Abstract
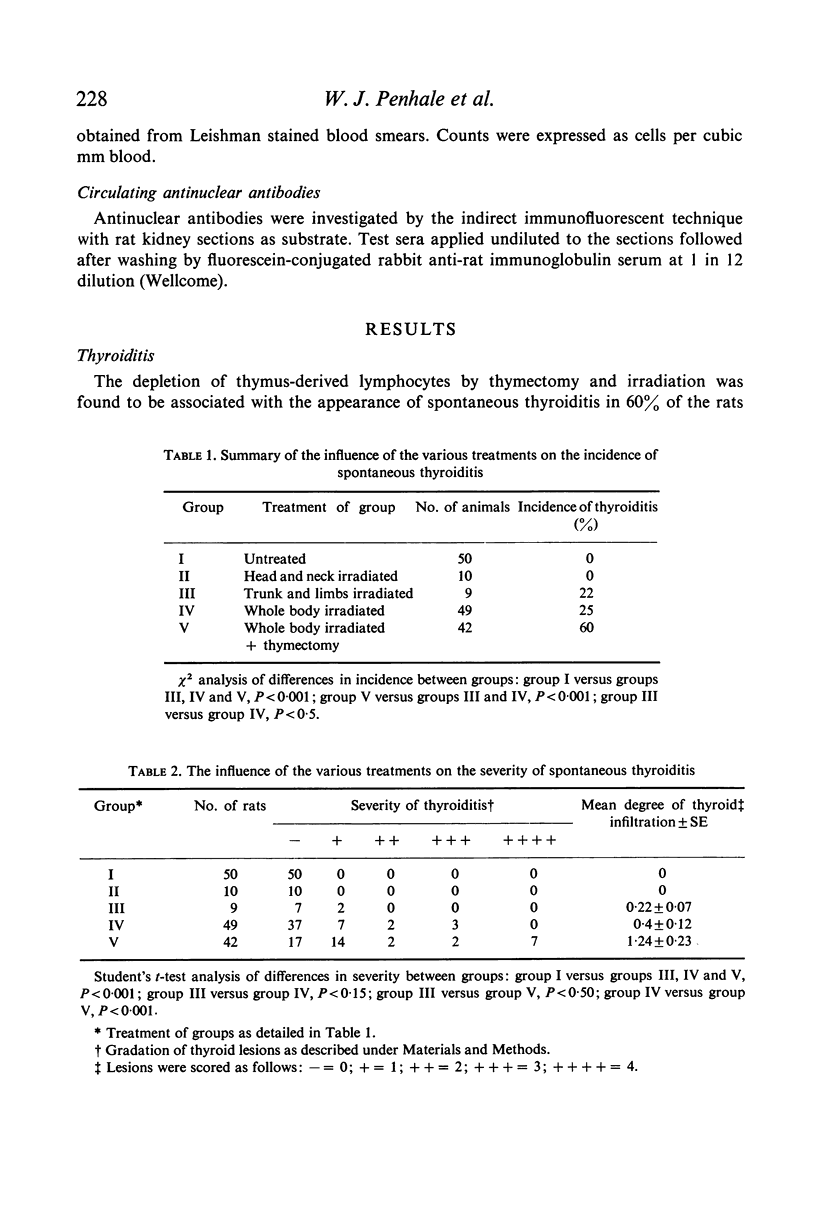
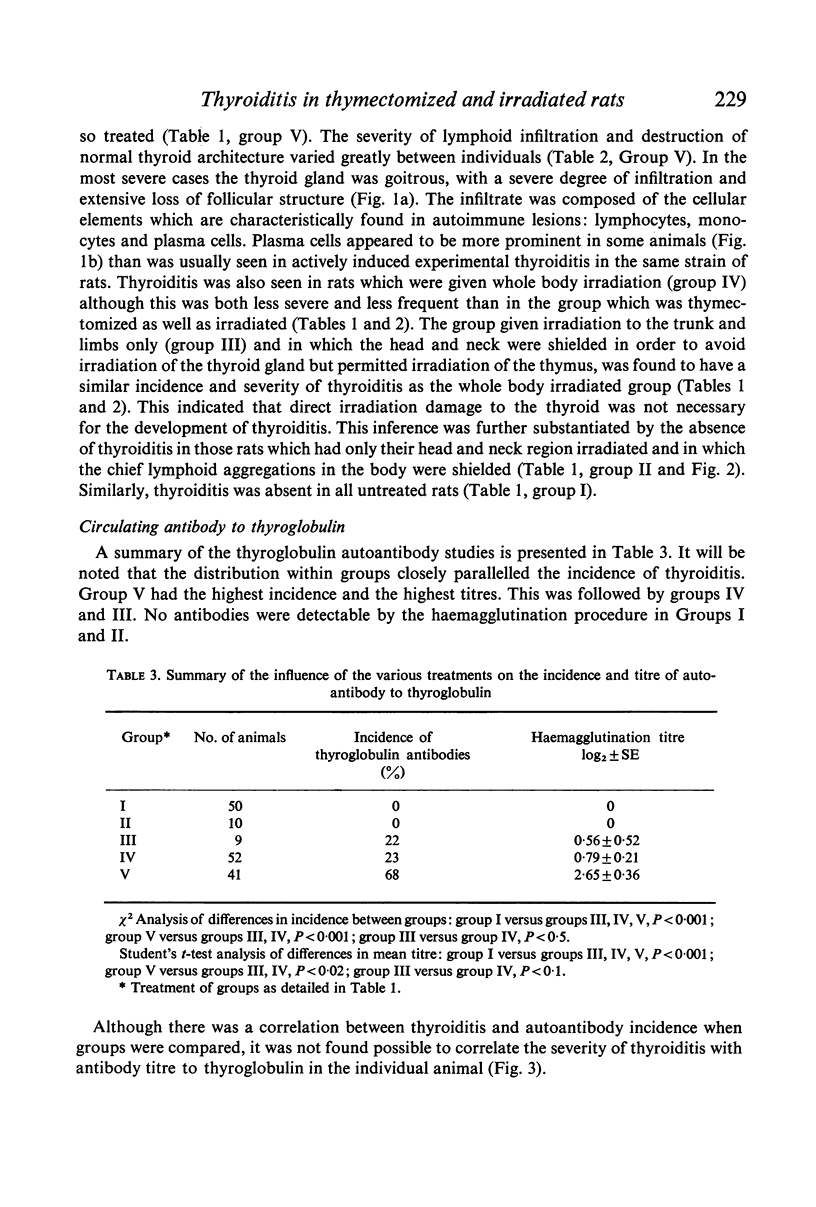
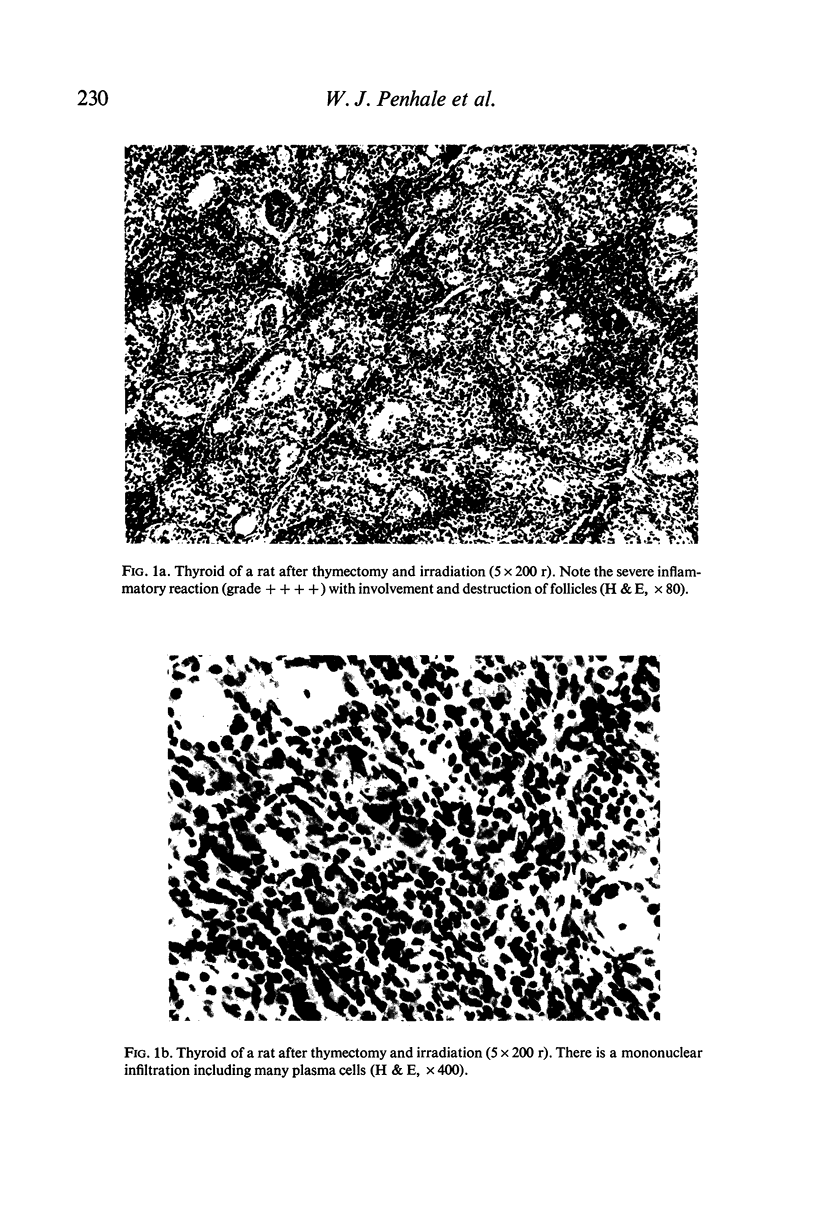
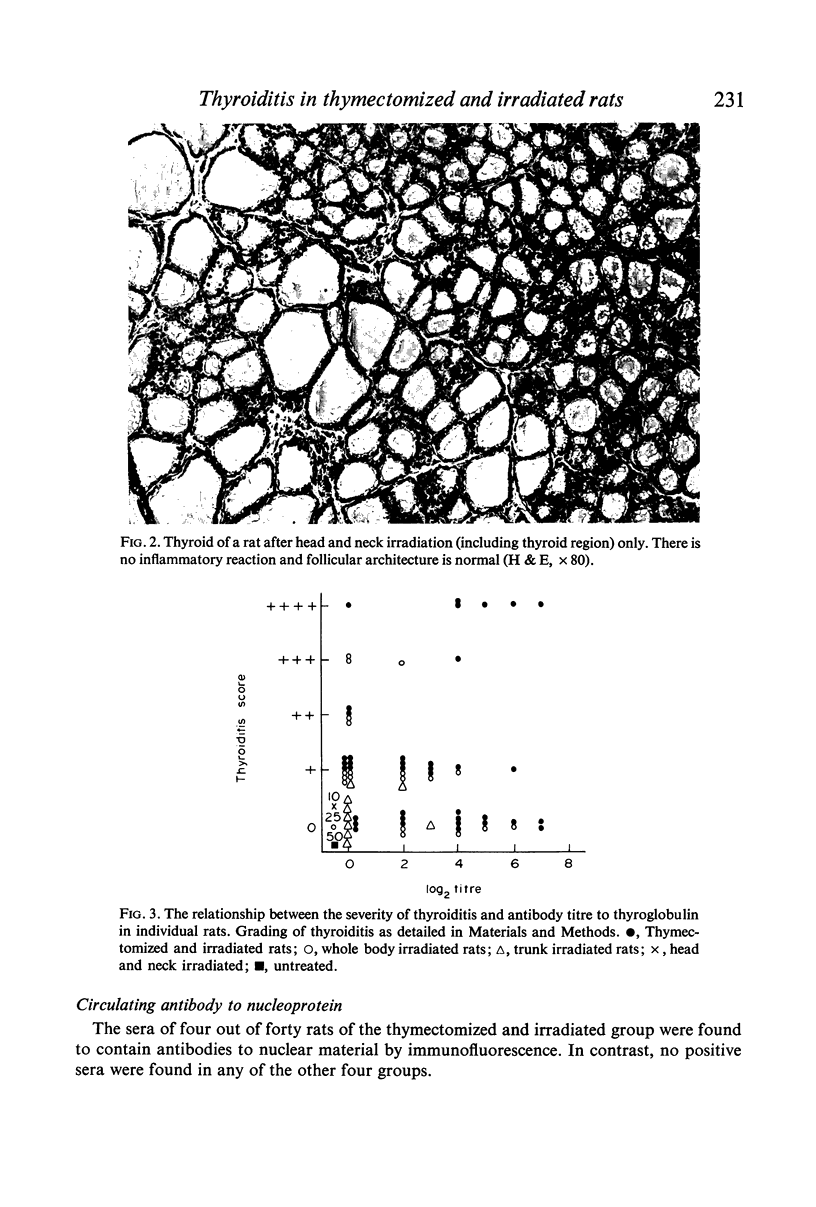
Thyroiditis and autoantibodies to thyroglobulin were found to develop spontaneously in 60% of