Abstract
IL-9 is a Th2 cytokine that exerts pleiotropic activities, and might be involved in the regulation of lung inflammatory processes. To characterize the activity of IL-9 on lung injury, we compared the pulmonary responses to bleomycin (blm) in IL-9 transgenic (Tg5) and wild-type (FVB) mice. Following intratracheal instillation of lethal doses of blm, the mortality rate was markedly reduced in Tg5 mice compared to their wild-type counterparts (ie, 25% mortality for Tg5 versus 85% for FVB mice, 21 days after instillation of 0.05U blm/mouse). Histological and biochemical analyses showed that blm induced less lung injury and less epithelial damage in Tg5 as compared to FVB animals. This protection of Tg5 mice was accompanied by an expansion of eosinophils and B cells in the lungs. In addition, TGF-β and prostaglandin-E2 (PGE2) levels in broncho-alveolar lavage fluid were also increased in transgenic mice. The contribution of B cells and eosinophils to the protective mechanism did not appear essential since eosinophil-deficient (IL-5 KO) and B-deficient (μMT) mice overexpressing IL-9 were also resistant to high doses of blm. We could rule out that TGF-β was a key factor in the protective effect of IL-9 by blocking this mediator with neutralizing antibodies. Indomethacin treatment, which inhibited PGE2 production in both strains, suppressed the protection in Tg5 mice, supporting the idea that IL-9 controls blm-induced lung injury through a prostaglandin-dependent mechanism.
Administration of bleomycin (blm) in rodents induces severe pneumonitis that can be fatal and serve as an experimental model to study the pathogenesis of lung injury and repair processes.1,2 While the precise mechanisms involved in the development of the disease remain incompletely understood, it is well admitted that acute cellular infiltrates including macrophages, granulocytes, and lymphocytes, as well as the sustained production of pro-inflammatory cytokines are the key events involved in the initiation and extension of blm-induced lung injury.3,4 For instance, TNF-α, IL-1β, and IL-6 have been reported by several authors to be secreted in the lung in response to blm treatment5–7 and their neutralization lead to less inflammatory cell accumulation and less subsequent pulmonary lesions.5,7–9 In addition, the pro-inflammatory Th1 cytokine interferon γ (IFN-γ) could also play an important detrimental role since IFN-γ-deficient mice presented less parenchymal inflammation, less weight loss and reduced mortality after blm-treatment.10 In contrast, it has been suggested that anti-inflammatory Th2 cytokines such as IL-4 and IL-10 might possess protective functions against blm-induced acute lung injury and lethality.11,12
IL-9 is a Th2 cytokine that exerts pleiotropic activities on several immune and non-immune cells such as T and B lymphocytes, eosinophils, mastocytes, and epithelial cells.13 This cytokine is involved in the regulation of lung inflammatory processes, including asthma14 and silica-induced pulmonary fibrosis.15 In addition, experimental observations demonstrated that IL-9 may be important in down-modulating some adverse inflammatory reactions.16 Here, we have investigated the role of IL-9 during blm-induced lung injury and lethality using transgenic mice overexpressing IL-9. The current report provides evidence that IL-9 confers a protective effect by up-regulating the production of prostaglandin (PG) mediators such as PGE2.
Materials and Methods
Mice
The animals were kept in a conventional animal facility and housed in positive-pressure air-conditioned units (25°C, 50% relative humidity) on a 12-hour light/dark cycle. Female mice weighing between 20 and 30 g were used in all experiments.
Transgenic mice overexpressing IL-9 (Tg5) and their control counterparts (FVB) were bred in the animal facility of the Ludwig Institute.17 B-deficient (μMT) and IL-5-deficient (IL-5−/−) mice on a C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME) and Professor F. Brombacher (University of Cape Town, South Africa), respectively.18,19
Mice overexpressing IL-9 (homo- or heterozygous for the IL-9 transgenic character) but deficient in B lymphocytes were generated by crossing Tg5 and μMT strains. The F1 generation was back-crossed with μMT to obtain the following phenotypes: control mice competent (B+IL-9N) or deficient in B cells (B−IL-9N) and IL-9 transgenic mice competent (B+IL-9+) or deficient in B cells (B−IL-9+). Animals carrying the IL-9 transgene and deficient in IL-5 were obtained by crossing IL-5-deficient with IL-9 transgenic mice following the same protocol. All of the IL-9 transgenic F2 mice showed high IL-9 concentrations in serum (mean of 0.2 μg/ml).
Bleomycin Administration
Bleomycin (Aventis, Brussels) was resuspended in sterile saline solution (NaCl 0.9%) at 1U/ml. Then blm was diluted in saline solution and administered in a volume of 60 μl (0.05U or 0.5U) by intratracheal instillation15 after anesthesia (sodium pentobarbital, 2 mg/mouse, intraperitoneally).
Bronchoalveolar Lavage (BAL) and Serum
After instillation of blm or saline, BAL and alveolar cell harvesting were performed as described previously.15 The BAL fluid was centrifuged (300 × g for 10 minutes at 4°C) and the cell-free supernatant used for biochemical measurements. Cell pellets were resuspended in saline and used to determine total cell numbers and differentials. These were done on the cells pelleted onto glass slides by cytocentrifugation and subjected to Diff-Quik staining (Dade, Brussels, Belgium). Polymorphonuclear and mononuclear cells were then counted by light microscopy at ×200 magnification (200 cells counted). To obtain serum, blood was collected by venous heart punction, incubated 30 minutes at 37°C, centrifuged at 100 × g for 10 minutes at 4°C and decanted.
