Abstract
We have previously demonstrated that vacuolar sorting receptor (VSR) proteins are concentrated on prevacuolar compartments (PVCs) in plant cells. PVCs in tobacco (Nicotiana tabacum) BY-2 cells are multivesicular bodies (MVBs) as defined by VSR proteins and the BP-80 reporter, where the transmembrane domain (TMD) and cytoplasmic tail (CT) sequences of BP-80 are sufficient and specific for correct targeting of the reporter to PVCs. The genome of Arabidopsis (Arabidopsis thaliana) contains seven VSR proteins, but little is known about their individual subcellular localization and function. Here, we study the subcellular localization of the seven Arabidopsis VSR proteins (AtVSR1–7) based on the previously proven hypothesis that the TMD and CT sequences correctly target individual VSR to its final destination in transgenic tobacco BY-2 cells. Toward this goal, we have generated seven chimeric constructs containing signal peptide (sp) linked to green fluorescent protein (GFP) and TMD/CT sequences (sp-GFP-TMD/CT) of the seven individual AtVSR. Transgenic tobacco BY-2 cell lines expressing these seven sp-GFP-TMD-CT fusions all exhibited typical punctate signals colocalizing with VSR proteins by confocal immunofluorescence. In addition, wortmannin caused the GFP-marked prevacuolar organelles to form small vacuoles, and VSR antibodies labeled these enlarged MVBs in transgenic BY-2 cells. Wortmannin also caused VSR-marked PVCs to vacuolate in other cell types, including Arabidopsis, rice (Oryza sativa), pea (Pisum sativum), and mung bean (Vigna radiata). Therefore, the seven AtVSRs are localized to MVBs in tobacco BY-2 cells, and wortmannin-induced vacuolation of PVCs is a general response in plants.
All eukaryotic cells contain an endomembrane system for the secretory pathway that is comprised of several functionally distinct membrane-bounded organelles, including the endoplasmic reticulum, Golgi apparatus, prevacuolar/endosomal compartment, and vacuole (Jiang and Rogers, 2003; Lam et al., 2005; Mo et al., 2006). These secretory organelles are defined by specific marker proteins present on organelle membranes. Plant secretory pathways are complicated because plant cells contain both lytic vacuole and protein storage vacuole (Neuhaus and Rogers, 1998; Robinson et al., 2005).
Soluble proteins reach lytic vacuoles and protein storage vacuoles because they contain vacuolar sorting determinants that can be recognized by vacuolar sorting receptor (VSR) proteins. BP-80 was the first VSR protein isolated from pea (Pisum sativum) due to its interaction with a vacuolar sorting determinant Asn-Pro-Ile-Arg (Kirsch et al., 1994), and several in vitro and in vivo studies demonstrated that BP-80 may function as a sorting receptor for transporting the Cys protease aleurain from Golgi to lytic vacuole in plant cells (Jiang and Rogers, 1998; Humair et al., 2001; Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). Similarly, two VSR homologs, the pumpkin (Cucurbita pepo) VSR protein PV72 and the Arabidopsis (Arabidopsis thaliana) AtVSR1, and a receptor-like protein, AtRMR, may function as sorting receptors for transporting storage proteins to the protein storage vacuole (Shimada et al., 1997; Paris and Neuhaus, 2002; Shimada et al., 2003; Neuhaus and Paris, 2005; Park et al., 2005).
Similar to mammalian cells and yeast, receptor-mediated protein sorting for transporting proteins from the trans-Golgi network (TGN) to vacuoles is mediated through an intermediate organelle termed the prevacuolar compartment (PVC) in plant cells (Bethke and Jones, 2000; Lam et al., 2005). In mammals, mannosyl 6-P receptors recruit acid hydrolases at the TGN and cause them to be selected into clathrin-coated vesicles for delivery of the receptor-ligand complex to endosomal/PVCs (Ghosh et al., 2003). Similarly, in plant cells, BP-80 is believed to function as a VSR that selects cargo proteins at the TGN for subsequent packaging into CCVs before delivery to the vacuole via a PVC, where the receptor is recycled back to TGN for another round of binding/selection of cargoes (Jiang and Rogers, 2003; daSilva et al., 2005; Lam et al., 2005). The recycling of VSR proteins from PVC to TGN is believed to be mediated by the newly characterized Arabidopsis retromer complexes that localize to PVCs and interact with VSR proteins (Oliviusson et al., 2006).
The subcellular localization of BP-80 and its homologs in plant cells has been studied using various approaches. Highly purified pea CCVs contained abundant BP-80 (Kirsch et al., 1994). Immunocytochemical electron microscope (immunoEM) studies with specific antibodies also localized BP-80 and its Arabidopsis homolog, AtELP, to both Golgi and a putative PVC in root-tip cells of pea and Arabidopsis, respectively (Ahmed et al., 1997; Paris et al., 1997). Further confocal immunofluorescence studies on the relative distribution of VSR proteins between Golgi and PVC in pea and tobacco (Nicotiana tabacum) cells demonstrated that BP-80 and its homologs were predominantly concentrated on the lytic PVCs at steady state (Li et al., 2002). These results indicate that VSRs can be used as markers to define PVCs and that VSR proteins recycle back to Golgi briefly for cargo selection and return to PVCs for cargo delivery (daSilva et al., 2005; Lam et al., 2005). A recent immunoEM study using VSR antibodies further identified the lytic PVC as multivesicular body (MVB) from high-pressure frozen/freeze-substituted tobacco BY-2 cells, where the VSR labeling was restricted to the boundary membrane of MVBs (Tse et al., 2004). Confirmation of MVBs as PVCs that are distinct from Golgi organelles comes from studying the different effects of the drugs wortmannin and Brefeldin A (BFA). Whereas BFA at low concentrations (5–10 μg/mL) caused Golgi apparatus to form typical BFA-induced aggregates, this drug did not affect MVBs in tobacco BY-2 cells (Tse et al., 2004). In contrast, wortmannin at 15 to 33 μm caused MVBs to swell and led to a reduction in the number of internal vesicles, while this drug did not cause any visible changes to Golgi apparatus in tobacco BY-2 cells (Tse et al., 2004).
BP-80 is a type I integral membrane protein with a single transmembrane domain (TMD) and cytoplasmic tail (CT). The molecular mechanism by which BP-80 reaches its final destination has been studied using a reporter system transiently expressed in tobacco suspension culture cells (Jiang and Rogers, 1998). When expressed in tobacco cells, this BP-80 reporter containing the BP-80 TMD and CT sequences colocalized with endogenous tobacco VSR proteins (Jiang and Rogers, 1998). Similarly, the yellow fluorescent protein (YFP)-BP-80 reporter containing the BP-80 TMD and CT sequences was found to localize to VSR-marked PVCs but separate from the Golgi apparatus in transgenic tobacco BY-2 cells (Tse et al., 2004). These results indicate that the TMD and CT regions are specific and sufficient for targeting BP-80 to its final destination (i.e. the PVC/MVB) in tobacco cells (Jiang and Rogers, 1998; Tse et al., 2004). More recently, the sorting determinants within BP-80 TMD and CT have been dissected using an elegant in vivo system that further confirmed BP-80 TMD/CT contained information necessary for efficient progress to the PVC in tobacco cells (daSilva et al., 2006). Because the TMD/CT regions among various BP-80 homologs (VSR proteins) are highly conserved (Hadlington and Denecke, 2000; Paris and Neuhaus, 2002; Neuhaus and Paris, 2005), it is thus reasonable to hypothesize that the TMD/CT sequences target individual VSRs to their final destinations in tobacco cells.
The Arabidopsis genome contains seven VSR proteins (defined as AtVSR1–7 in this study) with high conservation at the amino acid level, in particular in their TMD and CT regions (Hadlington and Denecke, 2000; Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). The expression of AtVSRs was detected in most Arabidopsis tissue types, including root, leaf, stem, flower, pollen, and seed (Laval et al., 1999; Paris and Neuhaus, 2002; Laval et al., 2003; Neuhaus and Paris, 2005). However, the seven AtVSRs are not equally expressed in these tissues and thus might suggest distinct functions in various tissues (Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). Transgenic Arabidopsis seeds with antisense AtVSR1 failed to germinate (Laval et al., 2003), indicating the essential role of VSR proteins in seed germination. While AtVSR1/AtELP was shown to bind to the N-terminal propeptide of Arabidopsis aleurain, involved the lytic vacuolar pathway (Ahmed et al., 2000), another study on an AtVSR knockout mutant indicated that AtVSR1 might function as a receptor for transporting storage proteins to seed protein storage vacuoles (Shimada et al., 2003). The foregoing suggests that there is no clear picture of the functional roles of AtVSR proteins in plants. Furthermore, relatively little is known about the subcellular localization of individual AtVSR in Arabidopsis. The aim of this study is to determine the subcellular localization of individual AtVSR proteins using a reporter system, which is based on the demonstration that the TMD/CT sequences of individual AtVSR target the protein to its final destination in transgenic tobacco BY-2 cells. Toward this goal, we have generated seven chimeric constructs containing the proaleurain signal peptide (sp) linked to green fluorescent protein (GFP) and the TMD/CT sequences of the seven individual AtVSR proteins, and transgenic tobacco BY-2 cell lines expressing these seven sp-GFP-TMD-CT fusions have been generated. Confocal immunofluorescence studies demonstrated that all GFP fusions exhibited typical punctate signals that mostly colocalized with VSR proteins in transgenic BY-2 cell lines. In addition, wortmannin induced the GFP-marked prevacuolar organelles to form small vacuoles, and VSR antibodies labeled these enlarged MVBs in all transgenic BY-2 cells. Wortmannin also induced VSR-marked PVCs to be vacuolated in other cell types, including Arabidopsis, rice (Oryza sativa), pea, and mung bean (Vigna radiata). Therefore, the seven GFP-AtVSR fusions are localized to PVCs/MVBs in tobacco BY-2 cells, and wortmannin-induced vacuolation of PVCs/MVBs is a general response in plants.
