Abstract
N-formyl peptides (e.g. fMLP; N-formyl-L-methionyl-L-leucyl-phenylalanine) are potent mediators for inflammatory reactions. We report functional expression in Xenopus oocytes of human fMLP-R98 cDNA, without co-expression of the promiscuous G-protein subunit, Gα-16.
Stimulation of voltage-clamped oocytes (−70 mV) with fMLP produced a dose-dependent biphasic inward current with fast and slow components. Analysis using GTP-γ-S and cholera and pertussis toxins suggested these currents are mediated by an endogenous G-protein of the Gq family.
The fast current reversed at −25 mV and was blocked by SITS (4-acetamido-4′-isothiocyanatostilbene-2,2′-disulphonic acid), suggesting the current is carried by Cl−. The slow current showed weak inward rectification, was Ca2+-dependent and blocked by Cd2+, 4-AP (4-aminopyridine) and haloperidol, suggesting activation of a mixed population of cation channels.
Comparative experiments with human neutrophils using flow cytometric analysis showed that the proportion of neutrophils activated by fMLP was reduced in the presence of SITS, in the absence of external calcium and in the presence of Cd2+, TEA (tetraethylammonium) and haloperidol but not 4-AP. In addition, the oxidative burst from activated neutrophils was reduced by SITS and by the absence of external calcium but not by Cd2+, TEA, 4-AP or haloperidol.
We suggest that in human neutrophils activation by fMLP is dependent on store-operated calcium influx that appears to be regulated by Cl− channels and linked, in part, to non-selective cation channels.
Keywords: fMLP, Xenopus oocytes, neutrophil, non-selective cation channel, chloride channel
Introduction
N-formylated peptides like fMLP (N-formyl-L-methionyl-L-leucyl-phenylalanine) play a major role as potent chemoattractants. They are believed to originate from either degraded bacterial or mitochondrial proteins (Carp, 1982; Marasco et al., 1984). The N-formyl peptide receptor is G-protein coupled and mediates anti-inflammatory reactions in human neutrophils and other tissues (Becker et al., 1998) such as the production of reactive oxygen derivatives (e.g. hydrogen peroxide) upon stimulation with fMLP.
First reports of the cloning and functional expression of the human fMLP receptor were first published in the early 1990s (Boulay et al., 1990; Murphy & McDermott, 1991). Boulay et al. (1990) identified two variants of the fMLP receptor from a CDM8 expression library derived using mRNA from human myeloid cells differentiated to the granulocyte phenotype. Both clones (fMLP-R26 and -R98), expressed in COS-7 cells, specifically bound a highly efficient hydrophilic derivative of fMLP that could be removed using excess fMLP. Boulay et al. (1990) suggested that these cDNAs represent allelic variations of the same gene.
The identity of the G-protein that couples the fMLP receptor to its intracellular responses has been investigated in a number of different cell types. Human leukaemia cells (HL-60) have been shown to express significant levels of pertussis toxin-insensitive Gα-16. However, this G-protein is down-regulated upon differentiation of HL-60 cells into neutrophils (Amatruda et al., 1991; Klinker et al., 1996). In human neutrophils, the intracellular responses to fMLP are affected to varying extents by pertussis toxin (reviewed in Amatruda et al., 1991). It is suggested that in human neutrophils the signal transduction upon stimulation with fMLP is mainly mediated by pertussis toxin-sensitive G-proteins of the Gi class (Bommakanti et al., 1995). Oocytes of the frog Xenopus laevis have been used in several studies to express the human fMLP receptor (fMLP-R98) once the receptor was cloned (Burg et al., 1995; Schultz et al., 1992). However, in these studies the receptor was only functionally active when a promiscuous G-protein of the Gq-class, Gα-16, was co-expressed with the fMLP receptor.
In this study we report, in contrast to previous studies, functional expression of the human receptor for fMLP (fMLP-R98) in Xenopus oocytes, without the need for exogenous Gα-16. Stimulation of the oocytes with fMLP produced a biphasic inward current. GTP-γ-S was used to establish that this current is mediated by G-proteins. Cholera and pertussis toxins were used to analyse which G-protein is responsible for the current response. Current-voltage analysis and ion channel blocking drugs were used to characterize the ionic basis of the currents elicited by stimulation with fMLP.
Our purpose in exploring the response of oocytes expressing the human fMLP receptor was to assess this preparation as a model system with which to analyse the interaction of drugs, especially general anaesthetics, with this receptor and associated intracellular signalling pathways. Accordingly we were interested in the similarity, or otherwise, of the response of neutrophils to stimulation with fMLP in the presence of the various channel-blocking drugs and with reduced external calcium concentration.
Methods
Materials
Oocytes were harvested from extra-large mature female Xenopus laevis (Blades Biological, U.K.). W. Bautsch (Hannover Medical School) donated the cDNAs, in plasmid vector pCDM8, coding for the human R98 variant of the fMLP receptor and the Gα-16 subunit. Ultracompetent E. coli MC1061/p3 were obtained from Invitrogen (The Netherlands). Other materials were purchased from the following companies: N-formyl-L-methionyl-L-leucyl-phenylalanine (fMLP), tetraethyl-ammonium (TEA), 4 aminopyridine (4-AP), 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfphonic acid (SITS), cadmium chloride, barium chloride and guanosine 5′-O-(3-thiotriphosphate) (GTP-γ-S), cholera toxin and pertussis toxin from Sigma (Germany and U.K.); lidocaine from Braun (Germany), haloperidol from Janssen-Cilag (U.K.); Dihydrorhodamine 123 (DHR) and carboxy-seminaphthorhodafluor-l-acetoxymethylester (SNARF1/AM) from Molecular Probes (U.S.A.); Dulbecco's PBS from Life Technologies (Germany); propidium iodide (PI) from Serva (Germany)
Characteristics of blood donors
This study was approved by the ethical committee of the University of Regensburg Medical School. After informed consent, venous blood was drawn from healthy donors with no history of infection 2 weeks prior to the experiments. Donors (n=6) had a mean age of 32 with a range of 27 to 38 years. No donors had to be excluded because of abnormal blood or differential leukocyte counts.
