of the Saccharomyces cerevisiae Rev1 protein. Due to the poor sequence conservation of the C termini between yeast Rev1 and its vertebrate homologs, it has been suggested that the C terminus of yeast Rev1 plays no part in the process of mutagenic bypass or protein-protein interactions (16, 29, 41). In this study, we assess the contribution of the C terminus of yeast Rev1 in DNA damage tolerance by overproducing a C-terminal fragment in cells exposed to DNA damage. We find that overproduction of the extreme C terminus of the yeast Rev1 protein confers a strong dominant-negative phenotype on the survival and reversion frequencies of otherwise wild-type cells exposed to DNA damage. We show that the dominant-negative phenotype of the Rev1 C terminus requires both REV1 and REV7, providing the first in vivo evidence for a critical function of the C terminus of Saccharomyces cerevisiae Rev1 in DNA damage tolerance. We use immunoprecipitation experiments to demonstrate that two novel regions of the Rev1 protein, the extreme C terminus and the BRCT region, independently interact with the Rev7 protein, the accessory subunit of Polζ. Consistent with an earlier observation (1) showing a direct interaction between the Rev1 polymerase-associated domain (PAD) and Rev7, we report that the Rev1 PAD also immunoprecipitates Rev7. Our results thus significantly expand the study of the interaction between Rev1 and Rev7 and have important implications for the process of translesion DNA synthesis.
MATERIALS AND METHODS
Yeast strains.
Strains are listed in Table 1. The strains used in this study are derivatives of W1588-4C (MATa leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5) and W1588-4A (MATα leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5), which are W303 strains corrected for RAD5 (47). Strains YLW20, YSD3, and YSD6 were constructed by gene replacement by PCR amplifying the rev1::kanMX4, rev7::kanMX4, and rev3::kanMX4 cassettes from yeast deletion strains YOR346W, YIL139C, and YPL167C, respectively (gifts from L. Samson, MIT), transforming the PCR products into the strain W1588-4A, W1588-4C, and YSD5, respectively, and selecting the transformants on YPD plates (38) containing 200 μg/ml G418 disulfate (Sigma). To construct strain YLW35, REV1 was PCR amplified along with its native promoter and 3′ untranslated region from the strain W1588-4C and subcloned into the low-copy pRS416 plasmid (39). The BRCT region was mutated using the QuikChange site-directed mutagenesis kit (Stratagene). The mutated rev1-1 fragment was subsequently subcloned into the integrating plasmid, pRS306 (39), digested with the restriction enzyme, SexAI, and transformed into strain YLW20 using standard transformation techniques (8). Transformants were selected on SD medium (38) lacking uracil.
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype |
|---|---|
| W1588-4C | MATaleu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5 |
| W1588-4A | MATα leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5 |
| YLW20 | Same as W1588-4A but rev1::KANMX4 |
| YLW35 | Same as YLW20 but PREV1-rev1-1:URA3 |
| YLW70 | Same as W1588-4C but bar1::LEU2 |
| YSD1 | Same as W1588-4A but trp1-1::PGAL1-HA-10HIS::TRP1 REV1-TEV-PROA-7HIS::HIS3MX6 |
| YSD2 | Same as W1588-4A but trp1-1::PGAL1-REV1CT239-HA- 10HIS::TRP1 REV1-TEV-PROA-7HIS::HIS3MX6 |
| YSD3 | Same as W1588-4C but rev7::KANMX4 |
| YSD4 | Same as W1588-4C but REV1-TEV-PROA-7HIS:: HIS3MX6 REV7-9MYC::KlTRP1 PDS1-HA- LEU2::pds1 bar1::URA3 |
| YSD5 | Same as YLW70 but REV7-13MYC::HIS3MX6 |
| YSD6 | Same as YSD5 but rev3::KANMX4 |
Strains YSD1 and YSD2 were constructed as follows. The KpnI and NotI fragment from the pAS311 construct (see below), containing either the GAL1 promoter alone or the GAL1 promoter together with the fragment encoding the Rev1 C-terminal 239-amino-acid region was ligated into the pRS404 plasmid (39). The resulting plasmid was digested with SnaB1 and transformed into strain W1588-4A harboring REV1 tagged at its endogenous locus with protein A. Transformants were selected on SD medium lacking tryptophan.
Strains YSD4 and YSD5 were constructed as follows. Cassettes containing the 13-Myc or 9-Myc sequence were amplified from the pFA6a-13Myc-His3MX6 (27) and pYM6-KlTRP (17) plasmids, respectively, using the forward primer 5′-GAGTGTATTCTCAATATGAAGAGGGCGAGAGCATTTTTGGATCTTTGTTTCGGATCCCCGGGTTAATTAA-3′ and reverse primer 5′-ACTTAGAGACATTTAATTTTAATTCCATTCTTCAAATTTCATTTTTGCACGAATTCGAGCTCGTTTAAAC-3′ (13-Myc) and the forward primer 5′-GTGTATTCTCAATATGAAGAGGGCGAGAGCATTTTTGGATCTTTGTTTCGTACGCTGCAGGTCGAC-3′ and reverse primer 5′-TAGAGACATTTAATTTTAATTCCATTCTTCAAATTTCATTTTTGCACATCGATGAATTCGAGCTCG-3′ (9-Myc). The 13-Myc cassette was transformed into strain YLW70, and transformants were selected on SD medium lacking histidine. Strains transformed with the 9-Myc-KlTRP cassette were selected on SD medium lacking tryptophan. For all fusion constructs, integrations at the appropriate genomic locus were confirmed by PCR and expression of the fusion proteins of expected molecular weights by immunoblotting with anti-Myc (Upstate) or PAP antibodies (Sigma).
Plasmids.
For overexpression studies, PCR-amplified REV1 fragments encoding the Rev1 C-terminal 426-amino-acid fragment (amino acids 560 to 985), the Rev1 C-terminal 239-amino-acid region (amino acids 747 to 985), the Rev1 PAD (amino acids 567 to 767), and the Rev1-BRCT region (amino acids from 155 to 268) were subcloned into the 2μm pAS311 plasmid that contains the GAL1 promoter and an HA-10HIS epitope tag (a gift from S. P. Bell, MIT). For all PCR amplifications of REV1, the pJN60-GST REV1 template was used (a gift from D. Hinkle, University of Rochester). REV7 was PCR amplified using the pMR7 template (from D. Hinkle, University of Rochester).
The pESC-Split TAP 2μm plasmid containing the oppositely oriented GAL1 and GAL10 promoters (used for experiments for which results are shown in Fig. 4 and 5), a kind gift from S. P. Bell (MIT), was constructed by PCR amplifying the coding sequence of the calmodulin-binding peptide (CBP) tag, a thrombin cleavage site, and the restriction sites NotI and AscI and inserting them into the NotI and PacI sites of the pESC-TRP plasmid driven by the GAL1 promoter. Similarly, the PCR-amplified coding sequence of the Tobacco etch virus cleavage site, protein A, and the restriction sites FseI and AsiSI were inserted into the BamHI and XhoI sites in front of the GAL10 promoter. A plasmid map is available on request. Appropriate REV1 fragments and REV7 were then inserted into this plasmid in frame with the protein A (ProA) and CBP tags, respectively.