FACS Analysis
BALF red blood cells were lysed by incubation for 5 minutes in 0.15 mol/L NH4Cl. Fluorescent labeling of cells resuspended in Hanks’ medium with 3% decomplemented fetal calf serum (FCS) and 10 mmol/L NaN3 was performed with rat fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (clone 53–6:7; ATCC, Manassas, VA) and anti-IgM (clone LOMM9 provided by Bazin, Université catholique de Louvain, Brussels, Belgium). For CD4 labeling, we used a biotinylated anti-CD4 (clone GK1.5, ATCC), followed by phycoerythrin (PE)-conjugated streptavidin (Becton-Dickinson, San Jose, CA). After staining, cells were fixed in paraformaldehyde (1.25%), and fluorescence intensity was measured on 104 cells/sample on a FACS-Scan apparatus (Becton-Dickinson). The lymphocyte population was gated according to side and forward scatters.
Immunoassays
TNF-α and PGE2 concentrations were measured by ELISA kits obtained respectively from Biosource International (Camarillo, CA) and Amersham (Bucks, UK) following the manufacturer’s protocols. The detection limit of these assays was 3 pg/ml and 16 pg/ml, respectively. IL-6 levels were estimated by a sensitive proliferation assay using the IL-6-dependent murine hybridoma cell line 7TD1.20 CC16 concentrations were measured by an automated latex immunoassay using rabbit antibodies against purified rat CC16 as previously described.21 Measurements of cysteinyl leukotriene in BALF were performed by using an immunoassay from R&D Systems (Minneapolis, MN) following the manufacturer’s protocols. The detection limit of this assay was less than 26.6 pg/ml.
Biochemical Measurements
LDH (lactate dehydrogenase) activity in BALF was assayed spectrophotometrically by monitoring the reduction of NAD+ at 340 nm in the presence of lactate.
After BAL, a part of the lungs was removed and homogenized in 50 mmol/L HEPES (pH 8.0) for the EPO activity determination. After a spun at 10,000 × g for 30 minutes at 4°C, the supernatant was removed and the pellet was resuspended in 0.5% cetyltrimethylammonium in distilled water. After another homogenization and centrifugation (10,000 × g for 30 minutes at 4°C), the obtained supernatant was diluted twofold, and 75 μl of EPO substrate freshly prepared was added (50 mmol/L HEPES, pH 8.0, 6 mmol/L KBr, 3 mmol/L O-phenylenediamine, and 8.8 mmol/L H2O2). The reaction was allowed to proceed at room temperature and stopped after 1.5 minutes with 75 μl of 4 N sulfuric acid containing 2 mmol/L resorcinol. EPO activity was measured at 490 nm.22
TGF-β Neutralization
Pan TGF-β polyclonal neutralizing antibodies (AB-100-NA)and normal rabbit IgG (AB-105-C) were purchased from R&D Systems. Groups of 10 mice were injected intravenously with a total of 150 μg of anti-TGF-β or rabbit IgG as control at days 0 and 6 after bleomycin treatment. This dosing is known to cause efficient neutralization of TGF-β.23,24 Because of the cost of the antibodies, this treatment was only applied to transgenic animals treated with blm.
Indomethacin Treatment in Drinking Water
An indomethacin (Sigma, St. Louis, MO) stock solution was made by dissolving 150 mg in 50 ml of ethanol. Three times per week throughout the experimental protocol, 2 ml of the indomethacin stock was added to 200 ml of water (FVB and Tg5). The water of the corresponding control mice was treated with 2 ml of ethanol for 200 ml of water.25 Based on a daily water consumption of 5 ml, this protocol provides a daily dose of 7.5 mg/kg indomethacin. Indomethacin treatment began 3 days before instillation of blm.
Histology
Lungs from FVB and Tg5 mice were excised and immersed in a Bouin solution (Meck-Belgabo, Leuven, Belgium). Then 5-μm-thick lung sections were performed and stained with hematoxylin and eosin. For ultra-thin sections, the lungs were fixed in a solution of 2.5% glutaraldehyde (Taab Reading, England) in 0.1 mol/L cacodylate buffer (Taab, pH 7.4), post-fixed for 1 hour in 1% osmium tetroxide, dehydrated in alcohol solutions of increasing strength, and embedded in LX112 resin (Ladd Research Industries, Williston, VT, USA). Then 0.5-μm-thick sections were cut from lung fragments and stained with toluidine blue.
Statistics
Treatment-related differences were evaluated using t-tests and one-way analysis of variance followed by pair-wise comparisons using the Student-Newman-Keuls test, as appropriate. Survival curves (Kaplan-Meier plots) were compared using a log rank test (Graph Pad Software Inc., San Diego, CA). A P value <0.05 was considered statistically significant.
Results
IL-9-Transgenic Mice Are Less Susceptible to Bleomycin-Induced Lung Injury
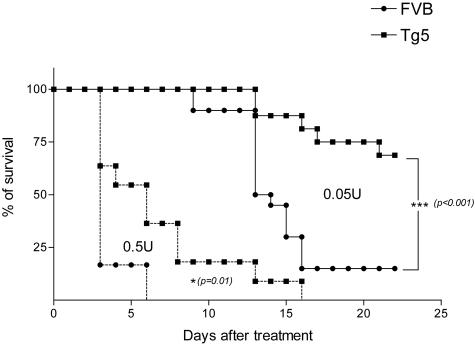
To characterize the activity of IL-9 in bleomycin-induced lung toxicity, we treated IL-9 transgenic (Tg5) and control (FVB) mice with 2 doses of blm (0.05 or 0.5U/mouse). Following instillation of the lowest dose, FVB mice began to die by day 10. Cumulative mortality in this group was 85% at day 21. Mortality rate in treated Tg5 mice was significantly lower (25% after 21 days) (Figure 1). After blm treatment (0.05/mouse), FVB mice lost more weight than Tg5 mice (day 6, mean ± SEM, n = 10 to 18, FVB saline = 24.2 ± 0.5 g; FVB blm = 20.5 ± 0.4 g and Tg5 saline = 24.9 ± 0.4 g; Tg5 blm = 24.1 ± 0.4 g). A protective effect was also observed in Tg5 animals at a higher dose (0.5U/mouse) (Figure 1). On the basis of these results, we focused our next analyses on the dose of 0.05U and on day 6, ie, before the occurrence of significant mortality.