randomly bred Wistar rats subjected to thymectomy and whole body irradiation after weaning. Antinuclear antibodies were found in approximately 10% of these animals. Thyroiditis was seen in rats which were whole body irradiated without thymectomy but at a much lower incidence (22%). Control rats given irradiation to the thyroid region alone showed no such effects. The histology of the thyroid gland was similar to that seen in Hashimoto thyroiditis in man.
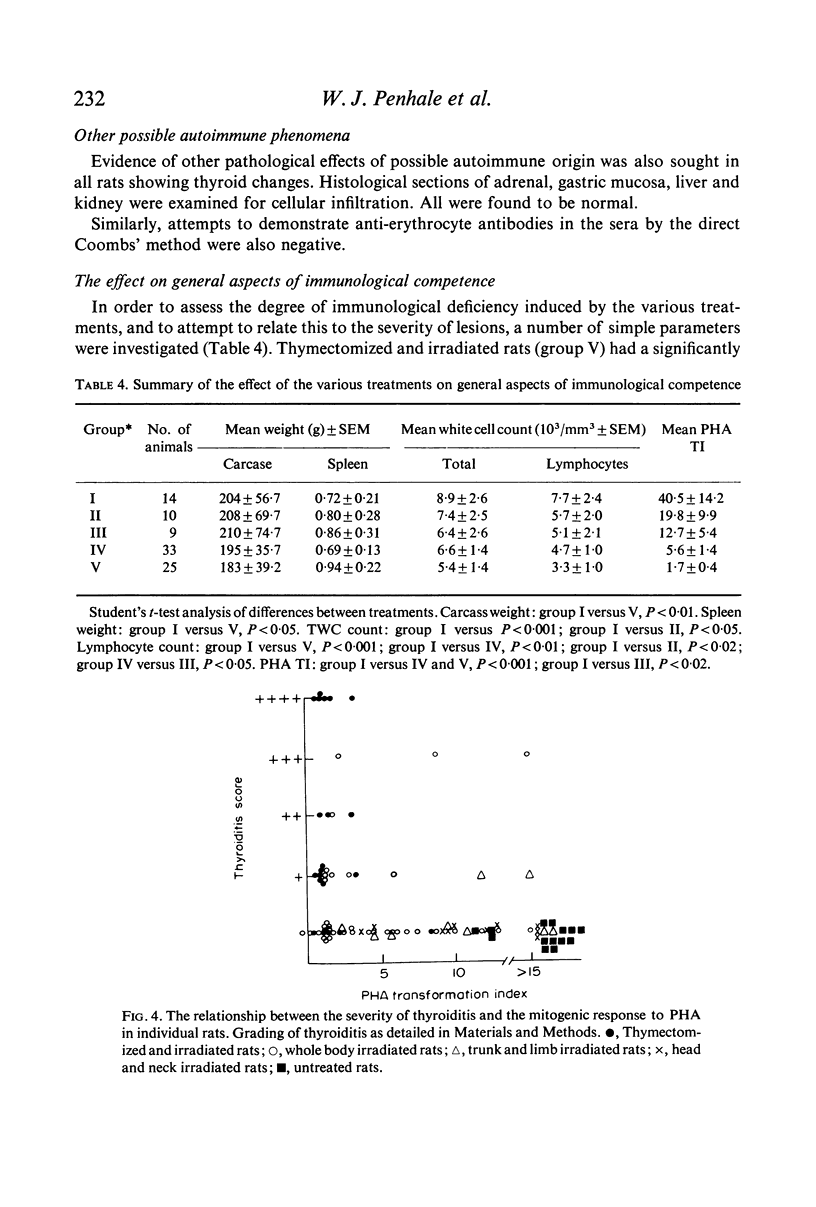
The majority of rats which developed severe thyroiditis were found to be lymphopenic and to have negligible mitogenic responses to phytohaemagglutinin as well as other changes which in view of the thymectomy and irradiation may be considered to be indicative of impaired T cell function.
