Abstract
The endothelium plays a critical role in the inflammatory process. The complement activation product, C5a, is known to have proinflammatory effects on the endothelium, but the molecular mechanisms remain unclear. We have used cDNA microarray analysis to assess gene expression in human umbilical vein endothelial cells (HUVECs) that were stimulated with human C5a in vitro. Chip analyses were confirmed by reverse transcriptase-polymerase chain reaction and by Western blot analysis. Gene activation responses were remarkably similar to gene expression patterns of HUVECs stimulated with human tumor necrosis factor-α or bacterial lipopolysaccharide. HUVECs stimulated with C5a showed progressive increases in gene expression for cell adhesion molecules (eg, E-selectin, ICAM-1, VCAM-1), cytokines/chemokines, and related receptors (eg, VEGFC, IL-6, IL-18R). Surprisingly, HUVECs showed little evidence for up-regulation of complement-related genes. There were transient increases in gene expression associated with broad functional activities. The three agonists used also caused down-regulation of genes that regulate angiogenesis and drug metabolism. With a single exception, C5a caused little evidence of activation of complement-related genes. These studies indicate that endothelial cells respond robustly to C5a by activation of genes related to progressive expression of cell adherence molecules, and cytokines and chemokines in a manner similar to responses induced by tumor necrosis factor-α and lipopolysaccharide.
The endothelium plays an important role in inflammation by actively participating in its initiation and propagation. Activation of the endothelium by lipopolysaccharide (LPS) or tumor necrosis factor (TNF)-α is known to promote the expression of adhesion proteins (eg, E-selectin, ICAM-1, VCAM-1) that initiate adhesive interactions with blood leukocytes. These adhesive interactions may cause leukocytes to encounter chemokines on the endothelial surface, which results in leukocyte activation and engagement of leukocyte integrins (LFA-1 and Mac-1, VLA-4) with their counter receptors (ICAM-1 and VCAM-1) on endothelial cells. The resulting firm attachments enable neutrophils to move through endothelial junctions. Thus, physical interactions between the endothelium and leukocytes are part of the inflammatory response, whereas additional contributions are provided by leukocytic receptors, which ligate inflammatory mediators such as C5a, TNF-α, and CXC chemokines.
TNF-α and LPS are initiators of the inflammatory response and are well known to activate endothelial cells. TNF-α is a proinflammatory mediator principally derived from phagocytic cells and is produced during systemic inflammatory responses, with resultant triggering of signaling cascades, culminating in cytokine and chemokine production, activation of phagocytic cells, and in some cases apoptosis.1 TNF-α effects on endothelial cells include synthesis-independent changes in cell shape that may contribute to vascular leakage at sites of inflammation as well as expression of proteins that regulate other parameters of the inflammatory response (eg, COX-2 and GTP cyclohydrolase-I). Similarly, LPS from gram-negative bacteria induces endothelial cells to express a variety of adhesion proteins, cytokines, and chemokines.2 LPS is also capable of inducing increased endothelial permeability. Blood-borne TNF-α and interleukin (IL)-1β seem to mediate LPS-induced endothelial permeability.3 Endothelial cells recognize the presence of microbial components such as LPS via a receptor complex that contains at least three cell surface components: CD14, Toll-like receptor-4 (TLR-4), and MD-2. Endothelial cell contact with LPS or TNF-α initiates nuclear factor (NF)-κB activation, which results in protein expression.3 There is little evidence of direct toxicity of LPS on cultured human endothelial cells.4,5 When transcriptional products are inhibited by cycloheximide (CHX),5 endothelial cells become sensitized to apoptotic signals.5–7 A similar phenomenon has been described for TNF-α.8 Exposure to TNF-α does not cause endothelial cell apoptosis, but the combination of TNF-α and CHX at appropriate concentrations leads to cell death.8 In both cases, anti-apoptotic gene induction is required to blunt the apoptotic pathway.6,8
C5a is a potent molecule generated from complement activation through cleavage of C5 and usually accompanied by formation of the membrane attack complex (C5b to C9). C5a increases endothelial P-selectin expression, secretion of von Willebrand factor, and smooth muscle contraction.9 Additionally, for myeloid cells C5a induces chemotaxis, release of superoxide anion, and production of inflammatory cytokines, (eg, IL-1, IL-6, IL-8, and TNF-α).10,11 These inflammatory mediators are produced as a result of increased transcriptional activity, indicating that C5a induces a primary gene response that does not require secondary protein signaling. In conjunction with the production of cytokines and chemokines, increased C5a receptor (C5aR, CD88) expression appears in many tissues in response to various inflammatory conditions.12–14 Studies have found C5aR expression on many nonmyeloid cells during events such as head trauma, ischemia, and bacterial meningitis.15,16 In addition, binding studies using antibodies directed against C5aR have shown elevations in C5aR protein expression in rat tissues during sepsis after cecal ligation and puncture.13 Such increases in receptor density on the endothelium suggest that C5a may substantially alter organ function.
Although the signaling pathways in endothelial cells used by the C5a receptor are becoming more defined, the transcriptional regulation of inflammatory genes associated with C5a-induced signaling remains to be determined. In this study, we have investigated gene expression of human umbilical vein endothelial cells (HUVECs) stimulated with human C5a, using cDNA-based microarray technology. We compared C5a-stimulated HUVECs to those stimulated with TNF-α and LPS. This study has provided the identification of genes activated in response to C5a and has confirmed the ability of C5a to induce a variety of proinflammatory genes in endothelial cells.
Materials and Methods
Endothelial Cell Culture
Primary HUVECs were isolated and plated in T-75 flasks coated with 1% gelatin (Sigma, St. Louis, MO). Cells were cultured in medium 199 (Life Technologies, Inc., Grand Island, NY) containing endothelial growth supplement (Sigma), 20% fetal bovine calf serum (Hyclone, Logan, UT), 5% penicillin/streptomycin (Life Technologies, Inc.), and 25 μg/ml heparin (Sigma). All experiments were performed with the first three to four cell passages. Uptake of acetylated low-density lipoprotein was > = 99%, indicating few if any nonendothelial cells in the monolayers.
Experimental Conditions for Microarray Analysis
Before experimentation, the recombinant C5a was tested for the presence of LPS with a Limulus amebocyte lysate assay that detects LPS at a concentration ≤25 pg/ml. The final concentration of C5a (50 ng/ml) used in experiments contained <3 pg LPS/ml. After HUVECs became confluent, the endothelial growth supplement was removed for 18 hours. To avoid secondary effects of newly synthesized proteins, at the beginning of each experiment the cells were treated with a nonapoptotic-inducing dose of CHX (10 μg/ml) for 10 minutes, then stimulated at 37°C with 50 ng/ml of C5a, 1 μg/ml of LPS (Escherichia coli, serotype 026:B6), or 1 ng/ml of human TNF-α, as indicated, for the times indicated. We used controls that had growth supplement removed for 18 hours followed by 30 minutes of treatment with CHX. Total RNA in HUVEC monolayers was collected using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. An additional phenol/chloroform step was performed to improve RNA purity. RNA was stored at −80°C. Before experimentation, the quality of each RNA sample was tested by agarose gel electrophoresis and staining with ethidium bromide.
Pretreatment of HUVECs with CHX was used to prevent a second cascade of gene activation because of early synthesis of proteins (eg, tissue factor) known to have gene-activating effects in HUVECs. Special conditions (lower concentrations of CHX) were used to prevent apoptosis that occurs at higher concentrations of CHX.
