Abstract
Wnt-5a has been shown to influence the metastatic behavior of human breast cancer cells, and the loss of Wnt-5a expression is associated with metastatic disease. We show here that NFAT1, a transcription factor connected with breast cancer metastasis, is activated by Wnt-5a through a Ca2+ signaling pathway in human breast epithelial cells. This activation was simultaneously counteracted by a Wnt-5a-induced Yes/Cdc42 signaling pathway. The observation that inhibition of the Wnt-5a/Yes/Cdc42 signal prolonged the duration of ionomycin-induced NFAT1 activation revealed the general importance of this pathway. The Wnt-5a-induced inhibition of NFAT1 did not require glycogen synthase kinase 3β, JNK, or Pak1 activity or modulation of the cytoskeleton. Instead, we observed that Wnt-5a induced a complex formation of NFAT1/casein kinase 1α, even upon treatment with ionomycin, which was blocked upon inhibition of the Wnt-5a/Yes/Cdc42 signaling pathway. Our results explain why Wnt-5a/Ca2+-induced NFAT activity is hard to detect and suggest a novel mechanism by which Wnt-5a can suppress tumor-specific, agonist-induced NFAT activity and thus the metastatic behavior of breast cancer cells.
Wnts are cysteine-rich secreted glycoproteins that exert their effects through auto- and paracrine signaling (39). They influence multiple processes during development and have also been implicated in carcinogenesis, including the development of breast cancer (13, 20, 25, 36, 58). Wnt ligands bind to the extracellular cysteine-rich domain of the Frizzled family of G-protein-coupled seven-transmembrane receptors, together with a coreceptor, LRP5 or LRP6 (37, 47, 54).
Wnt signaling is complex since the signals are transduced through several different pathways, depending on the Wnt type and the tissue analyzed (44, 47, 63). The signal pathway that has been best characterized to date is the canonical (β-catenin) Wnt pathway, which is highly conserved among species and is responsible for many important cellular processes (44, 47, 56, 63). Several noncanonical pathways (Wnt-5a, Wnt-11, and XWnt-4) have also been described, including the Wnt/Ca2+ signaling pathway, and the relationship between these pathways has been thoroughly discussed in a recent review (63). Briefly, signaling by Wnt-5a can trigger the noncanonical Wnt/Ca2+ pathway, leading to an increase in intracellular Ca2+ and activation of calcium-sensitive enzymes, such as calcineurin, and Ca2+/calmodulin-regulated kinase II (31, 57). Stimulation of calcium-sensitive enzymes such as these often leads to the activation of NFAT (for nuclear factor of activated T cells) transcription factor proteins in many cell types (5, 50), but it has been unclear whether the same applies to signaling by Wnt-5a and other Wnts (43, 53, 61). In addition, other noncanonical Wnt pathways have been described and shown to control planar cell polarity in Drosophila and convergent extension movements in vertebrates, and several investigators have reported that the Jun N-terminal kinase (JNK [6]) and small Rho-GTPases (RhoA, Rac1, or Cdc42 [35, 44]) are involved in these cascades (63).
Wnt-5a has been proposed to exert its effects via the Wnt/Ca2+ pathway, based on results obtained in both Xenopus (53) and mammalian cells; the latter is exemplified by receptor-blocking experiments in malignant melanomas where Wnt-5a can signal through frizzled receptor (Fz) 5 and thereby activate protein kinase C (PKC) (31, 65). There are conflicting results as to whether such Wnt/Ca2+-induced signaling leads to the activation of NFAT-dependent transcription or not (53, 61). Importantly, Wnt-5a has also been shown to activate other noncanonical pathways (47, 63, 68). Apparently, a single Wnt ligand can use divergent pathways in different cell types.
The transcription factor NFAT is expressed as five isoforms (NFAT1 to NFAT5; nomenclature proposed by Hoey et al. [22]; for a review, see reference 50) in various tissues. NFAT1 and NFAT5 have recently been implicated in invasive breast cancer and metastasis (23, 69). Activation of NFAT1 to NFAT4 comprises three steps: dephosphorylation, nuclear translocation, and an increase in affinity for DNA. The deactivation is regulated by active mechanisms of rephosphorylation and nuclear export. NFATs are phosphorylated on several serine residues, and the phosphorylation of distinct serines is probably brought about by different kinases, such as JNK, glycogen synthase kinase 3β (GSK-3β), casein kinase 1 (CK1α and CK1ɛ), and p21-activated kinase 1 (Pak1) (3, 4, 45, 67, 70).
We have previously shown that expression of the Wnt-5a protein is a predictor of longer disease-free survival in human breast cancer (25). Since both the loss of Wnt-5a (25) and induced NFAT activity (23, 69) have been implicated in invasive breast cancer, we investigated how Wnt-5a signaling might be associated with NFAT activity in breast epithelial cells.
MATERIALS AND METHODS
Cell culture.
We used the HB2 mammary epithelial cell line, which is a subclone of the MTSV-1.7 cell line originating from the laboratory of J. Taylor-Papadimitriou (ICRF, United Kingdom). We used the following clones, which had previously been produced in our laboratory (24): Wnt-5a-overexpressing cells stably transfected with a pLNCX Wnt-5a-HA vector (Wnt-5ahigh), Wnt-5a-antisense cells obtained by transfecting with a pLNCX vector containing the Wnt-5a cDNA in an antisense direction (3′ to 5′; Wnt-5alow), and Wnt-5a neo cells with an empty pLNCX vector (HB2 neo). We also used MCF-7 cells and MCF-7 cells stably transfected with the pLNCX Wnt-5a-HA vector (MCF-7 Wnt-5ahigh) (24), SYF cells lacking all Src-kinase family members and HEK293 cells (both from the American Type Culture Collection). The cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, and for HB2 cells 10 μg of bovine insulin and 5 μg of hydrocortisone/ml was added. The cells were maintained at 37°C in a humidified atmosphere with 5% carbon dioxide.
Chemicals and reagents.
Anti-CK1α and anti-NFAT1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and antibodies to phosphorylated JNK, total JNK, phosphorylated GSK-3β, phosphorylated (Ser 144) Pak1, and total Pak1 came from Cell Signaling (Beverly, MA). We purchased anti-total Yes and GSK-3β from BD Transduction Laboratories (Franklin Lakes, NJ), JNK inhibitor II from Calbiochem (La Jolla, CA), Fura-2 AM from Molecular Probes (Eugene, OR), anti-Cdc42 from Signal Transduction (Lexington, KY), and the G410 anti-phosphotyrosine antibody from Upstate Biotech, Inc. (Lake Placid, NY). The polyclonal rabbit Wnt-5a antibody directed against the C terminus of human Wnt-5a was developed in our laboratory (25). The Src-family kinase inhibitor 4-amino-5-(4-methyl-phenyl)-7-(t-butyl) pyrozolo-d-3,4-pyrimidine (PP1) was from Alexis Biochemicals (San Diego, CA), the casein kinase 1 inhibitor IC261 was from Santa Cruz Biotechnology (Santa Cruz, CA), and the PKC inhibitor bisindolylmaleimide-I (GFX), the Src-family kinase specific inhibitor Su6656, ionomycin, cyclosporine (CS), phorbol myristate acetate (PMA), and cytochalasin D were from Sigma-Aldrich (St. Louis, MO). Recombinant mouse Wnt-5a (0.4 to 1.6 μg/ml) and Wnt-3a (100 ng/ml) was from R&D Systems (Rochester, MN). We have experienced variations in efficiency of recombinant Wnt-5a, and therefore the recombinant Wnt-5a concentrations used in the present study vary between 0.4 and 1.6 μg/ml. All other laboratory reagents were obtained from Sigma Chemicals (St. Louis, MO).
Western blotting and immunoprecipitation.
Adherent cells grown to subconfluency on tissue culture-coated plates or cells plated on collagen-coated plates were lysed in hot Laemmli buffer and dithiothreitol (DTT) for whole-cell lysates. For immunoprecipitations, the cells were serum and/or insulin starved overnight; left unstimulated or stimulated as described; and lysed in 1 ml of lysis buffer containing 100 mM Tris-HCl (pH 7.5), 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, 50 mM NaCl, 4 mM Na3VO4, 20 μg of aprotinin/ml, 1 μg of leupeptin/ml, 2.5 mM benzamidine, and 2 mM Pefabloc (Roche). The supernatants were collected, and protein concentrations were measured. The lysates were precleared with protein-A Sepharose or protein-G Agarose (CK1α), and immunoprecipitations were performed with preconjugated beads, except for Yes immunoprecipitations that required preincubation with the antibody. The beads were washed five times in ice-cold wash buffer (50 mM HEPES [pH 7.4], 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 2 mM Na3VO4) and were boiled immediately in Laemmli buffer with DTT. The membranes were probed with antibodies to Wnt-5a (1:2,000), phospho-JNK (1:1,000), JNK (1:1,000), Cdc42 (1:1,000), phosphotyrosine (1:5,000), NFAT1 (1:200), Ack1 (1:1,000), phospho-GSK-3β (1:500), GSK-3β (1:1,000), phospho-Pak1 (1:1,000), Pak1 (1:1,000), or DDR1 (1:10,000), CK1α (1:500), and Yes (1:2,000) for 1 h at room temperature. The separated protein bands were visualized by incubating the membranes with horseradish peroxidase-conjugated secondary antibodies and with enhanced chemiluminescence. The chemicals used in the experiments were added at the times and concentrations indicated. For analysis of Wnt-5a protein levels in conditioned media, 15 ml of cell culture media from confluent Wnt-5ahigh and Wnt-5alow cells were centrifuged in Amicon Ultra Centrifugation Filter Devices (30,000 molecular weight cutoff; Millipore, Bedford, MA).