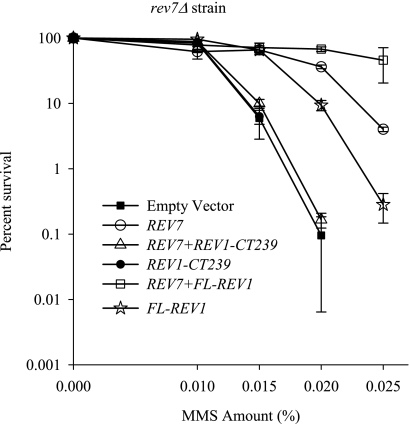
FIG. 4.
Effect of overproduction of the Rev1 C-terminal 239-amino-acid fragment on survival of the rev7Δ strain after DNA damage. MMS sensitivity of the rev7Δ strain carrying a plasmid expressing either Rev7 alone (REV7), the full-length Rev1 (FL-REV1), the Rev1 C-terminal 239-amino-acid region alone (REV1-CT239), coexpressing Rev7 and the C-terminal 239-amino-acid region of Rev1 (REV7+REV1-CT239), or coexpressing Rev7 and full-length Rev1 (REV7+FL-REV1). The empty expression plasmid is shown as a control. Error bars represent standard deviations of results from three independent colonies.
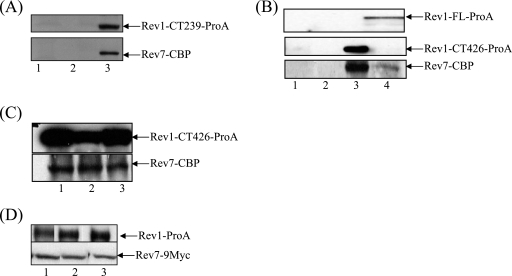
FIG. 5.
Physical interaction between Rev1 and Rev7. (A) Extracts from wild-type strains transformed with the empty plasmid (lane 1) or expressing Rev7-CBP alone (lane 2) or Rev7-CBP together with Rev1 C-terminal 239-amino-acid region tagged with ProA (lane 3) were immunoprecipitated using IgG-Sepharose beads, electrophoresed, blotted to polyvinylidene difluoride (PVDF), and probed with an anti-CBP antibody. (B) Extracts from wild-type strains transformed with the empty plasmid (lane 1) or expressing Rev7-CBP alone (lane 2), Rev7-CBP with the Rev1 C-terminal 426-amino-acid region tagged with ProA (lane 3), or Rev7-CBP with the full-length Rev1-ProA (Rev1-FL-ProA) (lane 4) were immunoprecipitated and immunoblotted as described above. (C) Wild-type strains coexpressing Rev7-CBP and the Rev1 C-terminal 426-amino-acid fragment tagged with ProA were left untreated (lane 1) or treated with 0.1% MMS for 30 min (lane 2). Extracts from these strains were immunoprecipitated and immunoblotted as for panel A. Samples in lane 3 were treated with 200 μg/ml of EtBr for 30 min on ice before the immunoprecipitation. (D) Strains YSD4 expressing Rev1-ProA and Rev7-9Myc were left untreated (lane 1) or treated with 0.1% MMS for 30 min (lane 2). Extracts from these strains were immunoprecipitated using IgG-Sepharose beads, electrophoresed, blotted to PVDF, and probed with an anti-Myc antibody. Samples in lane 3 were treated with 200 μg/ml of ethidium bromide for 30 min on ice before the immunoprecipitation.
MMS sensitivity and UV mutagenesis assays.
For methyl methanesulfonate (MMS) sensitivity assays, yeast strains transformed with plasmid constructs were grown in SD medium lacking tryptophan and supplemented with 2% raffinose for 2 days at 30°C. They were then subcultured into SD medium lacking tryptophan, supplemented with 2% galactose, and grown to 1 × 107 cells/ml. Appropriate dilutions were plated on SD medium plates lacking tryptophan supplemented with 2% galactose and the indicated amounts of MMS. Colonies were counted after 4 days at 30°C.
For UV mutagenesis assays, three independent colonies of strains YSD1 and YSD2 were grown for 2 days in SD medium lacking tryptophan and supplemented with 2% raffinose. They were then inoculated into synthetic complete (SC) medium with 2% galactose and grown to ∼1 × 108 cell/ml. Appropriate dilutions were plated on SC or SD lacking adenine medium plates with 2% galactose. Plates were irradiated at 1 J/m2/s using a G15T8 UV lamp (General Electric) at 254 nm. ADE2 revertants were counted after 7 days of growth at 30°C in the dark. The frequency of ADE2 reversion was calculated by subtracting the spontaneous value from the frequency at the 10-J/m2 UV dose.
Immunoprecipitations and Immunoblot analysis.
For the Rev1-Rev7 interaction studies, the W1588-4C strain transformed with the plasmids described in the legend to Fig. 4, were initially grown for 2 days in SD medium lacking tryptophan supplemented with 2% raffinose at 30°C. The cells were then subcultured and grown overnight at 30°C in SD medium lacking tryptophan and supplemented with 2% galactose to 1 × 107 cells/ml. The cells were centrifuged (4,000 × g, 5 min at 4° C) and washed once in water. All further steps were carried out at 4°C. Lysis and immunoprecipitations were carried out essentially as described previously (6), with some modifications. Briefly, cell pellets were resuspended in 0.5 to 1 ml ice-cold lysis buffer (50 mM HEPES, pH 7.7, 0.15 M NaCl, 1 mM Na-EDTA, 1% Triton X-100, 0.1% sodium deoxycholate supplemented with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail [Roche]) and lysed using a bead beater with 0.5 g of glass beads per sample. The lysate was centrifuged at 13,500 × g for 7 min. The supernatant was mixed with 50 μl immunoglobulin G (IgG)-Sepharose beads (Pharmacia) that were preequilibrated in lysis buffer. The supernatant and IgG beads were incubated on a rotating wheel for 2 h. The beads were washed three times with lysis buffer, and bound proteins were eluted using sodium dodecyl sulfate (SDS) loading buffer. For the immunoprecipitations described in the legend to Fig. 6, cells were lysed as described above and anti-Myc or anti-hemagglutinin (anti-HA) antibodies were used for immunoprecipitations as described previously (13).