Leukocyte preparation and quantification of H2O2 production
Leukocyte isolation with Histopaque® 1.077 (Sigma, Germany) separation medium and labelling of the neutrophils with DHR, SNARF1/AM and PI was carried out as described in detail in previous publications (Frohlich et al., 1996; Rothe & Valet, 1994). Cells were stimulated with fMLP for 15 min.
Analysis of neutrophil activation and oxidative burst was achieved using flow cytometry (FACScalibur, Becton Dickinson, U.S.A.) with argon ion laser excitation at 488 nm, measuring 10,000 cells of each stained sample. Data were acquired and processed using Cell Quest (Becton Dickinson, U.S.A.) and WinMDI 2.7 (Joseph Trotter, Scripps Institute, U.S.A.).
Amplification and purification of the plasmid vector
Amplification and purification of the plasmid vector pCDM8 was performed using ultracompetent E. coli (MC 1061/p3) and the Wizard SV Miniprep DNA Purification System (Promega, U.K.).
Preparation of cRNA
Plasmids containing the cDNA coding for the human fMLP receptor (R98) were linearized with HpaI and cRNA prepared using the mCAP mRNA capping kit (Stratagene, U.K.). The size of the cRNA was assessed using ethidium bromide stained agarose gel electrophoresis with RNA reference fragments of defined size.
Preparation of oocytes and injection of cRNA
Xenopus laevis were killed by decapitation followed by destruction of the spinal cord after immobilization in ice water for 5 min and pieces of ovary tissue were removed. The oocytes were manually stripped from the ovarian lobes using a soft plastic strip. Stage V and VI oocytes were selected, injected with 35 – 45 nl cRNA using a hydraulic microinjector and placed into sterile pots containing modified Barth's solution (mM): NaCl 100, KCl 1, NaHCO3 2, MgSO4 0.82, Ca(NO3)2 0.33, CaCl2 0.41, HEPES 10, pH 7.4) supplemented with penicillin (100 u cm−3) and streptomycin (100 μg cm−3).
Electrophysiological recording
Prior to use oocytes were defolliculated by incubation for 15 min in Ca2+-free Barth's solution containing collagenase (50 – 100 u cm−3) (Miledi & Woodward, 1989). Although not all somatic cells are removed by this method it is widely used (e.g. Kroll et al., 1991). Manual removal of the vitelline envelope does remove all somatic cells but leaves the oocytes very fragile. Unless the somatic cells are contributing to the response (which is not the case in these experiments) or there is a need to improve the speed of access to the plasma membrane surface, then this additional step is unnecessary.
Electrophysiological recordings were made using two-electrode voltage-clamp (Biologic, France: VF 180 amplifiers and CA 100 clamp amplifier) at a holding potential of −70 mV and superfusion with ND96 (mM: NaCl 96, KCl 2, CaCl2 1.8, MgCl2 1, HEPES 5, pH 7.4 with NaOH).
Experiments with ion channel blocking drugs and toxins
For all experiments with ion-channel blocking drugs on oocytes bath application of the drug (in ND96) was performed (with the oocyte at the holding potential of −70 mV) for 1.5 min before the oocyte was stimulated with fMLP, in the presence of the channel-blocking drug. For experiments with pertussis (500 ng ml−1) and cholera (25 μg ml−1) toxins oocytes were incubated for 120 min (at normal resting membrane potential) with the toxin. Afterwards they were voltage-clamped at −70 mV again and stimulated twice with fMLP. Controls were incubated in parallel in ND96.
For the complementary experiments with neutrophils the leukocyte-rich plasma suspension was incubated with the different ion-channel blocking agents 10 min prior to stimulation with fMLP.
Statistical analysis / data analysis
Current responses obtained from oocytes were recorded on a conventional potentiometric chart recorder and peak amplitudes were measured by hand. Currents recorded from individual oocytes were normalized, with respect to the appropriate control response from that oocyte, so that results could be pooled for analysis. Dose-response curves were constructed using non-linear curve fitting routines applied to a standard logistical equation (Origin V, Microcal Software Inc, U.S.A.).
Neutrophils were identified based on their typical forward (FSC) and side scatter (SSC) patterns and the SNARF1-related orange fluorescence (Figure 1). The SSC depends on the granularity of the cells, the FSC is related to their size. The rhodamine 123 fluorescence is proportional to the quantity of hydrogen peroxide produced by the cell. The percentage of the neutrophils activated after stimulation with fMLP, the mean fluorescence of the whole neutrophil population and the mean fluorescence of the activated cells were analysed. All experimental data obtained from neutrophil experiments are presented as non-normalized mean values with the standard error of the mean.
Figure 1.