GST pull-down assay.
The cDNA of the Cdc42-binding domain from PAK1B (PAKcrib; amino acids 56 to 267) was cloned into bacterial expression vector pGEX-2T (10) and was expressed in Escherichia coli as a fusion protein with glutathione S-transferase (GST). For GST pull-down assays, the cells were lysed in a buffer composed of 50 mM Tris (pH 7.5), 1% Triton X-100, 100 mM NaCl, 10 mM MgCl2, 5% glycerol, 2 mM Na3VO4, 20 μg of aprotinin/ml, 1 μg of leupeptin/ml, 2.5 mM benzamidine, and 2 mM pefabloc. The lysates were centrifuged for 30 s at 15,000 × g, the Triton X-100-soluble fractions were recovered, and protein concentrations were determined. The bacterial lysate containing the GST-PAK-crib fusion protein was added to the samples together with GST beads. After 1 h, the beads were collected and washed three times in 25 mM Tris (pH 7.5)-1% Triton X-100-100 mM NaCl-30 mM MgCl2-1 mM DTT-1 mM Na3VO4. The beads were then resuspended in Laemmli buffer and boiled for 5 min under reducing conditions. The precipitated proteins were separated by electrophoresis, and the membranes were probed with antibodies against Rac1 or Cdc42. The blots were reprobed with anti-GST antibodies, and blots examining the total Cdc42 or Rac1 levels were run in parallel.
Transient transfections and luciferase assays.
Wnt-5ahigh or Wnt-5alow cells were transfected with oligofectamine reagent (Invitrogen) according to the manufacturer's instructions. Each transfection was performed with 106 cells, 1 μg of reporter gene construct, and 0.2 μg of cytomegalovirus (CMV)-controlled Renilla reporter gene. The reporter genes used were human NFAT-pGL2 (AP1 independent) and a TATA-box cloned into pGL3 as a basic transcriptional background control (TATA-pGL3) or the TOP/FOPflash vectors. In cotransfection experiments, we used 1 μg of reporter gene construct, 1 μg of expression plasmid (dominant-negative Cdc42, Rac1 or wt hLRP6), and 0.2 μg of CMV-controlled Renilla. After 36 h, protein extracts were prepared, and luciferase assays were performed with a dual-luciferase reporter assay system (Promega). The obtained luciferase activity was normalized against the activity of the cotransfected Renilla reporter gene and the TATA background in the two different cell clones. Transfections for the JNK activation experiments were performed for 36 h, using 5 × 106 cells and 3 μg of dominant-negative Cdc42, Rac1, or empty vector. The expression levels were analyzed with anti-Myc antibodies. With the exception of cytochalasin D, treatment with all chemicals used in the transfection experiments (PP1, CS, GFX, BAPTA, PMA, and JNKinh) occurred for 36 h at the concentrations indicated in the figure legends. Cytochalasin D was used as described elsewhere (51). For the invasion assays SYF cells were transfected for 18 h using Oligofectamine (Invitrogen), Optimem (Gibco), and a control vector (empty vector) or the NFAT blocking vector VIVIT-green fluorescent protein (GFP) (NFAT regulatory domain originally produced by A. Rao [2] and purchased from Addgene; plasmid 11106). The cells were subsequently cultured in normal medium for 48 h and then harvested for invasion assays.
Immunofluorescence.
Wnt-5alow cells were seeded on glass coverslips and grown overnight to allow them to adhere. Thereafter, the Wnt-5alow cells were treated with recombinant mouse Wnt-5a (0.8 μg/ml; R&D Systems) for 1 h or were left untreated. Thereafter, the cells were washed with phosphate-buffered saline (PBS), fixed in freshly prepared 4% paraformaldehyde for 15 min, permeabilized in PBS-0.5% Triton X-100 for 15 min, and then blocked in 3% bovine serum albumin-PBS for 1 h at room temperature. Staining was performed with a mouse monoclonal anti-NFAT1 antibody (Affinity Bio Reagents) at 1:100 or isotype control antibodies in 1% bovine serum albumin-PBS for 1 h at room temperature, after which the cells were washed six times with PBS and then incubated with secondary anti-mouse Alexa 488 (1:500) antibodies. Next, the coverslips were washed six times in PBS, mounted in fluorescent mounting medium (Dako A/S), and examined and photographed in a Nikon Eclipse 800 microscope using a ×60 objective lens. Images were recorded with a scientific-grade, charge-coupled device camera (Hamamatsu, Japan) and were analyzed with HazeBuster deconvolution software (VayTek, Inc., Fairland, CT).
Calcium imaging.
HB2 or SYF cells were incubated with 4 μM fura-2/AM for 30 min, after which they were washed and placed in a chamber containing a physiologically balanced calcium medium (136 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.1 mM CaCl2, 1.2 mM KH2PO4, 5 mM NaHCO3, 5.5 mM glucose, 20 mM HEPES [pH 7.4]) at 37°C. The chamber was placed in a Nikon Diaphot microscope equipped with a Photon Technology International imaging system, and the cells were allowed to rest for 10 min. Fluorescent signals of cells were recorded before and after stimulation with Wnt-5a (0.8 μg/ml) or Wnt-3a (100 ng/ml) using excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. At least 40 ratios were recorded per minute. For each experiment, the fluorescence intensity from all cells in the field of vision was monitored, after which the calcium response of at least 10 single cells was calculated as the change in fluorescence intensity ratio (340/380 nm) using PTI image master software. Each single trace shown is a representative of at least three independent experiments. The Wnt-5a-induced calcium response was detected in more than 95% of the cells that were imaged.
Matrigel invasion assays.
Invasion assays were carried out using Matrigel invasion chambers (BD) with 8.0-μm-pore-size pore membranes in 24-well plates. HB2 Wnt-5alow and SYF cells were harvested, washed, and resuspended at a concentration of 105 cells per ml in serum-free culture media. Serum-containing medium (10% fetal calf serum [FCS]) was added to the lower well, and 0.5 ml (5 × 104 cells) of the cell suspension, containing recombinant Wnt-5a (0.8 μg/ml) where indicated, was added to the invasion chamber, and the cells were allowed to invade for 72 h (HB2 Wnt-5alow cells) or 36 h (SYF cells). Cells that had invaded through the Matrigel were fixed (4% paraformaldehyde), stained with crystal violet (Sigma Aldrich), and counted. The lower wells were always checked for cells to ensure that the experiments were stopped at the appropriate time for each cell type. SYF-cell transfection procedures were as described above, and the transfection efficiency (ca. 50%) was evaluated by using fluorescence microscopy (GFP).
RESULTS
Wnt-5a induces a Ca2+ signal and slight but distinct activation of NFAT.