RESULTS
Chimeric GFP Reporters as Tools to Study Subcellular Localization of AtVSR Proteins in Transgenic BY-2 Cells
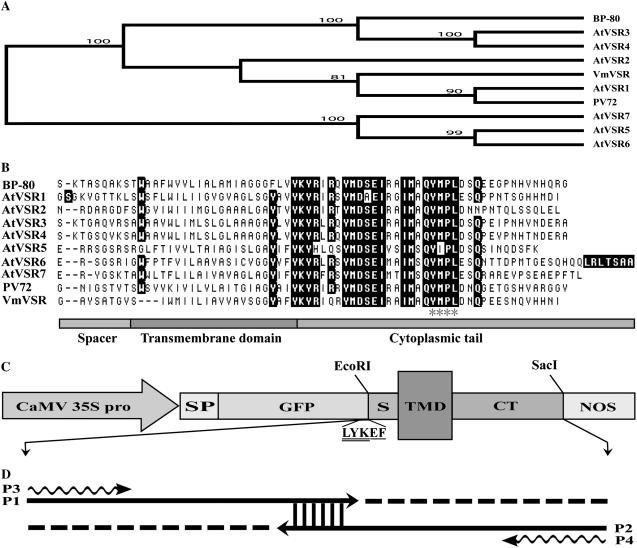
Phylogenetic analysis (Fig. 1A) based on full-length VSR protein sequences indicated that AtVSR1 and AtVSR2 are closely related to the pumpkin PV72 and black gram (Vigna mungo) VmVSR, while AtVSR3 and AtVSR4 are more closely related to the pea BP-80, and the other three AtVSR members are grouped into another cluster depending on their evolutional relationship. Alignment analysis also indicated that these VSRs are highly conserved within their TMD and CT regions. In addition, all VSRs contain a Tyr motif (mainly YMPL) in their CT regions (Fig. 1B) that might interact with AP-1 clathrin-adaptor protein complex of the clathrin-coated vesicles in the plant secretory pathway (Paris et al., 1997; Shimada et al., 1997; Sanderfoot et al., 1998; Lam et al., 2005).
Figure 1.
The AtVSR proteins and GFP fusions used to study the subcellular localization of AtVSRs. A, Phylogenetic analysis of AtVSR1 to 7 and the other three VSRs from pea (BP-80), pumpkin (PV72), and black gram (VmVSR). B, Alignment of amino acid sequences composed of the spacer, TMD, and CT regions of various VSRs. Black boxes indicate conserved amino acid residues among VSRs. C, Chimeric GFP constructs used in this study. Each fusion contains a signal peptide (SP) derived from a barley Cys protease proaleurain linked to GFP and sequences of spacer-TMD-CT derived from individual VSR protein. Such sp-GFP-spacer-TMD-CT fusion is under the control of the cauliflower mosaic virus 35S promoter and the 3′ NOS terminator. LYK and EF indicated the amino acid sequences of the last three amino acids of GFP and the EcoRI site, respectively. D, Overlapped primers extension methods used in this study to generate GFP fusion constructs with individual VSR spacer-TMD-CT sequences. Partially overlapped primers (P1 and P2) covering the whole sequences of spacer-TMD-CT of individual VSR were first used in self-priming PCR reaction to generate the target dsDNA sequences and used as template in the second PCR with primers P3 (with EcoRI site) and P4 (with SacI site) to amplify the full-length sequences of spacer-TMD-CT. Each unique spacer-TMD-CT was then inserted after the GFP sequences in frame via EcoRI and SacI sites as indicated.
To study the subcellular localization of the seven AtVSR proteins, we generated various chimeric constructs that contain sp sequences from the barley (Hordeum vulgare) proaleurain linked to GFP and TMD/CT sequences of individual AtVSR (termed GFP-AtVSR fusion in this study; Fig. 1C). Because the sequences (both genomic and cDNA) of AtVSRs are known, we thus designed overlapping primer pairs (P1/P2) that cover the full length of individual VSR TMD/CT sequences for self-priming PCR (Fig. 1D). The amplified double-strand DNA (dsDNA) of TMD/CT then served as template in further PCR using primers P3/P4 (Fig. 1D). Such cloning strategy does not need to use VSR DNA as template in PCR. Using this approach, we generated the seven GFP-AtVSR chimeric constructs for subsequent transformation into tobacco BY-2 cells.
Establishment of Transgenic Tobacco BY-2 Cell Lines Stably Expressing Seven GFP-AtVSR Reporters
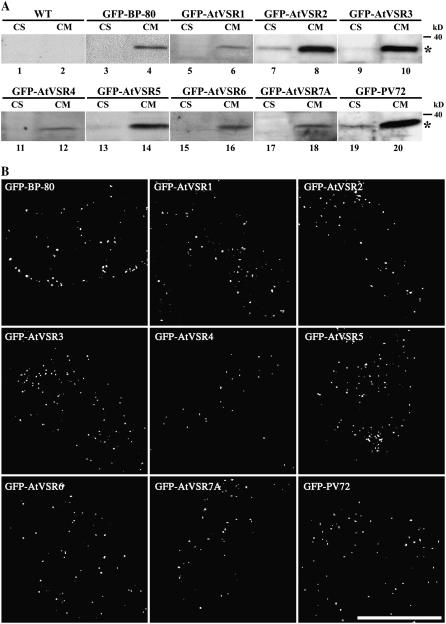
The seven GFP-AtVSR (termed GFP-AtVSR fusion) chimeric constructs were transferred into tobacco BY-2 cells via standard Agrobacterium tumefaciens-mediated transformation. As controls, we used transgenic BY-2 cell lines expressing the Golgi marker GONST1-YFP and the PVC marker GFP-BP-80 reporter (Tse et al., 2004). More than 50 individual transgenic BY-2 cell lines expressing each of the seven GFP-AtVSR1 to 7 fusions and the GFP-PV72 fusion were generated, where PV72 was likely a VSR for sorting storage proteins to PSVs in pumpkin seeds (Shimada et al., 1997). Cells from all these transgenic BY-2 cell lines expressing various GFP-VSR fusions gave typical punctate fluorescent signals (Fig. 2B), an indication of either Golgi or PVC localization (Tse et al., 2004). Most of these fluorescent signals colocalized with GFP antibody staining in confocal immunofluorescence of fixed cells (data not shown), indicating that the punctate fluorescent signals were derived from the GFP-VSR fusion proteins. The correct expression of the full-length GFP-VSR fusion proteins was further confirmed by western-blot analysis with GFP antibodies in these transgenic BY-2 cells. As shown in Figure 2A, a protein band of 37 kD corresponding to the full-length fusion protein was detected predominantly in the cell membrane fraction of all tested transgenic tobacco BY-2 cell lines expressing various GFP-VSR fusions (lanes 5–20), including the positive control BP-80 reporter (lanes 3 and 4). However, no such band was detected in wild-type BY-2 cells (lanes 1 and 2). These results demonstrated that all the GFP-AtVSR and GFP-PV72 fusions were expressed correctly in transgenic BY-2 cells. The difference in signal intensity of various GFP-VSR fusions detected by GFP antibodies in western-blot analysis (Fig. 2A) is presumably due to the different expression levels of these fusions in transgenic BY-2 cells.
Figure 2.
Analysis of transgenic tobacco BY-2 cell lines expressing various GFP-VSR fusions. A, Western-blot analysis of transgenic BY-2 cells. Cell soluble (CS) and cell membrane (CM) proteins were isolated from control wild-type (WT) and transgenic BY-2 cell lines expressing various GFP-VSR fusions as indicated, followed by protein separation via SDS-PAGE and western-blot analysis using GFP antibodies. Asterisks indicate positions of the full-length GFP-VSR fusion proteins. B, Confocal analysis of GFP signals in living transgenic BY-2 cells expressing various GFP-AtVSR fusions as indicated. Scale bar = 50 μm.
PVC Localization of GFP-AtVSR Fusions in Transgenic Tobacco BY-2 Cells
We previously demonstrated that VSR proteins are concentrated on PVCs (Li et al., 2002). Using VSR antibodies as markers in immunoEM, the lytic PVCs were identified as MVBs in tobacco BY-2 cells (Tse et al., 2004). In addition, the BP-80 reporter containing the BP-80 TMD/CT colocalized with endogenous tobacco VSR proteins (Jiang and Rogers, 1998; Tse et al., 2004). Therefore, both VSRs and the BP-80 reporter are PVC markers.
