Abstract
Activated Notch1 (AcN1) alleles cooperate with oncogenes from DNA tumor viruses in transformation of epithelial cells. AcN1 signaling has pleiotropic effects, and suggested oncogenic roles include driving proliferation through cyclin D1 or the generation of resistance to apoptosis on matrix withdrawal through a phosphatidylinositol 3-kinase (PI3K)-PKB/Akt-dependent pathway. Here, we extend the antiapoptotic role for AcN1 by showing inhibition of p53-induced apoptosis and transactivation. Chemical inhibitors of the PI3K pathway block AcN1-induced inhibition of p53-dependent apoptosis and nuclear localization of Hdm2. We show that expression of wild-type p53 does not inhibit synergistic transformation by AcN1 and human papillomavirus E6 and E7 oncogenes. We suggest that activation of Notch signaling may serve as an additional mechanism to inhibit wild-type p53 function in papillomavirus-associated neoplasia.
Human cervical tumors represent a major subset of female cancers in the developing world. These tumors are characterized by the persistent expression of the E6 and E7 oncogenes of the high-risk type 16 and 18 human papillomaviruses (HPV-16 and -18) (34). A range of observations have revealed the central roles of these two proteins in immortalization of human epithelial cells and their continuing requirement for persistent tumor progression in vivo (18, 21, 29). Invasive cervical tumors also consistently reveal the intracellular expression of Notch1 and Notch2 proteins, suggestive of deregulated Notch signaling (5, 33). Further, ligands of Notch are expressed in human cervical cancers (10). A functional role for deregulated Notch signaling has been suggested in the context of cervical neoplasias, as activated alleles of the Notch1 gene synergize with E6 and E7 oncogenes in in vitro transformation assays (24), analogous to the synergy with adenovirus E1A (4). Suggested roles for Notch1 in regulating epithelial tumor progression are induction of proliferation through transcriptional upregulation of cyclin D1 (25) and inhibition of apoptosis by activation of the phosphatidylinositol 3-kinase (PI3K)-PKB/Akt pathway (24).
One of the well-documented functions of the E6 protein is to bind and degrade p53 (34). This is believed to inhibit the function of the p53 pathway, as HPV-associated tumors have a low frequency of mutant p53 alleles. The major consequence of inhibiting p53 function is to inhibit a key inducer of apoptosis in transformed cells. Paradoxically, many cervical tumors often reveal detectable levels of p53 (22, 14), possibly reflecting incomplete degradation by E6. Alternative possibilities to explain the detection of the p53 protein include the presence of variants of E6 incapable of degrading p53 (16) and the presence of feedback loops that lead to high p53 levels consequent to E6 expression. In addition, there are reports suggesting that the E7 protein may have a role in stabilizing p53 levels (7).
In addition to being inhibited by viral oncogenes, p53 function is negatively regulated by cellular gene products such as Hdm2 (15). This process is regulated in part by a feedback loop since Hdm2 is a transcriptional target for p53. Activation of PI3K signaling through PKB/Akt can also activate Hdm2 and thus can negatively regulate p53 function (1, 9, 20), apart from modulating apoptosis by phosphorylating proteins on serine or threonine residues to potentiate cell survival proteins such as Bad, caspase 9, GSK3, etc. (3, 6). In this study, we have examined a potential link between activated Notch1 (AcN1) and p53 function mediated through the PI3K pathway.