Figure 1.
Survival rates of IL-9-transgenic (Tg5) and control (FVB) mice after intratracheal instillation of bleomycin (blm). Data represent composite deaths following administration of 0.05 U blm/mouse or 0.5 U blm/mouse in Tg5 and FVB mice (n = 10 to 20). ***, P < 0.001; *, P = 0.01 denote the level of significance of the differences between the mean values measured in blm-treated mice Tg5 and corresponding blm-treated FVB mice (log rank test). Similar results were obtained in three independent experiments for the dose of 0.05U/mouse.
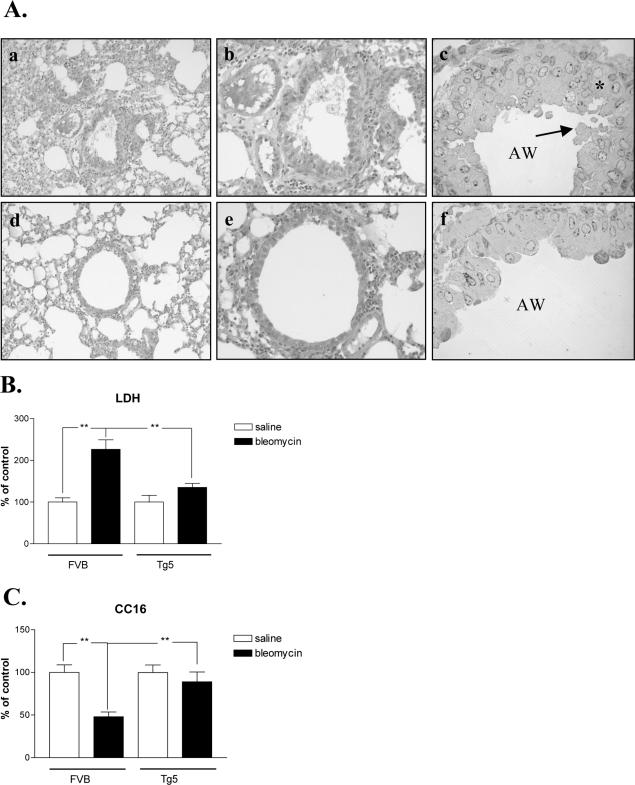
Histological studies revealed that the lung of blm-treated Tg5 mice showed markedly less damage and less cellular injury than that of FVB mice. Figure 2A shows that the lung (Figure 2A, panels a and b) and epithelial cells (Figure 2A, panel c) from blm-treated FVB mice presented classical or typical damages induced by bleomycin (interstitial pneumonitis, inflammatory cell infiltration, sloughy and disorganized epithelium, lack of cellular integrity, and marked presence of blebs) while in Tg5 animals (Figures 2A, panels d to f) lung damage was less severe and the integrity of epithelial cells was better conserved (no apparent bleb or cellular damage). These observations were supported by measurements of LDH activity in broncho-alveolar lavage fluid (BALF) which was significantly increased after blm treatment in FVB, but not Tg5 mice (Figure 2B). In addition, the reduction of CC16 (Clara cell protein) levels in BALF of FVB mice confirmed epithelial damage after blm treatment. In contrast, no significant change was noted in blm-treated Tg5 animals indicating that, in this strain, Clara cells were less affected (Figure 2C).
Figure 2.
A: Representative lung sections 6 days after bleomycin treatment (0.05U/mouse). a–b, top: Interstitial pneumonitis in blm-treated FVB mice (H&E staining, magnification ×100 and ×200, respectively). c: Disorganized epithelium (black star) as well as blebs (black arrow) were apparent in blm-treated FVB (blue toluidine staining, magnification ×1000; AW, airways). d–e, bottom: Lung damage was less severe in blm-treated IL-9 transgenic mice (Tg5 mice) (H&E staining, magnification ×100 and ×200, respectively). f: The integrity of epithelial cells was better conserved (no apparent bleb or cellular damage) (blue toluidine staining, magnification ×1000; AW, airways). B: LDH activity in BALF from blm-treated FVB and Tg5 mice. Bars represent means ± SEM of 4 to 6 animals. **, P < 0.01 (Student-Newman-Keuls multiple comparison test). Similar results were obtained two independent experiments. C: CC16 measurements were performed in BALF from blm-treated FVB and Tg5 mice. Bars represent means ± SEM of 4 to 6 animals. **, P < 0.01 (Student-Newman-Keuls multiple comparison test). Similar results were obtained in two independent experiments.
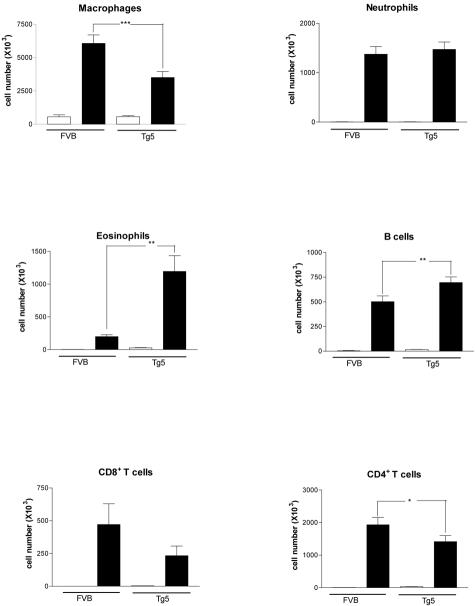
Cellular analyses performed on BAL revealed that the number of macrophages, CD4+ and CD8+ lymphocytes were less increased in blm-treated Tg5 mice in comparison with treated-FVB mice (Figure 3). However, BAL cells in Tg5 mice were characterized by a more marked recruitment of eosinophils and B lymphocytes compared to their wild-type counterparts (Figure 3). The pronounced presence of eosinophils in blm-treated Tg5 was confirmed by measuring EPO in lung tissue at day 6 (mean absorbance ± SEM, n = 5 to 6, FVB blm = 0.22 ± 0.02 OD; Tg5 blm = 1.2 ± 0.3 OD; P < 0.01).