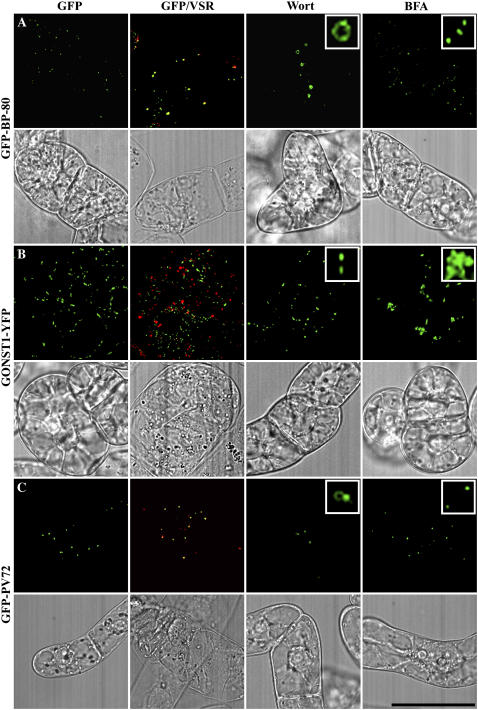
The properties of PVCs were also studied using transgenic BY-2 cells expressing the PVC marker GFP-BP-80 reporter versus the Golgi marker GONST1-YFP (Tse et al., 2004). BFA at low concentrations (5–10 μg/mL) caused the YFP-marked Golgi, but not the GFP-marked PVC, to form BFA-induced aggregates. In contrast, wortmannin caused GFP-marked PVCs to form small vacuoles, while YFP-marked Golgi remained unchanged (Tse et al., 2004). Therefore, PVC localization of the BP-80 reporter can be confirmed by the following three criteria: colocalization with VSR antibodies, formation of small vacuoles in response to wortmannin treatment (at 15–33 μm), and no change in BFA-treated cells (at 5–10 μg/mL).
We therefore used these three criteria to identify the organelles marked by various GFP-AtVSR1 to 7 fusions in transgenic tobacco BY-2 cells. Consistent with previous results, in transgenic BY-2 cells expressing the PVC marker GFP-BP-80, typical punctate fluorescent patterns were observed in living cells, and these punctate GFP signals mostly colocalized with VSR antibodies in confocal immunofluorescence (Fig. 3A). In addition, wortmannin treatment caused the GFP-marked PVCs to vacuolate, while BFA treatment did not cause any changes in GFP-marked PVCs (Fig. 3A). In contrast, in transgenic BY-2 cells expressing the Golgi marker GONST1-YFP, the punctate YFP signals were largely separated from VSR-labeled PVCs where BFA caused them to form aggregates, but the punctate YFP signals remained unchanged in the presence of wortmannin (Fig. 3B). Similarly, in transgenic cells expressing the GFP-PV72, most of the punctate GFP signals colocalized with VSR (Table I). While wortmannin induced the GFP-marked organelles to form small vacuoles, BFA did not cause any visible changes to these GFP-marked organelles (Fig. 3C). Therefore, GFP-PV72 localized to PVC in transgenic BY-2 cells.
Figure 3.
Characterization of PVC-localized GFP fusions in transgenic BY-2 cells. Transgenic cells expressing various GFP fusions were fixed prior to immunostaining with VSR antibodies to compare the localization between the GFP fusion (green) and VSR (red; GFP/VSR). Cells were also treated with wortmannin (Wort) at 16.5 μm for 2 h or treated with BFA at 10 μg/mL for 2 h before image collection for GFP-marked organelles in living cells. Colocalization of two signals was indicated by yellow color. The corresponding differential interference contrast images of the studied cells were included. Scale bar = 50 μm. A, The positive control cell line expressing GFP-BP-80, a PVC/MVB marker in tobacco BY-2 cells. B, The negative control cell line expressing the Golgi marker GONST1-YFP. C, New transgenic BY-2 cell line expressing GFP-PV72.
Table I.
Summary of various GFP fusions in transgenic BY-2 cells
| No. | Reporter Fusion | GFP/YFPa | Percentage of Colocalization with Anti-VSRb
|
+Wortc | +BFAd | |
|---|---|---|---|---|---|---|
| % | No. of Cells Studied | |||||
| 1 | GFP-BP-80 | Punctate | 70 | 9 | Ring | Punctate |
| 2 | GONST1-YFP | Punctate | 1 | 10 | Punctate | Aggregate |
| 3 | GFP-AtVSR1 | Punctate | 70 | 7 | Ring | Punctate |
| 4 | GFP-AtVSR2 | Punctate | 69 | 7 | Ring | Punctate |
| 5 | GFP-AtVSR3 | Punctate | 82 | 10 | Ring | Punctate |
| 6 | GFP-AtVSR4 | Punctate | 75 | 8 | Ring | Punctate |
| 7 | GFP-AtVSR5 | Punctate | 68 | 8 | Ring | Punctate |
| 8 | GFP-AtVSR6 | Punctate | 68 | 7 | Ring | Punctate |
| 9 | GFP-AtVSR7A | Punctate | 78 | 10 | Ring | Punctate |
| 10 | GFP-AtVSR7B | Punctate | 65 | 7 | Ring | Punctate |
| 11 | GFP-PV72 | Punctate | 75 | 8 | Ring | Punctate |
Fluorescent patterns detected in transgenic living cells.
Colocalization percentage of GFP/YFP signals with VSR antibodies as determined by confocal immunofluorescence.
Fluorescence patterns in transgenic living cells 2 h after wortmannin (Wort) treatment at 16.5 μm.
Fluorescence patterns in transgenic living cells 2 h after BFA treatment at 10 μg/mL.
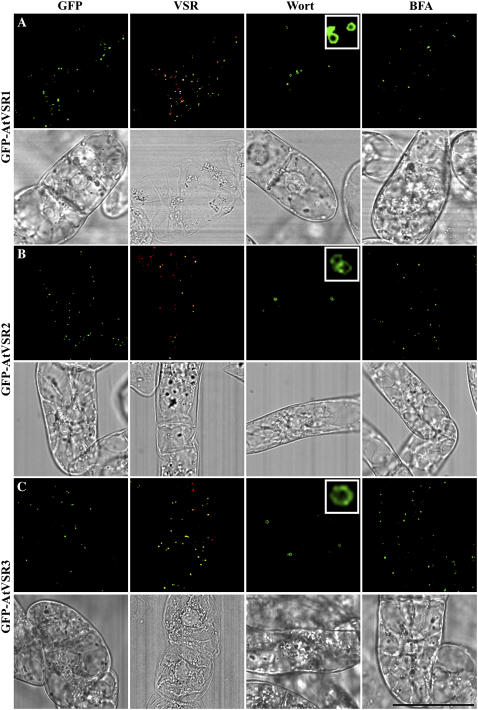
Similar results were obtained from the transgenic BY-2 cells expressing seven individual AtVSR fusions from GFP-AtVSR1 to GFP-AtVSR7 (Figs. 4–6). Most of the punctate GFP signals in these transgenic cell lines colocalized with VSR (Table I), and these GFP-marked organelles were also induced to form small vacuoles in the presence of wortmannin but did not respond to BFA treatment (Figs. 4–6). Therefore, all GFP-AtVSR fusions are most likely localized to PVCs in transgenic BY-2 cells. However, because the GFP-AtVSR1 to 7 fusions do not fully colocalize with VSR antibodies (Table I), we thus cannot exclude the possibility that different PVC types may exist in tobacco BY-2 cells (see “Discussion”).
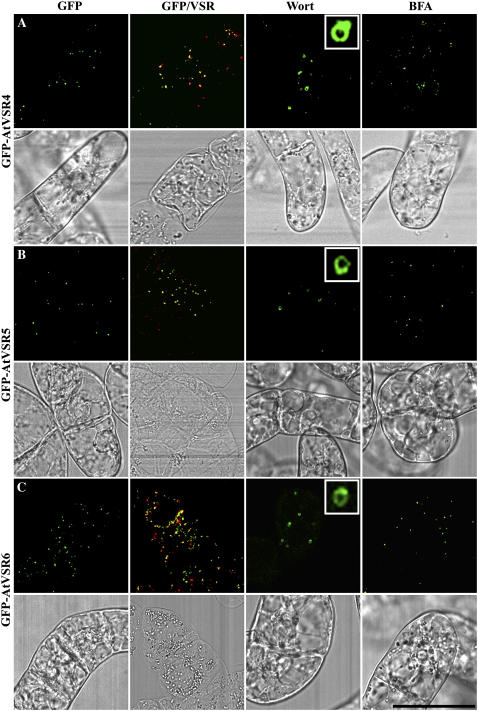
Figure 4.
PVC localization of GFP-AtVSR1-3 fusions in transgenic tobacco BY-2 cells. Transgenic tobacco BY-2 cells expressing GFP-AtVSR1-3 fusions (A–C), respectively, were fixed prior to immunostaining with VSR antibodies to compare the localization between the GFP fusion (green) and VSR (red; GFP/VSR). Cells were also treated with wortmannin (Wort) at 16.5 μm for 2 h or treated with BFA at 10 μg/mL for 2 h before image collection for respective GFP-marked organelles in living cells. Colocalization of two signals is indicated by a yellow color. The corresponding DIC images of the studied cells are included. Scale bar = 50 μm.
