Abstract
The cytopathic effect evidenced by cells infected with avian reovirus S1133 suggests that this virus may induce apoptosis in primary cultures of chicken embryo fibroblasts. In this report we present evidence that avian reovirus infection of cultured cells causes activation of the intracellular apoptotic program and that this activation takes place during an early stage of the viral life cycle. The ability of avian reoviruses to induce apoptosis is not restricted to a particular virus strain or to a specific cell type, since different avian reovirus isolates were able to induce apoptosis in several avian and mammalian cell lines. Apoptosis was also provoked in ribavirin-treated avian reovirus-infected cells and in cells infected with UV-irradiated reovirions, indicating that viral mRNA synthesis and subsequent steps in viral replication are not needed for apoptosis induction in avian reovirus-infected cells and that the number of inoculated virus particles, not their infectivity, is the critical factor for apoptosis induction by avian reovirus. Our finding that apoptosis is no longer induced when intracellular viral uncoating is blocked indicates that intraendosomal virion disassembly is required for apoptosis induction and that attachment and uptake of parental reovirions are not sufficient to cause apoptosis. Taken together, our results suggest that apoptosis is triggered from within the infected cell by viral products generated after intraendosomal uncoating of parental reovirions.
Apoptosis or programmed cell death is a controlled physiological process of cellular suicide that can be triggered by various intracellular and extracellular stimuli (38). Ultimately cells are fragmented into apoptotic bodies that are rapidly phagocytosed by neighboring cells or phagocytes, without concomitant inflammation or tissue damage (32); in contrast, cells that die by nonapoptotic mechanisms lyse and elicit an inflammatory response. Apoptotic cells display specific morphological and biochemical features that distinguish them from living cells and from necrotic cells (34). Apoptotic cells are characterized morphologically by detachment, shrinkage and rounding, plasma membrane blebbing, nuclear collapse, and chromatin condensation (20) and biochemically by fragmentation of chromatin, 28S rRNA, and proteins (1, 33) and by a loss of membrane phospholipid asymmetry, resulting in translocation of phosphatidylserine from the inner layer to the outer part of the plasma membrane (21). Many cellular insults as well as specific extracellular interactions of ligands with surface receptors cause activation of the intracellular apoptotic program (32), and therefore it is not surprising that apoptosis occurs in many virus-infected cells.
Apoptosis is induced by viruses from different families and genera, and the induction of apoptosis contributes directly to the cytopathogenesis of these viruses (for reviews, see references 29, 48, 51, and 55). Virus-induced apoptosis can be considered a defense mechanism by which multicellular organisms fight against viral infections; when triggered early in infection, apoptosis could limit virus replication and spread (24), and this is probably why several viruses encode antiapoptotic factors. These factors block the induction and/or execution of the apoptotic program, thus maximizing viral replication (for reviews, see references 24, 30, and 65). On the other hand, induction of apoptosis late in infection might be beneficial for viral replication by facilitating spread of progeny viral particles toward neighboring cells, without exposure to host immune defenses (51).
Avian reoviruses are icosahedral nonenveloped viruses that replicate in the cytoplasm of infected cells. They comprise a double-protein capsid shell and a genome consisting of 10 double-stranded RNA segments that express both structural and nonstructural proteins (54, 68). In poultry, they are involved in a variety of disease conditions, of which the most important is viral arthritis/tenosynovitis, a widespread syndrome that causes leg weakness (for reviews, see references 37, 54, and 67). In spite of the major economic losses they cause in the poultry industry, however, the molecular biology of the avian reoviruses is poorly characterized, because molecular virologists have paid much more attention to the mammalian reoviruses, considered the prototypes of the Orthoreovirus genus. Avian reoviruses differ from their mammalian counterparts in their lack of hemagglutinin and their ability to cause cell fusion (54).
For the past several years, our laboratory has been studying the replication of avian reoviruses in cultured cells. We have observed that infection of chicken embryo fibroblasts (CEF) with avian reovirus S1133 leads to extensive cytopathic effect, similar to the one described for apoptotic cells. In this work, we performed experiments first to assess whether avian reovirus-infected cells undergo apoptosis and then to determine at which stage in the virus life cycle apoptosis is induced. Our results demonstrate that avian reoviruses trigger activation of the intracellular death program very early in infection and that intracellular viral uncoating but not viral gene expression is required for apoptosis induction.
MATERIALS AND METHODS
Cells, viruses, and reagents.