Figure 3.
Cell types retrieved in BAL fluid of IL-9-transgenic mice (Tg5) and control mice (FVB) 6 days after blm (0.05U/mouse, filled bars) or saline (NaCl 0.9%, open bars) administration. Bar represent means ± SEM of 4 to 6 animals. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 denote the level of significance of the differences between mean values measured in blm-treated Tg5 mice and corresponding blm-treated FVB mice (Student-Newman-Keuls multiple comparison test).
Mediators of inflammation such as TNF-α and IL-6 were evaluated in serum. Levels of these pro-inflammatory cytokines were increased in blm-treated FVB mice in comparison to saline FVB animals. In contrast, no such difference was observed in blm-treated Tg5 mice respective to their saline situations (data not shown).
Eosinophils and B Lymphocytes Are Not Essential in the Resistance of Tg5 to Bleomycin
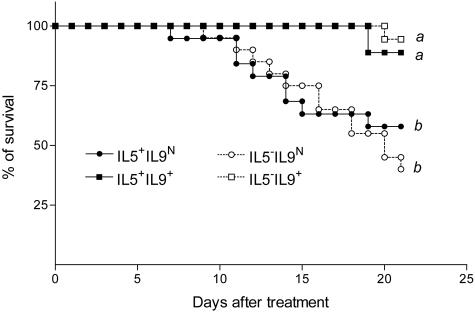
Because eosinophils were highly recruited in blm-treated Tg5 mice, we first examined their possible regulatory role in blm-treated IL-9-transgenic mice. IL-5 deficiency is associated with an incapacity to recruit eosinophils in tissue lesions. We therefore treated F2 IL-5-competent (IL-5+) or -deficient (IL-5−) IL-9-transgenic (IL-9+) or non-transgenic (IL-9N) mice with blm (0.05U/mouse) and estimated the mortality rate. As expected, 6 days after blm treatment, no eosinophils were detected in the BAL of IL-5-deficient mice overexpressing IL-9 or not. In contrast, corresponding IL-5-competent animals developed a blm-induced eosinophilia (data not shown). This observation was confirmed by EPO measurements in lung tissues after blm exposure (mean absorbance ± SEM, n = 3, IL-5−IL-9N 0.09 ± 0.001 a; IL-5−IL-9+ 0.09 ± 0.007 a; IL-5+IL-9N 0.13 ± 0.04 a and IL-5+IL-9+ 0.39 ± 0.05 b; different letters indicate statistically significant differences between groups (P < 0.01)). Again, IL-9-transgenic mice (IL-5+IL-9+ mice) were significantly protected against the toxic effects of blm when compared to control mice (IL-5+IL-9N) (Figure 4). This effect was still noted in the absence of eosinophilia (IL-5−IL-9+), indicating that eosinophil accumulation is probably not implicated in the protective mechanism of IL-9.
Figure 4.
Survival rates of IL-9-transgenic and control mice proficient or deficient in IL-5 after intratracheal instillation of bleomycin (blm). Data represent composite deaths following administration of 0.05 U blm/mouse (n = 15 to 20). Different letters indicate statistically significant differences between groups (P < 0.05) as analyzed by log rank test.
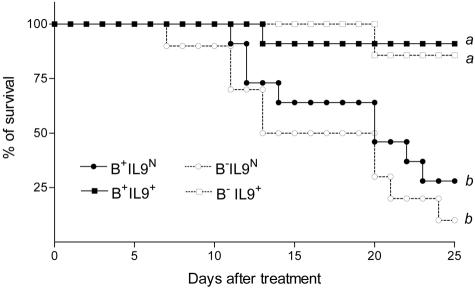
A similar protocol was performed to examine the participation of B lymphocytes. By studying the pulmonary responses to blm of F2 B-competent (B+) or -deficient (B−) mice overexpressing IL-9 or not, we found that the protective role of IL-9 was apparently not mediated by B cells. Indeed, IL-9-transgenic mice B-competent (B+IL-9+) or -deficient (B−IL-9+) were equally resistant to blm in comparison to control mice (B+IL-9N and B−IL-9N) (Figure 5).
Figure 5.
Survival rates of IL-9-transgenic mice and control mice proficient or deficient in B cells after intratracheal instillation of bleomycin (blm). Data represent composite deaths following administration of 0.05 U blm/mouse (n = 7 to 10). Different letters indicate statistically significant differences between groups (P < 0.05) as analyzed by log rank test.
TGF-β Is Not an Essential Mediator in the Protection against the Lethal Effect of Bleomycin
Overexpression of IL-9 is accompanied by an up-regulation of TGF-β. Indeed, levels of TGF-β in BALF were increased in saline Tg5 mice compared to saline FVB animals (mean ± SEM, n = 5 to 6, FVB = 11.1 ± 3.2pg/ml versus Tg5 = 23.3 ± 4.0pg/ml; P < 0.05). These biochemical data were mirrored by immunohistochemical analyses demonstrating more positive cells for TGF-β in the lung of Tg5 mice as compared to FVB animals (data not shown).