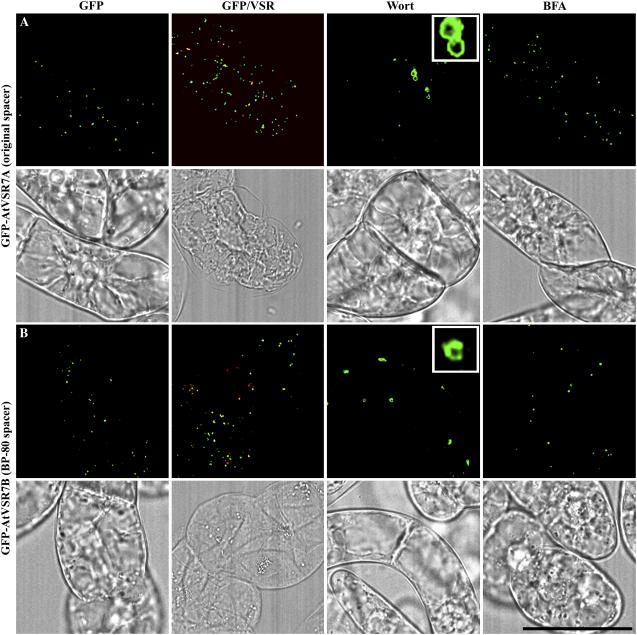
Figure 6.
The spacer sequences do not affect PVC localization of GFP-AtVSR7 in transgenic tobacco BY-2 cells. Transgenic tobacco BY-2 cells expressing GFP-AtVSR7A with original spacer or GFP-AtVSR7B with spacer sequences from BP-80 were fixed prior to immunostaining with VSR antibodies (red), respectively, to compare the localization between the GFP fusion (green) and VSR (red; GFP/VSR). Cells were also treated with wortmannin (Wort) at 16.5 μm for 2 h or treated with BFA at 10 μg/mL for 2 h before image collection for respective GFP-marked organelles in living cells. Colocalization of two signals is indicated by a yellow color. The corresponding DIC images of the studied cells are shown. Scale bar = 50 μm.
Figure 5.
PVC localization of GFP-AtVSR4-6 fusions in transgenic tobacco BY-2 cells. Transgenic tobacco BY-2 cells expressing GFP-AtVSR4-6 fusions (A–C), respectively, were fixed prior to immunostaining with VSR antibodies to compare the localization between the GFP fusion (green) and VSR (red; GFP/VSR). Cells were also treated with wortmannin (Wort) at 16.5 μm for 2 h or treated with BFA at 10 μg/mL for 2 h before image collection for respective GFP-marked organelles in living cells. Colocalization of two signals is indicated by a yellow color. The corresponding DIC images of the studied cells are shown. Scale bar = 50 μm.
The Spacer Sequences Did Not Affect PVC Localization of GFP-AtVSR7
The spacer region in front of the TMD of VSR is thought to be important for proper protein folding that would allow flexibility of the reporter linking to TMD. We previously used the BP-80 spacer in the chimeric reporter system to study trafficking of integral membrane proteins in tobacco cells (Jiang and Rogers, 1998; Tse et al., 2004). When expressed in tobacco cells, the BP-80 reporter colocalized with endogenous VSR proteins (Jiang and Rogers, 1998; Tse et al., 2004). Therefore, the TMD/CT sequences are sufficient and specific to target the reporter to PVCs.
However, in this study, we used the original spacer from individual AtVSRs to make the seven GFP-AtVSR1 to 7 fusions. To test the possible effect of spacer sequence on the PVC localization of GFP-AtVSR fusions and thus subcellular localization of AtVSRs, we generated two reporter fusions with the same TMD/CT sequences as AtVSR7, but their spacer sequences were derived from AtVSR7 and BP-80, respectively. AtVSR7 was chosen because it was relatively far away from BP-80 in terms of evolutionary distance and because BP-80 and AtVSR7 had less amino acid similarity, especially on the spacer region (Fig. 1B). Thus, we replaced the spacer sequences in GFP-AtVSR7 with that from BP-80 and compared their subcellular localization in transgenic BY-2 cells. As shown in Figure 6, transgenic BY-2 cells expressing either the GFP-AtVSR7A (with original spacer; Fig. 6A) or GFP-AtVSR7B (with BP-80 spacer; Fig. 6B) showed typical punctate fluorescent signals that mostly colocalized with VSR, thus representing PVCs. In addition, wortmannin treatment induced the GFP-marked PVCs to form small vacuoles, but BFA did not cause any visible changes to punctate GFP signals in both transgenic cell lines (Fig. 6, A and B). Therefore, it is likely that the spacer sequences from BP-80 do not affect PVC localization of GFP-AtVSR in transgenic BY-2 cells, and targeting of VSR proteins to PVCs depends solely on their TMD and CT sequences.
Wortmannin-Induced Vacuolated PVCs Contain VSRs in Tobacco BY-2 Cells
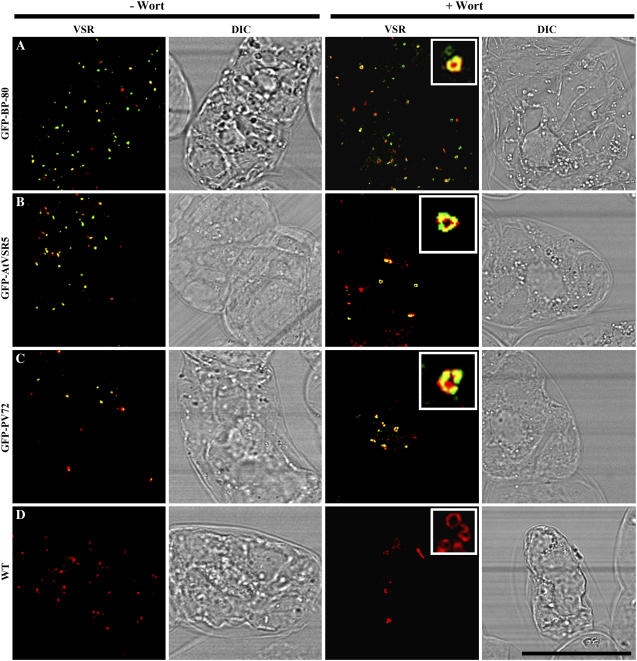
When expressed in transgenic tobacco BY-2 cells, the YFP-BP-80 reporter colocalized with endogenous VSRs (Tse et al., 2004). In addition, wortmannin induced the YFP-marked PVCs to form small vacuoles in transgenic BY-2 cells where the enlarged PVCs were still labeled by anti-VSRs (Tse et al., 2004). Similarly in this study, all organelles marked by GFP-AtVSR1 to 7 formed small vacuoles in response to wortmannin treatment (Figs. 3–6). To further test if VSR proteins are still present in these wortmannin-induced enlarged PVCs, confocal immunofluorescence with VSR antibodies was carried out in wortmannin-treated transgenic cells where their corresponding untreated cells were used as controls. As shown in Figure 7, in transgenic cells expressing the positive control GFP-BP-80 reporter, most of the punctate GFP-BP-80 signals (green) colocalized with VSRs (red) in the absence of wortmannin (Fig. 7A). When the cells were treated with wortmannin, the GFP-marked PVCs became vacuolated and these enlarged vacuoles colocalized with VSRs (Fig. 7A). Similarly, in transgenic BY-2 cells expressing either GFP-AtVSR5 or GFP-PV72, most of the punctate GFP signals in untreated cells and the vacuolated GFP signals in wortmannin-treated cells colocalized with VSRs (Fig. 7, B and C). Furthermore, in untransformed wild-type BY-2 cells, VSR antibodies detected typical punctate organelles and small vacuoles in untreated and wortmannin-treated cells, respectively (Fig. 7D). These results demonstrated that all GFP-VSR fusions were targeted to PVCs, and wortmannin specifically induced the vacuolation of PVCs in both wild-type and transgenic BY-2 cells.
Figure 7.
VSRs colocalized with PVC-derived vacuoles in wortmannin (Wort)-treated transgenic BY-2 cells. Transgenic tobacco BY-2 cells expressing three GFP fusions were treated with wortmannin at 16.5 μm for 2 h before both untreated (left) and wortmannin-treated (right) cells were fixed for subsequent immunolabeling with VSR antibodies. Yellow color indicates the colocalization of green and red signals. Scale bar = 40 μm.
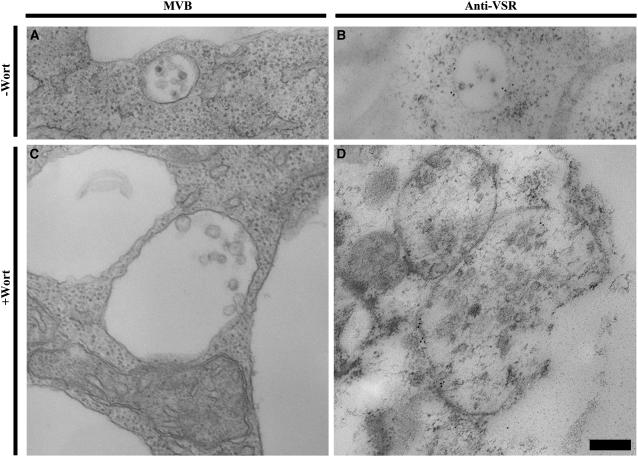
To further study the VSR-labeled structures in untreated and wortmannin-treated BY-2 cells, structural-EM with conventional chemical-fixed samples and immunoEM using VSR antibodies on high-pressure frozen/freeze-substituted samples were also carried out. As shown in Figure 8, in cells without wortmannin treatment, typical MVBs with sizes of about 200 to 400 nm in diameter that contain small but uniform internal vesicles were observed (Fig. 8A), and these MVBs were specifically labeled by VSR antibodies mainly on the membrane (Fig. 8B). However, in wortmannin-treated cells, many enlarged MVBs with sizes of over 500 nm in diameter were observed (Fig. 8C), and these enlarged MVBs were specifically labeled by VSR antibodies on their limiting membranes (Fig. 8D). These EM studies further confirmed the results of confocal microscopic observations (e.g. Fig. 7) and demonstrated that the GFP-marked PVCs were vacuolated in response to wortmannin treatment; these small vacuoles were labeled by VSR antibodies on their outer membranes.