Microarray Analysis
Gene expression analysis was done using techniques previously described by Dhanasekaran and colleagues.17 Briefly, purified polymerase chain reaction (PCR) products produced from sequence-verified clones (Research Genetics, Inc., Huntsville, AL) were spotted onto polylysine-coated glass microscope slides using an Omnigrid Robitic Arrayer (GeneMachines, San Carlos, CA) fashioned with quill-type pins (Majer, Tempe, AZ). Calibration and test print runs using salmon sperm diluted in 3× standard saline citrate were performed before final printing to ensure the highest quality spotting. HUVEC total RNA was used as a template for cDNA synthesis using reverse transcriptase (RT). Amino acyl coupling to dUTP provided a substrate for fluorescent labeling with Cy-3 (sample) and Cy-5 (cntrl). Fluorescently labeled cDNA was hybridized to 10 K cDNA microarray chips at 62°C for 14 to 16 hours. Fluorescent images of hybridized microarrays were obtained using a GenePix 4000A microarray scanner (Axon Instruments, Union City, CA).
Data Analysis
Primary analysis of the hybridized chip used GenePix Pro software (Axon Instruments) to adjust Cy5 and Cy3 signal intensities. Images were obtained by scanning of the grid of hybridization spots and linked to the corresponding gene list, allowing calculation of normalization factors that could be used to monitor signal ratio intensities. Poorly spotted genes, expressing weak or distorted signals, were automatically discarded by GenePix software. Subsequent analysis was performed manually by visualization of the scanned chip. Spots with diameters less than 50 μm or low-fluorescent signals intensities (<350) were discarded from consideration. This process removes ∼300 ± 25 genes (from a total of 10,000 hybridization spots) from each experimental microarray chip. After filtering data points, each scanned microarray was scaled by normalizing the median data to 1.0 using Microsoft Excel Macros (Microsoft, Redmond, WA). Normalized fluorescence ratios were placed into Microsoft data access. These data sets were exported into MB Eisen’s (University of California at Berkeley) cluster program. The first analysis used an arbitrary cutoff ratio of 2.6-fold to select genes significantly up- or down-regulated relative to the reference chip. Subsequent analysis increased the stringency of the filtering parameters to decrease gene volumes and identify only significantly up-regulated genes. All data were log2-transformed and hierarchically clustered with average linkage clustering then visualized using Tree View Program (http://rana.lbl.gov/EisenSoftware.htm).
RT-PCR
Replicate samples exposed to identical C5a-stimulating conditions were used for RT-PCR analysis. This was done to confirm microarray gene expression patterns after C5a stimulation of HUVECs. One μg of total RNA was reversed-transcribed with Superscript RT (Invitrogen) using random and poly dT primers for first-strand cDNA synthesis. The samples were washed using YM-30 microfilters (Microcon, Bedford, MA), then inverted into a new tube and centrifuged for 1 minute at 13,500 × g to collect the sample. This was repeated for each time point. Primers for each gene of interest were designed using Primer Select software and included: Bcl6: forward primer, 5′-AGCCCGAAATGCCCCCTACTT-3′, reverse primer, 5′-CCCCTGGTTTGGCATTCTGGT-3′; E-selectin: forward primer, 5′-TTATGATGAGGCCAGTGCTTATTG-3′, reverse primer, 5′-CTTTTTGCCTATTGTTGGGTTCAC-3′; ICAM-1: forward primer, 5′-GGGGAACCAGAGCCAGGAGACAC-3′, reverse primer, 5′-CGGGGGCATACAGGACACGAA-3′; MMP-12: forward primer, 5′-CGGGCAACTGGACACATCTACC-3′, reverse primer, 5′-GCTCCACGGGCAAAAACCAC-3′; UCP-3: forward primer, 5′-AGAACCATCGCCAGGGAGGAAGGA-3′, reverse primer, 5′-CACCGGGGAGGCCACCACTGT-3′; GAPDH: forward primer, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse primer, 5′-AGCCTTCTCCATGGTGGTGAAGA-3′. PCRs were run on Gene Amp PCR system 9700 (Applied Biosystems, Foster City, CA) using a hot-start method with the following conditions: 94°C for 5 minutes, 94°C for 1 minute, 60 to 65°C for 1 minute, 72°C for 1 minute, 72°C for 10 minutes. GAPDH reaction mixture was run for 26, 28, and 30 cycles to ensure that the reaction product was in the linear range. PCR products were examined in 1% agarose gel. The RT product volumes were readjusted and the reaction was repeated with GAPDH primers at 26 cycles. After RT product adjustment, the selected gene primers were amplified with 28 and 31 cycles, and then examined by agarose gel electrophoresis to confirm product size.
Western Blots
To examine the ability of C5a to induce proteins in HUVECs, we used Western-blotting techniques. HUVECs were grown to confluence, then stimulated with human C5a (50 ng/ml) at 37°C for 2, 8, or 18 hours. After stimulation, the supernatant fluids were removed and the monolayer was washed twice with phosphate-buffered saline. Cells were placed on ice and lysed using Nonidet P-40 buffer (50 mmol/L Tris-HCl, pH 8.0, 1% Nonidet P-40) containing protease inhibitors (Roche, Mannheim, Germany). The lysate was then collected with sterile cell scrapers and sonicated. The resulting cell lysate was centrifuged at 13,500 × g for 10 minutes and the supernatant fluid removed. This was repeated for each time point. The protein concentrations were estimated using a Bio-Rad protein colorimetric assay. The standardized supernatants were combined with sample buffer, sterile glycerol and stored at −80°C. The cell lysates were subjected to 10 and 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to methanol-activated polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk dissolved in TBST (1 mol/L Tris, pH 8, 1 mol/L NaCl, 0.1% Tween 20) for 1 hour. Immunoblotting was performed using a 1:2000 dilution of anti-mouse or anti-rabbit antibody [for ICAM-1, Bcl6 (Lab Vision, Fremont, CA)] and E-selectin (HyCult Biotechnology, Uden, The Netherlands). After an overnight incubation at 4°C, the membranes were washed three times with TBST and incubated with their respective secondary anti-rabbit or anti-mouse antibodies coupled to horseradish peroxidase for 2 hours at room temperature. The membranes were then washed twice with TBST followed by an additional wash of TBS. The immunoreactive bands were visualized using enhanced chemiluminescence and Western blotting detection agents according to the manufacturer’s directions.
Results
Gene Expression Signatures Induced by C5a, LPS, and TNF-α
We chose to examine the gene responses of endothelial cells stimulated with LPS, TNF-α, or C5a (see above for details) using a 10,000-spotted glass slide cDNA microarray chip to monitor gene expression responses. This provided a way to globally monitor at the transcriptional level changes in endothelial cells exposed to each of the three agonists. The chip contained many known genes in addition to expressed sequence tags. Genes were spotted in triplicate on each chip to serve as internal controls (see supplementary information for the complete annotated list at: http://www.pathology.med.umich.edu/chinnaiyan). cDNA was prepared using RT-PCR of total RNA collected from endothelial cells stimulated with C5a, TNF-α, or LPS at the times indicated. RNA from the common reference sample was collected from endothelial cells that were grown and maintained under identical conditions. cDNA derived from the stimulated cells was labeled with the fluorescent dye Cy5 along with cDNA from the unstimulated control that was labeled with the fluorescent dye Cy3.