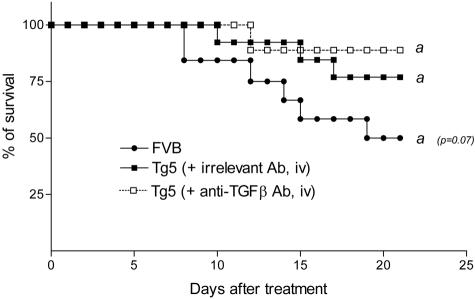
To test the importance of TGF-β in the modulation by IL-9 of the lung response to blm, IL-9-transgenic mice were treated with TGF-β neutralizing antibodies. No difference in terms of mortality rate was noted between Tg5 mice receiving blocking antibodies or Tg5 mice receiving irrelevant antibodies (Figure 6), indicating that TGF-β is not a key mediator in the protection conferred by IL-9.
Figure 6.
Effect of TGF-β neutralization (anti-TGFβ antibody, Ab, intravenously, iv) in IL-9-transgenic (Tg5) mice intratracheally instilled with bleomycin (blm). Data represent composite deaths following administration of 0.05 U blm/mouse (n = 10 to 13). Similar results were obtained when administrating neutralizing antibodies intraperitoneally (i.p) data not shown.
Prostaglandins Are Essential in the Limitation of blm-Induced Acute Toxicity in Transgenic Mice
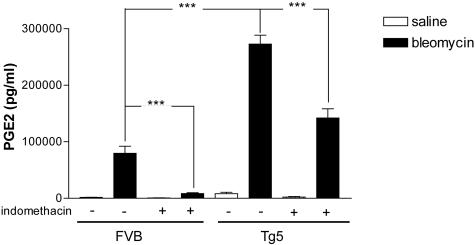
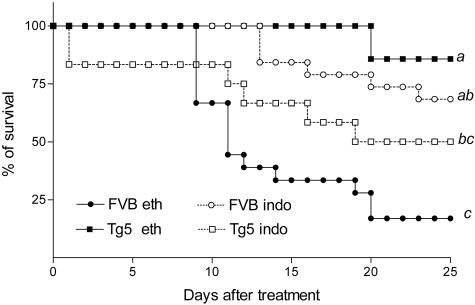
Prostaglandins (PG) and in particular PGE2 are involved in the pulmonary injury induced by blm.26 To explore a potential role for PG in the protection against blm-induced lung toxicity, we measured the levels of PGE2 in BALF of the different groups studied. While blm induced a significant increase of BALF PGE2 in FVB, this effect was more marked in Tg5 mice (Figure 7). The role of PG in our model was then investigated by blocking cyclooxygenase with indomethacin. This drug was administered in drinking water in saline or blm-treated mice, of the Tg5 or FVB strains (0.05U/mouse). Six days after the administration of blm, indomethacin treatment reduced PGE2 production by 87% and 48% in FVB and Tg5 mice, respectively (Figure 7). Indomethacin treatment significantly increased survival of blm-treated FVB mice (Figure 8) but induced significant mortality in blm-treated Tg5 mice. Six days after blm-treatment, the elevation of BALF LDH levels induced by blm was reduced by 38% in FVB and increased by 54% in Tg5 mice treated with indomethacin treatment. The reduction of CC16 levels in BALF was, however, not modified by indomethacin treatment (not shown). Since cysteinyl leukotriene have been involved in the pathogenesis of blm-induced lung injury,27,28 we also measured the levels of these eicosanoids in the BALF of the different strains, 6 days after blm treatment. While cysteinyl leukotriene levels were increased both in FVB and Tg5 mice, no clear difference that could be related to the contrasted susceptibility of these strains was observed (mean ± SEM, n = 4 to 6, FVB blm: 111 ± 14 ng/ml versus Tg5 blm: 156 ± 10 ng/ml in the indomethacin-treated group, and FVB blm: 126 ± 10 ng/ml versus Tg5 blm 317 ± 14 ng/ml in the control group).
Figure 7.
Prostaglandin E2 levels in BAL fluid (BALF) of bleomycin or saline-treated IL-9-transgenic (Tg5) and control (FVB) mice after indomethacin treatment (see Materials and Methods section). Bar represent means ± SEM (n = 4 to 6). ***, P < 0.001 (Student-Newman-Keuls multiple comparison test).
Figure 8.
Effect of indomethacin administration on bleomycin-induced mortality in IL-9-transgenic (Tg5) and control (FVB) mice. Data represent composite deaths following administration of 0.05 U blm/mouse (n = 12 to 18) and after indomethacin (indo) or ethanol (eth) treatment (see Materials and Methods section). Different letters indicate statistically significant differences between groups (P < 0.05) as analyzed by log rank test. Similar results were obtained in two independent experiments.
Discussion
In this report, we have shown that IL-9 protects against blm-induced lung injury and lethality. We demonstrated that the production of prostaglandin (PG)-related mediators such as PGE2 is up-regulated in IL-9 transgenic animals and that these factors contribute to down-modulate the adverse pulmonary responses to blm. These observations suggest a prostaglandin-dependent mechanism for the protective effect of IL-9 against blm-toxicity.
The toxic effect of blm occurs predominantly in the lung because the blm-inactivating enzyme (bleomycin hydrolase) is practically absent in this organ.29 An increased capacity to detoxify blm in IL-9 transgenic mice could be excluded because both strains expressed similar levels of bleomycin hydrolase transcripts in the lung tissue (unpublished observations).