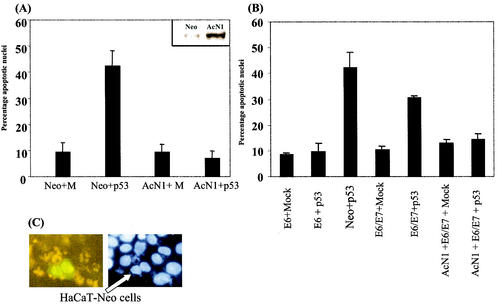
The human Notch1 was originally cloned from a chromosomal rearrangement generated in a human T-cell acute lymphoblastic leukemia cell line (8). The truncated allele generates a constitutively active intracellular form of Notch1 protein (2). We have shown that expression of AcN1 in HaCaT cells induces resistance to anoikis (apoptosis induced on matrix withdrawal) and sustains colony formation by HPV-16 E6 and E7 oncogenes in soft-agar assays (24). HaCaT is an HPV-negative immortalized line with mutant p53 alleles and stable expression of the mutant protein (17). Consistent with previous reports (23), we find that expression of wild-type p53 in HaCaT cells induced apoptosis (Fig. 1A). Transfection of wild-type p53 in HaCaT-Neo cells induced apoptosis in 40 to 45% of transfected cells by microscopy (Hoechst-stained cells were analyzed for chromatin condensation and nuclear fragmentation as in reference 24). However in HaCaT-AcN1 cells, no apoptosis induction was detected following expression of p53 (Fig. 1A). Similarly, HaCaT-Neo cells cotransfected with both AcN1 (AcN1-green fluorescent protein [GFP]) and p53 showed resistance to p53-induced apoptosis (Fig. 1C).
FIG. 1.
(A and B) Graphs showing percentages of apoptotic nuclei in transfected cells. (A) pCDNA3 (Mock [M]) and pCDNA3-AcN1 (AcN1) were used to generate HaCaT-Neo and HaCaT-AcN1 cells, respectively, by selecting with G-418 and pooling different clones. Inset, Notch1 overexpression in HaCaT-AcN1 stable cell lines compared to that in Neo-expressing cells. These cells were transfected with various combinations of plasmids. Mock (pCDNA3) or pCEP4-p53 along with pEGFPN1 was transfected in a ratio of 5:1 (2.5 μg of Mock or p53 plasmid with 0.5 μg of tracer GFP plasmid). GFP-positive cells were counted 24 h after transfection, counterstained with bisbenzamide (Hoechst), and assessed microscopically for nuclear condensation and chromatin fragmentation. Typically 100 GFP-positive cells were scored for apoptotic features. (B) HaCaT-E6 (lanes 1 and 2 [counting from the left]) and HaCaT-E6 and -E7 (lanes 4 and 5) cells expressing HPV-16 oncogenes were transfected with Mock (pCDNA3) or pCEP4-p53 along with pEGFPN1. HaCaT-AcN1 (lanes 6 and 7) cells were transfected with E6 and E7 plasmids along with Mock (pCDNA3) or pCEP4-p53. Standard deviations from the means of at least three independent experiments are shown for panels A and B. (C) HaCaT-Neo cells were transiently transfected with AcN1-GFP (fusion of AcN1 and GFP coding sequences in pEGFPN1 [gift from A. Sarin and H. Sade, National Centre for Biological Sciences], which was generated from a plasmid expressing AcN1 given by A. Rangarajan, Whitehead Institute) and the p53 vector in a ratio of 1:5 (0.5 μg of AcN1-GFP and 2.5 μg of p53). Adherent cells 14 h after transfection were fixed with 4% paraformaldehyde and observed under the fluorescence microscope. Ten minutes before fixation, bisbenzamide was added to the medium and the mixture was incubated at 37°C (Hoechst). Cells that were AcN1-GFP positive (nuclear GFP; arrow) were resistant to p53-induced cell death, while the controls transfected with pEGFPN1 and p53 showed around 40% cell death.
Expression of the E6 protein prevented the induction of apoptosis caused by p53 (Fig. 1B), as suggested from the well-documented link between these two proteins (26). There are conflicting reports on the role of E7 in regulating the function of p53. Consistent with the suggestion of Hickman et al. (12), we find that, on expression of both E7 and E6, there is p53-induced apoptosis of around 30% (Fig. 1B). However, AcN1 blocks the induction of p53-dependent apoptosis even in the additional presence of E7 (Fig. 1B).