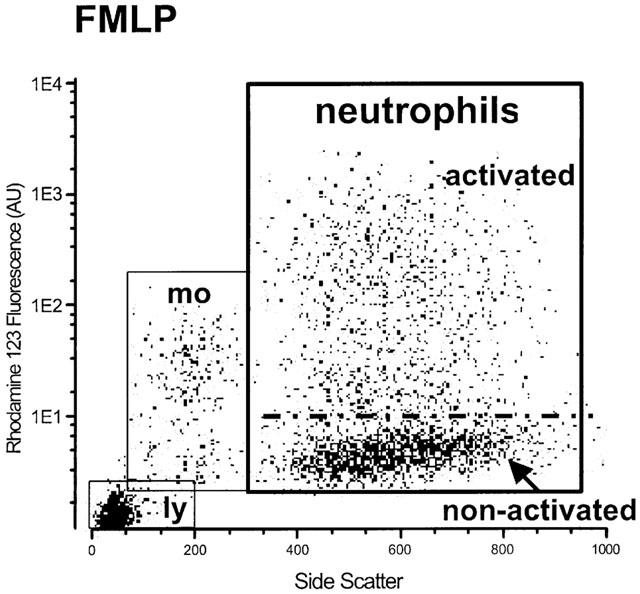
Flow cytometric analysis of the oxidative response in human neutrophils following stimulation with fMLP (100 nM). The Y-axis represents the production of hydrogen peroxide (rhodamine fluorescence) in arbitrary units (AU); the X-axis indicates the side scatter (SSC) as a marker of cellular granularity. The figure shows activated and non-activated neutrophils as well as monocytes (mo) and lymphocytes (ly).
The statistical difference between treatment groups in neutrophil and oocyte experiments was tested, where appropriate, using Student's t-test.
Results
fMLP receptors expressed in oocytes after single injection of fMLP-cRNA
Receptors, expressed in Xenopus oocytes by injection of fMLP-R98 cRNA alone, were stimulated by bath application of fMLP (100 nM) for 30 s (holding potential −70 mV). This resulted in a biphasic inward current (Figure 2). Current amplitudes following stimulation with 100 nM fMLP were, typically, 200 – 300 nA. Control oocytes (no cRNA injection) did not show any current response upon fMLP stimulation. Current-voltage analysis (Figure 2) showed that the fast current had a reversal potential around −25 mV and, at holding potentials more negative than −30 mV, showed outward rectification. The slow current showed weak inward rectification and it was not possible to establish the reversal potential.
Figure 2.
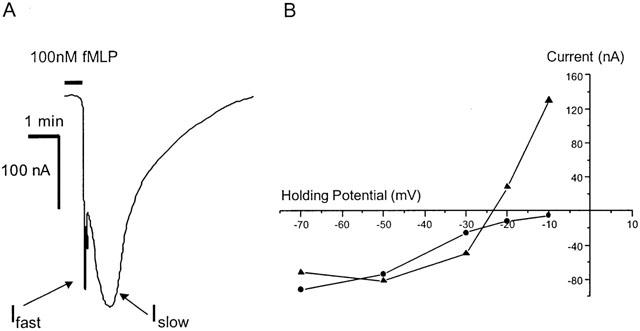
(A) An example of the biphasic current induced by stimulation (30 s) with fMLP (100 nM) of Xenopus oocytes injected with cRNA encoding the human fMLP-R98 receptor. The fast component (Ifast) typically had rise times of 3 to 10 s and the slow component (Islow) about 1 min. (B) An example of the current-voltage relationships for the fast current (solid triangles) and slow current (solid circles). The fast current exhibits a reversal potential at −24 mV and outward rectification at potentials less than −30 mV. The slow current exhibits weak inward rectification.
Analysis of the fMLP dose-response data from oocytes injected with fMLP-R98 cRNA alone gave EC50 values of 4.5 and 1.9 nM, with Hill coefficients of 1.0 for both, for the fast and slow currents, respectively (Figure 3).
Figure 3.
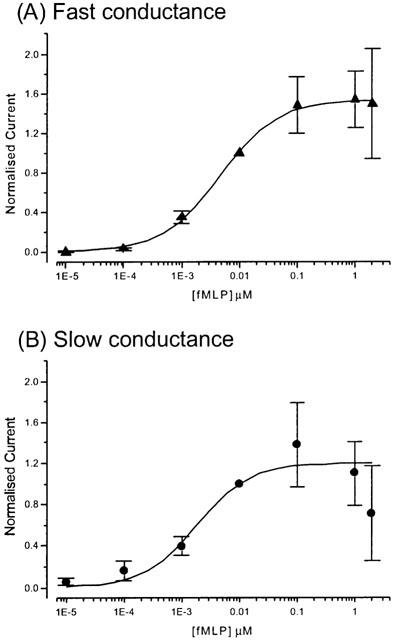
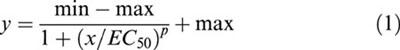 |
Injection of GTP-γ-S (40 nl at 200 μM) led to increases in the mean amplitudes of the fast and slow currents by approximately 30 fold (31.2±11.5; s.e.m., n=8) and 70 fold (72.6±29.6; s.e.m., n=8), respectively. To avoid an extended loss of holding potential, oocytes injected with GTP-γ-S were stimulated with fMLP using only 50 nM. Both the fast and slow currents remained unchanged on stimulation with fMLP (100 nM) after incubation of the oocytes with either cholera or pertussis toxin.
fMLP receptors expressed in oocytes after co-injection of cRNA encoding Gα-16
The currents obtained in oocytes co-injected with cRNA encoding both the fMLP receptor and the Gα-16 G-protein subunit after stimulation with fMLP (100 nM) showed variable responses and, in general, current amplitudes that were smaller (less than 150 nA) compared to single injected cells. Occasionally, monophasic outward currents (Figure 4A) were observed following stimulation with fMLP (100 nM), which had a reversal potential around −5 mV (Figure 4C). A combination of an inward and an outward current was also observed (Figure 4B,D). However, the majority of oocytes showed the biphasic inward current response as described above for oocytes expressing fMLP receptors alone. Accordingly, the remaining experiments were conducted using oocytes expressing fMLP receptors alone.