Primary cultures of CEF were prepared from 9- to 10-day-old chicken embryos and grown in medium 199 containing 10% tryptose phosphate broth and 7.5% calf serum. Mouse L fibroblasts, monkey kidney BSC-40 epithelial cells, and human epithelial HeLa cells were grown in monolayer cultures in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Propagation and titering of vaccinia virus were performed on BSC-40 cells. The avian reoviruses used were provided by Laboratorios Intervet S.A. (Salamanca, Spain) and were grown on semiconfluent monolayers of CEF. Conditions for growing, radiolabeling, purifying, and titrating these viruses have been described previously (25, 45). Generation of UV-inactivated avian reovirions was performed as previously described for mammalian reovirus (64). Polyclonal antibodies against the avian reovirus nonstructural protein μNS were generated in rabbits with a gel-purified recombinant S1133 μNS expressed in insect cells as the antigen.
Cell culture reagents were from Life Technologies (Barcelona, Spain), and microscopy reagents were from Polysciences, Inc. (Warrington, Pa.). The 35S cell-labeling mix was from Amersham Bioscience (Barcelona, Spain). All other reagents, enzymes, and inhibitors were from Sigma-Aldrich S.A. (Madrid, Spain).
Nuclear staining.
For nuclear staining, mock-infected or infected cells grown on glass cover slips were washed three times with cold phosphate-buffered saline (PBS) and fixed with methanol. Cover slips were kept at −20°C for 20 min, washed again with PBS, and incubated twice (5 min each) with PBS at room temperature. Nuclei were stained by incubation with Hoechst 33258 (50 ng/ml in PBS) for 10 min at room temperature in the darkness. Cells were then washed with PBS, dehydrated in 100% ethanol, and dried. Stained cells were mounted with Mowiol and visualized by fluorescence microscopy.
For the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling) assay, cells on cover slips were fixed with 4% paraformaldehyde and subsequently permeabilized and stained with an in situ cell death detection kit (TUNEL/fluorescein isothiocyanate [FITC] kit from Roche Applied Sciences, Barcelona, Spain) according to the manufacturer's instructions.
Virus infections, immunoprecipitation, and protein analysis.
Viral infections, virus purification, metabolic radiolabeling of cells with [35S]methionine-cysteine, preparation of cytoplasmic extracts, immunoprecipitation, and electrophoretic analysis of radiolabeled proteins were performed as described previously (25, 44, 45).
Analysis of integrity of cellular DNA and rRNA.
For the detection of oligonucleosomal DNA fragments, a monolayer of either mock-infected or infected cells on a 100-mm plate was scraped off into the culture medium, and cells were collected by low-speed centrifugation. The cell pellet was resuspended in PBS and centrifuged again, and cells in the final pellet were resuspended (2 × 107 cells/ml) and lysed in a buffer containing 10 mM Tris-HCl (pH 7.5), 10 mM EDTA, and 1% sodium dodecyl sulfate (SDS). Cell extracts were supplemented with 1 M NaCl, incubated for 12 h at 4°C, and centrifuged for 10 min at 10,000 × g and 4°C. The SDS concentration in the supernatant was adjusted to 0.5%, and the mixture was supplemented with 200 μg of proteinase K/ml and 50 μg of RNase A/ml and incubated for 1 h at 37°C. DNA was isolated by phenol-chloroform extraction, precipitation with 95% ethanol, and centrifugation. The DNA pellet was resuspended in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) containing 50 μg of RNase A/ml, incubated for 15 min at 37°C, and subsequently resolved by electrophoresis in 1.5% agarose gels (4 μg/well).
For analysis of the integrity of rRNAs, cell pellets were resuspended (3.3 × 107 cells/ml) in a buffer containing 10 mM Tris-HCl (pH 8.6), 0.14 M NaCl, 1.5 mM MgCl2, 0.5% Nonidet P-40, and 1 mM dithiothreitol, and cells were lysed by incubation in this buffer for 5 min at 4°C. After centrifugation for 90 s at 10,000 × g and 4°C, the supernatant was supplemented with an equal volume of 0.2 M Tris-HCl (pH 8.0), 25 mM EDTA, 0.3 M NaCl, 0.2% SDS, and 100 μg of proteinase K/ml and incubated for 30 min at 37°C. Cytoplasmic RNA was isolated by phenol and phenol-chloroform (24:1) extractions, followed by ethanol precipitation. After centrifugation, the RNA pellet was resuspended in water, incubated in RNA sample buffer (50% formamide, 2.2 M formaldehyde, and 40 μg of ethidium bromide per ml) for 5 min at 60°C and resolved in 1% agarose-0.22 M formaldehyde gels (4 μg/well).
Histone-associated DNA fragments were detected by immunoassay with the Cell Death Detection enzyme-linked immunosorbent assay (ELISA) kit from Roche Applied Sciences, following the manufacturer's instructions.
Flow cytometric analysis.