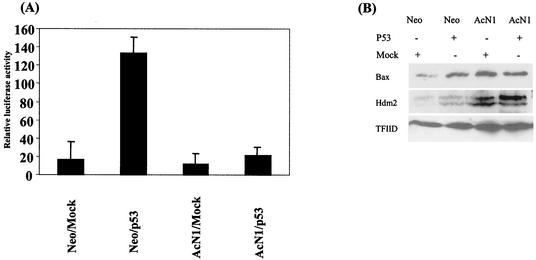
Induction of apoptosis by p53 can be through the transcriptional upregulation of proapoptotic proteins such as Bax or downregulation of prosurvival proteins such as Bcl2 and Bcl-xl (28). Transactivation-independent mechanisms that have been suggested include a direct interaction of the p53 protein in the mitochondria (11, 19, 27). We evaluated the activation of a p53-dependent reporter and find that AcN1 reduces the induction by sevenfold (Fig. 2A). Correspondingly, in the presence of p53, we find a 1.5-fold overexpression of the Bax protein in HaCaT-Neo cells and no increase in HaCaT-AcN1 cells (Fig. 2B). We evaluated the expression of Hdm2, another protein that is transcriptionally upregulated by p53, and find a similar phenomenon. There are 2- and 1.2-fold inductions of Hdm2 by p53 in the absence and presence of AcN1, respectively (Fig. 2B).
FIG. 2.
(A) Activation of the p53-dependent PG13-Luc reporter. HaCaT-Neo and HaCaT-AcN1 were transfected with either Mock (pCDNA3) or pCEP4-p53 (2 μg of DNA) along with the p53 reporter (0.5 μg of DNA). Renilla luciferase (0.2 μg of DNA) was used to normalize for transfection. Error bars, standard deviations from the means for three independent experiments. The assay was done 48 h after transfection. (B) Western blot showing Bax (N-20 [Santa Cruz Biotechnology]) and Hdm2 (AB-1 [Oncogene]) (Hdm2 dimer at 95 kDa) protein expression. Briefly, cells were lysed with 1% sodium dodecyl sulfate-1.0 mM sodium vanadate in 10 mM Tris (pH 7.4) lysis buffer heated to 95°C. Fifty micrograms of total protein, by the Bradford estimation method, was loaded into each well of a 10% polyacrylamide gel. Plasmids (1.5 μg of Mock [Neo] and p53 plasmids) were transfected in Neo- and AcN1-expressing cells. TFIID (SI-1 [Santa Cruz Biotechnology]) was used as a loading control.
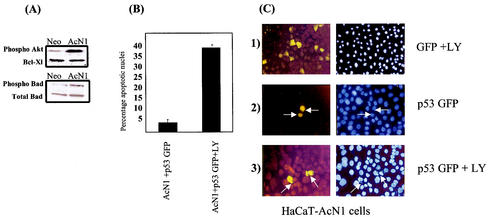
Constitutively activated forms of PKB/Akt cooperated with HPV-16 E6 and E7 oncogenes in in vitro transformation assays to the same extent as AcN1 (24). Further, we have earlier reported the detection of phosphorylated forms of PKB/Akt in detached cells in the presence of AcN1 (24). Addition of chemical inhibitors to PI3K induces anoikis and eliminates the phosphorylation of PKB/Akt (24). Here, we extended this analysis and find that, even in adherent cells, there is a markedly higher level of phosphorylated PKB/Akt consequent to AcN1 expression (Fig. 3A). We also evaluated levels of Bad, a downstream target of phosphorylated PKB/Akt. When phosphorylated by Akt, Bad is unable to interact with Bcl-xl (6), restoring Bcl-xl's antiapoptotic function. In the AcN1-expressing cells, levels of Bad phosphorylated at serine 136 were significantly higher (twofold) than those in Neo cells (Fig. 3A). However, there was no difference in levels of the antiapoptotic Bcl-xl protein and total Bad protein between Neo- and AcN1-expressing cells (Fig. 3A). Addition of LY294002 to HaCaT-AcN1 cells grown on coverslips and transfected with p53-GFP eliminated resistance to p53-induced apoptosis. Results of the assay are shown in Fig. 3B, and representative images are shown in Fig. 3C. Apoptosis was induced in around 40% of the p53-GFP-transfected AcN1 cells when treated with LY294002 (Fig. 3C). Similar results were obtained with wortmannin, another chemical inhibitor of PI3K (data not shown).