Figure 4.
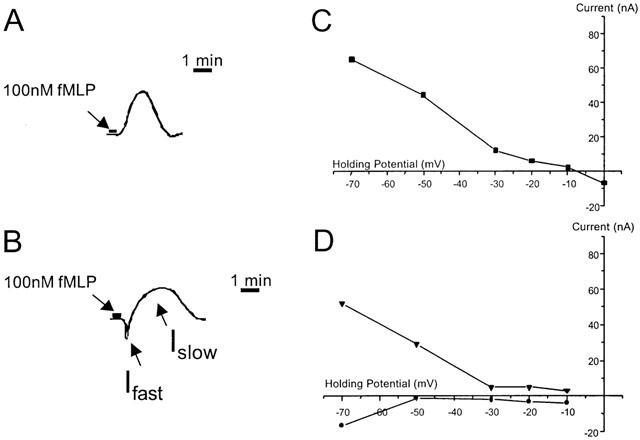
Examples of the monophasic outward currents (A) and combined inward (fast) / outward (slow) currents (B) induced by stimulation (30 s) with fMLP (100 nM) of Xenopus oocytes co-injected with cRNAs encoding Gα-16 G-protein subunit and the human fMLP-R98 receptor. The currents had rise times comparable with those obtained from oocytes injected with cRNA encoding fMLP-R98 alone (see Figure 2). The associated current-voltage relationships are also shown for the monophasic outward current (C) and the combined inward and outward currents (D).
Pharmacological manipulation of the response of fMLP receptors
The amplitude of the fMLP-induced fast current (100 nM fMLP) was reduced by about 80% in the presence of SITS (1 mM) (Figure 5; 0.16±0.05 (s.e.m., n=4); P<0.01).
Figure 5.
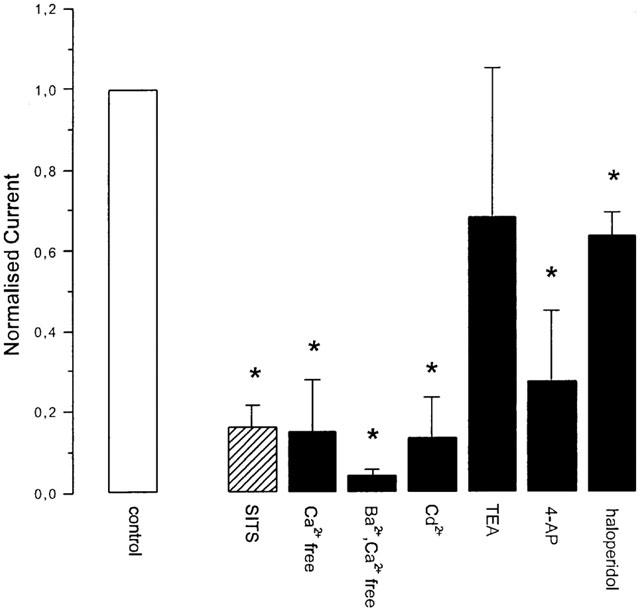
The effect of a variety of channel-blocking drugs ((mM): SITS 1, Ba2+ 2, Cd2+ 5, TEA 20, 4-AP 1, haloperidol 130 μM) on the amplitude of the fast and slow currents elicited by stimulation with 100 nM fMLP. The bars represent the mean normalized current (normalized to the current elicited prior to application of the channel-blocking drug) and the error bars represent the s.e.m. The striped bar represents the fast current and the solid bars represent the slow current. Significant changes (P<0.05, Student's t-test) in the amplitude of the currents are indicated by *. Detailed results are given in the text.
Stimulation with fMLP (100 nM) whilst perfusing the oocytes (at a holding potential of −70 mV) with a Ca2+-free ND96 resulted in a reduction in amplitude of the slow current by about 85% (Figure 5; 0.15±0.13 (s.e.m., n=3); P<0.01). When Ca2+ was replaced with Ba2+ the amplitude of the slow current was further reduced by some 10% (Figure 5; 0.042±0.0156 (s.e.m., n=5); P<0.01).
In the presence of Cd2+ (5 mM) the amplitude of the slow current was reduced by over 85% (Figure 5; 0.13±0.1 (s.e.m., n=4); P<0.01). In the presence of 4-aminopyridine (4-AP; 1 mM) the amplitude of the slow current was reduced by some 70% (Figure 5; 0.28±0.17 (s.e.m., n=6); P<0.01). The extent of the channel blockade by 4-AP increased progressively from 0 to 70% following four sequential stimulations with fMLP, separated by 2 min. In the presence of haloperidol (13 μM) the amplitude of the slow current was reduced by some 35% (Figure 5; 0.64±0.06 (s.e.m., n=6); P<0.01). The amplitude of the slow current was unchanged in the presence of TEA (20 mM).
Neutrophil responses
When stimulated with 100 nM fMLP in standard PBS, 49.3±2.6% (s.e.m., n=23) of the neutrophil population was activated, as measured by the production of hydrogen peroxide. The mean fluorescence of all neutrophils and of the activated neutrophils are given in Table 1.
Table 1.