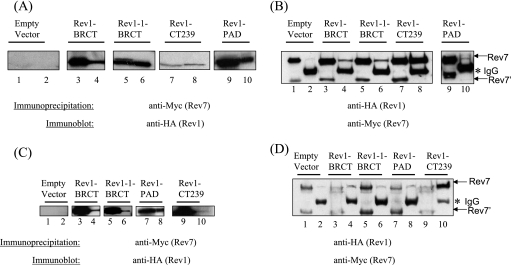
FIG. 6.
Interaction of Rev7 with the BRCT motif of Rev1 and the Rev1 PAD. (A) Extracts from the Rev7-13Myc strain (YSD5) expressing HA-tagged Rev1-BRCT, Rev1-1 BRCT, the Rev1 C-terminal 239-amino-acid fragment (Rev1 CT239), the Rev1 PAD, or the empty vector control were immunoprecipitated using the anti-Myc antibody. The immunoprecipitated protein complex was boiled in SDS loading buffer and analyzed by SDS-PAGE, followed by immunoblotting using an anti-HA antibody (lanes 2, 4, 6, 8, and 10). A portion of the extracts prior to immunoprecipitation were loaded as inputs (lanes 1, 3, 5, 7, and 9). (B) A reciprocal immunoprecipitation from panel A. Extracts from the strains were immunoprecipitated using the anti-HA antibody and subjected to immunoblot analysis using the anti-Myc antibody (lanes 2, 4, 6, 8, and 10). A portion of the extracts prior to immunoprecipitation were loaded as inputs (lanes 1, 3, 5, 7, and 9). The arrows indicate the positions of the two Rev-13Myc-specific bands described in the text (the upper band is designated Rev7 and the lower band is designated Rev7′). The asterisk indicates the position of the IgG heavy chain. (C) Extracts from a rev3Δ strain also harboring a 13Myc tag at the REV7 locus (YSD6) expressing HA-tagged Rev1-BRCT, Rev1-1 BRCT, the Rev1 PAD, the Rev1 C-terminal 239-amino-acid fragment (Rev1 CT239), or the empty vector control were immunoprecipitated using the anti-Myc antibody. The immunoprecipitated protein complex was boiled in SDS loading buffer and analyzed by SDS-PAGE, followed by immunoblotting using an anti-HA antibody (lanes 2, 4, 6, 8, and 10). A portion of the extracts prior to immunoprecipitation were loaded as inputs (lanes 1, 3, 5, 7, and 9). The arrows indicate the positions of the two Rev-13Myc-specific bands described in the text (the upper band is designated Rev7 and the lower band is designated Rev7′). The asterisk indicates the position of the IgG heavy chain. (D) A reciprocal immunoprecipitation from panel C. Extracts from the strains were immunoprecipitated using the anti-Myc antibody and subjected to immunoblot analysis using the anti-HA antibody (lanes 2, 4, 6, 8, and 10). A portion of the extracts prior to immunoprecipitation were loaded as inputs (lanes 1, 3, 5, 7, and 9).
To prepare cell extracts for the experiments described in the legends to Fig. 1D, 2D, and 2F, protein was extracted by trichloroacetic acid precipitation (17).
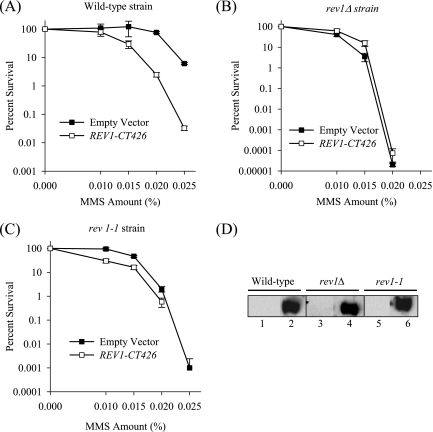
FIG. 1.
Effect of overproduction of the Rev1 C-terminal 426-amino-acid fragment on survival after DNA damage. MMS sensitivities of wild-type (A), rev1Δ (B), and rev1-1 (C) strains carrying either a high-copy plasmid overproducing the HA-tagged C-terminal 426-amino-acid fragment of Rev1 (REV1-CT426) or the empty vector control. Error bars represent the standard deviations of results from three independent colonies. (D) Immunoblot analysis of lysates from wild-type (W1588-4A), rev1Δ (YLW20), and rev1-1 (YLW35) strains transformed with either the empty plasmid control (lanes 1, 3, and 5) or a plasmid encoding the C-terminal 426-amino-acid fragment of Rev1 (lanes 2, 4, and 6) using anti-HA antibodies.
FIG. 2.
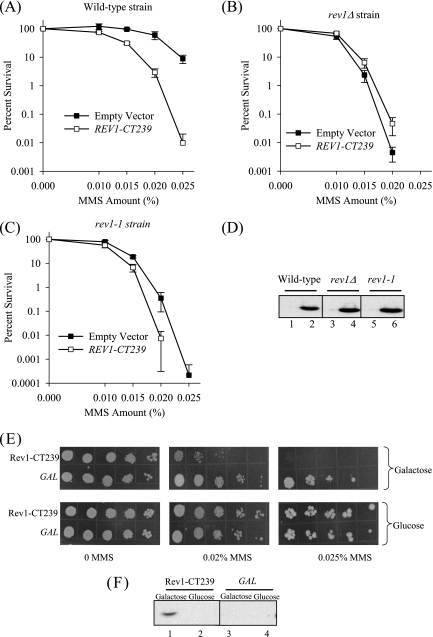
Effect of overproduction of the Rev1 C-terminal 239-amino-acid fragment on survival after DNA damage. MMS sensitivities of wild-type (A), rev1Δ (B), and rev1-1 (C) strains carrying either a high-copy plasmid overproducing the HA-tagged C-terminal 239-amino-acid fragment of Rev1 (REV1-CT239) or the empty vector control. (D) Immunoblot analysis of lysates from wild-type, rev1Δ, and rev1-1 strains transformed with either the empty plasmid control (lanes 1, 3, and 5) or a plasmid encoding the C-terminal 239 amino acid fragment of Rev1 (lanes 2, 4, and 6) using anti-HA antibodies. (E) Strains containing the GAL promoter fused to a fragment encoding the Rev1 C-terminal 239-amino-acid fragment (Rev1-CT239, strain YSD2) or the GAL promoter alone (GAL, strain YSD1), both integrated into the trp1-1 locus of a wild-type strain, were grown in the presence of glucose or galactose. Tenfold serial dilutions were spotted on selective medium plates containing galactose (upper panel) or glucose (lower panel) and the indicated amounts of MMS. (F) Trichloroacetic acid-precipitated proteins from strains YSD2 (lanes 1 and 2) and YSD1 (lanes 3 and 4), grown in the presence of galactose or glucose, were immunoblotted using an anti-HA antibody.