FIG. 3.
Blocking PI3K eliminates resistance to p53-induced apoptosis. (A) Western blot showing endogenous phospho-Akt (antibody against Ser472, Ser473, and Ser474 [BD transLab]), phospho-Bad (antibody against Ser136 [CST]), total Bad (CST), and Bcl-Xl (S-18 [Santa Cruz Biotechnology]) protein levels in HaCaT-Neo and HaCaT-AcN1 cell lysates. (B and C) PI3K inhibitor LY294002 eliminates resistance to p53-induced apoptosis. (B) Histogram showing apoptotic nuclei from HaCaT-AcN1 cells transfected with p53-GFP and treated with LY294002. Adherent cells were transfected with p53-GFP and treated as in Fig. 1C. (C) Representative images of the histogram in panel B. Neither pEGFPN1 (section 1) nor p53-GFP (section 2) induces apoptosis (arrows). Addition of LY294002 (20 μM) (section 3) induces apoptosis in these cells in the presence of p53-GFP (arrows).
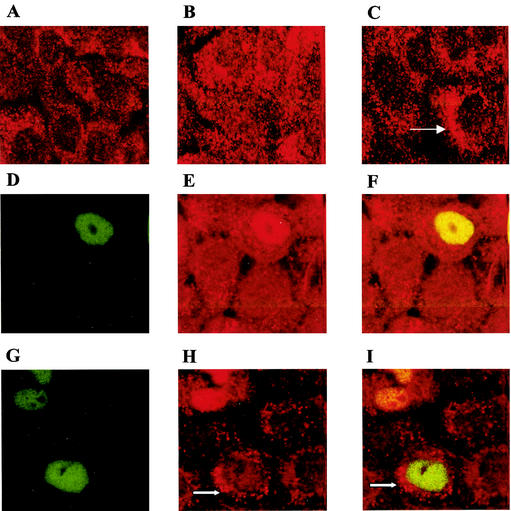
PKB/Akt has recently been shown to phosphorylate Hdm2 and thus inhibit p53 activation. By confocal imaging we show that HaCaT-Neo cells express endogenous Hdm2 predominantly in the cytoplasm (Fig. 4A), in keeping with very low levels of phosphorylated PKB/Akt. HaCaT-AcN1 cells exhibit the Hdm2 protein in both the nucleus and cytosol (Fig. 4B). Addition of LY294002 restores the predominantly cytosolic localization of Hdm2 (Fig. 4C). This suggests that activation of Hdm2 by PKB/Akt leads to nuclear localization. Hdm2 levels per se in HaCaT cells are fairly low, in keeping with the absence of wild-type p53 protein. Expression of wild-type p53-GFP shows colocalization with Hdm2 in the nucleus in all HaCaT-AcN1 p53-GFP-positive cells. On addition of LY294002, cytosolic Hdm2 can be detected in around 30% of p53-GFP-positive HaCaT-AcN1 cells. It should be noted that on overexpression of wild-type p53 in HaCaT, Hdm2 can be detected in the nucleus even in the absence of detectable phosphorylated PKB/Akt, possibly due to low levels of phosphorylated PKB/Akt in these cells.
FIG. 4.