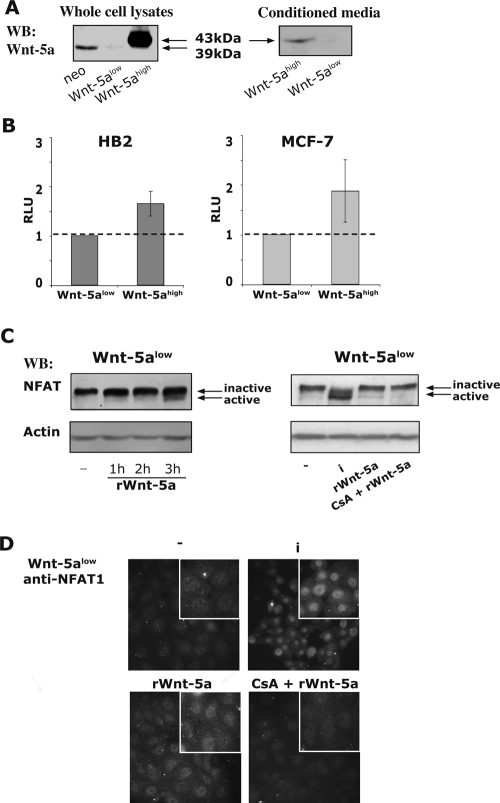
Normal HB2 mammary epithelial cells express Wnt-5a endogenously. Our present (Fig. 1A) and previous (24) results show that the Wnt-5a protein levels are modest in empty vector-transfected (HB2; neo), low in Wnt-5a antisense-transfected (Wnt-5alow), and high in Wnt-5a-overexpressing (Wnt-5ahigh) clones of HB2 epithelial cells, all three of which contain the Wnt receptors Fz1/2 and Fz5 (data not shown). Also, the produced Wnt-5a protein is properly processed and secreted and can be found in the concentrated conditioned media of Wnt-5ahigh cells but not of Wnt-5alow cells (Fig. 1A right). To ascertain a distinct difference in the level of Wnt-5a protein expression, we have here chosen to compare Wnt-5alow cells with Wnt-5ahigh cells. It has previously been reported that Wnt-5a can activate NFAT through the Wnt/Ca2+ noncanonical pathway in Xenopus (53), whereas no significant NFAT activity was detected in mammalian cells (61). To investigate whether Wnt-5a can activate NFAT via a noncanonical pathway in HB2 human breast epithelial cell lines, we compared basal activation levels of NFAT in Wnt-5alow and Wnt-5ahigh HB2 cells. To this end, we transiently transfected Wnt-5alow and Wnt-5ahigh cells with luciferase reporter vectors encoding NFAT sites, and the results indicate a moderate, although statistically significant, increase in basal activation levels of NFAT in the latter cells (Fig. 1B, left; P < 0.05). The luciferase reporter vector used had no AP1 sites and can be activated by NFAT alone. The differences between the cell lines were not due to clonal variations, since stably transfected MCF-7 Wnt-5ahigh cells also showed an increased NFAT activity compared to normal MCF-7 cells (Fig. 1B, right). We also detected an induced NFAT activation upon treatment of Wnt-5alow cells with recombinant mouse Wnt-5a (rWnt-5a; 0.8 μg/ml) as measured by faster-migrating, dephosphorylated, active NFAT1 bands on 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) gels (arrows; Fig. 1C), nuclear translocation of NFAT1, as measured by immunofluorescence (Fig. 1D) and by Luciferase reporter assays (see Fig. 3C). Ionomycin (labeled “i” in Fig. 1C) was used as a positive control (Fig. 1C and D), and the addition of CS inhibited the Wnt-5a-induced NFAT1 activation (Fig. 1C and D and 3C). We also analyzed the protein expression levels of other NFAT family members and found that NFAT5 was highly expressed, whereas NFAT2 is expressed at a moderate level but is surprisingly only weakly affected by ionomycin. NFAT4 is not expressed, and the expression of NFAT3 was not examined (data not shown).
FIG. 1.
(A) Western blot analysis of Wnt-5a expression levels in stably transfected HB2 human breast epithelial cells (left) and concentrated conditioned media (right). To ensure equal loading, protein measurements were done using a Coomassie protein assay reagent (Pierce, Rockford, IL). The blot shown is representative of several separate experiments. In the text, the studied clones are referred to as HB2 neo (empty vector), Wnt-5alow (Wnt-5a antisense), and Wnt-5ahigh (+Wnt-5a HA) cells. (B) Levels of NFAT activation in Wnt-5alow and Wnt-5ahigh HB2 (left) and MCF-7 (right) cells. The activation level of NFAT was measured by using luciferase reporter vectors containing NFAT sites (NFAT-pGL2; AP1 independent). Each transfection was done with approximately 106 cells, 1 μg of reporter gene construct, and 0.2 μg of CMV-controlled Renilla reporter gene. The cells were transfected overnight, after which conditioned medium from either Wnt-5alow or Wnt-5ahigh cells was added. The obtained luciferase activity (measured in relative luciferase units [RLU]) was normalized against the activity of the cotransfected Renilla reporter gene and the TATA-background in the two different cell clones. The statistically significant difference was analyzed by using the Student t test (P < 0.05). (C) Analysis of phosphorylation status of NFAT1 by SDS-6% PAGE upon treatment of Wnt-5alow cells with 1.6 μg of recombinant mouse Wnt-5a (rWnt-5a)/ml for the times indicated. Inactive NFAT is phosphorylated and migrates at 135 kDa, whereas active NFAT1 is dephosphorylated and migrates at 120 kDa. Ionomycin (i) is used as a positive control and CS (CsA) inhibits NFAT activity. (D) Nuclear localization of NFAT1 upon treatment of Wnt-5alow cells with 1.6 μg of recombinant Wnt-5a/ml for 1 h. Ionomycin (i) is used as a positive control, and CS inhibits NFAT activity.
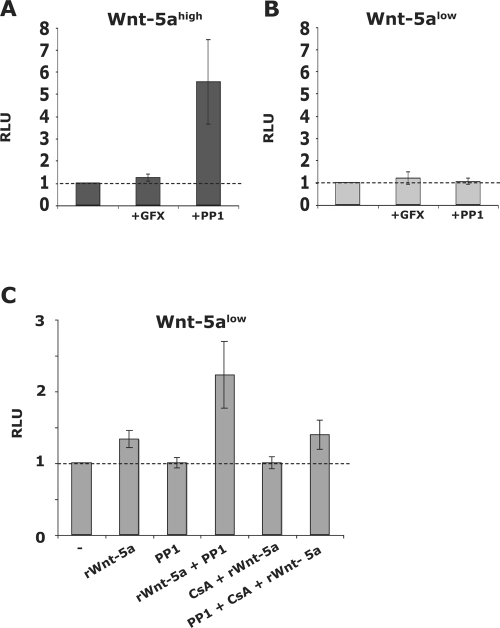
FIG. 3.
(A and B) Wnt-5ahigh (A; dark) and Wnt-5alow (B; light) cells were treated for 36 h with GFX (2 μM) or PP1 (10 μM) after which NFAT activation was evaluated as described above (GFX and PP1 added to the conditioned media). The basal levels of NFAT activation were set to 1 for each cell clone. The data are expressed as means ± the standard error of the mean (SEM) of 6 to 12 separate experiments. (C) Effect of rWnt-5a on NFAT activation alone or in combination with PP1 or CS in Wnt-5alow cells as measured by Luciferase reporter assays. Wnt-5alow cells were transfected and treated for 36 h with 0.8 μg of rWnt-5a/ml, 10 μM PP1, or 100 ng of CS/ml. The data are expressed as means ± the SEM of six experiments.
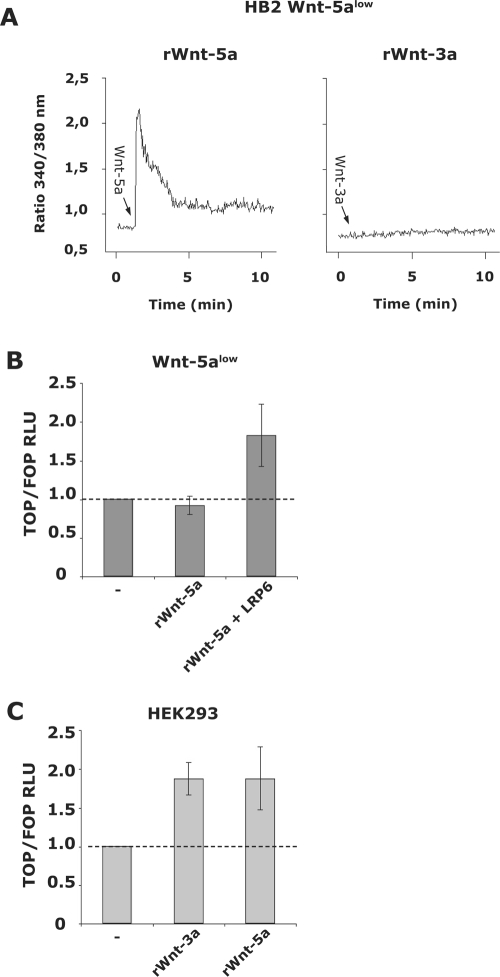
Importantly, recombinant Wnt-5a induced a dose-dependent, distinct, and sustained Ca2+ signal in Wnt-5alow cells, in contrast to recombinant Wnt-3a (Fig. 2A). However, recombinant Wnt-5a did not evoke a canonical signal in HB2 Wnt-5alow cells as measured by TOP/FOPflash reporters, unless the coreceptor LRP6 was overexpressed (Fig. 2B). Although both recombinant Wnt-5a and Wnt-3a stimulation led to canonical Wnt signaling in HEK293 cells, Wnt-3a did not induce canonical Wnt signaling in HB2 Wnt-5alow human breast epithelial cells (data not shown). Together, these data indicate that the recombinant Wnt-5a-induced Ca2+ signal in HB2 Wnt-5alow cells is indeed Wnt-5a specific.
FIG. 2.
(A) rWnt-5a (0.8 μg/ml; left) and rWnt-3a (100 ng/ml; right) induced Ca2+ signal in Wnt-5alow HB2 cells. (B) rWnt-5a (0.8 μg/ml; 24 h) induced canonical Wnt signaling as measured by luciferase assays using TOPflash and FOPflash reporters. The obtained luciferase activity (measured in RLU) was normalized against the activity of the cotransfected Renilla reporter gene. Wnt-5a only induces canonical signaling in human breast epithelial cells in the presence of the coreceptor LRP6. (C) rWnt-3a (100 ng/ml; 24 h) and rWnt-5a (0.8 μg/ml; 24 h) induced canonical Wnt signaling in HEK293 cells, as measured by luciferase assays using TOPflash and FOPflash reporters cotransfected with Renilla.