For immunoblotting, the protein samples were separated on SDS-polyacrylamide gel electrophoresis (PAGE), transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore), and probed with appropriate antibodies. Myc and HA-tagged proteins were detected using the mouse monoclonal antibody clone 4A6 (Upstate) and mono HA.11, clone 16B12 antibody (Covance), respectively. Rev7-CBP and ProA-tagged proteins were detected using the rabbit anti-CBP antibody (Upstate) and the rabbit PAP antibody (Sigma), respectively. All antibodies were used at a 1:1,000 dilution.
RESULTS
Overproduction of the PAD-containing C-terminal 426-amino-acid fragment of yeast Rev1 confers a strong dominant-negative phenotype on survival after DNA damage.
Recent studies in higher eukaryotes have demonstrated interactions between the C terminus of Rev1 and numerous translesion DNA polymerases (9, 29, 34, 40). These include Polκ, Polι, Polλ, Polη, and the Polζ accessory subunit, Rev7. We reasoned that, if the C terminus of yeast Rev1 was involved in mediating protein-protein interactions in a manner analogous to its higher eukaryotic counterparts, its overproduction would interfere with the activity of critical DNA damage resistance proteins. We expected that this would manifest as a severe defect in the survival of wild-type cells exposed to DNA damage. We therefore ectopically overproduced the C-terminal 426-amino-acid fragment of Rev1, including a portion of the thumb, linker, and PAD (30), tagged with HA, from a high-copy plasmid under the control of a GAL-inducible promoter. After induction in galactose, we assessed survival on selective medium plates containing various amounts of the DNA alkylating agent MMS. As shown in Fig. 1A, overproduction of the C-terminal 426-amino-acid fragment of Rev1 causes a decrease in the survival of otherwise wild-type cells exposed to MMS relative to the empty expression plasmid. This result strongly indicates that this region of Rev1 interferes with the activity of proteins involved in resistance to MMS. The decrease in survival after MMS treatment is due to a dominant-negative effect of the Rev1 C terminus, as overexpression in the rev1Δ strain (Fig. 1B) does not further sensitize the strain to MMS. Interestingly, overproduction of the Rev1 C terminus in the rev1-1 strain, which harbors a G193R point mutation in the BRCT motif, shows a modest exacerbation of the sensitivity of this strain to MMS (Fig. 1C), suggesting that the rev1-1 strain, already substantially defective in Rev1 activity, is further inhibited by overexpression of the C-terminal Rev1 fragment. Immunoblot analysis of lysates from the wild-type, rev1Δ, and rev1-1 strains using an anti-HA antibody confirmed the overexpression of the Rev1 C-terminal 426-amino-acid fragment (Fig. 1D).
The Rev1 PAD is not required for the dominant-negative phenotype of Rev1 C terminus-overproducing strains.
Recent experiments using purified proteins has demonstrated a requirement for the PAD of Rev1 to mediate a direct physical interaction with Rev7, the accessory subunit of yeast Polζ (1). To test whether overproduction of the extreme C terminus of yeast Rev1 (excluding the PAD) also confers a dominant-negative effect, we similarly expressed the last 239-amino-acid fragment of Rev1 in the wild-type, rev1Δ, and rev1-1 strains and plated the cells on selective medium plates containing MMS. As shown in Fig. 2A to C, overproduction of the C-terminal 239-amino-acid region of Rev1 causes a similar sensitization of wild-type yeast cells, a slight suppression of the MMS sensitivity of the rev1Δ strain, and a moderate exacerbation of the sensitivity of the rev1-1 strain. Immunoblot analysis of lysates from these strains with an anti-HA antibody revealed that the Rev1 C-terminal 239-amino-acid fragment was equally expressed in the wild-type, rev1Δ, and rev1-1 strains (Fig. 2D).
To rule out potential artifacts arising from the presence of the multicopy plasmid, we integrated a construct encoding the HA-tagged Rev1 C-terminal 239-amino-acid fragment under the control of the GAL promoter into the chromosomal trp1-1 locus of wild-type cells. To confirm that this strain still exhibited the dominant-negative phenotype after DNA damage, we induced expression of the Rev1 C-terminal 239-amino-acid region in galactose and spotted the cells on selective medium plates that contained various amounts of MMS. As shown in Fig. 2E (upper panel), overexpression of the fragment encoding the Rev1 C-terminal 239-amino-acid region causes a marked sensitization of the wild-type strain to MMS relative to a strain integrated with the GAL promoter alone. In contrast, repression of the GAL promoter by growing the cells in the presence of glucose abrogates the sensitivity of these cells to MMS treatment, suggesting that the dominant-negative effect on survival is due to overexpression of the Rev1 C terminus (Fig. 2E, lower panel). We verified the induction of the Rev1 C terminus in galactose by immunoblot analysis of lysates from these strains grown in glucose or galactose using an anti-HA antibody (Fig. 2F).
Taken together, the results presented in Fig. 1 and 2 suggest that the C terminus of yeast Rev1 is likely involved in protein-protein interactions that are crucial for the physiological role of Rev1. Intriguingly, we note that the dominant-negative phenotype associated with overproduction of the Rev1 C terminus also partially requires the Rev1 BRCT motif, as we observe a moderate exacerbation of the MMS sensitivity of the rev1-1 strain.
Wild-type yeast overproducing the Rev1 C-terminal 239-amino-acid fragment are defective in DNA damage-induced mutagenesis.
Rev1 function is required for the introduction of DNA damage-induced mutations in the genome, as rev1Δ strains are severely defective in DNA damage-induced mutagenesis (21). As REV1-dependent mutagenesis has been most thoroughly characterized in response to UV, we examined the UV sensitivity and UV-induced mutagenesis in the strain YSD2, which harbors the chromosomally integrated 239-amino-acid segment of Rev1 under the control of the GAL promoter. After induction in galactose, this strain is sensitive not only to MMS (Fig. 2E) but also to UV, relative to the strain YSD1, which carries an integration of the GAL promoter alone (data not shown).
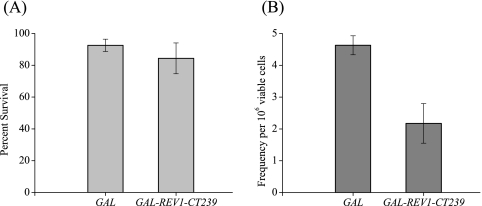
UV irradiation introduces lesions in DNA that are repaired by both error-free and error-prone mechanisms. The efficiency of the error-prone pathway is conveniently studied by assaying the reversion frequency of the ade2-1 allele after UV treatment. To determine if strain YSD2 was also defective in UV-induced mutagenesis, we exposed the cells to a low UV dose and measured the number of revertants per surviving cells. Even though the survival of either strain YSD1 or YSD2 was not significantly diminished after irradiation with this modest UV dose (Fig. 3A), strain YSD2 overproducing the Rev1 C-terminal 239-amino-acid region exhibits a decrease in the frequency of adenine revertants after UV treatment (Fig. 3B). These results strongly suggest that the overproduction of the Rev1 C terminus interferes with the ability of the cells to function in the mutagenic bypass of UV-induced DNA lesions.