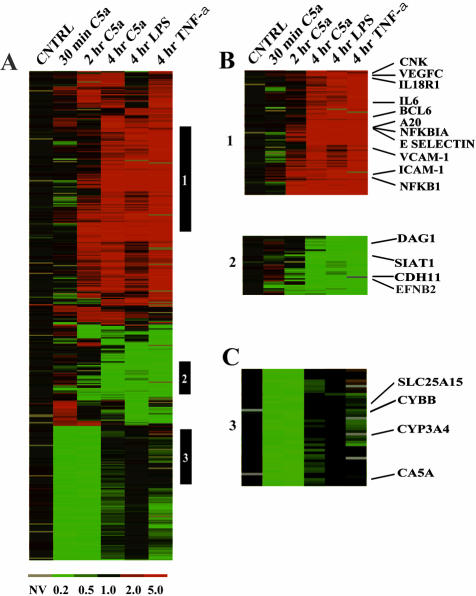
A hierarchical clustering algorithm was used to group genes displaying similar expression. Figure 1A represents an Eisen matrix gene cluster that includes the following conditions for stimulation: C5a stimulation for 30 minutes, 2 hours, and 4 hours; LPS stimulation for 4 hours; and TNF-α stimulation for 4 hours. We arbitrarily considered that gene activation changes of <2.6-fold were not significant. The control (cntrl) (Figure 1A, column 1) represents a labeling control in which identical samples were labeled with Cy3 and Cy5. Figure 1 represents a cluster of 1244 genes that were commonly induced (B1) or repressed (B2) after cell exposure to C5a, LPS, or TNF-α. The vertical bars (frame A, labeled 1, 2, and 3) highlight specific gene clusters. These clusters are enlarged in frames B1, B2, and C3 so that specific genes could be visualized.
Figure 1.
Microarray cluster of 1260 genes. Gene activation values of <2.6-fold increases above control values were not considered to be of significance (see text). HUVECs were stimulated with LPS (1 μg/ml) for 4 hours; TNF-α (1 ng/ml) for 4 hours; and C5a (50 ng/ml) for 30 minutes, 2 hours, and 4 hours. The control (cntrl) represents a labeling control between two identically stimulated samples. A: The black vertical bar 1 is a cluster of genes up-regulated in response to stimulation by C5a, LPS, or TNF-α. The black vertical bar 2 represents a cluster of genes down-regulated in response to 4 hours of stimulation with C5a, LPS, or TNF-α. The black vertical bar 3 represents genes that are transiently down-regulated in response to C5a at 30 minutes and 2 hours. B: Expanded sections are from black vertical bars 1 and 2. Induced genes selected have cytokine/chemokine and transcription-related functions. The selected repressed genes are associated with cytoskeletal functions. C: An expanded section is from black vertical bar 3. Transiently repressed genes in response to 50 ng/ml of C5a were associated with metabolic functions.
The clustering profile resulted in three major groups including: genes induced, genes repressed, or genes transiently repressed in HUVECs exposed to C5a, TNF-α, or LPS. The first cluster, designated 1 in frame B, contained induced genes with up to 4 hours of exposure to C5a, LPS, or TNF-α. In this cluster, we chose to focus on genes that were associated with cytokine/chemokine signaling and cellular adhesion molecules. These included CNK (cytokine inducible kinase), IL-18R-1, and IL-6. CNK has been linked to the cell cycle, especially the G2 phase. Our data suggest that this transcript is consistently up-regulated by all three stimuli. IL-6, which was induced by all three stimuli (especially C5a) underscores the potential role of IL-6 in inflammatory responses of endothelial cells.
In addition, well-known adhesion molecule genes (eg, ICAM-1, E-selectin, and VCAM-1) were induced in endothelial cells by all three stimuli. This implies that endothelial cells express adhesion molecules during inflammation, facilitating leukocyte adherence to the endothelium and transmigration into the extravascular space. Furthermore, E-selectin was highly induced by each of the three stimuli, with >20-fold increases in expression (Table 1). VEGFC was another prominent gene that was induced by all three stimuli at 4 hours, possibly facilitating an endothelial proliferative response, which could be critical in wound healing. When we examined other genes within this cluster, we found expressions of proinflammatory genes together with genes that have repressive functions. Several of the genes that were induced at 4 hours (Figure B1) encode proinflammatory proteins, such as NF-κB1. NF-κB1 is involved in the transcription of many early response genes in endothelial cells (eg, ICAM-1 and E-selectin). In contrast, several genes within cluster B1 induced by each of the three stimuli at 4 hours function to repress transcription (eg, Bcl6, NF-κBIA) or inhibit processes such as apoptosis (eg, A20). The proto-oncogene Bcl6 encodes a transcriptional repressor protein that has been shown in Bcl6-deficient mice to be a negative regulator of inflammation.18,19 NF-κBIA (IκBα) negatively regulates NF-κB activation. A20 selectively inhibits caspase 8 activation and TNF-α receptor-induced apoptosis in Jurkat T cells.20 A20 does not directly inhibit caspase 8 activity but rather targets a component of the signaling pathway upstream of caspase 8 that is peculiar to TNF-α receptor.21 The capacity of each stimulus to induce similar genes demonstrates the conserved behavior of endothelial cells during responses to structurally distinct stimuli.
Table 1.