Flow cytometric analysis of the oxidative response in human neutrophils following stimulation with fMLP (100 nM) in the presence of a variety of ion-channel blocking drugs
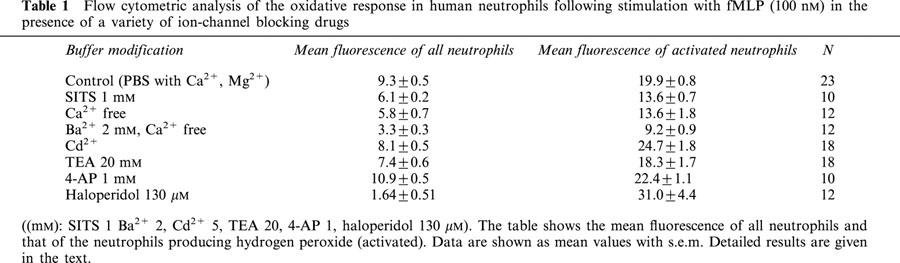
In the absence of calcium the proportion of neutrophils activated was reduced by some 56% (Figure 6, 21.9±1.8%; s.e.m., n=12, P<0.01). When calcium was replaced by barium (2 mM) the proportion of neutrophils activated was reduced by 87% (Figure 6, 6.3±0.3%; s.e.m., n=12, P<0.01).
Figure 6.
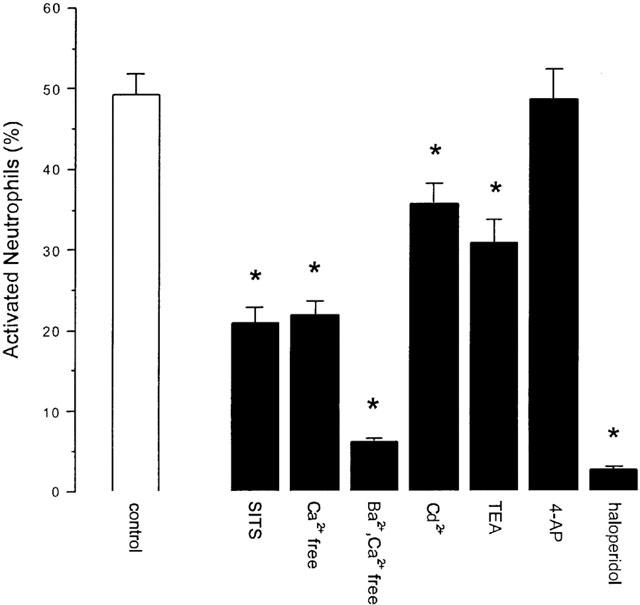
The effect of a variety of channel-blocking drugs ((mM): SITS 1, Ba2+ 2, Cd2+ 5. TEA 20, 4AP 1, haloperidol 130 μM) on the proportion of neutrophils activated to produce hydrogen peroxide (neutrophil respiratory burst) following stimulation with 100 nM fMLP. The bars represent the mean percentage of neutrophils activated and the error bars represent the s.e.m. Significant changes (P<0.05, Student's t-test) in the percentage activation are indicated by *. Detailed results are given in the text.
In the presence of the chloride channel-blocking drug SITS (1 mM) the proportion of neutrophils activated was reduced by 58% (Figure 6, 20.9±1.8%; s.e.m., n=10). Cadmium (5 mM), TEA (20 mM) and haloperidol (130 μM) reduced the proportion of neutrophils activated by 27, 37 and 98%, respectively (Figure 6, 35.8±2.5%, n=18, P<0.05; 30.9±2.8, n=18, P<0.05; 1.64±0.51, n=12, P<0.01; all s.e.m.). 4-AP was without effect (Figure 6).
Treatment of neutrophils with SITS (1 mM) or replacement of Ca2+ with Ba2+ significantly reduced both the mean fluorescence of all neutrophils and the fluorescence of the activated neutrophils (see Table 1). Treatment with Cd2+ (5 mM), TEA (20 mM) and 4-AP (1 mM) did not affect the mean fluorescence, either the total or that of the activated neutrophils (Table 1). In the presence of haloperidol (130 μM) the mean fluorescence of the activated neutrophils increased (31±4.4, s.e.m., n=12, P<0.01).
Discussion
We have demonstrated functional expression of human fMLP receptors (fMLP-R98) in oocytes after injection of the cRNA for that receptor alone. This is in marked contrast to previous studies that reported that co-expression of Gα-16 was necessary for functional expression (Burg et al., 1995; Schultz et al., 1992) although the same receptor variant was used.
The functional response (biphasic inward current) obtained in our experiments is specifically initiated by an agonist-receptor interaction because no response to fMLP could be induced in non-injected oocytes. The EC50 for fMLP in oocytes expressing fMLP-R98 after single injection of the cRNA encoding this receptor (4.5 and 1.8 nM for the fast and slow currents, respectively) is comparable to the EC50 obtained for the fMLP stimulated oxidative response in human neutrophils (13 nM, unpublished results). Earlier studies of fMLP binding to human neutrophils indicated the presence of both high and low affinity binding sites with Kd values of 2 and 180 nM, respectively (Atkinson et al., 1988). However, the stimulation of chemotaxis in neutrophils is apparently more sensitive with an EC50 of 0.07 nM (Prossnitz & Ye, 1997).
We conclude that the signal transduction in oocytes is mediated by an endogenous G-protein because application of GTP-γ-S resulted in a considerable increase in amplitude of both the fast and slow currents following stimulation with fMLP. It is likely the G-protein is a member of the Gq-class because it is not susceptible to either pertussis or cholera toxin.
Direct comparison of our results with those reported previously (Burg et al., 1995) is difficult. However, the shape of the current response described by Burg et al. (1995) is similar to those we obtained (see Figure 2). No assumption about the direction of the currents recorded by Burg et al. (1995) can be made from the orientation of their figure (no details are given in the text) because, in other publications from this research group, similar graphical presentation of currents have used both upward and downward orientations to illustrate currents obtained upon stimulation of C5a receptors, in equivalent experiments (Klos et al., 1994; Kroll et al., 1991).