A member of the Src kinase family inhibits Wnt-5a-induced activation of NFAT.
Collagen-induced activation of the receptor tyrosine kinase DDR1 requires a Wnt-5a induced activation of a nonreceptor tyrosine Src kinase (8). To investigate whether Wnt-5a-induced stimulation of Src is an upstream event in NFAT activation, we treated the cells with the Src inhibitor PP1. Unexpectedly, this treatment resulted in increased NFAT activity in Wnt-5ahigh but not in Wnt-5alow cells (Fig. 3A and B). The experiments were repeated with recombinant Wnt-5a treatment of Wnt-5alow cells. As shown in Fig. 3C, recombinant Wnt-5a indeed induced a slight, CS-sensitive activation of NFAT in Wnt-5alow cells. Notably and in line with the previous experiments, recombinant Wnt-5a in combination with PP1 gave rise to a prominent NFAT activation (Fig. 3C) that was inhibited upon cotreatment with CS. In some cell types, Wnt-5a signaling has been linked to the activation of PKC (21, 30, 32, 65). However, in our experiments, the discrete NFAT activation was affected neither by the inhibition of PKC (with GFX; Fig. 3A and B) nor by the downregulation of that protein (prolonged treatment with PMA; data not shown), indicating that Wnt-5a activates NFAT through a PKC-independent pathway.
Wnt-5a-induced activation of Yes and Cdc42 counteracts its Ca2+-induced activation of NFAT.
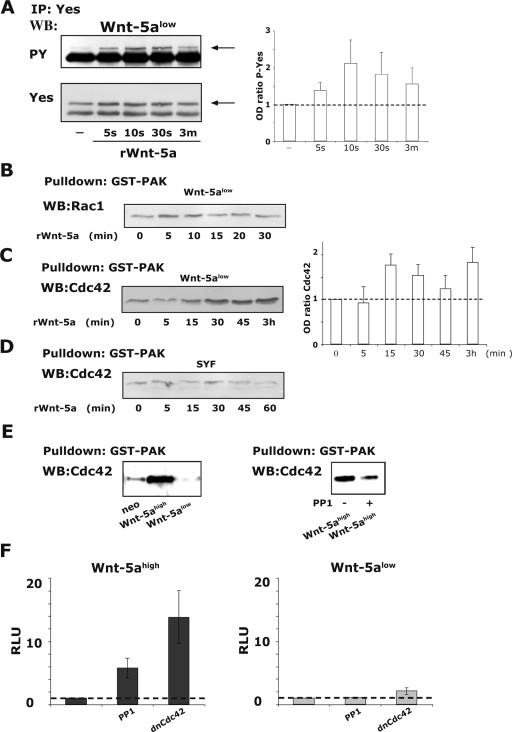
To evaluate which Src-kinase is activated by Wnt-5a, we performed Wnt-5a-induced tyrosine phosphorylation studies of the Src-kinases Src, Fyn, and Yes. Neither Src nor Fyn was activated by recombinant Wnt-5a as measured by immunoprecipitation and immunoblotting for phosphotyrosine or by phospho Src-enzyme-linked immunosorbent assays (data not shown). However, the Src kinase Yes was phosphorylated by recombinant Wnt-5a already after 5 s and peaked within the first minute (Fig. 4A).
FIG. 4.
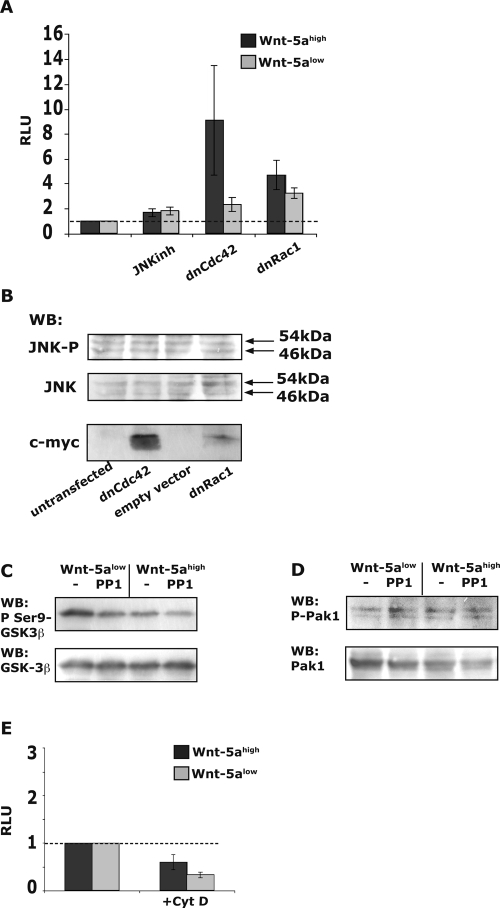
(A) rWnt-5a induced activation of the Src-kinase Yes in Wnt-5alow cells as measured by determining the phosphotyrosine (PY) levels (arrow; top) of immunoprecipitated Yes. The membranes were reprobed for total Yes (arrow; bottom). The ratio of the optical density (OD) from several experiments is expressed as means ± the SEM (right). (B) rWnt-5a-induced Rac1 activation in Wnt-5alow cells, as measured by GST-pulldown assays of active Rac1. (C) rWnt-5a-induced Cdc42 activation in Wnt-5alow cells, as measured by GST-pulldown assays of active Cdc42. The ratio of the OD from several experiments is expressed as means ± the SEM (right). (D) rWnt-5a-induced Cdc42 activation in SYF cells, as measured by GST-pulldown assays of active Cdc42. Wnt-5a does not activate Cdc42 in Src-family kinase negative SYF cells. (E) Activation status of Cdc42 in adherent cells expressing various levels of Wnt-5a (neo, Wnt-5ahigh and Wnt-5alow; left). The effect on Cdc42 activity upon the inhibition of Yes (10 μM PP1, 1 h) in Wnt-5ahigh cells (right). The blots shown are representative of three separate experiments. (F) Levels of NFAT activity in Wnt-5ahigh (dark) and Wnt-5alow (light) cells treated for 36 h with 10 μM PP1 or transfected with 1 μg of dnCdc42. Transfection and calculation of RLU values were done as described in Fig. 1. Basal levels of NFAT activation in both types of cells were set to 1. The data are expressed as means ± the SEM of six separate experiments.
Small Rho GTPases have previously been implicated in noncanonical Wnt signaling (44). Furthermore, the small Rho GTPase Cdc42 can both be activated by Src-kinases (12, 62, 64) and activate many of the kinases that have previously been reported as regulators of NFAT activity (1, 11, 40, 62, 67). In light of this, we investigated the degree to which Rac1 and Cdc42 is activated in human breast epithelial cells. We used the GST-PAK-crib binding assay to measure the relative amounts of active Rac1 and Cdc42 upon treatment of Wnt-5alow cells with recombinant Wnt-5a and found that while Rac1 might be slightly activated (Fig. 4B), Cdc42 is indeed activated (Fig. 4C). All blots were reprobed for GST, and the total levels of Rac1 and Cdc42 were analyzed in separate experiments (data not shown). It is of interest to note that the levels of actin-bound Cdc42 increased upon addition of Wnt-5a, as judged by analysis of the Cdc42 levels in the Triton-insoluble fractions (data not shown). The mouse embryonic fibroblast cell line SYF lacks all Src-kinase family members (26). We therefore analyzed whether Wnt-5a could activate Cdc42 in these cells. As shown in Fig. 4D, we did not detect an activation of Cdc42 upon Wnt-5a treatment in Src-family kinase-negative SYF cells. The cells expressed both Frizzled-2 and Frizzled-5, and Wnt-5a was able to induce a Ca2+ signal in these cells (data not shown and see also Fig. 8B). The Cdc42 activation levels (Fig. 4E, left) were also higher in HB2 breast epithelial cells with high Wnt-5a expression levels, as measured in resting subconfluent cells. Furthermore, the inhibition of Src-kinases (Yes) with PP1 led to decreased activation of Cdc42 in Wnt-5ahigh-expressing cells (Fig. 4E, right). This suggests that Wnt-5a-induced signaling in mammary epithelial cells includes stimulation of both the nonreceptor tyrosine kinase Yes and Cdc42.
FIG. 8.