Hdm2 localization in HaCaT-Neo (A) and HaCaT-AcN1 (B to I) cells. Briefly, cells grown on coverslips were fixed in 4% paraformaldehyde for 10 min at room temperature, permeabilized for 1 h in phosphate-buffered saline (PBS) containing 10% goat serum and 0.3% Triton X-100, and incubated overnight with the Hdm2 (AB-1 [Oncogene]) antibody at 4°C. An anti-mouse red fluorescent antibody was then added after washes with 1× PBS, and the cells were incubated for 1 h. The cells were washed again in 1× PBS and visualized under a confocal microscope. (A) HaCaT-Neo cells have a predominantly cytosolic localization of Hdm2. (B) HaCaT-AcN1 cells have Hdm2 localized both in the nucleus and the cytosol. (C) All HaCaT-AcN1 cells treated with LY294002 have Hdm2 localized in the cytoplasm (arrow). (D to F) Wild-type p53-GFP was visualized in the nucleus in transfected AcN1 cells (D; green). Hdm2 is overexpressed in these cells (E; red) and colocalizes with p53 (F; yellow). (G to I) LY294002 was added to HaCaT-AcN1 cells transfected with p53-GFP. p53-GFP remains localized in the nucleus (G; green). Hdm2 (H; red) localizes in the cytosol in 30% of the GFP-positive cells 10 h after transfection (arrow). No colocalization seen in these cells (arrow [I]).
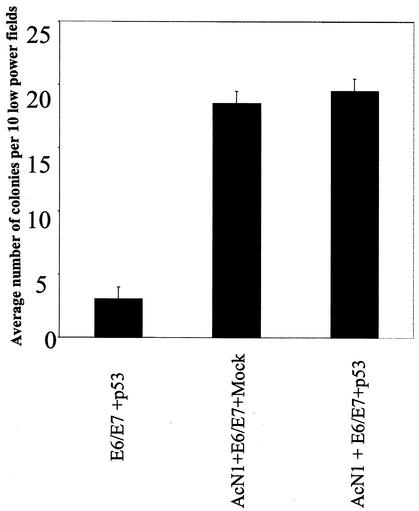
The results obtained so far show that AcN1 can inhibit p53-induced apoptosis and that this is dependent on a PI3K-PKB/Akt-dependent pathway. We suggest that there is a bifurcation downstream of PKB/Akt, with two components of a prosurvival mechanism that includes both the upregulation of genes such as the Bad gene and subcellular relocalization of Hdm2. The importance of these observations lies in the potential inability of p53 to block transformation by HPV-16 E6 and E7 oncogenes in the presence of AcN1. Expression of AcN1 along with E6 and E7 typically yields a three- to fivefold increase in soft-agar colonies (24) of HaCaT cells. Expression of p53 does not block this increase (Fig. 5).
FIG. 5.
Histogram showing numbers of colonies observed in in vitro soft-agar transformation assays. Details of soft-agar colony formation assay are given in reference 24. Values are means of two independent experiments with standard deviations. Bar 1 (counting from the left), HaCaT-E6/E7 cells transfected with p53; bars 2 and 3, HaCaT-AcN1 cells transfected with E6 and E7 and Mock and with p53, respectively.
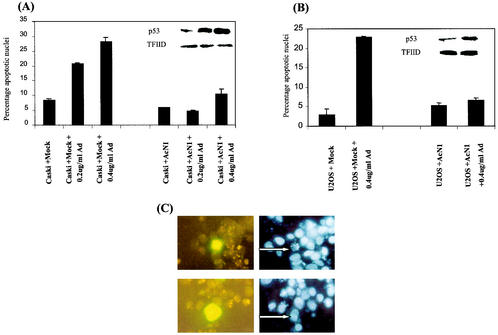
To extend the above observations with cell lines that express endogenous wild-type p53, we used both Caski and U2OS cells (Fig. 6) and induced p53-dependent apoptosis with doxorubicin (Adriamycin) (31). Caski and U2OS are cell lines derived from an HPV-16-positive cervical tumor and osteosarcoma, respectively. Caski cells have been shown to accumulate p53 on treatment with actinomycin D and leptomycin B, resulting in apoptotic death of these cells (13). Here, apoptosis dependent on p53 expression was induced by doxorubicin. AcN1 or control (Mock-) transfected cells were identified by coexpressing GFP. Doxorubicin at 0.2 and 0.4 μg/ml in Caski cells led to 20 and 25 to 30% apoptosis, respectively (Fig. 6A). Expression of AcN1 in these cells reduces apoptosis to 5 and 10% with 0.2 and 0.4 μg of doxorubicin/ml, respectively (Fig. 6A). U2OS cells were insensitive to the lower doxorubicin concentration of 0.2 μg/ml. However, around 20% of the transfected cells showed distinct apoptotic morphology at 0.4 μg of doxorubicin/ml, and AcN1 reduced apoptosis in these cells to 5% (Fig. 6B). Insets in Fig. 6A and B show induction of p53 in these cells after addition of doxorubicin, and representative pictures are shown in Fig. 6C. The results in Fig. 6 show that expression of activated Notch1 blocks p53-dependent apoptosis induced by doxorubicin in transformed cell lines irrespective of the expression of HPV oncogenes.