We cannot explain why Burg et al. (1995) required the expression of the additional exogenous G-protein subunit to achieve functional expression. It is possible that diet or environmental factors in the maintenance of the Xenopus laevis play a part. However, conditions were standard; a 12-h light:dark cycle, a water temperature of 20°C and never more than six animals in each 80 litre tank. They were routinely fed on minced heart, although animals fed on Xenopus pellets also expressed functional receptors using the fMLP-R98 cRNA alone. It should be noted that in common with expression of different RNAs, both by us and by other groups (personal communication), functional expression during the months June to September was poor. The turnover of the endogenous G-protein that provides the coupling in our experiments seems complex. If oocytes were injected with fMLP-R98 cRNA more than 24 h after harvesting, functional expression was very erratic. This was observed even when oocytes from the same batch, which had been injected approximately 6 h after harvesting, had reliably expressed functional receptors. However, oocytes that reliably expressed functional receptors exhibited stable responses for up to 4 days. These observations may also contribute to the difference observed between the results we report and those of Burg et al. (1995). In summary, we found functional expression of fMLP receptors in oocytes most reliable without co-expression of the promiscuous G-protein.
The current-voltage analysis strongly suggests that the fast current is carried by Cl− because the chloride equilibrium potential in Xenopus oocytes is −23 mV (Faber & Korn, 1987). This is reinforced by the finding that the current is almost completely blocked by the chloride channel blocking drug SITS (Mitchell et al., 1997). The rapid activation of this current suggests that the ion channel may be directly activated by a G-protein subunit following stimulation of the receptor. These features, together with its activation by GTP-γ-S, suggests the channel may resemble the Maxi Cl− channel (Sun et al., 1992; Mitchell et al., 1997).
The properties exhibited by the slow current, weak inward rectification, Ca2+-sensitivity and blockade by Cd2+, 4-AP and haloperidol, suggest the involvement of a cation channel but do not immediately suggest a single ion channel. The time course of the response suggests it may arise as a result of the activation of an intracellular signalling pathway that leads to channel opening perhaps by phosphorylation by protein kinases or by direct activation by Ca2+. Oocytes are known to contain five channels that can pass K+ ions, three that are highly selective for K+ and two non-selective channels. The highly selective K+ channels are a large-conductance Ca2+-activated K+ channel (Krause et al., 1996), a slowly inactivating delayed rectifier K+ channel (Lu et al., 1990; Parker & Ivorra, 1990) and a Na+-activated K+ channel (Egan et al., 1992). The non-selective channels are a stretch-activated channel (Lane et al., 1993; Methfessel et al., 1986) and a channel that is regulated by the external concentration of divalent cations (Arellano et al., 1995), both of which are permeable to K+ and Na+. In addition, it is highly likely that Xenopus oocytes express Ca2+-activated non-selective (CAN) channels permeable to Na+ and K+ since these are found widely expressed in numerous tissues (including oocytes) of many different species (Swandulla & Partridge, 1990). In general, CAN channels show linear I – V relationships and slow activation. Our working hypothesis, therefore, is that in the oocyte stimulation of the fMLP receptor activates an endogenous Gq-protein that in turn activates inositol 1,4,5-triphosphate to cause the release of intracellular Ca2+. The released Ca2+ is then free to activate one, or more, of the Ca2+-activated cation channels.
One of the intracellular signalling pathways activated by stimulation of neutrophils with fMLP is the activation of PLC (phospholipase C) leading to the generation of IP3 and the release of intracellular calcium (Frohlich et al., 1998). In turn, store-operated calcium influx (SOCI) has been associated with the activation of neutrophils by a variety of inflammatory mediators (Hauser et al., 2001). Reducing [Ca2+]o by replacing calcium in the bathing medium with barium almost abolished neutrophil activation by fMLP. It has also been suggested that a part of the final pathway for the production of the oxidative burst is the initiation of NADPH-oxidase (nicotinamide adenine dinucleotide phosphate) by PKC (protein kinase C) (Frohlich et al., 1998). The activity of the PKC isoforms found in human neutrophils (α, β and γ) are calcium sensitive (Prossnitz & Ye, 1997). If SOCI is restricted then the activity of PKC would be reduced. This would be expected, in turn, to reduce the fluorescence of the activated neutrophils, as was observed. In human microglia SOCI is regulated by Cl− channels (McLarnon, et al., 2000). If this mechanism operates in human neutrophils then, following the argument used above, it would explain the reduction in activation and reduced fluorescence of activated neutrophils in the presence of the Cl−-channel blocking drug SITS.
Schumann et al. (1992) performed experiments using the whole-cell configuration of the patch-clamp technique to voltage-clamp single neutrophils. They showed that human neutrophils possess potassium and non-selective cationic channels. Stimulation of the neutrophils with fMLP (100 nM, 30 s) activated cationic currents that showed voltage dependence and weak outward rectification. Activation of the non-selective cationic channels in human neutrophils was shown to involve both Ca2+-dependent and -independent mechanisms. von tscharner et al. (1986) reported two separate voltage-dependent, non-selective cation channels in human neutrophils that were indirectly coupled to the fMLP receptor and whose activation was Ca2+-dependent. The varied effect of Cd2+, TEA and haloperidol on the response of neutrophils to stimulation with fMLP may reflect varying effects on non-specific cation channels that are contributing to the calcium influx.