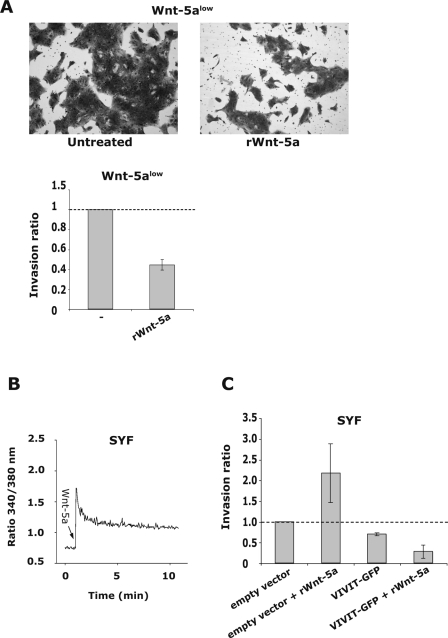
(A) HB2 Wnt-5alow cells were subjected to Matrigel invasion assays. Addition of rWnt-5a (0.8 μg/ml; 72 h) inhibited the invasive capacity of human breast epithelial cells. The top pictures show two representative fields of the Matrigel invasion membranes, and the lower graph represents the mean ratio ± the SD of counted cells that have invaded. (B) rWnt-5a (0.8 μg/ml)-induced Ca2+-signal in SYF cells. (C) The invasive capacity of Src family kinase lacking SYF cells is increased upon treatment with rWnt-5a (0.8 μg/ml; 36 h). The SYF cells were transfected with an empty vector (control) or a negative NFAT regulatory domain vector, VIVIT-GFP (see Materials and Methods). Expression of the inhibitory NFAT-domain lead to a decreased invasion of the SYF cells and also disrupted the Wnt-5a induced increase in invasion.
We next transfected Wnt-5ahigh cells and Wnt-5alow cells with dominant-negative Cdc42 (dnCdc42) and evaluated the levels of NFAT activation and, in support of our hypothesis, the results (Fig. 4F, left) were similar to those obtained upon the addition of PP1. Thus, the fact that PP1 augments the stimulation of NFAT suggests that the Wnt/Ca2+ pathway can activate NFAT in the absence of an antagonizing Wnt pathway.
Cytoplasmic localization of NFAT1 is regulated by Wnt-5a.
As previously mentioned NFAT activity is mainly regulated by phosphorylation of serine residues, thus leading to its cytoplasmic sequestration. Whether this process is an active nuclear export mechanism or an active inhibition of nuclear import is not fully understood (50). However, one way of measuring whether Wnt-5a indeed inhibits NFAT activity is to investigate the effects of Wnt-5a on the nuclear export of already-activated NFAT.
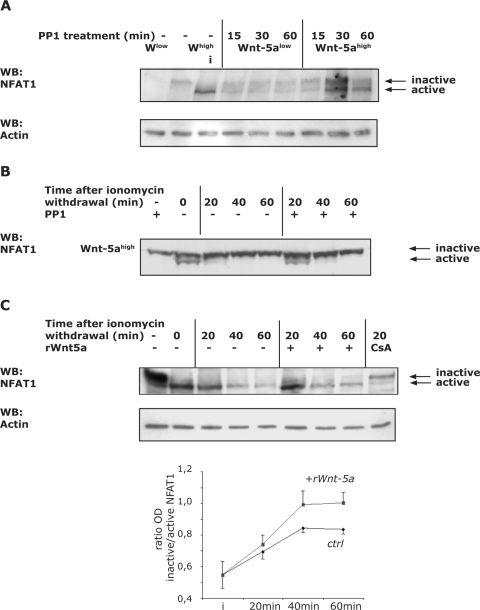
First, we investigated whether the addition of PP1 alone affected NFAT activity in Wnt-5ahigh cells compared to Wnt-5alow cells, as measured by Western blot analysis. The phosphorylated form of NFAT1 appears to have a molecular mass of approximately 135 kDa, whereas the active, dephosphorylated form, which is sequestered in the nucleus, is about 120 kDa (23, 52, 55). In line with our previous results we found that treatment with PP1 alone caused a slight activation of NFAT1 in both types of cells, an effect that was more pronounced in the Wnt-5ahigh cells (Fig. 5A). Ionomycin activation of NFAT was used as a positive control. Western blotting indicated that the activation reached steady-state levels after approximately 2 to 3 h (data not shown). The degree of activation in the Wnt-5ahigh cells cannot be directly compared to the strong activation observed in the luciferase assays, which measure the accumulated effects of Wnt-5a secretion, Wnt/Ca2+ signaling, and subsequent NFAT activation over a period of 36 h.
FIG. 5.
(A) Wnt-5a/PP1-induced activation of NFAT1. Wnt-5ahigh and Wnt-5alow cells were cultured to confluence in six-well plates. PP1 (10 μM) was added for the indicated times or, as a positive control, cells were treated only with ionomycin (“i” in lane 3, 1 μM, 15 min). Negative control cells were left untreated (lanes 1 and 2). Proteins were separated by SDS-6% PAGE. Active NFAT1 leads to stronger immunodetection, and therefore equal loading was controlled with β-actin (bottom). (B) Nuclear export of activated NFAT1 in Wnt-5ahigh cells. Wnt-5ahigh cells were cultured to confluence in 12-well plates and were either pretreated with PP1 (10 μM) or left untreated. The Wnt-5ahigh or Wnt-5ahigh/PP1-containing medium was removed and kept for later use. Ionomycin-containing fresh medium (1 μM) was added to the wells for 5 min and was subsequently replaced with the Wnt-5a- or Wnt-5a/PP1-containing media for the times indicated. The positive control was treated with ionomycin only (lane 2). Whole-cell lysates were prepared by adding hot Laemmli buffer. Equal protein loading was controlled as described in panel A. (C) Nuclear export of activated NFAT1 in Wnt-5alow cells. Wnt-5alow cells were cultured to confluence in 12-well plates and were either pretreated with rWnt-5a (1.6 μg/ml) or left untreated. The medium was removed and kept for later use. Ionomycin-containing fresh medium (1 μM) was added to the wells for 5 min and was subsequently replaced with the Wnt-5a-lacking or -containing media for the times indicated. The positive control was treated with ionomycin only (lane 2), and the negative control was cotreated with CS (lane 9). Whole-cell lysates were prepared by adding hot Laemmli buffer. Equal protein loading was controlled as described in panel A. The ratio (OD) of inactive/active NFAT1 from several experiments is expressed as means ± the SEM (lower).
Second, we performed experiments to investigate the effects of Wnt-5a on nuclear export of already-activated NFAT1. Ionomycin is an ionophore that potently activates NFAT. Wnt-5ahigh cells were preincubated in the absence or presence of PP1 and subsequently stimulated with 1 μM ionomycin for 5 min, after which the ionomycin-containing medium was replaced with the Wnt-5a conditioned medium lacking (−) or containing (+) PP1 (Fig. 5B), and the extent of nuclear export after withdrawal of ionomycin was analyzed by Western blotting. Indeed, we noticed that NFAT1 activation was prolonged in PP1-treated Wnt-5ahigh cells (Fig. 5B), indicating that the noncanonical, Yes/Cdc42-associated Wnt-5a signal has the capacity to also counteract NFAT activation induced by other agonists. Notably, the ionomycin-induced NFAT activation was sustained in untreated Wnt-5alow cells (Fig. 5C) compared to Wnt-5ahigh cells (Fig. 5B). The Wnt-5a-induced inactivation of NFAT1 was also measured using recombinant Wnt-5a (Fig. 5C). Using the same set of experiments but without the addition of PP1, we could see that recombinant Wnt-5a indeed counteracted the ionomycin-induced NFAT activity in Wnt-5alow cells. CS was used as a control.
Neither JNK, GSK-3β, Pak1, nor Akt is part of the counteracting Wnt-5a signaling.
Since serine/threonine kinases phosphorylate NFAT and hence regulate its activation, we sought to investigate potential Wnt-5a/Yes/Cdc42-activated serine/threonine kinases. It has previously been shown that JNK can phosphorylate and thereby inactivate NFAT (4, 15, 34). The RhoGTPase Cdc42 is a signaling molecule that has been shown to be dependent on Src for its activation (62) and also to act upstream of JNK (40). Furthermore, it has been reported that Cdc42 and the serine/threonine kinase JNK may be involved in noncanonical Wnt pathways (41), although existing data are contradictory (63). Nevertheless, we initially hypothesized that JNK is activated by Wnt-5a via Yes and Cdc42 and that it inhibits NFAT activity induced by an agonist-induced Ca2+ signal. First, we investigated the effects of inhibiting JNK on Wnt-5a-induced NFAT activation. The inhibition of JNK did not influence the activation of NFAT in Wnt-5ahigh cells compared to Wnt-5alow cells (Fig. 6A). The results also show that dnRac1 had a much smaller effect on NFAT activation compared to dnCdc42. More importantly, in dnRac1-transfected cells we only found a minute difference in NFAT activation levels between Wnt-5alow and Wnt-5ahigh cells compared to that found in dnCdc42-transfected cells. This indicates that other factors, unrelated to Wnt-5a, can affect NFAT activity via Rac1.
FIG. 6.