Figure 8.
ImmunoEM localization of VSRs to vacuolated MVBs in wortmannin (Wort)-treated BY-2 cells. Untreated or wortmannin-treated (at 16.5 μm for 2 h) BY-2 cells were either chemically fixed and prepared for structural-EM observation (A and C) or subjected to high-pressure frozen/freeze substitution for subsequent immunoEM study with VSR antibodies (B and D). A and C, Examples of normal MVB and enlarged MVB from untreated and wortmannin-treated BY-2 cells, respectively. B and D, Examples of normal MVB and enlarged MVB that were labeled by VSR antibodies in untreated and wortmannin-treated cells, respectively. Bar = 200 nm.
Wortmannin-Induced Vacuolation of PVCs Is a General Response in Plant Cells
Many plants contain VSRs (Fig. 1). Our results indicated that all tested GFP-VSR fusions containing TMD/CT sequences from different plant species expressed in transgenic BY-2 cells formed small vacuoles in response to wortmannin treatment (Figs. 3–7); this was further confirmed by immunoEM studies using VSR antibodies (Fig. 8). Therefore, we carried out experiments to find out if such wortmannin-induced vacuolation of PVCs is a general response in plant cells.
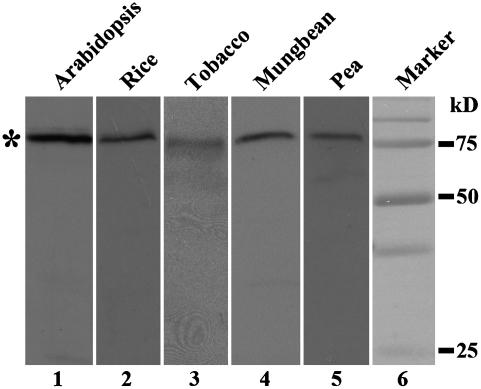
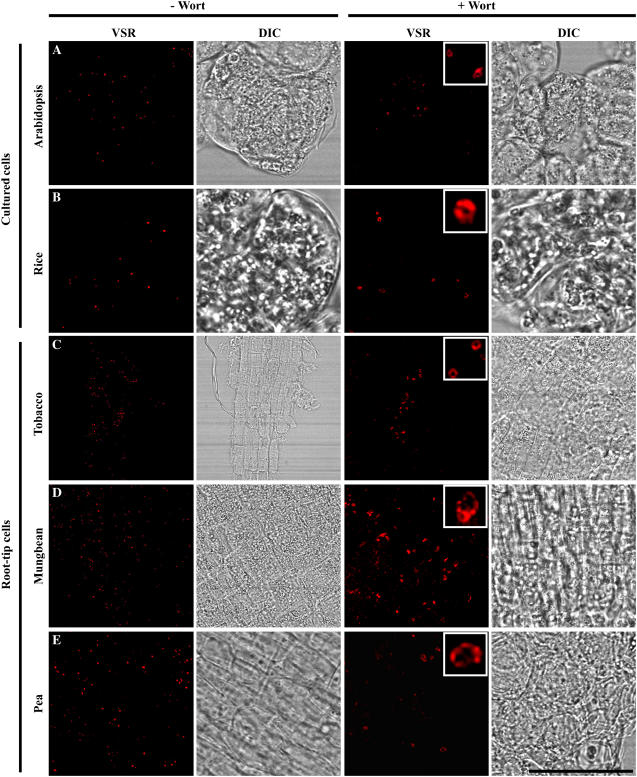
Western-blot analysis with VSR antibodies was first carried out with protein samples extracted from various plants, including Arabidopsis, rice, tobacco, pea, and mung bean. As shown in Figure 9, VSR antibodies detected a single band at 80 kD in all these tested protein samples, indicating that VSR proteins are universally present in these plant cells. To further study the VSR-marked organelles in these cell types, confocal immunofluorescence with VSR antibodies was then carried out in both cultured cells (rice and Arabidopsis) and root tip cells (pea, mung bean, and tobacco). As shown in Figure 10, typical punctate signals were detected with VSR antibodies in all these cells (Fig. 10, A–E, left), a result consistent with the detection of PVCs by VSR antibodies in these cell types. In addition, when these cells were treated with wortmannin prior to fixation and subsequent immunostaining with VSR antibodies, enlarged and small vacuoles were detected in all these wortmannin-treated cell types (Fig. 10, A–E, right), a result further demonstrating that these VSR-labeled organelles were PVCs in these cell types and that wortmannin-induced vacuolation of PVCs is a general response in plant cells.
Figure 9.
Western-blot analysis of VSR proteins in various plant cells. Total proteins were extracted from suspension-cultured cells of Arabidopsis and rice, as well as seeds of tobacco, mung bean, and pea, followed by SDS-PAGE and western-blot detection using VSR antibodies. Asterisk indicates positions of VSR proteins.
Figure 10.
Wortmannin (Wort)-induced VSR-marked PVCs to form small vacuoles in various plant cell types. Suspension-cultured cells of rice and Arabidopsis or root-tip cells from germinating seedlings of pea, mung bean, and tobacco were treated with wortmannin (at 16.5 μm for 2 h) before both untreated and wortmannin-treated cells were fixed for confocal immunofluorescence, using VSR antibodies to detect normal PVCs in untreated cells or enlarged PVCs in wortmannin-treated cells. Scale bar = 50 μm.
DISCUSSION
The Arabidopsis genome contains seven VSR proteins with little information on their individual subcellular localization and function in plants. Several studies indicated that different members of the AtVSR proteins might function as distinct sorting receptors for targeting hydrolytic enzymes and storage proteins to the lytic vacuoles and protein storage vacuoles, respectively, in plants (Paris et al., 1997; Humair et al., 2001; Shimada et al., 2003). It thus seems that individual AtVSR proteins might have different functions in transporting different ligand proteins to distinct destinations (Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). As a first step to fully understand the functional roles of all AtVSR proteins in plants, we studied the subcellular localization of the seven AtVSR proteins individually using a reporter system and demonstrated that the seven GFP-AtVSR fusions localized to PVCs in transgenic tobacco BY-2 cells.
A Reporter System to Study Subcellular Localization of VSR Proteins in Transgenic Tobacco BY-2 Cells
Due to its easy culture and transformation, as well as correct protein folding and targeting, transgenic tobacco BY-2 cells expressing various reporter fusion proteins have been successfully used to study the subcellular localization of expressed proteins as well as the organelle dynamics that are marked by the reporter in living cells, including various secretory organelles such as endoplasmic reticulum, Golgi, and PVCs (Ritzenthaler et al., 2002; Tamura et al., 2004; Tse et al., 2004; Robinson et al., 2005; Yang et al., 2005). In this study, we hypothesized that the TMD and CT sequences of AtVSRs targeted individual AtVSR protein to its final destination in Arabidopsis. This hypothesis is based on a previous study demonstrating that a reporter containing the BP-80 TMD/CT sequences colocalized with the endogenous VSR proteins when the reporter was transiently expressed in tobacco protoplasts of suspension-cultured cells. This BP-80 reporter trafficked from Golgi to a putative PVC, where the reporter was processed into the mature form (Jiang and Rogers, 1998). This result was further confirmed by recent studies in which the GFP-BP-80 reporter was shown to colocalize with endogenous tobacco VSR proteins within PVCs/MVBs in transgenic tobacco BY-2 cells (Tse et al., 2004) as well as in tobacco leaf protoplasts (daSilva et al., 2005). All these results indicate that the BP-80 TMD/CT sequences are sufficient to target the BP-80 receptor to PVCs in tobacco BY-2 cells. Therefore, tobacco BY-2 cells are good systems for studying the subcellular localization of VSR proteins using the reporter system.
PVC Localization of the Seven GFP-AtVSR Fusions in Transgenic BY-2 Cells
The seven Arabidopsis VSR proteins are highly conserved at their TMD and CT regions (Fig. 1B; Supplemental Fig. S1; Hadlington and Denecke, 2000; Paris and Neuhaus, 2002; Neuhaus and Paris, 2005), with a conserved YXXΦ sorting motif involved in the clathrin-coated, vesicle-mediated sorting pathway (Happel et al., 2004). Because TMD and CT sequences are important for PVC targeting of BP-80, such conservation within the TMD/CT regions of AtVSR1 to 7 indicates that these seven AtVSR proteins may share a similar mechanism of protein sorting in Arabidopsis (Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). It is thus reasonable to hypothesize that the TMD and CT sequences of AtVSRs target the receptor proteins to their final destination correctly in transgenic tobacco BY-2 cells. This hypothesis is further supported by a recent study using an in vivo assay system of tobacco protoplasts that demonstrated that the BP-80 CT contained multiple targeting signals for localization and recycling of BP-80 in PVC of tobacco cells (daSilva et al., 2006).