Failure to restore a functional epithelial barrier has been identified as a major contributing event to the progression of lung injury and inflammation.30 In particular, it has been suggested that apoptosis of pneumocytes is a key preliminary event in the extension of lung lesions observed in blm models.31 Clara cells are particularly sensitive to blm-induced injury since a dramatic loss in their numbers was observed in different strains of mice treated with blm.32,33 By measuring BALF CC16 levels, we found that Clara cell cytotoxicity was less severe in blm-treated Tg5 than in corresponding FVB mice. These biochemical data were comforted by the histological analysis (Figure 2A), showing that epithelium integrity was less affected after blm treatment in transgenic than in FVB mice. Among the pleiotropic functions of IL-9, it has been shown that this cytokine possesses anti-apoptotic properties.34 We can thus speculate that overexpression of IL-9 in blm-treated mice may confer a relative protection of the epithelium against injury by inhibiting epithelial cell apoptosis and may limit the initial pathogenic stages and their subsequent lung disorders.
The importance of recruited pulmonary T lymphocytes in acute and lethal inflammatory reaction has been reported in different animal models. For instance, recruited CD4+, CD8+, and NK lymphocytes are responsible for the lethal respiratory lesions observed in experimental pulmonary disorders induced respectively by Toxoplasma infection,35 viral contamination,36 or co-administration of IL-18 and IL-2.37 The exact role of T lymphocytes and their subpopulations in blm-induced injury remains relatively unclear. Indeed, reports using nude mice and administration of blm have led to conflicting conclusions.38–40 However, several experimental studies using blocking antibodies clearly demonstrated that the recruited CD3+, CD4+, and CD8+ lymphocytes directly contribute to the development of the deleterious effects of blm.5,11,41 It is likely that the mechanisms by which T cells act include their cytotoxic capacities and/or their ability to produce inflammatory cytokines such as TNF-α and IFN-γ as well as oxidants and proteases.11,42,43 Our data showed a reduction of the number of CD4+ and CD8+ T cells as well as the levels of pro-inflammatory cytokines such as TNF-α and IL-6 in blm-treated IL-9 transgenic mice. Therefore, we may suggest that this reduced recruitment of T cells and synthesis of pro-inflammatory cytokines may have contributed to limit blm-induced lung injury in IL-9 transgenic mice.
Accumulation of eosinophils in the tissue lesions is a hallmark of numerous inflammatory diseases. It is generally accepted that these immune cells play a key role in the pathogenesis of inflammation because eosinophils are able to release high levels of toxic products (proteases and oxidants) as well as mediators amplifying the inflammation such as TNF-α, IL-12, GM-CSF, and chemokines.44 However, this concept was recently challenged by reports showing that eosinophils may possess strong anti-inflammatory functions mediated by the production of anti-inflammatory cytokines.45 The presence of numerous lung eosinophils in IL-9 transgenic mice leads us to test the hypothesis that these cells could play a protective role in these mice. To assess the contribution of the eosinophils, we used IL-5−/− mice that are deficient in eosinophils. Our data presented in Figure 4 indicate that eosinophils are not essential for the protective effect of IL-9.
In a previous study,46 we have demonstrated that IL-9 is an important regulator of B lymphocytes. Although B cells are found to be increased in the lung of blm-treated Tg5 mice, a protective role against the acute effect of blm appears, however, unlikely because B-deficient transgenic animals were also resistant to blm (Figure 5).
TGF-β is a multipotent cytokine that modulates cellular proliferation, apoptosis and is involved in immunoregulation processes.47 This factor is also involved in the control of inflammation and possesses strong anti-inflammatory functions.48 BALF of Tg5 mice contained much more TGF-β than that from FVB but neutralization by specific antibody injection in Tg5 mice indicated that this cytokine is probably not a major mediator of their resistance to blm. Consistently, Nakao et al,49 in a murine model of lung fibrosis induced by blm, showed that inhibition of TGF-β signaling prevented the induction of lung fibrosis but did not affect the severity of lung inflammation.
Because prostaglandins (PG) and in particular PGE2 were present at high levels in inflamed lesions and could induce inflammatory changes when directly injected into the lung, they were initially considered as pro-inflammatory mediators.50 The pro-inflammatory properties of PG were confirmed in experiments using drugs inhibiting cyclooxygenases (COX-1 and -2), which catalyze the synthesis of numerous PG mediators.51 For instance, in a murine model of endotoxemia, inhibition of PG production by a specific COX-2 inhibitor (NS-398) lead to increased survival, supporting the direct participation of PGs in the acute inflammatory process.52 The availability of PG-related-deficient mice (COX-1, COX-2, and prostanoid receptor knock-outs) has further confirmed the pro-inflammatory functions for PG in different models.51 For instance, the genetic ablation of COX2 prevented the development of inflammation in a model of autoimmune arthritis53 or in a model of hepatocellular toxicity.54 It is known that prostaglandins and in particular PGE2 are up-regulated in experimental blm-induced lung injury.55 Reports have concluded that inhibition of prostaglandin synthesis by indomethacin was associated with a marked reduction of blm-lung injury, suggesting that PG contribute to exacerbate the lesions during the early phase of this model.56,57 We also observed that PGE2 was significantly up-regulated in BALF from blm-treated FVB mice and that administration of indomethacin was associated with a marked reduction of PGE2 production and blm toxicity in FVB mice.
While PGs appear to promote acute inflammation in the majority of experimental models, important exceptions have, however, been observed. Indeed, recent evidences suggest that PGs also serve critical down-regulatory functions in models of injury and inflammation.50 For instances, by using COX1- and COX2-deficient mice, PGs have been reported to attenuate the inflammatory response in models of allergic airway disease.58 Interestingly, in models of drug-induced liver injury and mortality, Reilly and co-authors59 have demonstrated a protective role for COX-derived products such as PGE2 and PGD2. Recently, it has been proposed that the existence of four subtypes of receptors for PGE2 (EP1–4) could explain the multiplicity of the biological responses elicited by this eicosanoid and how these responses might be diverse and sometimes opposite.50
Inhibition of PG production by indomethacin treatment in Tg5 mice exacerbated lung injury and death induced by administration of blm, suggesting that in IL-9 transgenic mice, PG contributes to limit blm-induced lung injury. It is probable that this effect is mediated, at least in part, by PGE2 because this mediator was dramatically up-regulated in blm-treated Tg5 and indomethacin treatment reduced PGE2 levels and reverted resistant-Tg5 mice into a sensitive strain.