(A) Analysis of NFAT activation levels in Wnt-5ahigh (dark) and Wnt-5alow (light) cells treated with JNK inhibitor II (5 μM, 36 h) or transfected with dnCdc42 or dnRac1 (1 μg per transfection) as a control. Transfection and calculation of RLU values were done as described in Fig. 1. The basal level of NFAT activation for each cell clone was set to 1. The data are expressed as means ± the SEM of 6 to 18 separate experiments. (B) The effects of transfection with dnCdc42 or dnRac1 vectors on activation of JNK were investigated. HB2 neo cells (5 × 106) were transfected with 3 μg of empty vector, dnCdc42, or dnRac1 myc-tagged plasmids. The cells were harvested after 36 h. Western blot analysis of phospho-JNK was performed, and the blots were reprobed for total JNK (middle) and myc-expression to ensure efficient transfection efficiency (bottom). The blots shown are representative of five separate experiments. (C) The activation status of GSK-3β (P-Ser 9) was investigated by analyzing the lysates of cells treated with 10 μM PP1 during a prolonged incubation (overnight) to better mimic the luciferase assays. (D) The activation status of Pak1 (P-Ser 144) was investigated as described in panel C. (E) Effect of cytochalasin D on levels of NFAT activation. The transfections and luciferase experiments were performed as described in Fig. 1. Immediately after transfection, cells were kept for 1 h in normal medium or medium containing 5 μM cytochalasin D and thereafter in conditioned medium for an additional 36 h. The experiment was also performed with exposure to cytochalasin D for 12 h before analysis, which gave similar results. Basal levels of NFAT activation in both types of cells were set to 1. The data are expressed as means ± the SEM of six separate experiments.
We also examined the possibility that JNK activation is related to the level of Cdc42 activity. To this end, we transfected HB2 neo cells with dnCdc42 or dnRac1 constructs and analyzed the levels of phosphorylation of JNK. The results revealed that inhibition of Cdc42 did not cause any significant changes in JNK activity (Fig. 6B). We analyzed the expression level of the myc-tagged dnCdc42 and dnRac1 in each experiment and measured JNK phosphorylation at different levels of myc expression and at different times after transfection. We also performed these experiments in Wnt-5ahigh cells (data not shown). The results obtained using these approaches showed that the activation of JNK is not affected by Wnt-5a-induced stimulation of Cdc42. Using the same experimental strategy, we also found that neither p38 nor extracellular signal-regulated protein kinase phosphorylation/activation was affected by Wnt-5a-induced stimulation of Cdc42 (data not shown). This agrees well with a previous study concerning Dishevelled-induced stimulation of JNK in mammalian cells that showed that neither Cdc42 nor Rac1 was involved in Dishevelled-induced JNK activation (33).
It is well established that GSK-3β is a NFAT kinase, and there is evidence that this protein is stimulated by Cdc42 (11). Therefore, we chose to analyze the status of GSK-3β activation in cells treated with PP1 or the alternative Src-kinase inhibitor Su6656 (Su6). PP1 (Fig. 6C), but not Su6 (data not shown), caused a slight reduction of GSK-3β phosphorylation as measured in whole-cell lysates of Wnt-5ahigh and Wnt-5alow cells. More importantly, the PP1-induced decrease in GSK-3β phosphorylation on Ser-9 cannot explain the PP1-induced activation of NFAT because that would have required inactive GSK-3β (phosphorylated). Another NFAT kinase is Pak1 (67). Pak1 is activated by both Cdc42 and Rac1 (42). However, neither PP1 (Fig. 6D) nor Su6 (data not shown) affected the phosphorylation-status of Pak1 (Ser 144 in the CRIB domain). We also analyzed the Akt phosphorylation levels in these sets of experiments but found no differences (data not shown).
Small Rho GTPases, such as Cdc42, are all well-known modulators of the actin cytoskeleton (35). It is difficult to imagine that Cdc42 can have a direct effect on NFAT1 serine-phosphorylation and activity. However, it was recently suggested that actin polymerization has a direct impact on the nuclear sequestration of NFAT (51). Therefore, we also studied whether using cytochalasin D, to disrupt filamentous actin, would affect Wnt-5a-induced levels of NFAT activity. We found no effect of cytochalasin D in either the Wnt-5alow or the Wnt-5ahigh cells (Fig. 6E).
CK1α binds to NFAT1 upon Wnt-5a stimulation.
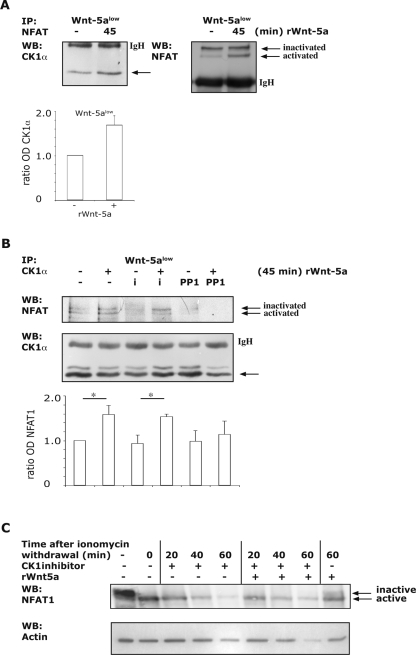
As mentioned above, NFAT proteins have specific serine-rich motifs that are phosphorylated upon inactivation. It has been reported that the different motifs are phosphorylated by distinct kinases and the motif required for nuclear import is regulated primarily through phosphorylation by CK1 (45). The CK-family of proteins is constitutively active serine/threonine kinases that act through complex formation, rather than being activated by phosphorylation (27). Thus, in resting T cells, it was shown that CK1 and NFAT formed a cytoplasmic complex that upon activation dissociated, resulting in the subsequent nuclear localization of NFAT. Interestingly, the involvement of CK proteins has been implicated in both canonical and noncanonical Wnt pathways (16, 18, 19, 27, 59, 60). However, it has not yet been studied whether Wnt proteins can induce complex formation between NFAT and CK1 and thereby inhibit the activation of NFAT. To analyze whether CK1α is a downstream effector of Wnt-5a signaling, we stimulated Wnt-5alow cells with recombinant Wnt-5a and immunoprecipitated NFAT1. As shown in Fig. 7A, we could detect a modest but consistent increase in CK1α binding to NFAT1 upon stimulation with recombinant Wnt-5a. We simultaneously detected a clear activation of NFAT1 as shown by an increase of the faster-migrating band (Fig. 7A, right). We confirmed this association through immunoprecipitations of CK1α and immunoblotting for NFAT1 (Fig. 7B) before and after recombinant Wnt-5a stimulation. Treatment with ionomycin has previously been shown to both activate NFAT but, more importantly, to disrupt the CK1α/NFAT1 binding (45). As shown in Fig. 7B, we demonstrate that upon the addition of recombinant Wnt-5a, the ionomycin-induced disruption of the CK1α/NFAT complex was avoided. In accordance with this, when we inhibited Yes we did not detect the Wnt-5a induced increase in complex formation between CK1α and NFAT1 (Fig. 7B), again indicating that the Wnt-5a-induced Yes/Cdc42 pathway is responsible for the inhibition of NFAT activity. Finally, we examined the ability of recombinant Wnt-5a to counteract ionomycin-induced NFAT activity in Wnt-5alow cells treated with a CK1α inhibitor (Fig. 7C). Indeed, addition of the CK1 inhibitor prevented Wnt-5a from inactivating NFAT1, as measured by nuclear export experiments and using Wnt-5a as a positive control.
FIG. 7.
(A) rWnt-5a (0.8 μg/ml)-induced CK1α and NFAT1 complex formation in Wnt-5alow cells. NFAT1 was immunoprecipitated, and Western blotting was performed with antibodies specific for CK1α (left) or reprobed for NFAT1 (right). The mouse isotype control antibodies immunoprecipitated CK1α weakly, and several experiments were therefore performed to ascertain that the enhanced binding of CK1α to NFAT1 was consistent (bottom; histogram of OD measurements of several experiments; error bars indicate the standard deviation). (B) rWnt-5a induced CK1α and NFAT1 complex formation in Wnt-5alow cells. CK1α was immunoprecipitated, and Western blotting was performed with antibodies specific for NFAT1 (top) and reprobed for CK1α (bottom). The goat isotype control antibodies did not precipitate CK1α or NFAT1 (data not shown). Ionomycin (1 μM) was used as a control and was added for 15 min at the end of the experiments. PP1 was added at 10 μM for 45 min. The data shown is a representative of 5 separate experiments. (bottom; histogram of OD measurements of five experiments; error bars indicate the SEM). (C) Nuclear export of activated NFAT1 in Wnt-5alow cells. Wnt-5alow cells were treated as described in Fig. 5C, except that a CK1 inhibitor was used to block the Wnt-5a-induced NFAT inactivation. The positive control was treated with ionomycin only (lane 2), and the negative control was treated with rWnt-5a only (lane 9). Whole-cell lysates were prepared by adding hot Laemmli buffer. Equal protein loading was controlled using an antibody towards β-actin.
Wnt-5a decreases the invasive capacity of human breast epithelial cells.
In order to examine the biological significance of Wnt-5a-induced effects on NFAT, we used Matrigel invasion assays. Wnt-5a has previously been shown to inhibit tumor cell migration (7). As shown in Fig. 8A, the addition of recombinant Wnt-5a to Wnt-5alow HB2 cells indeed suppressed the capacity of these cells to invade Matrigels as well. However, when the same set of experiments was performed using the Src-family kinase-negative SYF cells, the addition of Wnt-5a rather induced the invasive capacity (Fig. 8B). To explore whether this possibly could be caused by Wnt-5a-induced NFAT activation, we transfected the cells with an NFAT regulatory domain (VIVIT-GFP) that should disrupt NFAT activation. In agreement with our hypothesis, VIVIT both decreased the basal invasive capacity of SYF cells and disrupted the Wnt-5a induced increase in Matrigel invasion (Fig. 8B). The control cells (ctrl) were transfected with an empty vector, and the transfection efficiency was ca. 50%.