In this study, we used three well-established confocal immunofluorescence criteria to distinguish PVCs from Golgi organelles in transgenic tobacco BY-2 cells (Tse et al., 2004). First, PVCs colocalized with VSR antibodies and Golgi remained separated from VSR-marked organelles. Second, in BY-2 cells treated with BFA at low concentrations (5–10 μg/mL), Golgi organelles formed typical BFA-induced aggregates but PVCs remained unchanged. Third, wortmannin induced PVCs but not Golgi to be vacuolated, and these PVC-derived vacuoles colocalized with VSR antibodies in BY-2 cells.
Indeed, when the seven GFP-AtVSR fusions were stably transformed into tobacco BY-2 cells, they all exhibited typical punctate fluorescent signals (Figs. 3–5); most of these punctate signals colocalized with VSR proteins and formed small vacuoles in response to wortmannin treatment, indicating their PVC localization in transgenic tobacco BY-2 cells (Figs. 3–5; Tse et al., 2004). These results demonstrated that the seven GFP-AtVSR fusions and, thus, the seven AtVSRs located to PVCs in tobacco BY-2 cells. Therefore, it is most likely that the seven AtVSR proteins are localized to PVCs in Arabidopsis plants. However, this hypothesis needs to be tested via further studies and confirmation of PVC localization of the seven GFP-AtVSR fusions in transgenic Arabidopsis plants. We are in the process of generating transgenic Arabidopsis plants via Agrobacterium-mediated transformation. In addition to their use in studies of PVC localization, transgenic plants expressing the seven GFP-AtVSR fusions will also be useful for studying specific ligand-VSR interactions in Arabidopsis because the truncated GFP-AtVSR fusion will compete against normal protein sorting mediated by the endogenous VSR proteins in Arabidopsis and thus cause secretion of cargo proteins (daSilva et al., 2005).
Distinct PVCs?
Plant cells contain at least two functionally and biochemically distinct vacuoles: lytic vacuole and protein storage vacuole (Neuhaus and Rogers, 1998; Vitale and Raikhel, 1999); it is thus possible that two distinct PVCs exist for these two vacuole types. In addition, multiple vesicular transport pathways are responsible for transporting proteins to vacuoles (Jiang and Rogers, 2003). The generation of transgenic tobacco BY-2 cells expressing the GONST1-YFP and the YFP-BP-80 reporters has allowed us to characterize and identify the lytic PVC as a MVB (Tse et al., 2004; Lam et al., 2005). Similarly, the seven GFP-AtVSR fusions were found to locate to VSR-marked PVCs in transgenic tobacco BY-2 cells in this study. However, these GFP-AtVSR fusions might not locate to the same PVCs in BY-2 cells for the following reasons. First, the VSRat-1 antibodies used in this study to detect PVCs/MVBs in BY-2 cells were generated using CHO cell-derived truncated VSRat-1 recombinant proteins lacking TMD/CT as antigens to inject rabbits (Tse et al., 2004); thus, these VSRat-1 antibodies recognize several members of VSRs in BY-2 cells because the N-terminal regions of known plant VSRs are conserved. Second, when the localization of individual GFP-AtVSR fusion was compared with VSRat-1 antibodies, a majority (around 70%–80%) of the GFP signals were labeled with the antibodies, indicating PVC/MVB localization of the GFP-AtVSR fusion in transgenic BY-2 cells. However, because VSRat-1 might label more than one VSR, the other 20% to 30% of GFP signals lacking anti-VSR labeling might represent different PVCs in tobacco BY-2 cells. Indeed, a recent proteomic analysis on localization of organelle proteins indicated that VSRs might have different subcellular localization in Arabidopsis culture cells (Dunkley et al., 2006). Third, chemical fixation of cells might reduce the fluorescent signals of the reporter in fixed cells as compared to living cells.
To address the hypothesis of distinct PVC/MVB populations in BY-2 cells, pairs of XFP-AtVSR fusions can be compared directly in tobacco BY-2 cells. Toward this goal, we have recently tested and developed a transient expression system with protoplasts of BY-2 cells via polyethylene glycol-mediated or electroporation-mediated transformation. Preliminary results indicated that either full or partial colocalization of pairs of XFP-VSR could be observed when they were transiently coexpressed in protoplasts of wild-type BY-2 cells (data not shown). However, further detailed studies comparing various combinations of pairs of XFP-VSR fusions transiently expressed in BY-2 cells and, later, in Arabidopsis cells are necessary before a conclusion could be made on the existence and nature of distinct PVCs/MVBs in BY-2 and Arabidopsis cells. Furthermore, to reduce the effects of transient expression in protoplasts, stably transformed BY-2 cell lines coexpressing pairs of XFP-VSR fusions might also be needed for future study to address the important question of distinct PVCs/MVBs.
VSR Spacer Sequences Did Not Affect PVC Localization of GFP-AtVSR Fusions in Transgenic Tobacco BY-2 Cells
BP-80 and its homolog VSR proteins all contain a Ser/Thr-rich region (defined as spacer region in this study) in front of their TMD regions, which may be important for proper protein folding and protein flexibility (Paris et al., 1997; Jiang and Rogers, 1998; Paris and Neuhaus, 2002; Neuhaus and Paris, 2005). A reporter system was originally developed to study trafficking of integral membrane protein, where a reporter protein was fused with the spacer-TMD-CT of BP-80, and the resulting BP-80 reporter was transiently expressed in tobacco suspension culture cells (Jiang and Rogers, 1998). Inclusion of the spacer region in the reporter fusion would allow free movement and flexibility of the reporter linked to TMD. When expressed in tobacco cells, the BP-80 reporter colocalized with endogenous VSR proteins in PVCs/MVBs (Jiang and Rogers, 1998; Tse et al., 2004). Therefore, the BP-80 TMD/CT was sufficient to target the BP-80 reporter to PVC/MVBs marked by endogenous VSR proteins in tobacco cells (Jiang and Rogers, 1998; Tse et al., 2004).
In this study, all seven GFP-AtVSR fusions contain the original spacer sequences from the individual Arabidopsis VSR protein (Fig. 1). These seven GFP-AtVSR fusions were found to localize to PVCs in transgenic tobacco BY-2 cells (Figs. 3–5). In addition, when the original spacer sequences in GFP-AtVSR7 were replaced with the spacer sequences of BP-80 (Jiang and Rogers, 1998), the resulting GFP-AtVSR7B fusion was also found to localize to PVC in transgenic tobacco BY-2 cells (Fig. 6). These results demonstrated that the TMD-CT sequences of individual AtVSR were sufficient and specific for PVC targeting of each AtVSR in tobacco BY-2 cells, while the spacer sequences had little effect on the PVC localization of GFP-AtVSR fusions in transgenic BY-2 cells. However, further experiments with replacement by a spacer from a differently localized protein or by a random sequence will need to be carried out so a solid conclusion can be made.
When a construct containing sp-GFP fused with the TMD and the first five residues of the CT of BP-80 was expressed in tobacco leaf epidermal cells, this GFP fusion colocalized completely with a YFP-tagged Golgi marker (Brandizzi et al., 2002). Because the TMD and CT sequences of BP-80 were sufficient and specific for targeting the BP-80 reporter to its final destination (Jiang and Rogers, 1998; Tse et al., 2004; daSilva et al., 2005; this study), it is thus possible that the CT sequence is also essential for PVC targeting of the receptor. In fact, a recent study demonstrated that the BP-80 CT might contain multiple sorting signals for PVC targeting and recycling in tobacco cells (daSilva et al., 2006). Therefore, the full-length sequences of BP-80 CT might be essential for efficient PVC targeting of the GFP-BP-80 reporter in tobacco cells.
PVC Localization of GFP-PV72 and GFP-AtVSR1 Fusions in Transgenic Tobacco BY-2 Cells
BP-80 is a receptor for the lytic vacuole pathway (Paris et al., 1997; Humair et al., 2001; Tse et al., 2004; daSilva et al., 2005). However, members of VSRs might also function as receptors for the PSV pathway. For example, the pumpkin VSR protein PV72 was believed to function as a sorting receptor mediating the transport of 12S globulin storage proteins to PSVs via precursor-accumulating vesicles in pumpkin seeds (Shimada et al., 1997, 2002; Hara-Nishimura et al., 1998; Watanabe et al., 2002). Similarly, using Arabidopsis T-DNA knockout mutants for VSR proteins as tools to study ligand-receptor interaction, AtVSR1 was demonstrated to function as a sorting receptor for transporting storage proteins to PSVs in Arabidopsis seeds (Shimada et al., 2003). In addition to seeds, PSV-like structures and PSV-like pathways might also exist in vegetative plant cells. For example, the PSV-like pathway marked by trafficking of the α-tonoplast intrinsic protein reporter was distinct from the lytic vacuole pathway marked by traffic of the BP-80 reporter in tobacco suspension culture cells (Jiang and Rogers, 1998), and PSV-like structures marked by the overexpressed seed storage protein phaseolin were identified via confocal immunofluorescence in Arabidopsis cells where RMR might function as a sorting receptor for PSV (Park et al., 2004, 2005). However, to our knowledge, no PSV native to vegetative tissues or suspension culture cells has been identified so far.