HUVEC Gene Expression Ratios
| Gene | Name | Control 0 versus 0 | C5a 4 hours | TNF 4 hours | LPS 4 hours |
|---|---|---|---|---|---|
| Cytokine/chemokine associated genes | |||||
| TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | 1.048 | 9.119 | 32.208 | 20.003 |
| TNFAIP2 | Tumor necrosis factor, alpha-induced protein 2 | 0.987 | 6.739 | 15.707 | 8.303 |
| EGR1 | Early growth response 1 | 1.1 | 6.439 | 13.455 | 8.891 |
| GRO1 | GRO1 oncogene (melanoma growth stimulating activity, alpha) | 0.958 | 6.329 | 43.025 | 16.269 |
| VEGFC | Vascular endothelial growth factor C | 1.063 | 5.277 | 2.705 | 4.533 |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (p105) | 1.081 | 5.265 | 7.38 | 4.351 |
| CSF1 | Colony-stimulating factor 1 (macrophage) | 0.991 | 4.843 | 10.561 | 2.191 |
| NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer alpha | 0.904 | 3.711 | 9.408 | 6.848 |
| SCYA2 | Small inducible cytokine 2 (chemokine ligand 2) | 0.92 | 3.191 | 4.667 | 4.09 |
| GADD34 | Growth arrest and DNA-damage-inducible 34 | 0.948 | 2.998 | 3.985 | 2.258 |
| PLAB | Prostate differentiation factor | 0.979 | 2.976 | 1.172 | 1.102 |
| PRKR1R | Repressor of (P58 repressor) | 0.981 | 2.907 | 3.458 | 1.934 |
| IFNGR2 | Interferon gamma receptor 2 (interferon gamma transducer 1) | 1.138 | 2.885 | 2.881 | 2.779 |
| SCYA7 | Small inducible cytokine A7 (monocyte chemotactic protein 3) | 0.83 | 2.873 | 4.361 | 2.629 |
| CNK | Cytokine-inducible kinase | 1.146 | 2.85 | 2.34 | 1.918 |
| TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | 1.098 | 2.796 | nv | 4.536 |
| FLT1 | VEGF receptor 1 | nv | 2.605 | 0.959 | 1.479 |
| IL6 | Interleukin 6 (interferon, beta 2) | 1.138 | 2.358 | 7.836 | 4.211 |
| SCYA13 | Small inducible cytokine subfamily A (Cys-Cys), member 13 | 0.901 | 2.349 | 4.226 | 1.656 |
| IL18R1 | Interleukin 18 receptor 1 | 1.037 | 2.255 | 21.959 | 3.139 |
| TGIF | TG-interacting factor (TALE family homeobox) | 1.041 | 2.157 | 3.28 | 1.859 |
| NFE2L2 | Nuclear factor (erythroid-derived 2)-like 2 | 0.892 | 1.944 | 3.052 | 1.884 |
| TGIF | TG-interacting factor (TALE family homeobox) | 1.01 | 1.89 | 3.483 | 1.517 |
| SCYA4 | Small inducible cytokine A4 (homologous to mouse Mip-1b) | nv | 1.359 | 3.265 | 1.555 |
| IL1R1 | Interleukin 1 receptor, type 1 | 0.976 | 1.319 | 2.738 | 0.903 |
| TRAF6 | TNF receptor-associated factor 6 | nv | 1.26 | 2.922 | 1.247 |
| TGFB2 | Transforming growth factor, beta 2 | 1.56 | 1.192 | 0.936 | 0.282 |
| TNFAIP6 | Tumor necrosis factor, alpha-induced protein 6 | 0.962 | 1.108 | 8.47 | 1.786 |
| FGF18 | Fibroblast growth factor 18 | 0.97 | 1.097 | 6.205 | 1.04 |
| ISG15 | Interferon-stimulated protein, 15 kDa | 1.342 | 1.051 | 3.076 | 1.137 |
| TNFR11 | Tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | 1.393 | 1.015 | 4.665 | 1.08 |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 0.891 | 0.964 | 3.27 | 1.004 |
| Cell adhesion-associated genes | |||||
| SELE | Selectin E (endothelial adhesion molecule 1) | 1.142 | 23.598 | 74.38 | 54.27 |
| VCAM1 | Vascular cell adhesion molecule 1 | 0.943 | 8.446 | 11.283 | 4.106 |
| ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 1.058 | 8.442 | 15.417 | 6.068 |
| CDH13 | Cadherin 13, H-cadherin (heart) | 1.079 | 0.733 | 0.856 | 0.292 |
| Complement-associated genes | |||||
| MBL2 | Mannose-binding lectin (protein C) 2, soluble (opsonic defect) | 1.164 | 2.72 | 6.075 | 5.956 |
| DAF | Decay accelerating factor for complement (CD55, Cromer blood group system) | 0.927 | 1.386 | 0.947 | 0.746 |
| XPA | Xeroderma pigmentosum, complementation group A | 0.802 | 1.137 | 0.924 | 0.893 |
| C5 | Complement component 5 | 0.831 | 1.133 | 1.408 | 1.176 |
| ITGAM | Integrin, alpha M complement component receptor 3, alpha | 1.079 | 1.002 | 2.079 | 1.417 |
The second cluster (B2) contains genes that were down-regulated in endothelial cells after exposure to C5a, LPS, or TNF-α for 4 hours. Although this cluster consisted of genes having many known as well as unknown links to inflammation, we chose to focus on genes associated with the cytoskeleton and angiogenesis. DAG1 (dystroglycan 1) may act as a laminin 1 receptor. It has been shown to be implicated in several forms of muscular disease and also may function to control angiogenesis.22,23 CDH 11 (cadherin 11) is a protein that forms tight junctions, maintains structural integrity and controls intercellular permeability. EFNB2 (Ephrin B2) is thought to be involved in cell-to-cell signaling and seems to be important for angiogenic functions.24,25 Interestingly, Ephrin B2 has been shown to activate downstream kinases that can inhibit the secretion of vascular endothelial growth factor (VEGF) through MAPK suppression.24,25 SIAT1 (sialyltransferase 1) encodes for ST6Gal I. This sialyltransferase mediates the transfer of α2,6-linked sialic acids to Galβ1,4GlcNAc terminal of N-linked glycoproteins.26 In general, it seems that the net effect of 4 hours of stimulation with C5a, LPS, or TNF-α is the down-regulation of genes that negatively regulate angiogenesis.
The third cluster (C3) emphasizes transiently repressed genes after stimulation of endothelial cells with C5a for 30 minutes and 2 hours. From this cluster, we identified several genes associated with mitochondrial oxidative and metabolic functions. SLC25A15 (ORNT1) encodes the mitochondrial ornithine transporter involved in urea cycle function and is involved in progression of hyperornithinemia, hyperammonemia, and homocitrullinuria.27 The CYBB gene encodes the gp91(phox) protein. The product of this gene is the large subunit of flavocytochrome b558, gp91phox, which forms the catalytic core of the antimicrobial superoxide-generating enzyme, NADPH oxidase.28 CYP3A4 (cytochrome P-450 3A) is a drug-metabolizing enzyme involved in the inactivation of anti-cancer drugs.29 CA VA (carbonic anhydrase VA) is localized in the mitochondria and may play an important role in ureagenesis and gluconeogenesis.30 These data suggest that C5a during the first 2 hours represses induction of metabolic enzymes that would induce metabolic imbalances. Collectively, our data from Figure 1 suggests that HUVECs have a large number of early universal response genes, which are activated or repressed. Also, C5a repressed genes that are primarily related to metabolic responses.
Comparative Gene Activation Responses to C5a, TNF-α, and LPS
A limited comparative display of gene expression ratios is shown in Table 1 featuring HUVEC responses to C5a, TNF-α, and LPS after 4 hours of stimulation. Three clusters describing the functional groups of genes are represented. The uppermost part of the table contains cytokine- and chemokine-related proinflammatory genes; the middle cluster is cell adhesion-related genes, and the lowest cluster represents complement-related genes. We chose to compare endothelial cytokine/chemokines, cell adhesion, and complement gene expression affected by C5a, TNF-α, and LPS stimulation. All functional groups were sorted in descending order by the C5a gene expression column (in bold). Especially robustly expressed were TNF-α genes, VEGCF, EGR1, GRO1, and NF-kB1, C5F1, and SC4A2. Although TNF-α seemed to be the most potent stimulus, in several cases C5a was as potent as LPS. This clustering indicates the up-regulation of cytokines and chemokines and their receptors (32 genes) in stimulated HUVECs. Also found were anti-apoptotic genes (A20 TNFA1P3 and TNFA1P2) that are linked to Fas L and TNF-α stimulation. Additionally, several cytokine genes were induced, such as SCYA2, SCYA7, SCYA4, and SCYA13. This family of genes is important for expressing chemotactic activities for myeloid cells during inflammation.
Of the vascular adhesion genes, E-selectin was the most robustly up-regulated by each stimulus after 4 hours of stimulation (Table 2, middle section). C5a induced a 24-fold increase, TNF-α a 74-fold increase, and LPS a 54-fold increase. In the case of VCAM-1 and ICAM-1, TNF-α was the most effective stimulus, followed by C5a, then LPS. As is indicated in Figure 1 and Table 1, it is clear that C5a is a potent agonist for gene activation in HUVECs.
Table 2.