FIG. 3.
Effect of overproduction of the Rev1 C-terminal 239-amino-acid fragment on survival after UV exposure and UV-induced reversion of the ade2-1 allele. (A) Strains YSD1 (GAL) and YSD2 (GAL-REV1-CT239) were grown to ∼1 × 108 cells/ml in the presence of galactose. Appropriate dilutions were plated on SC medium plates and irradiated with a 10-J/m2 UV dose. Colonies were counted after 3 days at 30°C. The error bars represent standard deviations of results from three independent colonies. (B) Strains YSD1 and YSD2, grown to ∼1 × 108 cells/ml in the presence of galactose, were plated undiluted on selective medium plates lacking adenine. The cells were irradiated with a 10-J/m2 UV dose and incubated in the dark for 6 days. The frequency of adenine revertants per surviving fraction corrected for spontaneous revertants are represented. The error bars represent standard deviations of results from three independent colonies.
REV7 is required for the dominant-negative phenotype of the Rev1 C terminus on cell survival after DNA damage.
The extreme C-terminal 100 amino acids of mouse and human Rev1 interact with Rev7, the accessory subunit of the Rev7-Rev3 complex that together comprise Polζ (9, 29, 34). To determine if yeast REV7 is required for the ability of the overproduced Rev1 C terminus to sensitize cells to DNA damage, we expressed the Rev1 C-terminal 239-amino-acid fragment (tagged with protein A) in the rev7Δ strain and assessed the viability of the cells after MMS treatment. As shown in Fig. 4, expression of the Rev1 C-terminal 239-amino-acid fragment does not exacerbate the sensitivity of the rev7Δ strain after MMS treatment (note that the survival curve for the rev7Δ strain transformed with the plasmid encoding the Rev1 C-terminal fragment overlaps with that of the empty plasmid control and is thus hidden in Fig. 4). This result suggests that the REV1-dependent function that is inhibited by expressing the C terminus of Rev1 is also REV7 dependent.
If the yeast Rev1 C terminus is involved in a functional interaction with Rev7, we predicted that coexpression of the two proteins would interfere with the ability of Rev7 to complement the MMS-sensitive phenotype of the rev7Δ strain. We therefore constructed plasmids under the control of the oppositely oriented GAL1 and GAL10 promoters to coexpress CBP-tagged Rev7 and the Rev1 C-terminal 239-amino-acid fragment tagged with ProA. After induction in galactose, cells were spotted on plates containing various amounts of MMS. As expected, expression of Rev7 alone complements the lethality of the rev7Δ strain after MMS treatment relative to cells transformed with the empty vector control (Fig. 4). However, coexpression of the Rev1 C terminus and Rev7 completely blocks the ability of Rev7 to complement the MMS-induced lethality of the rev7Δ strain. In contrast, coexpression of Rev7 and the full-length Rev1 protein rescues the lethality of this strain in response to MMS. Intriguingly, expression of the full-length Rev1 alone partially rescues the MMS sensitivity of the rev7Δ strain (Fig. 4). These data strongly support our inference that the C terminus of Rev1 is involved not only in a functional interaction with Rev7 but in a physical interaction as well.
The C terminus of Rev1 interacts with Rev7.
To examine whether the Rev1 C terminus and Rev7 interact as components of a complex, we used the plasmid system described above to coexpress Rev7 and the C-terminal 239-amino-acid region of Rev1 (excluding the PAD), the PAD-containing C-terminal 426-amino-acid fragment, or the full-length Rev1 in wild-type cells. Following galactose induction, we performed coimmunoprecipitations using IgG-Sepharose beads followed by immunoblot analysis using an anti-CBP antibody. As shown in Fig. 5A, IgG beads immunoprecipitate Rev7 from cells coexpressing the C-terminal 239-amino-acid region of Rev1 and Rev7 (lane 3), whereas Rev7 is not immunoprecipitated from cells transformed with either the empty vector (lane 1) or Rev7 alone (lane 2). These results thus provide the first demonstration that the extreme C-terminal 239-amino-acid fragment of S. cerevisiae Rev1 (excluding the PAD) can mediate an interaction with Rev7.
Since the dominant-negative phenotype resulting from overproduction of the Rev1 C terminus was also exhibited by the PAD-containing C-terminal 426-amino-acid fragment (Fig. 1), we tested if this region likewise interacts with Rev7. We therefore performed immunoprecipitations from cells coexpressing Rev7 and either the C-terminal 426-amino-acid fragment of Rev1 or the full-length Rev1 protein. Consistent with the observations of Acharya et al. (1), Rev7 is immunoprecipitated from cells coexpressing Rev7 and either the Rev1 C-terminal 426-amino-acid fragment (Fig. 5B) or the full-length Rev1 (lane 4) but not from cells transformed with either the empty vector or Rev7 alone (lanes 1 and 2, respectively). Thus, consistent with an earlier study (1), the C-terminal 426-amino-acid fragment of yeast Rev1 (including the PAD) interacts with Rev7, although our immunoprecipitation experiments do not show that the interaction is direct.
To rule out the possibility that the interaction between Rev7 and Rev1 is mediated indirectly via independent binding of the two proteins to DNA, extracts from cells coexpressing Rev1 and Rev7 were treated before immunoprecipitation with ethidium bromide (EtBr), a reagent that disrupts DNA-protein interactions by distorting DNA structure by intercalation (18). However, despite the presence of the DNA-binding Rev1 PAD, the efficiency of interaction between Rev1 and Rev7 is not influenced by the addition of EtBr (Fig. 5C, lane 3), indicating that the interaction between Rev1 and Rev7 is independent of DNA. MMS treatment of the strains expressing Rev7 and the C-terminal 426-amino-acid fragment of Rev1 does not significantly alter the interaction between the two proteins (Fig. 5C, lane 2), implying that the association between the two proteins occurs independently of DNA damage.
To confirm that the interaction between Rev1 and Rev7 also occurs at endogenously expressed levels, we tagged Rev1 and Rev7 at their C termini with the protein A and 9-Myc epitope tags, respectively, by homologous recombination at the REV1 and REV7 loci. Upon immunoblot analysis using an anti-Myc antibody, we observed two bands specific to Rev7: one of the expected molecular size, Rev7-9Myc (∼45 kDa), and another of a lower molecular size (∼35 kDa) (data not shown). Since we observe similarly sized proteins using a ProA-tagged Rev7 (data not shown) and Rev7-13Myc (Fig. 6B), we speculate that the lower-migrating band is an N-terminal truncation of the Rev7 protein. MMS treatment of the Rev7-9Myc strain does not change the levels of this smaller Rev7 fragment nor is its expression altered during the yeast cell cycle (data not shown). The significance and mechanism of regulation of this truncated Rev7 product is unclear and is currently under investigation.