In this study, both GFP-PV72 and GFP-AtVSR1 were found to localize to VSR-marked PVCs in transgenic tobacco BY-2 cells (Figs. 3 and 4). The PVC localization of GFP-PV72 in transgenic BY-2 cells is consistent with a previous study (Shimada et al., 2002) in which sp-GFP was fused to the TMD and CT regions of pumpkin PV72. When this sp-GFP-PV72 fusion (identical to GFP-PV72 in this study) was expressed in tobacco BY-2 cells, it localized to punctate structures whose appearance was consistent with either Golgi or PVCs (Mitsuhashi et al., 2000). Further, immunofluorescent analysis demonstrated that this sp-GFP-PV72 fusion colocalized with endogenous AtELP/AtVSR1 protein in Arabidopsis suspension cells (Shimada et al., 2002), evidence of PVC localization (Li et al., 2002; Tse et al., 2004). Similarly, the GFP-BP-80 fusion (a marker for the lytic PVC) and the other GFP-AtVSR fusions were also found to localize to VSR-marked PVCs in transgenic tobacco BY-2 cells (Figs. 3–6). These results demonstrate that all GFP-VSR fusions (BP-80, AtVSR, and PV72) localized to the same VSR-marked PVCs in transgenic BY-2 cells.
Because these VSRs might function in either the lytic vacuole pathway (e.g. BP-80) or PSV pathway (e.g. PV72) and no visible PSV has been identified in BY-2 cells, we hypothesize that pathways leading to the lytic vacuole and PSV share the same VSR-marked PVCs in tobacco BY-2 cells. However, we do not know if these VSR-marked PVCs might actually contain two distinct PVC populations for the lytic vacuole and PSV, respectively (Lam et al., 2005). The PVC/MVB localization of GFP-PV72 and GFP-AtVSR1 fusion proteins in transgenic tobacco BY-2 cells may indicate the existence of the PSV pathway in BY-2 cells. It would be of great interest to identify the native PSV in vegetative tissues or suspension culture cells using storage protein markers that can be found in both seeds and vegetative tissues or culture cells. Toward this goal, we have recently generated antibodies against a storage protein (termed S2), and these S2 antibodies detected the same protein (based on Mr) in seeds and vegetative tissues such as leaves and stems of mung bean. In addition, S2 antibodies specifically labeled PSVs and dense vesicles in developing mung bean cotyledons (J. Wang and L. Jiang, unpublished data). It would thus be interesting to identify the S2-labeled structures in leaves or shoots of mung bean via immunoEM.
Wortmannin-Induced Vacuolation of PVC Is a General Response in Plant Cells
An inhibitor of phosphatidylinositol 3-kinase, wortmannin, was described to be involved in transport of endocytosed components from endosomes to lysosomes (Arcaro and Wymann, 1993; Kjeken et al., 2001). It can alter the biogenesis of MVBs and induce the vacuolation of multivesicular endocytic compartments in human cells (Fernandez-Borja et al., 1999; Houle and Marceau, 2003). Similarly, wortmannin also caused swelling of MVBs/PVCs in tobacco BY-2 cells (Tse et al., 2004). In this study, PVC organelles marked by the GFP-AtVSR fusions formed small vacuoles in response to wortmannin treatment in transgenic tobacco BY-2 cells, a result consistent with our previous study (Tse et al., 2004). Furthermore, such response is not limited to tobacco culture BY-2 cells. When root tips of tobacco, pea, and mung bean were treated with wortmannin, followed by immunolabeling with VSR antibodies, the VSR-marked PVCs were also found to form small vacuoles in these wortmannin-treated cells. Therefore, wortmannin-induced vacuolation of PVCs/MVBs is a general response of plant cells, and wortmannin is a useful tool for identifying PVCs and studying PVC-mediated traffic in plant cells.
CONCLUSION
Several factors may contribute to the PVC localization of GFP-AtVSR fusions in BY-2 cells. First, PVCs marked by VSR antibodies may contain two populations of PVCs for lytic vacuole and PSV, respectively. Second, there is no visible PSV in BY-2 cells; thus, proteins for the PSV pathway may end up to PVC for PSV in BY-2 cells. Third, in plants like Arabidopsis, the expression of VSRs is temporally and spatially regulated, resulting in expression of selected VSRs in specific tissues or during different developmental stages. Fourth, in vitro binding studies demonstrated that VSR proteins bind to vacuolar sorting determinants of both proteases and storage proteins for their delivery to the lytic vacuole and PSV, respectively. The N-terminal regions of the seven AtVSR proteins are relatively conserved with a 30-amino acid (from 64 to 92) distinct region. This distinct domain of individual AtVSR may be responsible for binding specific cargo protein for transportation. Alternatively, the conserved N-terminal regions of AtVSR may bind to both lytic enzymes and storage proteins, providing that these cargo proteins localized to the same transport vesicles or organelles in vivo.
The PVC localization of these GFP-AtVSR fusions is likely to reflect the subcellular localization of AtVSR proteins when they are expressed in BY-2 cells and, thus, the localization of AtVSRs in Arabidopsis plants, even though this conclusion needs to be confirmed by future study on transgenic Arabidopsis plants expressing the seven GFP-AtVSR fusions. Therefore, this study will serve as a first step to further investigate the functions of the seven Arabidopsis VSR proteins and PVC-mediated protein trafficking in plants. Several questions can be asked in future studies. For example, what are the ligands of individual AtVSRs? Do the seven AtVSR traffic through the same PVCs or distinct PVCs leading to either lytic vacuole or PSV? Do the seven AtVSR all express in the whole plant? Our current research aims to answer some of these questions.
MATERIALS AND METHODS
General methods for construction of recombinant plasmids, characterization of cloned inserts, and maintenance of tobacco (Nicotiana tabacum) BY-2 culture cells have been described previously (Jiang and Rogers, 1998; Tse et al., 2004).
Oligonucleotides
The oligonucleotide/primer sequences (from 5′ to 3′) used in self-priming PCR to amplify dsDNA fragments for further cloning are listed in Supplemental Table S1.
Sequence alignment and tree drawing were performed using the ClustalW and unweighted pair-group method using arithmetic averages algorithm with default parameters in the MacVector 7.2 (Accelrys) analysis package. The trees were statistically evaluated with a bootstrap analysis with 1,000 bootstraps.
Plasmid Construction
The amino acid and nucleotide sequences of VSR of Arabidopsis (Arabidopsis thaliana), pumpkin (Cucurbita pepo), and black gram (Vigna mungo) were obtained from BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/) using the pea (Pisum sativum) BP-80 amino acid sequence (Paris et al., 1997). To generate various chimeric constructs that contain sp sequence from the barley (Hordeum vulgare) proaleurain linked to GFP (Tse et al., 2004) and the TMD/CT sequences of individual AtVSR (these constructs were termed GFP-AtVSR fusions in this study; Fig. 1C), two pairs of primers were designed and used to generate dsDNA containing the spacer-TMD/CT sequences of individual VSR. P1/P2 are overlapped primer pairs (with 30-nucleotide overlap) that cover the full-length spacer-TMD/CT sequences of individual VSR to be used for self-priming PCR to generate the dsDNA fragment of spacer-TMD/CT sequences (Fig. 1D), which in turn will serve as templates for further PCR using primer pairs P3/P4 to generate full-length spacer-TMD/CT sequences of individual VSR (Fig. 1D). P1 and P3 contain an EcoRI site, while P2 and P4 contain a stop codon and SacI site. Individual spacer-TMD-CT DNA fragments from the above PCR were gel purified and digested with EcoRI/SacI before being cloned into pSYFP491K (Tse et al., 2004) via the same restriction sites to replace the spacer-BP-80 TMD/CT sequences. The resulting constructs were then cloned into the binary vector pSYFP491K (Tse et al., 2004) via HindIII/SacI sites before they were transferred into Agrobacterium for BY-2 cell transformation. As a result, all newly generated GFP-VSR fusions contain 35S promoter-sGFP-spacer-TMD-CT-Nos 3′ terminator (Tse et al., 2004). All constructs were checked by both restrictions mapping and DNA sequencing.
Transformation of BY-2 Cells
All chimeric constructs were transferred into Agrobacterium tumefaciens (strain LBA4404) via electroporation and used to transfect wild-type BY-2 cells as previously described (Tse et al., 2004). BY-2 cells are maintained in Murashige and Skoog (MS) medium by subculturing once a week and culturing at room temperature in a shaker set at 125 rpm. Transfected BY-2 cells with Agrobacterium were transferred onto MS media (Sigma) containing kanamycin (50 μg/mL) and cefotaxin (250 μg/mL) for 3 to 4 weeks for colony formation. Resistant BY-2 cell colonies (approximately 100–200 from each construct) were subjected to screening for their GFP signals via confocal microscopy. Selected cell lines (5–10 per construct) were further transferred into MS liquid medium with kanamycin to initiate suspension culture to be used for subsequent analysis. Transgenic tobacco BY-2 cells were maintained in both liquid and solid culture via subculturing once per week.