FIG. 6.
Activated Notch1 blocks apoptosis induced in the context of endogenous p53. Caski cells (cervical cancer-derived cell line; A) and U2OS cells (osteosarcoma cell line; B) were transfected with Mock (pCDNA3) or AcN1 expression vector along with a tracer AcNI-GFP vector in the ratio of 5:1 as mentioned previously. A total of 3 μg of total DNA was transfected in each 35-mm-diameter dish with Lipofectamine. Twenty-four hours after transfection, doxorubicin (Ad) was added to a final concentration of 0.2 or 0.4 μg/ml to induce p53 expression in these cells. Fourteen hours after addition of doxorubicin, cells were trypsinized and stained with bisbenzamide (Hoechst) and GFP-positive cells were assessed microscopically for nuclear condensation and/or chromatin fragmentation. Typically 100 GFP-positive cells were scored for apoptotic features. U2OS cells are not sensitive to 0.2 μg/ml. Values are means of two independent experiments with standard deviations. Insets show Western blot analysis for p53 expression (FL-393 [Santa Cruz Biotechnology]) from lysate prepared from a parallel set. (A, inset) Lane 1 (counting from the left), endogenous p53 expression in Caski cells; lanes 2 and 3, graded increase in p53 levels with increasing concentration of doxorubicin. TFIID is used as a loading control. (B, inset) Lane 1, endogenous p53 expression in U2OS cells; lane 2, increase in p53 levels with increasing concentration of doxorubicin. TFIID is used as a loading control. (C) Upper sections show representative pictures of AcN1-GFP-positive U2OS cells (cotransfected with the Mock plasmid) clearly showing apoptotic nuclei (arrow) after induction of p53 by doxorubicin. Bottom sections shows AcN1-transfected U2OS cells resistant to apoptosis, as marked by normal nuclei (arrow) after induction of p53 by doxorubicin. Left and right sections show GFP and Hoechst UV fluorescence, respectively.
Features of deregulated Notch signaling are consistently observed in the progression of high-grade cervical tumor precursors to invasive tumors (5). In addition to the cooperative transformation that we have noted in HaCaT cells, we have recently analyzed d1DR cells, which are immortalized by using the HPV-16 genome. On coexpression of AcN1 and HPV 16 E6 and E7 oncogenes, these cells generate progressive tumors in vivo (O. Chakrabarti et al., unpublished data). Consistent with these observations, the use of an antisense construct against Notch1 in Caski cells, which are derived from a cervical tumor, inhibited growth in vivo (32). In contrast, recent observations from Talora et al. suggest that Notch1, in contrast to Notch2, may be downregulated in late-stage cervical tumors (30). They suggest that adenovirus Notch1 may negatively regulate the HPV-16 promoter, with consequent growth arrest (30), in cervical tumor-derived lines including Caski. Thus the role of the Notch pathway may involve a complex interplay of signals that both sustain and inhibit aspects of tumor progression.
In this study, we have shown that, in an immortalized human epithelial cell line, activation of Notch1 signaling prevents apoptosis induced on p53 overexpression. Our results extend the link between activated Notch signaling and consequent induction of the PI3K-PKB/Akt pathway as a mediator of apoptosis resistance. Further, in transformed cells, we show that activated Notch1 signaling prevents cell death induced by a DNA-damaging agent.