In summary, we have shown that functional expression of fMLP receptors in Xenopus oocytes can be achieved using fMLP-R98 cRNA alone. Once expressed, these receptors couple to an endogenous G-protein that mediates a biphasic inward current, comprising a fast current carried by Cl− and a slower current carried by cations. In human neutrophils activation by fMLP is dependent on store-operated calcium influx which appears to be regulated by Cl− channels and linked, in part, to non-selective cation channels.
Xenopus oocytes have been used extensively in experiments aimed at understanding the mechanisms underlying the effects of general anaesthetics. These experiments have included many directed at the effect of anaesthetics on G-protein coupled receptors (see for example, Durieux, 1995; Hollmann et al., 2000). The attraction of the oocyte expression system as a model cell in which to study the effects of anaesthetics on the fMLP receptor is the potential to manipulate, independently, the receptor and the intracellular signalling pathway so as to dissect the separate effects of anaesthetics on these components. Hence our concern to characterize the nature of the response in detail. Whilst we accept that there are differences between the transduction of receptor binding to physiological effect in the oocyte and the neutrophil, we believe that a significant advance in our understanding of the interaction of anaesthetics and the fMLP receptor and its signalling pathway will result from experiments using the oocyte as a model system.
Acknowledgments
We thank Dr W. Bautsch (Hannover Medical School) for the gift of the cDNAs coding for the human fMLP (R98 variant) and for Gα-16.
Abbreviations
- 4-AP
4-aminopyridine
- DHR
dihydrorhodamine
- fMLP
N-formyl-L-methionyl-L-leucyl-phenylalanine
- FSC
forward scatter
- GTP-γ-S
guanosine 5′-O-3-thiotriphosphate
- PI
propidium iodide
- SITS
4-acetamido-4′-isothiocyanatostilbene-2,2′-disulphonic acid
- SNARF1/AM
carboxy-seminaphthorhodafluor-1-acetoxymethylester
- SSC
side scatter
- TEA
tetraethylammonium
References
- AMATRUDA T.T., STEELE D.A., SLEPAK V.Z., SIMON M.I. G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARELLANO R.O., WOODWARD R.M., MILEDI R. A monovalent cationic conductance that is blocked by extracellular divalent cations in Xenopus oocytes. J. Physiol. Lond. 1995;484:593–604. doi: 10.1113/jphysiol.1995.sp020689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON Y.H., MARASCO W.A., LOPEZ A.F., VADAS M.A. Recombinant human tumor necrosis factor-alpha. Regulation of N-formylmethionylleucylphenylalanine receptor affinity and function on human neutrophils. J. Clin. Invest. 1988;81:759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER E.L., FOROUHAR F.A., GRUNNET M.L., BOULAY F., TARDIF M., BORMANN B.J., SODJA D., YE R.D., WOSKA J.R., Jr, MURPHY P.M. Broad immunocytochemical localization of the formylpeptide receptor in human organs, tissues, and cells. Cell Tissue Res. 1998;292:129–135. doi: 10.1007/s004410051042. [DOI] [PubMed] [Google Scholar]
- BOMMAKANTI R.K., DRATZ E.A., SIEMSEN D.W., JESAITIS A.J. Extensive contact between Gi2 and N-formyl peptide receptor of human neutrophils: mapping of binding sites using receptor-mimetic peptides. Biochemistry. 1995;34:6720–6728. doi: 10.1021/bi00020a017. [DOI] [PubMed] [Google Scholar]
- BOULAY F., TARDIF M., BROUCHON L., VIGNAIS P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- BURG M., RAFFETSEDER U., GROVE M., KLOS A., KOHL J., BAUTSCH W. G alpha-16 complements the signal transduction cascade of chemotactic receptors for complement factor C5a (C5a-R) and N-formylated peptides (fMLF-R) in Xenopus laevis oocytes: G alpha-16 couples to chemotactic receptors in Xenopus oocytes. FEBS Lett. 1995;377:426–428. doi: 10.1016/0014-5793(95)01379-2. [DOI] [PubMed] [Google Scholar]
- CARP H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J. Exp. Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURIEUX M.E. Halothane inhibits signaling through ml muscarinic receptors expressed in Xenopus oocytes. Anesthesiology. 1995;82:174–182. doi: 10.1097/00000542-199501000-00022. [DOI] [PubMed] [Google Scholar]
- EGAN T.M., DAGAN D., KUPPER J., LEVITAN I.B. Na+-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res. 1992;584:319–321. doi: 10.1016/0006-8993(92)90913-t. [DOI] [PubMed] [Google Scholar]
- FABER D.S., KORN H. Voltage-dependence of glycine-activated Cl− channels: A potentiometer for inhibition. J. Neurosci. 1987;7:807–811. doi: 10.1523/JNEUROSCI.07-03-00807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROHLICH D., ROTHE G., SCHWALL B., SCHMITZ G., HOBBHAHN J., TAEGER K. Thiopentone and propofol, but not methohexitone nor midazolam, inhibit neutrophil oxidative responses to the bacterial peptide fMLP. Eur. J. Anaesthesiol. 1996;13:582–588. doi: 10.1046/j.1365-2346.1996.d01-405.x. [DOI] [PubMed] [Google Scholar]