Interestingly, we note that Rev1-ProA immunoprecipitates only the higher-migrating band of Rev7-9Myc (Fig. 5D, lane 1). The presence of the lower-migrating Rev7 band notwithstanding, we observe an interaction between Rev1 and Rev7 at endogenous expression levels. Pretreatment of the cells with MMS (lane 2) or treatment of the lysates with ethidium bromide (lane 3) does not alter the interaction between the two proteins, suggesting that the interaction between Rev1 and Rev7 is independent of DNA damage or DNA. These data further extend our previous conclusions by demonstrating that the endogenous protein levels of Rev1 and Rev7 are sufficient to mediate the interaction.
The Rev1 BRCT motif also interacts with Rev7.
The yeast Rev1 N-terminal BRCT motif is required for mutagenesis and resistance to DNA damaging agents, as the G193R point mutation in the yeast Rev1 BRCT motif (the rev1-1 mutation) abrogates its function in DNA damage-induced mutagenesis (19). Our results concerning the interaction between full-length Rev1 and Rev7 did not exclude the possibility that the interaction between Rev1 and Rev7 might involve a region of Rev1 distinct from the C terminus. To determine if the N-terminal BRCT region of Rev1 might also be involved in the interaction with Rev7, we expressed either the HA epitope-tagged BRCT region of Rev1 or the Rev1-1 protein in a Rev7-13Myc strain. We performed immunoprecipitations using an anti-Myc antibody and immunoblot analysis using an anti-HA antibody. As shown in Fig. 6A, Rev7-13Myc immunoprecipitates the HA-tagged BRCT region of Rev1 (lane 4). Surprisingly, the Rev1-1 BRCT domain is also pulled down by Rev7-13Myc (lane 6). The Rev1 C-terminal 239-amino-acid fragment used as a positive control also interacts efficiently with Rev7-13Myc (lane 8), whereas cells transformed with the empty expression vector do not immunoprecipitate Rev1 (lane 1). Conversely, we immunoprecipitated the HA-tagged BRCT Rev1, BRCT Rev1-1, and the Rev1 C-terminal 239-amino-acid region with anti-Myc antibodies (Fig. 6B). Taken together, these results thus define the BRCT motif of Rev1 and the extreme C-terminal 239-amino-acid fragment as novel regions necessary for the Rev7 interaction.
The Rev1 PAD interacts with Rev7.
The Rev1 PAD was recently shown to directly interact with Rev7 (1). Using the purified Rev1 and Rev7 proteins and the yeast two-hybrid assay, Acharya et al. demonstrated the involvement of the Rev1 PAD in a direct interaction with Rev7 (1). Although deletion of the PAD supports an interaction with Rev7 (Fig. 5A), we wished to determine if this domain coimmunoprecipitates with Rev7 independent of the extreme C-terminal 239-amino-acid fragment. We therefore created a construct similar to that of Acharya et al. (1) that expresses the HA-tagged Rev1 PAD (amino acids from 567 to 767) in a Rev7-13Myc strain and performed immunoprecipitations from crude extracts using anti-HA and anti-Myc antibodies. As shown in Fig. 6A (lane 10), Rev7 efficiently immunoprecipitates the Rev1 PAD, whereas Rev7 is not immunoprecipitated from cells transformed with the empty vector (data not shown). Similarly, the Rev1 PAD pulls down Rev7-13Myc (Fig. 6B, lane 10), whereas cells transformed with the empty vector control do not (data not shown). This result therefore demonstrates that, consistent, with results using purified proteins, the Rev1 PAD forms a complex with Rev7 (1).
Rev1 interacts with Rev7 independently of Rev3.
To test whether the interaction between Rev1 and Rev7 is mediated by Rev3, the catalytic subunit of Polζ, we expressed the HA-tagged BRCT Rev1, BRCT Rev1-1, the Rev1 PAD, and the C-terminal 239-amino-acid fragment in the rev3Δ strain that also harbors a 13-Myc tag at the REV7 locus. Immunoprecipitations from crude extracts using anti-Myc and anti-HA antibodies reveal that the interaction between Rev7 and (i) the Rev1 BRCT and the Rev1-1 BRCT regions, (ii) the Rev1 PAD, and (iii) the Rev1 C-terminal 239-amino-acid fragment occur even in the absence of Rev3, indicating that Rev3 is not required for the interaction between Rev1 and Rev7 (Fig. 6C and D).
DISCUSSION
In the current study we demonstrate a functional interaction between the Saccharomyces cerevisiae Rev1 C terminus and Rev7. Analogous to the interacting region for Rev7 identified in higher eukaryotes, we provide evidence suggesting that the Rev7 protein interacts with the extreme Rev1 C-terminal fragment excluding the PAD. We also note that Rev7 forms a complex with a Rev1 C-terminal construct that contains the PAD (Fig. 5 and 7), a region that increases the DNA binding surface of Rev1 and is thus crucial for polymerase activity (26, 30, 37, 43). As expected from a recent study (1), the Rev1 PAD by itself immunoprecipitates the Rev7 protein (Fig. 6A). However, in contrast to the results of Acharya et al. (1), we find that the extreme C-terminal 239-amino-acid fragment of Rev1 (independent of the PAD) is also present in a complex with Rev7 (Fig. 6A and B and 7). We ascribe the differences between our findings and those of Acharya et al. (1) to the different techniques used to detect the interactions; a directed yeast two-hybrid assay and purified Rev7 and Rev1 truncated proteins were used in their analysis, whereas we immunoprecipitated the two proteins from cell extracts. Furthermore, the use of different protein tags between our analysis and theirs could also account for the changes in binding, as Acharya et al. only observed the binding of Rev1 to glutathione S-transferase (GST)-Rev7 but not the binding of Rev7 to the GST-Rev1 PAD (1).
FIG. 7.
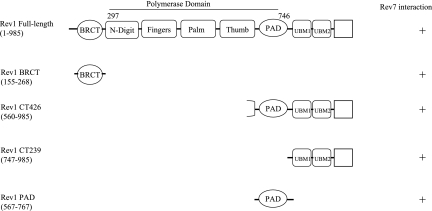
Schematic depiction of Rev1 protein fragments required for binding to Rev7. The 985-amino-acid Rev1 protein contains a BRCT motif at the N terminus (4). The polymerase domain (amino acids 297 to 746) consists of the fingers, palm, thumb, and PAD motifs conserved among other Y-family polymerases. In addition, the Rev1 “N-digit” interacts with the incoming dCTP (30). The C-terminal region of Rev1 is comprised of two copies of the ubiquitin-binding motif (UBM1 and UBM2) (3).