C5a-Induced Expression
| Time course for gene activation in C5a-stimulated HUVECs | C5a
|
|||
|---|---|---|---|---|
| 30 minutes | 2 hours | 4 hours | ||
| Adhesion genes | ||||
| VCAM1 | Vascular cell adhesion molecule 1 | 0.588 | 2.539 | 8.446 |
| ICAM1 | Intercellular adhesion molecule 1 (CD54) | 0.916 | 3.588 | 8.442 |
| SELE | Selectin E (endothelial adhesion molecule 1) | 0.987 | 2.333 | 23.598 |
| MBL2 | Mannose-binding lectin (protein C) 2 | 0.978 | 1.642 | 2.72 |
| Transcription genes | ||||
| NFIX | Nuclear factor I/X (CCAAT-binding transcription factor) | 3.296 | nv | 1.606 |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells | 1.035 | 4.096 | 5.265 |
| ATF3 | Activating transcription factor 3 | 1.197 | 2.241 | 5.695 |
| HIVEP1 | Human immunodeficiency virus type I enhancer-binding protein 1 | 1.005 | 2.722 | 2.69 |
| NFE2L2 | Nuclear factor (erythroid-derived 2)-like 2 | 1.263 | 3.048 | 1.944 |
| PRKRIR | Protein-kinase, (P58 repressor) | 0.828 | 1.425 | 2.907 |
| ZNF162 | Zinc finger protein 162 | 1.311 | 1.134 | 3.666 |
| NFKBIA | Nuclear factor of kappa light polypeptide gene inhibitor, alpha | 1.001 | 1.454 | 3.711 |
| ZFP36 | Zinc finger protein homologous to Zfp-36 in mouse | 1.07 | 1.504 | 2.77 |
| BCL6B | B-cell CLL/lymphoma 6, member B | 1.251 | 1.799 | 3.066 |
| BCL6 | B-cell CLL/lymphoma 6 (zinc finger protein 51) | 1.147 | 1.355 | 4.633 |
In the lowest cluster in Table 1, we evaluated genes that are complement-related (eg, C1q, C1s, C2, C3, C5, C6, C8, factor D, factor I, properdin, C4-binding protein, decay accelerating factor, C3aR, and so forth), some 22 in all. Of all of these genes, mannose-binding lectin-2 (MBL2) was significantly up-regulated (2.7- to 6.1-fold increase) by C5a, TNF-α, or LPS (Table 1). Why HUVECs lacked elevated gene expression levels for complement-related genes under these conditions used is unclear. It is possible that activation of complement-related genes would secondarily occur but would be precluded by the current experimental design that uses pretreatment of HUVECs with CHX.
Functional Clustering
Next, we investigated gene expression patterns developing in C5a-stimulated HUVECs as a function of time. We focused on transcriptional and cellular adhesion genes (described in Figure 1) that had gene expression ratios ≥2.60 at 30 minutes, 2 hours, and 4 hours. A 2.60-fold change was arbitrarily chosen as the cutoff. This analysis produced gene clusters of activation for each functional group, being described as early (30 minutes), mid (2 hour), or late (4 hour) gene induction (Table 2). The vascular adhesion molecule genes that were greater than 2.6-fold included E-selectin, ICAM-1, VCAM-1, and MBL2 (mannose-binding lectin). E-selectin, ICAM-1, and VCAM-1 showed a concerted increase in gene expression 2 and 4 hours after C5a stimulation, whereas MBL2 gene expression was >2.60 only at 4 hours. Vascular adhesion molecules were most profoundly up-regulated at 4 hours. NF-κB1 was one of the transcriptional genes induced maximally at 4 hours by C5a (Table 2). Other transcription-associated genes displayed various patterns of induction with respect to time. NFIX (nuclear factor I/X) was transiently induced very early (at 30 minutes). NFI (nuclear factor I) represents a family of transcription factors that have been shown to repress von Willebrand factor promoter activity.31 There was robust expression of several transcriptional factors 2 to 4 hours after stimulation with C5a, such as ATF3 (activating transcription factor 3), which is a known stress response gene. Overexpression of ATF3 suppresses TNF-α-induced cell death of HUVECs.32 EGR1 is another transcriptional regulator that recognizes and binds to the DNA sequence 5′-CGCCCCCGC-3′ (EGR-site). It can activate the transcription of target genes whose products are required for mitogenesis and differentiation.33 EGR-1 functions as an angiogenic regulator and promotes proliferation.34 PRKRIR is a cellular inhibitor of PKR. It blocks the PKR-inhibitory function of p58ipk, resulting in restoration of kinase activity and suppression of cell growth.35 Additionally, there was induction of Bcl6, which may act as a sequence-specific repressor of transcription and has been shown to modulate the transcription of STAT-dependent IL-4 responses of B cells.36 The data suggest that adhesion and transcriptional related genes act in a temporal manner to modify inflammatory responses of HUVECs after contact with C5a.
Transient Gene Activation
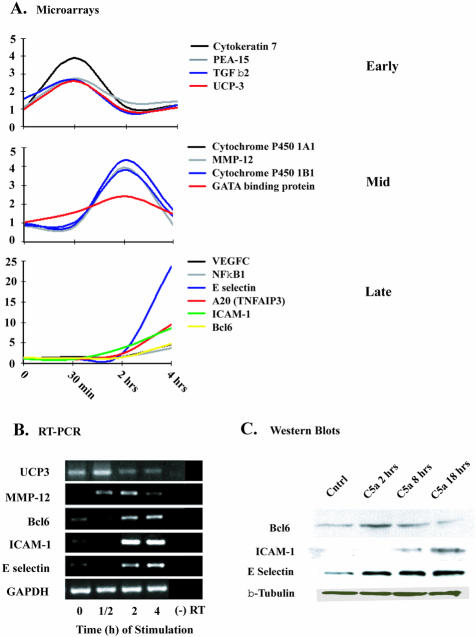
The temporal behavior displayed in Table 2 was the basis for investigating transient or progressive gene expression at 30 minutes, 2 hours, and 4 hours after C5a stimulation. To visualize transient patterns, we used Microsoft Access software (Microsoft) to query genes from Figure 1 that increased transiently (ie, gene expression ratios ≥2.6) at each time point. By using this selection method, the gene numbers of interest decreased from a total of 1244 genes to 19, 16, and 139 for the 30minute, 2-hour, and 4-hour time points, respectively. Figure 2A contains selected genes that were induced early (by 30 minutes), at the midpoint (2 hours), and late (4 hours). The gene patterns revealed broad functional classifications that varied between time points. Genes such as UCP-3 and PEA-15 peaked transiently at 30 minutes. UCP-3 belongs to the coupling protein family. The specific physiological role of the protein is unknown, but UCP-3 is known to be involved in energy balance and defense against generation of reactive oxygen species.37 PEA-15 is a small cytosolic protein that contains a death effector domain and may block the ability of Ras to suppress integrin activation without inhibiting ERK.38 Additionally, PEA-15 protects against TNF-α-induced apoptosis in astrocytes.39 Collectively, this cluster of genes seems to function in a cell-protective manner.
Figure 2.
Semiquantitative gene and protein expression based on microarray, RT-PCR, and Western blotting techniques. A: Lines represent groups of clustered genes induced after 30 minutes (early), 2 hours (mid), or 4 hours (late) stimulation with C5a (50 ng/ml). B: RT-PCR confirmation of selected gene expression in response to C5a (50 ng/ml) stimulation is shown. Data are representative of n = 3. C: Western blots for protein expression of selected genes are displayed. Cell lysates were collected after 2, 8, 18 hours after stimulation with C5a. Control lysates were from cells without C5a stimulation. Loading conditions were confirmed by β-tubulin content.