Expression of HPV-16 E6 and E7 oncogenes with activated alleles of the Notch1 gene sustains transformation even in the presence of p53. This is consistent with the inhibition of p53 function by activated Notch1 in the process of transformation. In addition, the introduction of activated Notch1 alleles inhibits the induction of apoptosis induced by DNA damage in an HPV-16-positive cervical tumor line. Collectively, the results in this study strengthen the evidence of a potential role for Notch signaling in the context of neoplasias such as cancers of the cervix. It would be informative to analyze the link between the various Notch genes and the p53 pathway in diverse cellular contexts.
Acknowledgments
We thank Hubert Stöppler and Melissa Conrad Stöppler for pMSII ref-E6/E7, Denise Galloway for the pLXSN-E6 plasmid, Jon C. Aster for pcDNA3-AcN1, Tyler Jacks for p53-GFP plasmids, and Hadassah Sade and Apurva Sarin for the gift of AcN1-GFP, which was modified from a plasmid given by Annapoorni Rangarajan. We also thank Karthikeyan Veeraraghavalu for the blot showing levels of Notch1 in AcN1 and Neo cells and Vignesh Kumar G and Sai Padmavathy Ravikumar for technical assistance.
Pradip Nair was supported by a DST fast-track young scientist grant.
REFERENCES
- 1.Ashcroft, M., R. L. Ludwig, D. B. Woods, T. D. Copeland, H. O. Weber, E. J. MacRae, and K. H. Vousden. 2002. Phosphorylation of HDM2 by Akt. Oncogene 21:1955-1962. [DOI] [PubMed] [Google Scholar]
- 2.Aster, J. C., and W. S. Pear. 2001. Notch signaling in leukemia. Curr. Opin. Hematol. 8:237-244. [DOI] [PubMed] [Google Scholar]
- 3.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 4.Capobianco, A. J., P. Zagouras, C. M. Blaumueller, S. Artavanis-Tsakonas, and J. M. Bishop. 1997. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol. 17:6265-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel, B., A. Rangarajan, G. Mukherjee, E. Vallikad, and S. Krishna. 1997. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J. Gen. Virol. 78:1095-1101. [DOI] [PubMed] [Google Scholar]
- 6.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 7.Eichten, A., M. Westfall, J. A. Pietenpol, and K. Munger. 2002. Stabilization and functional impairment of the tumor suppressor p53 by the human papillomavirus type 16 E7 oncoprotein. Virology 295:74-85. [DOI] [PubMed] [Google Scholar]
- 8.Ellisen, L. W., J. Bird, D. C. West, A. L. Soreng, T. C. Reynolds, S. D. Smith, and J. Sklar. 1991. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66:649-661. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb, T. M., J. F. Leal, R. Seger, Y. Taya, and M. Oren. 2002. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 21:1299-1303. [DOI] [PubMed] [Google Scholar]
- 10.Gray, G. E., R. S. Mann, E. Mitsiadis, D. Henrique, M. L. Carcangiu, A. Banks, J. Leiman, D. Ward, D. Ish-Horowitz, and S. Artavanis-Tsakonas. 1999. Human ligands of the Notch receptor. Am. J. Pathol. 154:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haupt, Y., S. Rowan, E. Shaulian, K. H. Vousden, and M. Oren. 1995. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 9:2170-2183. [DOI] [PubMed] [Google Scholar]
- 12.Hickman, E. S., S. Bates, and K. H. Vousden. 1997. Perturbation of the p53 response by human papillomavirus type 16 E7. J. Virol. 71:3710-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hietanen, H., S. Lain, E. Krausz, C. Blattner, and D. P. Lane. 2000. Activation of p53 in cervical carcinoma cells by small molecules. 97:8501-8506. [DOI] [PMC free article] [PubMed]