The interaction between Rev1 and Rev7 is independent of Rev3, the large catalytic subunit of Polζ, as the interactions between the various regions of Rev1 occur even in a rev3Δ strain (Fig. 6C and D). Although this result would be consistent with a direct interaction between Rev1 and Rev7, we cannot exclude the possibility that other proteins may mediate the interaction, since we immunoprecipitated the proteins from a whole-cell lysate. Intriguingly, a recent study has described a condition under which the association between Rev1 and Rev7 occurs only in wild-type cells but not in a rev3Δ strain (14). Hirano and Sugimoto (14) performed immunoprecipitations in yeast cells arrested in G2/M with nocodazole to rule out interactions that depended on DNA replication. Our assays were performed using asynchronous logarithmically growing yeast cells, a factor that could explain the differences between our results and those of Hirano and Sugimoto (14).
The functional importance of the interaction between the Rev1 extreme C terminus and Rev7 is supported by the in vitro observation that a deletion of the C-terminal ∼200-amino-acid fragment of yeast Rev1 abolishes its stimulatory effect on Polζ-catalyzed extension from opposite the acetylaminofluorine-dG DNA lesion (10). Though the catalytic dCMP transferase activity of this truncated Rev1 protein remains intact, it is nevertheless unable to synergistically perform lesion bypass together with Polζ from across the acetylaminofluorine-dG DNA adduct, pointing to a structural role for Rev1 in assembling this TLS complex (10). Furthermore, deletion or point mutations of the C terminus abolishes the ability of Rev1 to complement the DNA damage sensitivity of the rev1Δ strain (19; S. D'Souza, L. Waters, and G. C. Walker, unpublished observations).
Our observation that elevated levels of the Rev1 C terminus confer a severe defect on the viability and induced mutagenesis of wild-type strains exposed to DNA damage provides a demonstration of the physiological importance of this region in vivo. Though overproduction of the Rev1 BRCT motif does not exhibit a similar effect (data not shown), we do find that the phenotype conferred by overproduction of the Rev1 C terminus partially requires the Rev1 BRCT motif, as we observe a moderate exacerbation of the survival of the rev1-1 strain to DNA damage (Fig. 1C and 2C). Since the Rev1-1 protein is thought to be deficient in interacting with key partners, the exacerbation of the MMS sensitivity of the rev1-1 strain suggests that high levels of the Rev1 C-terminal fragment further interfere with these interactions. Consistent with this notion, we observe an interaction between the BRCT motif of Rev1 and Rev7 (Fig. 6 and 7). The G193R rev1-1 mutation, however, still supports an interaction with Rev7, suggesting that the exacerbation of the rev1-1 defect does not directly affect the Rev1-Rev7 interaction but rather a Rev7-dependent component of Rev1 function. Intriguingly, while this manuscript was in review, Hirano and Sugimoto (14), while studying the association of Polζ and Rev1 with regions near a double-strand break, demonstrated that the rev1-1 mutation does not impair Rev1 association with Rev7. However, the association of the Rev1-1 protein to a double-strand break was significantly reduced, as was the association of Rev7 to the double-strand break in rev1-1 mutant strains, suggesting that the BRCT motif of Rev1 plays a role in assembling a complex of proteins after DNA damage.
The requirement for REV7 for the dominant-negative effect of the Rev1 C terminus indicates that this phenotype is mediated by a titration of Rev7 by elevated levels of the Rev1 C terminus. However, overexpression of Rev7 in wild-type cells that co-overproduce the Rev1 C terminus does not alleviate the MMS-sensitive phenotype of these strains (data not shown). This suggests that, in addition to Rev7, one or more additional proteins required for the tolerance to DNA damage are also sequestered by elevated levels of the Rev1 C terminus. A clue to the identity of some of these proteins and the regulation of their interaction with Rev1 comes from a recent study showing that the Rev1 C terminus possesses a ubiquitin-binding domain conserved evolutionarily in all Y-family TLS polymerases (3). These domains are believed to play crucial roles in TLS by regulating the localization of TLS polymerases to replication factories after DNA damage. Thus, the overproduction of the yeast Rev1 C terminus in wild-type cells exposed to DNA damage could possibly prevent Rev1 binding to ubiquitin, thereby altering its capacity to function in DNA lesion bypass.
The Rev7 protein is composed almost entirely of a unique HORMA domain (Hop1, Rev7, and MAD2), thought to be involved in protein-protein interactions and chromatin binding (2, 42). The human homolog of Rev7 (MAD2B) inhibits the anaphase-promoting complex, a ubiquitin ligase that controls sister chromatid separation and exit from mitosis (5). These observations, which suggest a role for yeast Rev7 during G2/M, coupled with the finding that the level of the Rev1 polymerase is highest at the G2/M phase of the cell cycle (46), prompted us to evaluate whether its interacting partner, Polζ, is subject to the same manner of fluctuation. However, unlike Rev1, Rev3 and Rev7 levels remain constant throughout the cell cycle (data not shown). Although Rev7 has no effect on either the catalytic efficiency or the nucleotide incorporation specificity of Rev1 in vitro (1), the presence of DNA damage in the template could alter the function of the Rev1-Rev7 complex. Thus, it remains possible that Polζ acts during S phase, while a Rev1-Polζ complex in G2 serves a role in filling unrepaired gaps in the DNA (28) that persist after S phase is complete.
Acknowledgments
We thank the members of the laboratories of G. C. Walker and S. P. Bell for helpful suggestions. In particular, we are grateful to L. Simmons, K. Gibson, B. Davies, V. Godoy, L. Waters, R. Woodruff, B. Minesinger, D. Jarosz, M. Wiltrout, S. Simon, and H. Kobayashi for critical reading of the manuscript. We also thank J. Randell and H. Rodriguez for the construction of the pESC-Split TAP 2μm plasmid (used in experiments for which results are shown in Fig. 4 and 5) and L. Waters for constructing strains YLW20, YLW35, and YLW70. We acknowledge S. P. Bell (MIT) for the generous gifts of the pESC-Split TAP and pAS311 plasmids, D. Hinkle (University of Rochester) for the pMR7 and pJN60 plasmids, and L. Samson (MIT) for the YOR346W, YIL139C, and YPL167C strains.
This work was supported by an American Cancer Society Research Professorship to G.C.W.
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Acharya, N., L. Haracska, R. E. Johnson, I. Unk, S. Prakash, and L. Prakash. 2005. Complex formation of yeast Rev1 and Rev7 proteins: a novel role for the polymerase-associated domain. Mol. Cell. Biol. 25:9734-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., and E. V. Koonin. 1998. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 23:284-286. [DOI] [PubMed] [Google Scholar]
- 3.Bienko, M., C. M. Green, N. Crosetto, F. Rudolf, G. Zapart, B. Coull, P. Kannouche, G. Wider, M. Peter, A. R. Lehmann, K. Hofmann, and I. Dikic. 2005. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310:1821-1824. [DOI] [PubMed] [Google Scholar]
- 4.Callebaut, I., and J. P. Mornon. 1997. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 400:25-30. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J., and G. Fang. 2001. MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev. 15:1765-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncker, B. P., K. Shimada, M. Tsai-Pflugfelder, P. Pasero, and S. M. Gasser. 2002. An N-terminal domain of Dbf4p mediates interaction with both origin recognition complex (ORC) and Rad53p and can deregulate late origin firing. Proc. Natl. Acad. Sci. USA 99:16087-16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2006. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, D.C.