DISCUSSION
We have previously shown that expression of Wnt-5a predicts longer disease-free survival in human breast cancer (25) and that the metastasis-suppressing activity of this protein may be related to increased DDR1-dependent cell adhesion and decreased migration (8). The pronounced difference in prognosis between patients who have tumors that do or do not express Wnt-5a strongly suggests that, in addition to DDR1 activation, Wnt-5a also affects other processes that influence invasiveness. One such target could be NFAT, a transcription factor that has been associated with metastatic behavior of mammary cancer cells (23, 69). However, although it is a common view that Wnt-5a can induce a Ca2+ signal, as recently outlined in a review concerning noncanonical Wnt signaling and Ca2+ (28), the Wnt-5a induced Ca2+ signal per se has to our knowledge thus far only been monitored in thyroid cells (29). In line with this, the reports concerning a Wnt-5a-specific activation of NFAT are contradictory (53, 61). Recently, a study was published that suggested that Wnt-5a do not have Ca2+ signaling properties in HEK293 cells (38). The Ca2+ results from this latter study are in direct contradiction to the Wnt-5a induced Ca2+ responses reported in the present study. However, there are several significant differences between the assays performed in their study and ours (see Materials and Methods). To further elucidate how Wnt-5a might suppress breast cancer metastasis, we investigated the effect of Wnt-5a on NFAT activity in human mammary epithelial cells in detail.
Our results indicate that Wnt-5a signaling does regulate NFAT1 activity in mammary cells, as shown by a discrete but statistically significant higher basal activation level of NFAT1 in Wnt-5a-overexpressing HB2 cells. The activation of NFAT1 in breast epithelial cells occurs via a Wnt-5a-induced Ca2+/calmodulin pathway. More importantly, however, we also discovered a novel Wnt-5a-induced Yes/Cdc42/CK1α-dependent mechanism that counteracts both Wnt-5a-induced and Ca2+-induced NFAT1 activation in general. The findings suggest that the Wnt-5a/Ca2+ induced NFAT1 activity is continually antagonized by the Wnt-5a-induced formation of CK1α/NFAT1 complexes in human mammary epithelial cells. In addition, the present observations of simultaneous stimulatory and inhibitory Wnt-5a signals might explain why previous reports concerning Wnt-5a-induced NFAT activation in mammalian cells did not detect significant activation of NFAT by Wnt-5a (61).
In concurrence with some reports concerning Wnt-induced activation of JNK (33, 61), but in contrast to others (17, 41), we did not find a relationship between Wnt-5a-induced activation of Cdc42 and JNK. Likewise, we found no relationships between Cdc42 and other previously suggested proteins that can regulate NFAT activity, including GSK-3β (3, 11, 43, 45), p38 (14), extracellular signal-regulated kinase (49), Pak1 (67), and Akt (48, 69). In a recent study (69), Akt was found to counteract NFAT activity in breast cancer cells and hence inhibited breast cancer cell invasion, but that study did not address the effects of Wnt-5a. It is well known that the activation of Cdc42 leads to modulation of the actin cytoskeleton. However, such a mechanism is not involved in Cdc42-induced regulation of NFAT, although this has been proposed to occur in other cells (51).
As previously mentioned, recent studies have shown that the canonical and noncanonical Wnt signaling pathways overlap or antagonize each other (63, 66). Wnt-5a belongs to the group of nontransforming Wnts and has been implicated in antagonizing the transforming capacities of canonical Wnt signaling in mammary cells (24, 46). Accordingly, it is reasonable to conclude that the loss of Wnt-5a expression in breast tumors leads to a more malignant phenotype. It does not, however, directly explain why the Wnt-5a negative tumors are more metastatic (9, 25). We have previously shown that loss of Wnt-5a in breast cancer cells leads to inactivation of the DDR1 receptor and thereby reduced adhesion and increased motility (8, 24), thus suggesting one mechanism whereby Wnt-5a could reduce the metastatic potential in breast cancer. In addition, it was recently demonstrated that increased activation of NFAT induced by certain tumor-specific integrins elevates the motility, and thus the invasive potential, of mammary tumor cells (23, 69). In the present study we show that the invasive capacity of human breast epithelial cells is reduced upon treatment with recombinant Wnt-5a. In contrast, in SYF cells lacking all Src-kinase family members the Wnt-5a-induced Ca2+-signal leads to an increased invasive capacity, most likely reflecting an increased NFAT activity. This is supported by the fact that the expression of the negative NFAT regulatory domain VIVIT (2) both reduced the invasive capacity of SYF cells and abrogated the Wnt-5a-induced increase in invasive capacity.
It is intriguing to consider how altered activity of NFAT could relate to the metastatic ability of breast tumor cells, since the subset of genes that are induced by NFAT in tumors remain to be determined. Nevertheless, the present findings suggest that the loss of Wnt-5a, along with the eradication of the antagonizing effect of the protein on NFAT activity, can result in an excessive activation of NFAT by other activating receptors such as tumor-specific integrins, leading to augmented tumor cell motility and invasion. In accordance with this, Wnt-5a counteracted Ca2+-induced activation of NFAT1 by ionomycin. Consequently, the loss of Wnt-5a protein expression and reduced Yes/Cdc42/CK1α signaling may increase the metastatic potential of breast cancer by excessive NFAT activation.
Acknowledgments
This study was supported by the Swedish Cancer Foundation (T.A.), the Medical Research Council (T.A.), the U-MAS Research Foundations (T.A. and K.L.), the Crafoord Foundation (K.L.), and the Royal Physiographic Society in Lund (J.D. and K.L.). K.L. was supported by a fellowship from the Swedish Society for Medical Research (SSMF).
We thank Lena Axelsson for help with invasion assays, J. Taylor-Papadimitriou for providing the HB2 cells, T. Leanderson for the NFAT-pGL2 plasmid, M. Sigvardsson for the TATA-pGL3 plasmid, P. Aspenström for the dnCdc42 and dnRac1 plasmids, B. Williams for the hLRP6 plasmid. We also thank Patricia Ödman for linguistic revision of the manuscript and T. Leanderson and Jill Howlin for scientific discussions and linguistic revision of the manuscript.