- FROHLICH D., ROTHE G., WITTMANN S., SCHMITZ G., SCHMID P., TAEGER K., HOBBHAHN J. Nitrous oxide impairs the neutrophil oxidative response. Anesthesiology. 1998;88:1281–1290. doi: 10.1097/00000542-199805000-00020. [DOI] [PubMed] [Google Scholar]
- HAUSER C.J., FEKETE Z., ADAMS J.M., GARCED M., LIVINGSTON D.H., DEITCH E.A. PAF-mediated Ca2+ influx in human neutrophils occurs via store-operated mechanisms. J. Leukoc. Biol. 2001;69:63–68. [PubMed] [Google Scholar]
- HOLLMANN M.W., FISCHER L.G., BYFORD A.M., DURIEUX M.E. Local anesthetic inhibition of ml muscarinic acetylcholine signaling. Anesthesiology. 2000;93:497–509. doi: 10.1097/00000542-200008000-00030. [DOI] [PubMed] [Google Scholar]
- KLINKER J.F., WENZEL-SEIFERT K., SEIFERT R. G-Protein-coupled receptors in HL-60 human leukemia cells. Gen. Pharmacol. 1996;27:33–54. doi: 10.1016/0306-3623(95)00107-7. [DOI] [PubMed] [Google Scholar]
- KLOS A., MATJE C., RHEINHEIMER C., BAUTSCH W., KOHL J., MARTIN U., BURG M. Amino acids 327–350 of the human C5a-receptor are not essential for [125I]C5a binding in COS cells and signal transduction in Xenopus oocytes. FEBS Lett. 1994;344:79–82. doi: 10.1016/0014-5793(94)00350-5. [DOI] [PubMed] [Google Scholar]
- KRAUSE J.D., FOSTER C.D., REINHART P.H. Xenopus laevis oocytes contain endogenous large conductance Ca2+-activated K+ channels. Neuropharmacology. 1996;35:1017–1022. doi: 10.1016/0028-3908(96)00134-7. [DOI] [PubMed] [Google Scholar]
- KROLL B., EMDE M., JEROMIN A., PENNER L., RECHKEMMER G., KRETZSCHMAR T., KLOS A., KOHL J., BAUTSCH W. Functional expression of a human C5a receptor clone in Xenopus oocytes requires additional RNA. FEBS Lett. 1991;291:208–210. doi: 10.1016/0014-5793(91)81285-g. [DOI] [PubMed] [Google Scholar]
- LANE J.W., MCBRIDE D.W., Jr, HAMILL O.P. Ionic effects on amiloride block of the mechanosensitive channel in Xenopus oocytes. Br. J. Pharmacol. 1993;108:116–119. doi: 10.1111/j.1476-5381.1993.tb13449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU L., MONTROSE RAFIZADEH C., HWANG T.C., GUGGINO W.B. A delayed rectifier potassium current in Xenopus oocytes. Biophys. J. 1990;57:1117–1123. doi: 10.1016/S0006-3495(90)82632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARASCO W.A., PHAN S.H., KRUTZSCH H., SHOWELL H.J., FELTNER D.E., NAIRN R, BECKER E.L., WARD P.A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- MCLARNON J.G., HELM J., GOGHARI V., FRANCIOSI S., CHOI H.B., NAGAI A., KIM S.U. Anion channels modulate store-operated calcium influx in human microglia. Cell Calcium. 2000;28:261–268. doi: 10.1054/ceca.2000.0150. [DOI] [PubMed] [Google Scholar]
- METHFESSEL C., WITZEMANN V., TAKAHASHI T., MISHINA M., NUMA S., SAKMANN B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflügers Arch. –Eur. J. Physiol. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- MILEDI R., WOODWARD R.M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J. Physiol. 1989;416:601–622. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL C.H., WANG L., JACOB T.J.C. A large-conductance chloride channel in pigmented ciliary epithelial. J. Membr. Biol. 1997;158:167–175. doi: 10.1007/s002329900254. [DOI] [PubMed] [Google Scholar]
- MURPHY P.M., MCDERMOTT D. Functional expression of the human formyl peptide receptor in Xenopus oocytes requires a complementary human factor. J. Biol. Chem. 1991;266:12560–12567. [PubMed] [Google Scholar]
- PARKER I., IVORRA I. A slowly inactivating potassium current in native oocytes of Xenopus laevis. Proc. R. Soc. Lond. B Biol. Sci. 1990;238:369–381. doi: 10.1098/rspb.1990.0005. [DOI] [PubMed] [Google Scholar]
- PROSSNITZ E.R., YE R.D. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol. Therap. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- ROTHE G., VALET G. Flow cytometric assays of oxidative burst activity in phagocytes. Meth. Enzymol. 1994;233:539–548. doi: 10.1016/s0076-6879(94)33059-x. [DOI] [PubMed] [Google Scholar]
- SCHULTZ P., STANNEK P., VOIGT M., JAKOBS K.H., GIERSCHIK P. Complementation of formyl peptide receptor-mediated signal transduction in Xenopus laevis oocytes. Biochem. J. 1992;284:207–212. doi: 10.1042/bj2840207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUMANN M.A., TANIGAKI T., HELLER D.N., RAFFIN T.A. Ca(2+)-dependent and Ca(2+)-independent mechanisms modulate whole-cell cationic currents in human neutrophils. Biochem. Biophys. Res. Commun. 1992;185:531–538. doi: 10.1016/0006-291x(92)91657-c. [DOI] [PubMed] [Google Scholar]
- SUN X.P., SUPPLISSON S., TORRES R, SACHS G., MAYER E. Characterization of large-conductance chloride channels in rabbit colonic smooth muscle. J. Physiol. Lond. 1992;448:355–382. doi: 10.1113/jphysiol.1992.sp019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANDULLA D., PARTRIDGE L.D.Non-specific cation channels Potassium Channels: Structure, classification, function and therapeutic potential 1990Chichester: Ellis Horwood Ltd; 167–180.ed., Cook, N.S. pp [Google Scholar]
- VON TSCHARNER V., PROD'HOM B., BAGGIOLINI M., REUTER H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. Nature. 1986;324:369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