- 8.Geitz, R. D., and R. A. Woods. 2002. Guide to yeast genetics and molecular and cell biology, vol. 350. Academic Press, San Diego, Calif.
- 9.Guo, C., P. L. Fischhaber, M. J. Luk-Paszyc, Y. Masuda, J. Zhou, K. Kamiya, C. Kisker, and E. C. Friedberg. 2003. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 22:6621-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, D., Z. Xie, H. Shen, B. Zhao, and Z. Wang. 2004. Translesion synthesis of acetylaminofluorene-dG adducts by DNA polymerase zeta is stimulated by yeast Rev1 protein. Nucleic Acids Res. 32:1122-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haracska, L., S. Prakash, and L. Prakash. 2002. Yeast Rev1 protein is a G template-specific DNA polymerase. J. Biol. Chem. 277:15546-15551. [DOI] [PubMed] [Google Scholar]
- 12.Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. Burgers, S. Prakash, and L. Prakash. 2001. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 15:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 14.Hirano, Y., and K. Sugimoto. 2006. ATR homolog Mec1 controls association of DNA polymerase zeta-Rev1 complex with regions near a double-strand break. Curr. Biol. 16:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, R. E., M. T. Washington, L. Haracska, S. Prakash, and L. Prakash. 2000. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature 406:1015-1019. [DOI] [PubMed] [Google Scholar]
- 16.Kannouche, P. L., and A. R. Lehmann. 2004. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3:1011-1013. [PubMed] [Google Scholar]
- 17.Knop, M., K. Siegers, G. Pereira, W. Zachariae, B. Winsor, K. Nasmyth, and E. Schiebel. 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15:963-972. [DOI] [PubMed] [Google Scholar]
- 18.Lai, J. S., and W. Herr. 1992. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc. Natl. Acad. Sci. USA 89:6958-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larimer, F. W., J. R. Perry, and A. A. Hardigree. 1989. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J. Bacteriol. 171:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence, C. 1994. The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do, and how does it do it? Bioessays 16:253-258. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence, C. W. 2004. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv. Protein Chem. 69:167-203. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence, C. W., and R. B. Christensen. 1978. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. I. rev1 mutant strains. J. Mol. Biol. 122:1-21. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence, C. W., G. Das, and R. B. Christensen. 1985. REV7, a new gene concerned with UV mutagenesis in yeast. Mol. Gen. Genet. 200:80-85. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence, C. W., T. O'Brien, and J. Bond. 1984. UV-induced reversion of his4 frameshift mutations in rad6, rev1, and rev3 mutants of yeast. Mol. Gen. Genet. 195:487-490. [DOI] [PubMed] [Google Scholar]
- 25.Lemontt, J. F. 1971. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics 68:21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling, H., F. Boudsocq, R. Woodgate, and W. Yang. 2001. Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell 107:91-102. [DOI] [PubMed] [Google Scholar]
- 27.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 28.Lopes, M., M. Foiani, and J. M. Sogo. 2006. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21:15-27. [DOI] [PubMed] [Google Scholar]
- 29.Murakumo, Y., Y. Ogura, H. Ishii, S. Numata, M. Ichihara, C. M. Croce, R. Fishel, and M. Takahashi. 2001. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 276:35644-35651. [DOI] [PubMed] [Google Scholar]
- 30.Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash, and A. K. Aggarwal. 2005. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science 309:2219-2222. [DOI] [PubMed] [Google Scholar]
- 31.Nelson, J. R., P. E. Gibbs, A. M. Nowicka, D. C. Hinkle, and C. W. Lawrence. 2000. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol. 37:549-554. [DOI] [PubMed] [Google Scholar]
- 32.Nelson, J. R., C. W. Lawrence, and D. C. Hinkle. 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382:729-731. [DOI] [PubMed] [Google Scholar]
- 33.Nelson, J. R., C. W. Lawrence, and D. C. Hinkle. 1996. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science 272:1646-1649. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi, E., Y. Murakumo, N. Kanjo, J. Akagi, C. Masutani, F. Hanaoka, and H. Ohmori. 2004. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9:523-531. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka, C., D. Loakes, and K. Negishi. 2002. The role of deoxycytidyl transferase activity of yeast Rev1 protein in the bypass of abasic sites. Nucleic Acids Res. Suppl. 2:87-88. [DOI] [PubMed] [Google Scholar]
- 36.Plosky, B. S., and R. Woodgate. 2004. Switching from high-fidelity replicases to low-fidelity lesion-bypass polymerases. Curr. Opin. Genet. Dev. 14:113-119. [DOI] [PubMed] [Google Scholar]
- 37.Prakash, S., R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74:317-353. [DOI] [PubMed] [Google Scholar]
- 38.Sherman, F. 2002. Guide to yeast genetics and molecular and cell biology, vol. 350. Academic Press, San Diego, Calif.
- 39.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi, R., M. Oshige, M. Uchida, G. Ishikawa, K. Takata, K. Shimanouchi, Y. Kanai, T. Ruike, H. Morioka, and K. Sakaguchi. 2004. Purification of Drosophila DNA polymerase zeta by REV1 protein-affinity chromatography. Biochem. J. 382:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tissier, A., P. Kannouche, M. P. Reck, A. R. Lehmann, R. P. Fuchs, and A. Cordonnier. 2004. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amsterdam) 3:1503-1514. [DOI] [PubMed] [Google Scholar]
- 42.Torpey, L. E., P. E. Gibbs, J. Nelson, and C. W. Lawrence. 1994. Cloning and sequence of REV7, a gene whose function is required for DNA damage-induced mutagenesis in Saccharomyces cerevisiae. Yeast 10:1503-1509. [DOI] [PubMed] [Google Scholar]
- 43.Trincao, J., R. E. Johnson, C. R. Escalante, S. Prakash, L. Prakash, and A. K. Aggarwal. 2001. Structure of the catalytic core of S. cerevisiae DNA polymerase eta: implications for translesion DNA synthesis. Mol. Cell 8:417-426. [DOI] [PubMed] [Google Scholar]
- 44.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 45.Washington, M. T., I. G. Minko, R. E. Johnson, L. Haracska, T. M. Harris, R. S. Lloyd, S. Prakash, and L. Prakash. 2004. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase zeta. Mol. Cell. Biol. 24:6900-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters, L. S., and G. C. Walker. 2006. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G2/M phase rather than S phase. Proc. Natl. Acad. Sci. USA 103:8971-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, X., E. G. Muller, and R. Rothstein. 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2:329-340. [DOI] [PubMed] [Google Scholar]