In terms of gene activation peaking at 2 hours, MMP-12 and cytochrome P4501A1 (CYP1A1) were prominent examples. MMP-12 has been implicated in numerous biological functions including degradation of elastin, fibrinogen, and laminin, most of these functions being associated with macrophages.40 CYP1A1 is a cytochrome P-450 isozyme that has been linked to metabolism of xenobiotics, creating cytotoxic and/or mutagenic products.41 Many of the late induced genes are known to be NF-κB-dependent, such as E-selectin, ICAM-1, VCAM-1, A20, and NF-κBIA (described above). Products of late-induced genes (4 hours and beyond) demonstrated many interactions that are known to be involved in the inflammatory response. The progressive induction of NF-κB1 may suggest that NF-κB signaling is not only used to activate its own gene transcript but also other common NF-κB-associated genes. The especially high expression of E-selectin suggests that this gene maybe a primary target for C5a in the endothelial cell. Interestingly, our microarray data did not display a change in gene expression for P-selectin (data not shown). P-selectin has been shown to be expressed very rapidly (15 minutes) in response to C5a on endothelial cells,42 inferring that the increased expression is because of storage granule translocation rather than to a transcriptional product.
To extend the microarray gene expression data, we chose to validate the profiles of selected genes described in Figure 2A. The data shown in Figure 2B represent semiquantitative RT-PCR profiles of HUVECs after C5a stimulation at the three time points. UCP-3 (uncoupling protein-3) showed a relatively high basal expression in the control group, which increased after 30 minutes of exposure to C5a, then greatly diminished. This confirms the microarray data. MMP-12 (metalloelastase) showed a moderate increase in expression at 0.5 and 2 hours, with a sharp decline by 4 hours. The genes, Bcl6, ICAM-1, and E-selectin, showed increases in expression at 2 hours, continuing through the 4-hour time point. These data confirm the patterns found in the microarray analyses.
Protein synthesis of ICAM-1, Bcl6, and E-selectin was evaluated using Western blotting (Figure 2C). We chose the transcriptional regulator (Bcl6) that has not previously linked to C5a or to endothelial transcriptional control mechanism(s) and also focused on adhesion proteins (ICAM-1 and E-selectin), which play prominent roles in endothelial-cell-neutrophil adhesion. Western blot analysis showed that HUVECs expressed a low basal level of Bcl6, with strong expression at 2 hours and then gradually diminishing thereafter. ICAM-1 and E-selectin showed a progressive induction of protein in response to C5a, with sustained expression at 8 and 18 hours. Interestingly, the Western blot analysis demonstrated a significant lag time between gene activation and elevated protein levels for ICAM-1, suggesting that additional secondary protein products might be required. Because it is known that CHX can stabilize mRNA for many transcripts, the delayed expression of ICAM-1 protein may be because of CHX-induced stabilization of ICAM-1 mRNA.
Discussion
Elevated levels of proinflammatory mediators can create chaotic systemic reactions (as found in sepsis) and may lead to multiorgan failure and/or death.43 During an inflammatory response, complement activation products such as C3a and C5a may contribute to progression of the inflammatory response by specifically ligating to receptors found on surrounding tissues (including the endothelium), as well as to receptors on blood leukocytes. C3a and C5a induce vasodilation, increase the permeability of small blood vessels, and contract smooth muscle.44 Ligation of C5aR on endothelial cells can cause a sustained pertussis toxin-sensitive cytoskeletal response, resulting in cell retraction, increased paracellular permeability, and eosinophil transmigration.44,45 With this in mind, we investigated gene responses of HUVECs to stimulation by C5a and other inflammatory mediators (TNF-α and LPS), anticipating that such information may provide critical clues to the mechanism(s) involved in physiological responses to these mediators.
HUVECs were stimulated with 50 ng/ml of C5a, based on an earlier study demonstrating that this concentration provided optimal transcription that could not be because of any contaminating LPS.45 We monitored gene expression using microarray technology. To do this, we compared a C5a-induced gene expression time course (0, 30 minutes, 2 hours, 4 hours) to the 4-hour gene expression profiles induced by TNF-α and LPS, which are well known activators of endothelial cells. Grouping the data in this manner enabled the visualization of large early response gene clusters (Figure 1; A to C). Because HUVECs were pretreated with subapoptotic doses of CHX, each cluster consisted of hundreds of induced or repressed primary response genes independent of protein synthesis for activation. At 4 hours all three stimuli induced in HUVECs many proinflammatory and anti-inflammatory genes linked to a variety of known and unknown functions (Figure 1). These included genes that encode signal transduction proteins (eg, CNK, NFκB1), secreted proteins (eg, VEGFC, IL-6), and integral membrane proteins (eg, ICAM-1, VCAM-1, E-selectin, and IL-18R1). It was somewhat surprising that for certain genes C5a was nearly as active as an agonist as were TNF-α and LPS. A closer examination of the induced genes within this cluster suggested multiple positive feedback loops. For example, C5a, LPS, and TNF-α induced VEGFC along with ICAM-1 and VCAM-1. It has been shown that the stimulation of HUVECs by VEGF for 18 hours up-regulates ICAM-1 and VCAM-1 expression and significantly increases PMN adhesion in response to C5a.46 Another example of a positive feedback loop involves two IL family members, IL-6 and IL-18R1. IL-6 acts as a potent stimulus for increased C5aR expression on myeloid and nonmyeloid tissues.47 Thus, transcription leading to translation and secretion of IL-6 may form a positive feedback loop that increases C5aR expression on endothelial cells. The IL-18R system and its signal transduction pathways are analogous to those of the IL-1R. Basal levels of IL-18R on endothelial cells are reported to be increased in atherosclerotic conditions,48 which involve local production of cytokines such as TNF-α or interferon-γ. Among other functions, macrophages generate IL-18 in response to interferon-γ. The ability of C5a to induce the IL-18R gene may suggest that complement activation acts as a link between macrophages and endothelial cells during atherosclerosis.
Agonists such as TNF-α and LPS have been shown to activate proapoptotic and anti-apoptotic genes in endothelial cells. We observed similar gene activations in our experimental conditions for not only TNF-α and LPS, but also C5a. Figure 1, B1, highlights several anti-apoptotic mediators that increased expression, including NFκBIA, A20, and Bcl6. NFκBIA (IκBα) has been proposed to conceal the nuclear localization signal of Rel proteins, thereby preventing nuclear binding of NF-κB. The A20 gene encodes a protein that prevents cell death of microvascular endothelial cells when exposed to the combination of LPS and CHX. This process requires soluble CD14.6 The protein (Bcl6) can interact with a variety of POZ-containing proteins that function as transcription co-repressors. This suggests that C5a, like LPS and TNF-α, induces factors that block apoptosis, possibly to counter caspase and other apoptotic-promoting activity.19
In addition to the large early response cluster, there were a number of repressed genes (Figure 1, B2). This cluster contained genes that were universally repressed by C5a, LPS, and TNF-α. We observed that SIAT1 was highly down-regulated by each stimulus. It has been reported that the B-cell-restricted molecule, CD22, has a domain that specifically recognizes α2,6-linked sialic acid residues.26 Thus, down-regulation of SIAT1 may decrease the likelihood of B-cell adherence during the early stages of inflammation.