Three hypotheses can be offered to explain how indomethacin conferred protection (in FVB) and, in contrast, enhanced lethality (in Tg5) after bleomycin administration. First, the type of pulmonary response may vary with the concentration of the mediator, with a predominant pro-inflammatory activity of PGE2 at low concentrations (FVB mice) and an anti-inflammatory activity at higher concentrations (Tg5 mice). Interestingly, PGE2 levels were lowered by indomethacin treatment in Tg5 mice to a level similar to that measured in FVB mice in the absence of indomethacin treatment (Figure 7) and similar mortality rates were observed in these cases (Figure 8).
Second, it is plausible that the expression of EP receptor subtypes is different in FVB and Tg5 mice, and hence that the biological activity of PGE2 differs in both strains.
Third, it is also possible that the origin of PGE2 is different in both strains. While indomethacin almost completely abrogated PGE2 production in blm-treated FVB mice, this same treatment only partly reduced the levels of this eicosanoid in blm-treated transgenic mice (Figure 7). Therefore, while it cannot be excluded that the indomethacin-dosing regime induced incomplete cyclooxygenase inhibition in Tg5 mice, it is also possible that a significant part of PGE2 produced in Tg5 mice is formed independently of these enzymes. The latter would be consistent with the recent demonstration that PGE2 can be formed nonenzymatically from isoprostanes derived from the free radical-catalyzed peroxidation of arachidonate indicate.60
In summary, our results support a protective role of IL-9 in lung injury induced by blm and suggest that overexpression of IL-9 reverts the deleterious activity of PGE2 in this model.
Acknowledgments
The authors thank Xavier Dumont, Yousof Yakoub, Francine Uwambayineme, Monique Stevens, and Nicole Botterman for their excellent technical assistance.
Footnotes
Address reprint requests to F. Huaux, Unit of Industrial Toxicology and Occupational Medicine, Faculty of Medicine, UCL, Clos Chapelle-aux-Champs, 30.54, 1200 Brussels Belgium. E-mail: huaux@toxi.ucl.ac.be.
Supported in part by the Fonds de la Recherche Scientifique Médicale and the Actions de Recherche Concertées, Communauté française de Belgique-Direction de la Recherche Scientifique. François Huaux is a Postdoctoral Researcher with the Fonds National de la Recherche Scientifique (FNRS), Belgium.
References
- Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- Thrall RS, Barton RW. A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1984;129:279–283. [PubMed] [Google Scholar]
- Nettelbladt O, Scheynius A, Bergh J, Tengblad A, Hallgren R. Alveolar accumulation of hyaluronan and alveolar cellular response in bleomycin-induced alveolitis. Eur Respir J. 1991;4:407–414. [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Phan SH, Kunkel SL. C-C chemokines: novel mediators of the profibrotic inflammatory response to bleomycin challenge. Am J Respir Cell Mol Biol. 1996;15:693–702. doi: 10.1165/ajrcmb.15.6.8969262. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Hao H, Cohen DA, Jennings CD, Bryson JS, Kaplan AM. Bleomycin-induced pulmonary fibrosis is independent of eosinophils. J Leukoc Biol. 2000;68:515–521. [PubMed] [Google Scholar]
- Zhang K, Gharaee Kermani M, McGarry B, Remick D, Phan SH. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol. 1997;158:954–959. [PubMed] [Google Scholar]
- Ma JY, Barger MW, Hubbs AF, Castranova V, Weber SL, Ma JK. Use of tetrandrine to differentiate between mechanisms involved in silica-versus bleomycin-induced fibrosis. J Toxicol Environ Health A. 1999;57:247–266. doi: 10.1080/009841099157692. [DOI] [PubMed] [Google Scholar]
- Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001;24:545–555. doi: 10.1165/ajrcmb.24.5.4064. [DOI] [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Arai T, Abe K, Matsuoka H, Yoshida M, Mori M, Goya S, Kida H, Nishino K, Osaki T, Tachibana I, Kaneda Y, Hayashi S. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am J Physiol. 2000;278:L914–L922. doi: 10.1152/ajplung.2000.278.5.L914. [DOI] [PubMed] [Google Scholar]
- Renauld J-C, van Snick J. Interleukin-9. Thomson A, Lotze MT, editors. London: Academic Press; The Cytokine Handbook. 2003:pp 347–356. [Google Scholar]
- Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arras M, Huaux F, Vink A, Delos M, Coutelier JP, Many MC, Barbarin V, Renauld JC, Lison D. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am J Respir Cell Mol Biol. 2001;24:368–375. doi: 10.1165/ajrcmb.24.4.4249. [DOI] [PubMed] [Google Scholar]
- Grohmann U, van Snick J, Campanile F, Silla S, Giampietri A, Vacca C, Renauld JC, Fioretti MC, Puccetti P. IL-9 protects mice from gram-negative bacterial shock: suppression of TNF-alpha, IL-12, and IFN-gamma, and induction of IL-10. J Immunol. 2000;164:4197–4203. doi: 10.4049/jimmunol.164.8.4197. [DOI] [PubMed] [Google Scholar]
- Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci USA. 1986;83:9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatek T, Hermans C, Broeckaert F, Wattiez R, Wiedig M, Toubeau G, Falmagne P, Bernard A. Quantification of Clara cell protein in rat and mouse biological fluids using a sensitive immunoassay. Eur Respir J. 1998;11:726–733. [PubMed] [Google Scholar]
- Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase: application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198:1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takagawa S, Katayama I, Nishioka K. Anti-sclerotic effect of transforming growth factor-beta antibody in a mouse model of bleomycin-induced scleroderma. Clin Immunol. 1999;92:6–13. doi: 10.1006/clim.1999.4720. [DOI] [PubMed] [Google Scholar]
- McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- Peebles RS, Jr, Dworski R, Collins RD, Jarzecka K, Mitchell DB, Graham BS, Sheller JR. Cyclooxygenase inhibition increases interleukin 5 and interleukin 13 production and airway hyperresponsiveness in allergic mice. Am J Respir Crit Care Med. 2000;162:676–681. doi: 10.1164/ajrccm.162.2.9911063. [DOI] [PubMed] [Google Scholar]
- Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M, Bailie M, Marshall T, Wilke C, Phan SH, Toews GB, Moore BB. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med. 2002;165:229–235. doi: 10.1164/ajrccm.165.2.2104050. [DOI] [PubMed] [Google Scholar]
- Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA. 2004;101:3047–3052. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma T, Holland JF, Masuda H, Waligunda JA, Goldberg GA. Microbiological assay of bleomycin: inactivation, tissue distribution, and clearance. Cancer. 1974;33:1230–1238. doi: 10.1002/1097-0142(197405)33:5<1230::aid-cncr2820330507>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Li XP, Zhang HY, Soledad-Conrad V, Zhuang JJ, Uhal BD. Bleomycin-induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am J Physiol. 2003;284:L501–L507. doi: 10.1152/ajplung.00273.2002. [DOI] [PubMed] [Google Scholar]
- Daly HE, Baecher-Allan CM, Barth RK, D’Angio CT, Finkelstein JN. Bleomycin induces strain-dependent alterations in the pattern of epithelial cell-specific marker expression in mouse lung. Toxicol Appl Pharmacol. 1997;142:303–310. doi: 10.1006/taap.1996.8056. [DOI] [PubMed] [Google Scholar]
- Daly HE, Baecher-Allan CM, Paxhia AT, Ryan RM, Barth RK, Finkelstein JN. Cell-specific gene expression reveals changes in epithelial cell populations after bleomycin treatment. Lab Invest. 1998;78:393–400. [PubMed] [Google Scholar]
- Louahed J, Renauld JC, Demoulin JB, Baughman G, Bourgeois S, Sugamura K, van Snick J. Differential activity of dexamethasone on IL-2-, IL-4-, or IL-9- induced proliferation of murine factor-dependent T cell lines. J Immunol. 1996;156:3704–3710. [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN- gamma, and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Zhao MQ, Stoler MH, Liu AN, Wei B, Soguero C, Hahn YS, Enelow RI. Alveolar epithelial cell chemokine expression triggered by antigen- specific cytolytic CD8(+) T cell recognition. J Clin Invest. 2000;106:R49–R58. doi: 10.1172/JCI9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kato S, Oizumi K, Kinoshita M, Inoue Y, Hoshino K, Akira S, McKenzie AN, Young HA, Hoshino T. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood. 2002;99:1289–1298. doi: 10.1182/blood.v99.4.1289. [DOI] [PubMed] [Google Scholar]
- Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- Helene M, Lake Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65:187–195. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- Schrier DJ, Phan SH, McGarry BM. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis: a biochemical evaluation. Am Rev Respir Dis. 1983;127:614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Sharma SK, MacLean JA, Pinto C, Kradin RL. The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. Am J Respir Crit Care Med. 1996;154:193–200. doi: 10.1164/ajrccm.154.1.8680680. [DOI] [PubMed] [Google Scholar]
- Li G, Siddiqui J, Hendry M, Akiyama J, Edmondson J, Brown C, Allen L, Levitt S, Poulain F, Hawgood S. Surfactant protein-A-deficient mice display an exaggerated early inflammatory response to a beta-resistant strain of influenza A virus. Am J Respir Cell Mol Biol. 2002;26:277–282. doi: 10.1165/ajrcmb.26.3.4584. [DOI] [PubMed] [Google Scholar]
- Huaux F, Liu TJ, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol. 2003;171:5470–5481. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- Dombrowicz D, Capron M. Eosinophils, allergy, and parasites. Curr Opin Immunol. 2001;13:716–720. doi: 10.1016/s0952-7915(01)00284-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J Immunol. 2003;170:5756–5763. doi: 10.4049/jimmunol.170.11.5756. [DOI] [PubMed] [Google Scholar]
- Vink A, Warnier G, Brombacher F, Renauld JC. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med. 1999;189:1413–1423. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doestchman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Reddy RC, Chen GH, Tateda K, Tsai WC, Phare SM, Mancuso P, Peters-Golden M, Standiford TJ. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am J Physiol. 2001;281:L537–L543. doi: 10.1152/ajplung.2001.281.3.L537. [DOI] [PubMed] [Google Scholar]
- Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, III, Toews GB. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin- dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979;95:117–130. [PMC free article] [PubMed] [Google Scholar]
- Mall G, Zimmermann P, Siemens I, Burkhardt A, Otto HF. Prevention of bleomycin-induced fibrosing alveolitis with indomethacin: stereological studies on rat lungs. Virchows Arch A Pathol Anat Histopathol. 1991;419:339–347. doi: 10.1007/BF01606525. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, Pohl LR. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol. 2001;14:1620–1628. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- Gao L, Zackert WE, Hasford JJ, Danekis ME, Milne GL, Remmert C, Reese J, Yin H, Tai HH, Dey SK, Porter NA, Morrow JD. Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostoglandins independent of cyclooxygenase. J Biol Chem. 2003;278:28479–28489. doi: 10.1074/jbc.M303984200. [DOI] [PubMed] [Google Scholar]