REFERENCES
- 1.Adam, L., R. Vadlamudi, M. Mandal, J. Chernoff, and R. Kumar. 2000. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem. 275:12041-12050. [DOI] [PubMed] [Google Scholar]
- 2.Aramburu, J., M. B. Yaffe, C. Lopez-Rodriguez, L. C. Cantley, P. G. Hogan, and A. Rao. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133. [DOI] [PubMed] [Google Scholar]
- 3.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 4.Chow, C. W., M. Rincon, J. Cavanagh, M. Dickens, and R. J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278:1638-1641. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. [DOI] [PubMed] [Google Scholar]
- 6.Davis, R. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 7.Dejmek, J., A. Dejmek, A. Safholm, A. Sjolander, and T. Andersson. 2005. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 65:9142-9146. [DOI] [PubMed] [Google Scholar]
- 8.Dejmek, J., K. Dib, M. Jonsson, and T. Andersson. 2003. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int. J. Cancer 103:344-351. [DOI] [PubMed] [Google Scholar]
- 9.Dejmek, J., K. Leandersson, J. Manjer, A. Bjartell, S. O. Emdin, W. F. Vogel, G. Landberg, and T. Andersson. 2005. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin. Cancer Res. 11:520-528. [PubMed] [Google Scholar]
- 10.Edlund, S., M. Landstrom, C. Heldin, and P. Aspenstrom. 2002. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell 13:902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421:753-756. [DOI] [PubMed] [Google Scholar]
- 12.Fukuhara, T., K. Shimizu, T. Kawakatsu, T. Fukuyama, Y. Minami, T. Honda, T. Hoshino, T. Yamada, H. Ogita, M. Okada, and Y. Takai. 2004. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J. Cell Biol. 166:393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles, R. H., J. H. van Es, and H. Clevers. 2003. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653:1-24. [DOI] [PubMed] [Google Scholar]
- 14.Gomez del Arco, P., S. Martinez-Martinez, J. L. Maldonado, I. Ortega-Perez, and J. M. Redondo. 2000. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275:13872-13878. [DOI] [PubMed] [Google Scholar]
- 15.Gomez, M., L. Bosc, A. Stevenson, M. Wilkerson, D. Hill-Eubanks, and M. Nelson. 2003. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 and Crm-1. J. Biol. Chem. 278:46847-46853. [DOI] [PubMed] [Google Scholar]
- 16.Ha, N. C., T. Tonozuka, J. L. Stamos, H. J. Choi, and W. I. Weis. 2004. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol. Cell 15:511-521. [DOI] [PubMed] [Google Scholar]
- 17.Habas, R., I. B. Dawid, and X. He. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17:295-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagen, T., and A. Vidal-Puig. 2002. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem. Biophys. Res. Commun. 294:324-328. [DOI] [PubMed] [Google Scholar]
- 19.Hammerlein, A., J. Weiske, and O. Huber. 2005. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/β-catenin complex. Cell Mol. Life Sci. 62:606-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatsell, S., T. Rowlands, M. Hiremath, and P. Cowin. 2003. Beta-catenin and Tcfs in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 8:145-158. [DOI] [PubMed] [Google Scholar]
- 21.He, X., J. Saint-Jeannet, Y. Wang, J. Nathans, I. Dawid, and H. Varmus. 1997. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275:1652-1654. [DOI] [PubMed] [Google Scholar]
- 22.Hoey, T., Y. L. Sun, K. Williamson, and X. Xu. 1995. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity 2:461-472. [DOI] [PubMed] [Google Scholar]
- 23.Jauliac, S., C. Lopez-Rodriguez, L. Shaw, L. Brown, A. Rao, and A. Toker. 2002. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4:540-544. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson, M., and T. Andersson. 2001. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J. Cell Sci. 114:2043-2053. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson, M., J. Dejmek, P. Bendahl, and T. Andersson. 2002. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 62:409-416. [PubMed] [Google Scholar]
- 26.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knippschild, U., A. Gocht, S. Wolff, N. Huber, J. Lohler, and M. Stoter. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal 17:675-689. [DOI] [PubMed] [Google Scholar]
- 28.Kohn, A. D., and R. T. Moon. 2005. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38:439-446. [DOI] [PubMed] [Google Scholar]
- 29.Kremenevskaja, N., R. von Wasielewski, A. S. Rao, C. Schofl, T. Andersson, and G. Brabant. 2005. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24:2144-2154. [DOI] [PubMed] [Google Scholar]
- 30.Kuhl, M., K. Geis, L. C. Sheldahl, T. Pukrop, R. T. Moon, and D. Wedlich. 2001. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/β-catenin and Wnt/Ca2+ signaling. Mech. Dev. 106:61-76. [DOI] [PubMed] [Google Scholar]
- 31.Kuhl, M., L. Sheldahl, M. Park, J. Miller, and R. Moon. 2000. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16:279-283. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl, M., L. C. Sheldahl, C. C. Malbon, and R. T. Moon. 2000. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275:12701-12711. [DOI] [PubMed] [Google Scholar]
- 33.Li, L., H. Yuan, W. Xie, J. Mao, A. M. Caruso, A. McMahon, D. J. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 34.Liang, Q., O. Bueno, B. Wilkins, C. Kuan, Y. Xia, and J. Molkentin. 2003. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22:5079-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozano, E., M. Betson, and V. M. Braga. 2003. Tumor progression: small GTPases and loss of cell-cell adhesion. Bioessays 2 5:452-463. [DOI] [PubMed] [Google Scholar]
- 36.Lustig, B., and J. Behrens. 2003. The Wnt signaling pathway and its role in tumor development. J. Cancer Res. Clin. Oncol. 129:199-221. [DOI] [PubMed] [Google Scholar]
- 37.Mao, J., J. Wang, B. Liu, W. Pan, G. r. Farr, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 38.Mikels, A. J., and R. Nusse. 2006. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. 2002. The Wnts. Genome Biol. 3:3001.13001.15. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minden, A., A. Lin, F. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi, T., K. Kawachi, S. Kamakura, N. Masuyama, H. Yamanaka, K. Matsumoto, A. Kikuchi, and E. Nishida. 1999. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274:30957-30962. [DOI] [PubMed] [Google Scholar]
- 42.Morreale, A., M. Venkatesan, H. R. Mott, D. Owen, D. Nietlispach, P. N. Lowe, and E. D. Laue. 2000. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat. Struct. Biol. 7:384-388. [DOI] [PubMed] [Google Scholar]
- 43.Murphy, L., and C. Hughes. 2002. Endothelial cells stimulate T-cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the Wnt/glycogen synthase kinase-3 beta pathway. J. Immunol. 169:3717-3725. [DOI] [PubMed] [Google Scholar]
- 44.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamura, H., C. Garcia-Rodriguez, H. Martinson, J. Qin, D. M. Virshup, and A. Rao. 2004. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol. 24:4184-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson, D., and D. Gibo. 1998. Antisense wnt-5a mimics wnt-1-mediated C57MG mammary epithelial cell transformation. Exp. Cell Res. 241:134-141. [DOI] [PubMed] [Google Scholar]
- 47.Pandur, P., D. Maurus, and M. Kuhl. 2002. Increasingly complex: new players enter the Wnt signaling network. Bioessays 24:881-884. [DOI] [PubMed] [Google Scholar]
- 48.Patra, A. K., S. Y. Na, and U. Bommhardt. 2004. Active protein kinase B regulates TCR responsiveness by modulating cytoplasmic-nuclear localization of NFAT and NF-κB proteins. J. Immunol. 172:4812-4820. [DOI] [PubMed] [Google Scholar]
- 49.Porter, C. M., M. A. Havens, and N. A. Clipstone. 2000. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J. Biol. Chem. 275:3543-3551. [DOI] [PubMed] [Google Scholar]
- 50.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 51.Rivas, F. V., J. P. O'Keefe, M. L. Alegre, and T. F. Gajewski. 2004. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol. Cell. Biol. 24:1628-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruff, V. A., and K. L. Leach. 1995. Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J. Biol. Chem. 270:22602-22607. [DOI] [PubMed] [Google Scholar]
- 53.Saneyoshi, T., S. Kume, Y. Amasaki, and K. Mikoshiba. 2002. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295-299. [DOI] [PubMed] [Google Scholar]
- 54.Schweizer, L., and H. Varmus. 2 May 2003, posting date. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 4:4. [Online.] http://www.biomedcentral.com/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott, J. E., V. A. Ruff, and K. L. Leach. 1997. Dynamic equilibrium between calcineurin and kinase activities regulates the phosphorylation state and localization of the nuclear factor of activated T cells. Biochem. J. 324(Pt. 2):597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seidensticker, M., and J. Behrens. 2000. Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta 1495:168-182. [DOI] [PubMed] [Google Scholar]
- 57.Sheldahl, L., D. Slusarski, P. Pandur, J. Miller, M. Kuhl, and R. Moon. 2003. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 161:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smalley, M., and T. Dale. 2001. Wnt signaling and mammary tumorigenesis. J. Mammary Gland Biol. Neoplasia 6:37-52. [DOI] [PubMed] [Google Scholar]
- 59.Sobrado, P., A. Jedlicki, V. H. Bustos, C. C. Allende, and J. E. Allende. 2005. Basic region of residues 228-231 of protein kinase CK1α is involved in its interaction with axin: binding to axin does not affect the kinase activity. J. Cell Biochem. 94:217-224. [DOI] [PubMed] [Google Scholar]
- 60.Takada, R., H. Hijikata, H. Kondoh, and S. Takada. 2005. Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells 10:919-928. [DOI] [PubMed] [Google Scholar]
- 61.Topol, L., X. Jiang, H. Choi, L. Garrett-Beal, P. Carolan, and Y. Yang. 2003. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 162:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tu, S., W. Wu, J. Wang, and R. Cerione. 2003. Epidermal growth factor-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J. Biol. Chem. 278:49293-49300. [DOI] [PubMed] [Google Scholar]
- 63.Veeman, M., J. Axelrod, and R. Moon. 2003. A second canon: functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 5:367-377. [DOI] [PubMed] [Google Scholar]
- 64.Versteeg, H. H., I. Hoedemaeker, S. H. Diks, J. C. Stam, M. Spaargaren, P. M. van Bergen En Henegouwen, S. J. van Deventer, and M. P. Peppelenbosch. 2000. Factor VIIa/tissue factor-induced signaling via activation of Src-like kinases, phosphatidylinositol 3-kinase, and Rac. J. Biol. Chem. 275:28750-28756. [DOI] [PubMed] [Google Scholar]
- 65.Weeraratna, A., Y. Jiang, G. Hostetter, K. Rosenblatt, P. Duray, M. Bittner, and J. Trent. 2002. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1:279-288. [DOI] [PubMed] [Google Scholar]
- 66.Weidinger, G., and R. T. Moon. 2003. When Wnts antagonize Wnts. J. Cell Biol. 162:753-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yablonski, D., L. P. Kane, D. Qian, and A. Weiss. 1998. A Nck-Pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 17:5647-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamanaka, H., T. Moriguchi, N. Masuyama, M. Kusakabe, H. Hanafusa, R. Takada, S. Takada, and E. Nishida. 2002. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoeli-Lerner, M., G. K. Yiu, I. Rabinovitz, P. Erhardt, S. Jauliac, and A. Toker. 2005. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20:539-550. [DOI] [PubMed] [Google Scholar]
- 70.Zhu, J., F. Shibasaki, R. Price, J. C. Guillemot, T. Yano, V. Dotsch, G. Wagner, P. Ferrara, and F. McKeon. 1998. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell 93:851-861. [DOI] [PubMed] [Google Scholar]