- 14.Huang, L. W., Y. Y. Chou, S. L. Chao, T. J. Chen, and T. T. Lee. 2001. p53 and p21 expression in precancerous lesions and carcinomas of the uterine cervix: overexpression of p53 predicts poor disease outcome. Gynecol. Oncol. 83:348-354. [DOI] [PubMed] [Google Scholar]
- 15.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., Y. Zhao, G. Meng, M. Zeng, S. Srinivasan, L. M. Delmolino, Q. Gao, G. Dimri, G. F. Weber, D. E. Wazer, H. Band, and V. Band. 2002. Human papillomavirus oncoprotein E6 inactivates the transcriptional coactivator human ADA3. Mol. Cell. Biol. 22:5801-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman, T. A., R. Modali, P. Boukamp, J. Stanek, W. P. Bennett, J. A. Welsh, R. A. Metcalf, M. R. Stampfer, N. Fusenig, E. M. Rogan, et al. 1993. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis 14:833-839. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani, F., and L. Banks. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874-7887. [DOI] [PubMed] [Google Scholar]
- 19.Marchenko, N. D., A. Zaika, and U. M. Moll. 2000. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 275:16202-16212. [DOI] [PubMed] [Google Scholar]
- 20.Mayo, L. D., and D. B. Donner. 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98:11598-11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munger, K., J. R. Basile, S. Duensing, A. Eichten, S. L. Gonzalez, M. Grace, and V. L. Zacny. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888-7898. [DOI] [PubMed] [Google Scholar]
- 22.Nair, P., N. K. Nair, P. G. Jayaprakash, and M. R. Pillai. 1999. Decreased programmed cell death in the uterine cervix associated with high risk human papillomavirus infection. Pathol. Oncol. Res. 5:95-103. [DOI] [PubMed] [Google Scholar]
- 23.Paramio, J. M., C. Segrelles, S. Lain, E. Gomez-Casero, D. P. Lane, E. B. Lane, and J. L. Jorcano. 2000. p53 is phosphorylated at the carboxyl terminus and promotes the differentiation of human HaCaT keratinocytes. Mol. Carcinog. 29:251-262. [DOI] [PubMed] [Google Scholar]
- 24.Rangarajan, A., R. Syal, S. Selvarajah, O. Chakrabarti, A. Sarin, and S. Krishna. 2001. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology 286:23-30. [DOI] [PubMed] [Google Scholar]
- 25.Ronchini, C., and A. J. Capobianco. 2001. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic). Mol. Cell. Biol. 21:5925-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 27.Schuler, M., and D. R. Green. 2001. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 29:684-688. [DOI] [PubMed] [Google Scholar]
- 28.Somasundaram, K. 2000. Tumor suppressor p53: regulation and function. Front. Biosci. 5:D424-D437. [DOI] [PubMed] [Google Scholar]
- 29.Storey, A., and L. Banks. 1993. Human papillomavirus type 16 E6 gene cooperates with EJ Ras to immortalize primary human cells. Oncogene 8:919-924. [PubMed] [Google Scholar]
- 30.Talora, C., D. C. Sgroi, C. P. Crum, and G. P. Dotto. 2002. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 16:2252-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tergaonkar, V., M. Pando, O. Vafa, G. Wahl, and I. Verma. 2002. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell 1:493-503. [DOI] [PubMed] [Google Scholar]
- 32.Weijzen, S., P. Rizzo, M. Braid, R. Vaishnav, S. M. Jonkheer, A. Zlobin, B. A. Osborne, S. Gottipati, J. C. Aster, W. C. Hahn, M. Rudolf, K. Siziopikou, W. M. Kast, and L. Miele. 2002. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat. Med. 8:979-986. [DOI] [PubMed] [Google Scholar]
- 33.Zagouras, P., S. Stifani, C. M. Blaumueller, M. L. Carcangiu, and S. Artavanis-Tsakonas. 1995. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc. Natl. Acad. Sci. USA 92:6414-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]