Next, we identified genes that encode proteins implicated in cytoskeletal or intercellular functions. CDH 11 is involved in the formation of cell-to-cell contacts. It is highly expressed in nervous tissue, bone, endometrium, placenta, and other mesenchymal tissues during development.49 Although its role in inflammation is speculative, the loss of cadherin 11 in endothelial cells may lead to increased vascular permeability. DAG1 was another down-regulated gene that forms part of the dystrophin-associated protein complex, which links the cytoskeleton to the extracellular matrix. α-Dystroglycans may function as laminin receptors. It was suggested that in normal endothelial cells decreased DAG1 expression might aid in the angiogenic process.22 Ephrin B2 was another down-regulated gene that has angiogenic implications. This ligand, which is itself a transmembrane protein, is known to be involved in defining boundaries between arteries and vein. There is conflicting evidence as to the exact role ephrin B2 plays in angiogenesis. Yet, these receptor-ligand interactions have been shown to suppress VEGF- and angiopoietin-1-induced Ras/mitogen-activated protein kinase pathways.24 Thus, the ability of C5a to induce a down-regulation of ephrin B2 may promote angiogenic effects of VEGF.
In contrast to progressively induced genes that are a part of the large gene clusters (Figure 1, A and B), we found a number of genes that displayed transient expression. We examined a cluster of genes that were transiently down-regulated in response to C5a (Figure 1C). Genes were selected that were known to be associated with cellular metabolism. SLC25A15 encodes an ornithine transporter that is critical for the urea cycle. CYBB encodes gp91phox protein that translocates to the membrane for the production of ROS under resting and stimulated conditions.28 CYP3A4 was also transiently repressed. This gene is a member of drug metabolizing enzymes that are down-regulated in the liver by cytokines. Jover and colleagues29 suggested that IL-6 activation of gp130 receptor subunit is required for IL-6-induced down-regulation of CYP3A4. Therefore, the ability of C5a to transiently repress CYP3A4 fits with the widespread ability of inflammatory mediators to repress this family of drug-metabolizing enzymes.
To visualize the temporal patterns of gene activation, we selected genes from Figure 1 that encode adhesion molecules and transcriptional factors (Table 2). Adhesion proteins are highly expressed on endothelial cells during inflammatory responses; comparing the temporal expression of these genes with transcriptional factors may provide information about the signal transduction pathways resulting from C5a receptor ligation. We grouped the genes in order of activation and labeled each group as early (30 minutes), mid (2 hours), or late (4 hours). We found that gene activation occurred in stages. Genes that encode adherence proteins such as ICAM-1, VCAM-1, and E-selectin showed increased expression at 2 hours (mid) and remained elevated through 4 hours (late) of exposure to C5a. These represent the main adherence proteins responsible for the extravasation of leukocytes, monocytes, and other immune cells from the peripheral circulation. Thus, early transcriptional commitments suggest an importance in endothelial response mechanisms. MBL2, a complement-related gene, was induced at 4 hours (Table 1). The genes that encode transcription factors also demonstrated a temporal activation scheme. Several of the transcriptional factor genes that became activated after 2 hours of stimulation with C5a (EGR1 and ATF3) are known to be immediate early response genes that are associated with many inflammatory conditions.32,33 Much like the adhesion genes, these genes also remain induced through 4 hours. There was another group of genes that became induced after 4 hours and represented late genes. This group seemed to be dominated by anti-apoptotic genes such as NFκBIA, Bcl6, and PRKRIR.
In Figure 1, transient patterns of gene activation resulting from C5a stimulation were evaluated. Gene responses were classified as early, mid, or late expression groups, similar to Table 1. The selected genes (Figure 2A) were representatives from each group. The complete list can be found in supplemental Figure 2. Figure 2A demonstrates the complexity of gene activation responses to C5a. The activation patterns, especially at 30 minutes and 2 hours, fell into multiple functional categories, many with no previously known link to effects of complement activation products. When exposure to C5a increased to 4 hours, inflammation and stress response-related genes showed intensified expression. Figure 2B shows RT-PCR confirmation of the transiently induced genes. The gene expression levels for Bcl6, ICAM-1, and E-selectin increased abruptly at 2 hours and corresponded to the microarray data shown in Table 2. We wanted to investigate the expression of Bcl6, ICAM-1, and E-selection proteins so that we could compare the temporal patterns of gene expression with those of protein expression (Figure 2C). Bcl6 showed a transient increase at 8 hours and then returned to basal levels. C5a caused an ICAM-1 protein increase at 18 hours, whereas E-selectin protein expression increased progressively from 2 to 18 hours. This confirms the findings of other studies that show protein levels of E selectin increasing earlier than those for ICAM-1. Although more experiments need to be done to link Bcl6 expression with expression of other proteins, these data suggest that Bcl6 is transiently increased but has no effect on the ultimate reduction in expression of ICAM-1 or E-selectin.
Identifying the chronological role in gene expression patterns for gene expression may provide important clues as to the functions these genes play in pathophysiological roles. In response to a stimulus such as C5a, endothelial cells have a predictable molecular response, which can be used to identify the patterns of gene activation. By dissecting gene activation in this manner, potential rate-limiting gene candidates may be identified, which might lead to clinical interventions for inflammatory disorders.
Footnotes
Address reprint requests to Peter A. Ward, M.D., Department of Pathology, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor, MI 48109-0602. E-mail: pward@umich.edu.
Supported by the National Institutes of Health (GM-02950 and HL-31963).