Antibodies
A recombinant protein representing the lumenal portion (lacking the TMD and CT) of VSRAt-1 (Paris et al., 1997) was expressed in Drosophila melanogaster S2 cells (Cao et al., 2000) and purified for use as antigen to generate VSRat-1 antibodies (Cao et al., 2000; Li et al., 2002). Affinity-purified recombinant proteins were used for immunization of two rabbits at the animal house of the Chinese University of Hong Kong (Tse et al., 2004). Antibodies were purified by affinity chromatography using a column made with recombinant protein coupled to CNBr Sepharose (Sigma) as described (Paris et al., 1997). GFP antibodies were purchased from Molecular Probes or generated using recombinant GFP as antigens to inject rabbits at the animal house of the Chinese University of Hong Kong and affinity purified. Secondary or lissamine rhodamine- or FITC-conjugated affinity-purified anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories. For western-blot analysis, GFP antibodies and VSR antibodies were used at 4 μg/mL.
Drug Treatment
Stock solutions of wortmannin (Sigma) at 2.5 mg/mL in dimethyl sulfoxide and BFA (Sigma) at 1 mg/mL in dimethyl sulfoxide were used. Both drugs were diluted in MS liquid medium to appropriate working concentrations before incubation with BY-2 cells. For each drug treatment, BY-2 cells were mixed with drugs in working solutions in MS media at 1:1 ratio to ensure minimal variation. Treated samples were then harvested at the indicated time for subsequent confocal and EM analysis as described (Tse et al., 2004). Each treatment was repeated at least twice with similar results.
Confocal Immunofluorescence Studies
Fixation and preparation of cultured cells (tobacco BY-2, Arabidopsis, rice [Oryza sativa]), root tips (pea, mung bean [Vigna radiata], and tobacco), and their labeling and analysis by epifluorescence and confocal immunofluorescence have been described previously (Jiang and Rogers, 1998; Jiang et al., 2000; Li et al., 2002). The settings for collecting confocal images within the linear range were as described (Jiang and Rogers, 1998). For immunolabeling, anti-VSR polyclonal rabbit antibody at a final concentration of 4 μg/mL was used and incubated at 4°C overnight. All confocal fluorescence images were collected using a Bio-Rad Radiance 2100 system. Images were processed using Adobe Photoshop software as previously described (Jiang and Rogers, 1998). The extent of colocalization of two signals in confocal immunofluorescence images from BY-2 cells was quantitated as described previously (Jiang and Rogers, 1998; Jiang et al., 2000).
Electron Microscopy of Resin-Embedded Cells
The general procedures for conventional thin sectioning of chemically fixed samples of BY-2 cells and immunoEM localization of antibodies with high-pressure freezing/frozen substitution BY-2 samples were performed essentially as described previously (Ritzenthaler et al., 2002). Immunolabeling of London Resin White sections was carried out using VSR antibodies at 1:100 dilution (40 μg/mL) and gold-coupled secondary antibodies at 1:50 dilution. Aqueous uranyl acetate/lead citrate poststained sections were examined in a JOEL JEM-1200EX II transmission EM operating at 80 kV.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g52850 (Arabidopsis AtVSR1), At2g30290 (Arabidopsis AtVSR2), At2g14740 (Arabidopsis AtVSR3), At2g14720 (Arabidopsis AtVSR4), At2g34940 (Arabidopsis AtVSR5), At1g30900 (Arabidopsis AtVSR6), At4g20110 (Arabidopsis AtVSR7), and AB006809 (pumpkin PV72).
Supplemental Materials
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of plant VSR proteins.
Supplemental Table S1. Oligonucleotides used in this study.
Supplementary Material
Acknowledgments
We are grateful to Mr. Jason LAM (CUHK) for sharing the HPF block of BY-2 cells for immunoEM study. We also thank Prof. David Robinson and Dr. Stefan Hillmer (University of Heidelberg) for their continuous support in our TEM studies. We also sincerely thank the two anonymous reviewers for their insightful comments on the manuscript.
This work was supported by the Research Grants Council of Hong Kong (grant nos. CUHK4156/01M, CUHK4260/02M, CUHK4307/03M, and CUHK4580/05M) and by the National Natural Science Foundation of China (grant no. 30529001 to L.J.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Liwen Jiang (ljiang@cuhk.edu.hk).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ahmed SU, Bar-Peled M, Raikhel NV (1997) Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol 114: 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol 149: 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A, Wymann MP (1993) Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL (2000) Vacuoles and prevacuolar compartments. Curr Opin Plant Biol 3: 469–475 [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris N (2002) The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14: 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Rogers SW, Butler J, Beevers L, Rogers JC (2000) Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12: 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Foresti O, Denecke J (2006) Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18: 1477–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley TP, Hester S, Shadforth IP, Runions J, Weimar T, Hanton SL, Griffin JL, Bessant C, Brandizzi F, Hawes C, et al (2006) Mapping the Arabidopsis organelle proteome. Proc Natl Acad Sci USA 103: 6518–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J (1999) Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol 9: 55–58 [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4: 202–212 [DOI] [PubMed] [Google Scholar]
- Hadlington JL, Denecke J (2000) Sorting of soluble proteins in the secretory pathway of plants. Curr Opin Plant Biol 3: 461–468 [DOI] [PubMed] [Google Scholar]
- Happel N, Honing S, Neuhaus JM, Paris N, Robinson DG, Holstein SE (2004) Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J 37: 678–693 [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura II, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10: 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle S, Marceau F (2003) Wortmannin alters the intracellular trafficking of the bradykinin B2 receptor: role of phosphoinositide 3-kinase and Rab5. Biochem J 375: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humair D, Hernandez Felipe D, Neuhaus JM, Paris N (2001) Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor. Plant Cell 13: 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Phillips TE, Rogers SW, Rogers JC (2000) Biogenesis of the protein storage vacuole crystalloid. J Cell Biol 150: 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Rogers JC (1998) Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J Cell Biol 143: 1183–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Rogers JC (2003) Sorting of lytic enzymes in the plant Golgi apparatus. Annu Plant Rev 9: 114–140 [Google Scholar]
- Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91: 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeken R, Mousavi SA, Brech A, Griffiths G, Berg T (2001) Wortmannin-sensitive trafficking steps in the endocytic pathway in rat liver endothelial cells. Biochem J 357: 497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Jiang L, Oliviusson P, Heinzerling O, Robinson DG (2005) Plant prevacuolar compartments and endocytosis. Plant Cell Monogr 1: 37–61 [Google Scholar]
- Laval V, Chabannes M, Carriere M, Canut H, Barre A, Rouge P, Pont-Lezica R, Galaud J (1999) A family of Arabidopsis plasma membrane receptors presenting animal beta-integrin domains. Biochim Biophys Acta 1435: 61–70 [DOI] [PubMed] [Google Scholar]
- Laval V, Masclaux F, Serin A, Carriere M, Roldan C, Devic M, Pont-Lezica RF, Galaud JP (2003) Seed germination is blocked in Arabidopsis putative vacuolar sorting receptor (atbp80) antisense transformants. J Exp Bot 54: 213–221 [DOI] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L (2002) BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol 43: 726–742 [DOI] [PubMed] [Google Scholar]
- Mitsuhashi N, Shimada T, Mano S, Nishimura M, Hara-Nishimura I (2000) Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol 41: 993–1001 [DOI] [PubMed] [Google Scholar]
- Mo BX, Tse YC, Jiang L (2006) Plant prevacuolar/endosomal compartments. Int Rev Cytol 253: 96–129 [DOI] [PubMed] [Google Scholar]
- Neuhaus JM, Paris N (2005) Plant vacuoles: from biogenesis to function. Plant Cell Monogr 1: 63–82 [Google Scholar]
- Neuhaus JM, Rogers JC (1998) Sorting of proteins to vacuoles in plant cells. Plant Mol Biol 38: 127–144 [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Neuhaus JM (2002) BP-80 as a vacuolar sorting receptor. Plant Mol Biol 50: 903–914 [DOI] [PubMed] [Google Scholar]
- Paris N, Rogers SW, Jiang L, Kirsch T, Beevers L, Phillips TE, Rogers JC (1997) Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol 115: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim SJ, Vitale A, Hwang I (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134: 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Lee D, Lee GJ, Hwang I (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenfuhr A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Oliviusson P, Hinz G (2005) Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic 6: 615–625 [DOI] [PubMed] [Google Scholar]
- Sanderfoot AA, Ahmed SU, Marty-Mazars D, Rapoport I, Kirchhausen T, Marty F, Raikhel NV (1998) A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc Natl Acad Sci USA 95: 9920–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I (1997) A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol 38: 1414–1420 [DOI] [PubMed] [Google Scholar]
- Shimada T, Watanabe E, Tamura K, Hayashi Y, Nishimura M, Hara-Nishimura I (2002) A vacuolar sorting receptor PV72 on the membrane of vesicles that accumulate precursors of seed storage proteins (PAC vesicles). Plant Cell Physiol 43: 1086–1095 [DOI] [PubMed] [Google Scholar]
- Tamura K, Yamada K, Shimada T, Hara-Nishimura I (2004) Endoplasmic reticulum-resident proteins are constitutively transported to vacuoles for degradation. Plant J 39: 393–402 [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Raikhel NV (1999) What do proteins need to reach different vacuoles? Trends Plant Sci 4: 149–155 [DOI] [PubMed] [Google Scholar]
- Watanabe E, Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2002) Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J Biol Chem 277: 8708–8715 [DOI] [PubMed] [Google Scholar]
- Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG (2005) Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17: 1513–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.