It is concluded that these findings support the hypothesis that thymus-derived lymphocytes play an important part in the control of organ specific autoimmune disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Wick G. The influence of thymectomy on the development of erythrocyte-specific antinuclear factors in obese strain (OS) and normal white leghorn chickens. Immunology. 1973 Mar;24(3):545–549. [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Calder E. A., Penhale W. J., McLeman D., Barnes E. W., Irvine W. J. Lymphocyte-dependent antibody-mediated cytotoxicity in Hashimoto thyroiditis. Clin Exp Immunol. 1973 Jun;14(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- Clinton B. A., Weigle W. E. Cellular events during the induction of experimental thyroiditis in the rabbit. J Exp Med. 1972 Dec 1;136(6):1605–1615. doi: 10.1084/jem.136.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J. The effect of x-rays on circulating lymphocytes of mice quantitated by PHA-responsiveness. Clin Exp Immunol. 1971 Apr;8(4):603–615. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen H. J., Van Alten P. A., Good R. A. Decreased lymphoid cell multiplication in the post-thymectomy state. Transplantation. 1969 Jan;7(1):1–11. doi: 10.1097/00007890-196901000-00001. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Transfer of experimental autoimmune thyroiditis by serum from thyroidectomized donors. J Exp Med. 1969 Aug 1;130(2):263–285. doi: 10.1084/jem.130.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune responses of mice. 3. A raised tolerance threshold in NZB thymus cells. Immunology. 1971 Dec;21(6):1037–1043. [PMC free article] [PubMed] [Google Scholar]
- Teague P. O., Friou G. J. Antinuclear antibodies in mice. II. Transmission with spleen cells; inhibition or prevention with thymus or spleen cells. Immunology. 1969 Nov;17(5):665–675. [PMC free article] [PubMed] [Google Scholar]
- Thivolet J., Monier J. C., Ruel J. P., Richard M. H. Antinuclear autoantibodies in Swiss mice thymectomized at birth. Nature. 1967 Jun 10;214(5093):1134–1136. doi: 10.1038/2141134a0. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Transfer of experimental autoimmune thyroiditis of the mouse by serum. J Immunol. 1971 Apr;106(4):1139–1142. [PubMed] [Google Scholar]
- Weigle W. O. Recent observations and concepts in immunological unresponsiveness and autoimmunity. Clin Exp Immunol. 1971 Oct;9(4):437–447. [PMC free article] [PubMed] [Google Scholar]
- Welch P., Rose N. R., Kite J. H., Jr Neonatal thymectomy increases spontaneous autoimmune thyroiditis. J Immunol. 1973 Feb;110(2):575–577. [PubMed] [Google Scholar]
- Wick G., Kite J. H., Jr, Cole R. K., Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. 3. The effect of bursectomy on the development of the disease. J Immunol. 1970 Jan;104(1):45–53. [PubMed] [Google Scholar]