References
- Madge LA, Poder JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70:317–325. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- Heumann D, Glauser MP, Calandra T. Molecular basis of host-pathogen interaction in septic shock. Curr Opin Microbiol. 1998;1:49–55. doi: 10.1016/s1369-5274(98)80142-2. [DOI] [PubMed] [Google Scholar]
- Nooteboom A, Van Der Linden CJ, Hendriks T. Tumor necrosis factor-alpha and interleukin-1beta mediate endothelial permeability induced by lipopolysaccharide-stimulated whole blood. Crit Care Med. 2002;30:2063–2068. doi: 10.1097/00003246-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Cordon-Cardo, Bayoumy CS, Garzotto M, McLoughlin, Gallily MR, Edwards CK, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 2001;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman TH, Harlan JM. Human endothelial cell response to lipopolysaccharide, interleukin-1, and tumor necrosis factor is regulated by protein synthesis. Cell Immunol. 1989;119:41–52. doi: 10.1016/0008-8749(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Hu X, Yee E, Harlan JM, Wong F, Karsan A. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood. 1998;92:2759–2765. [PubMed] [Google Scholar]
- Choi KB, Wong F, Harlan JM, Chaudhary PM, Hood L, Karsan A. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem. 1998;273:20185–20188. doi: 10.1074/jbc.273.32.20185. [DOI] [PubMed] [Google Scholar]
- Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J, Fitting C, Haeffner-Cavaillon N. Recombinant C5a enhances interleukin-1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur J Immunol. 1990;20:253–257. doi: 10.1002/eji.1830200204. [DOI] [PubMed] [Google Scholar]
- Denk A, Matthais G, Schimid S, Berberich I, Ritz O, Lindemann D, Ludwig S, Wirth T. Activation of NF-κB via the IκB kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276:28451–28458. doi: 10.1074/jbc.M102698200. [DOI] [PubMed] [Google Scholar]
- Farkas I, Baranyi L, Liposits Z, Yamamoto T, Okada H. Complement C5a anaphylatoxin fragment causes apoptosis in TGW neuroblastoma cells. Neuroscience. 1998;86:903–911. doi: 10.1016/s0306-4522(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma VJ, Markiewski MM, Mastellos D, Strey CW, Pierson CL, Lambris JD, Zetoune FS, Ward PA. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110:101–108. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnum SR. Complement in central nervous system inflammation. Immunol Res. 2002;26:7–13. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- Nataf S, Stahel PF, Davoust N, Barnum SR. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 1999;22:397–402. doi: 10.1016/s0166-2236(98)01390-3. [DOI] [PubMed] [Google Scholar]
- Barnum S, Ames R, Maycox P, Hadingham S, Meakin J, Harrison D, Parsons A. Expression of the complement C3a and C5a receptors after permanent focal ischemia. An alternative interpretation. Gila. 2002;38:169–173. doi: 10.1002/glia.10069. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- Toney LM, Cattoretti G, Graf JA, Merghoub T, Pandolfi PP, Dalla-Favera R, Ye BH, Dent AL. BCL-6 regulates chemokine gene transcription in macrophages. Nat Immunol. 2000;3:189–190. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- Dent AL, Vasanwala FH, Toney LM. Regulation of gene expression by the proto-oncogene BCL6. Crit Rev Oncol Hematol. 2002;41:1–9. doi: 10.1016/s1040-8428(01)00164-0. [DOI] [PubMed] [Google Scholar]
- Tam WF, Sen R. IκB family members function by different mechanisms. J Biol Chem. 2001;276:7701–7704. doi: 10.1074/jbc.C000916200. [DOI] [PubMed] [Google Scholar]
- He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa H, Ninomiya H, Kitamura Y, Fujiwara K, Masaki T. Vascular endothelial cells that express dystorglycan are involved in angiogenesis. J Cell Sci. 2001;115:1487–1496. doi: 10.1242/jcs.115.7.1487. [DOI] [PubMed] [Google Scholar]
- Meier T, Ruegg M. The role of dystroglycan and its ligands in physiology and disease. News Physiol Sci. 2000;15:255–259. doi: 10.1152/physiologyonline.2000.15.5.255. [DOI] [PubMed] [Google Scholar]
- Kim I, Ryu Y, Kwak H, Ahn S, Oh J, Yancopoulos G, Gale N, Koh G. EphB ligand, ephrinB2, suppresses the VEGF and angiopoietin-1 induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. EMBO J. 2002;16:1126–1130. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- Oike Y, Ito Y, Hamada K, Zhang XQ, Miyata K, Arai F, Inada T, Araki K, Nakagata N, Takeya M, Kisanuki YY, Yanagisawa M, Gale NW, Suda T. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;100:1326–1333. [PubMed] [Google Scholar]
- Lo N, Lau J. Transcription of the β-galactoside α 2,6-sialytransferase gene (SIAT1) in B-lymphocytes: cell type-specific expression correlates with presence of the divergent 5′-untranslated sequence. Glycobiology. 1999;9:907–914. doi: 10.1093/glycob/9.9.907. [DOI] [PubMed] [Google Scholar]
- Inoue I, Saheki T, Kayanuma K, Uono M, Nakajima M, Takeshita K, Koike R, Yuasa T, Miyatake T, Sakoda K. Biochemical analysis of decreased ornithine transport activity in the liver mitochondria from patients with hyperornithinemia, hyperammonemia and homocitrullinuria. Biochim Biophys Acta. 1988;964:90–95. doi: 10.1016/0304-4165(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Murakami H, Kita K, Anraku Y. Cloning of cybB, the gene for cytochrome b561 of Escherichia coli K12. Mol Gen Genet. 1984;198:1–6. doi: 10.1007/BF00328692. [DOI] [PubMed] [Google Scholar]
- Jover R, Bort R, Gomez-Lechon J, Castell J. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. EMBO J. 2002;16:1799–1801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- Henry RP. Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol. 1996;58:523–538. doi: 10.1146/annurev.ph.58.030196.002515. [DOI] [PubMed] [Google Scholar]
- Jahroudi N, Ardekani AM, Greenberger JS. An NF1-like protein functions as a repressor of the von Willebrand factor promote. J Biol Chem. 1996;3271:21413–21421. doi: 10.1074/jbc.271.35.21413. [DOI] [PubMed] [Google Scholar]
- Kawauchi J, Zhang C, Nobori K, Hashimoto Y, Adachi MT, Noda A, Sunamori M, Kitajima S. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J Biol Chem. 2002;277:39025–39034. doi: 10.1074/jbc.M202974200. [DOI] [PubMed] [Google Scholar]
- Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- Gale M, Jr, Blakely CM, Hopkins DA, Melville MW, Wambach M, Romano PR, Katze MG. Regulation of interferon-induced protein kinase PKR: modulation of P58IPK inhibitory function by a novel protein, P52rIPK. Mol Cell Biol. 1998;18:859–871. doi: 10.1128/mcb.18.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartatik T, Okada S, Okabe S, Arima M, Hatano M, Tokuhisa T. Binding of BAZF and Bcl6 to STAT6-binding DNA sequences. Biochem Biophys Res Commun. 2001;284:26–32. doi: 10.1006/bbrc.2001.4931. [DOI] [PubMed] [Google Scholar]
- Jezek P, Garlid K. Mammalian mitochondrial uncoupling proteins. Int J Biochem Cell Biol. 1998;30:1163–1168. doi: 10.1016/s1357-2725(98)00076-4. [DOI] [PubMed] [Google Scholar]
- Formstecher E, Ramos J, Fauquet M, Calderwood D, Hsieh J, Canton B, Nguyen X, Barnier J, Camonis J, Ginsberg M, Chneiweiss H. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNF alpha-induced apoptosis. J Neurosci. 1999;19:8244–8251. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkela E, Bohling T, Herva R, Uria A, Saarialho-Kere U. Human Macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone. 2001;29:487–493. doi: 10.1016/s8756-3282(01)00595-6. [DOI] [PubMed] [Google Scholar]
- Delescluse C, Lemaire G, de Sousa G, Rahmani R. Is CYP1A1 induction always related to AHR signaling pathway? Toxicology. 2000;153:73–82. doi: 10.1016/s0300-483x(00)00305-x. [DOI] [PubMed] [Google Scholar]
- Bless NM, Tojo SJ, Kawarai H, Natsume Y, Lentsch AB, Padgaonkar VA, Czermak BJ, Schmal H, Friedl HP, Ward PA. Differing patterns of P-selectin expression in lung injury. Am J Pathol. 1998;153:1113–1122. doi: 10.1016/S0002-9440(10)65655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Lomas JL, Grutkoski PS, Chung CS. Pathological aspects of apoptosis in severe sepsis and shock? Int J Biochem Cell Biol. 2003;35:7–15. doi: 10.1016/s1357-2725(02)00099-7. [DOI] [PubMed] [Google Scholar]
- Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209–222. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- Schindler R, Gelfand J, Dinarello C. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide of IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- Zhang H, Issekutz AC. Growth factor regulation of neutrophil-endothelial cell interactions. J Leukoc Biol. 2001;70:225–232. [PubMed] [Google Scholar]
- Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Skalli O. Identification of cadherin-11 down-regulation as a common response of astrocytoma cells to transforming growth factor-α. Differentiation. 2000;66:165–172. doi: 10.1046/j.1432-0436.2000.660402.x. [DOI] [PubMed] [Google Scholar]