At the end of the infection, adherent and floating cells were collected and centrifuged at 500 × g for 10 min. Pelleted cells were then resuspended in PBS, centrifuged, and resuspended again in a minimum volume of PBS. Cells were then permeabilized by the dropwise addition of 80% ice-cold ethanol to a final concentration of 70% and incubated overnight at 4°C. Cells were centrifuged, resuspended in PBS at a final concentration of 106 cells/ml, and subsequently incubated for 30 min at 37°C with 50 μg of propidium iodide/ml and 100 μg of RNase A/ml (Sigma). Aliquots of 106 cells were subjected to flow cytometric analysis with a FACSCalibur flow cytometer equipped with an argon-ion laser (488 nm), and the data were acquired and analyzed with CELLQuest software (Becton Dickinson).
To detect translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane, adherent and floating cells were collected and assayed with Vybrant apoptosis assay kit #3 (Molecular Probes, Eugene, Oreg.), following the manufacturer's instructions.
RESULTS
Avian reovirus-infected CEF undergo apoptosis.
The infection of monolayers of CEF with avian reovirus S1133 causes a strong cytopathic effect. Thus, whereas mock-infected cells show the typical fibroblast-like morphology, most of the avian reovirus-infected cells manifest shrinkage, rounding, and detachment from the plate. Furthermore, infected cells, but not their uninfected counterparts, suffer nucleus damage, with chromatin condensation and margination (Fig. 1A). Since the morphology of avian reovirus-infected cells is very similar to that reported for apoptotic cells, we next performed different assays to assess the apoptotic state of avian reovirus-infected cells. For this, CEF monolayers were infected with 20 PFU of avian reovirus S1133/cell, and, at 24 h postinfection, infected cells were examined for different biochemical hallmarks of apoptosis.
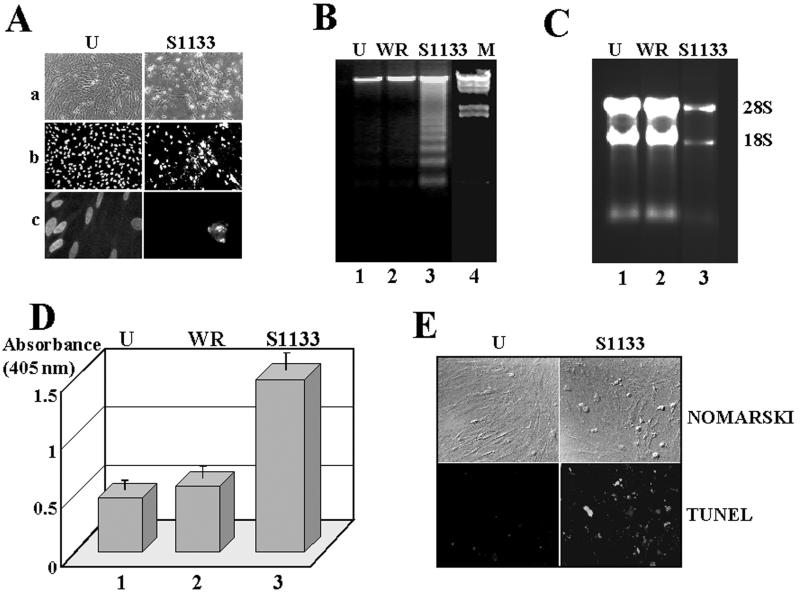
FIG. 1.
Morphological and biochemical analysis. CEF cells were mock infected (U) or infected with 20 PFU of either vaccinia virus (WR) or the avian reovirus S1133 (S1133) per cell for 24 h. (A) Cells were either visualized with a light microscope (a) or stained with Hoechst and visualized with a fluorescent microscope at 450 nm after the samples had been excited at 330 nm (b and c). Magnification: (a and b) ×10; (c) ×60. (B) DNA extracted from these cells and λ DNA digested with both HindIII and EcoRI (M) were analyzed by electrophoresis on 1.5% agarose gels and stained with ethidium bromide. (C) Electrophoretic analysis of total cytoplasmic RNA isolated from these cells. Positions of 28S and 18S rRNAs are shown on the right. (D) ELISA detection of histone-associated DNA fragments from cell extracts. The values shown are means of four independent experiments, and error bars indicate standard deviations of the means. (E) Cells were fixed and permeabilized and subsequently stained with a Boehringer Mannheim TUNEL/FITC kit according to the manufacturer's instructions. TUNEL-positive cells were observed by fluorescence (TUNEL) or by visible light (Nomarski) at a magnification of ×20.
Internucleosomal DNA fragmentation into an oligonucleosome-length DNA ladder (Fig. 1B) and rRNA degradation (Fig. 1C) were evident in avian reovirus-infected CEF but not in either uninfected cells or vaccinia virus-infected cells. Apoptosis in avian reovirus-infected cells could also be observed by ELISA detection of cytoplasmic histone-associated DNA fragments (Fig. 1D) and by in situ visualization at the single-cell level by the TUNEL assay (Fig. 1E). These results clearly demonstrate that S1133-infected CEF undergo apoptosis.
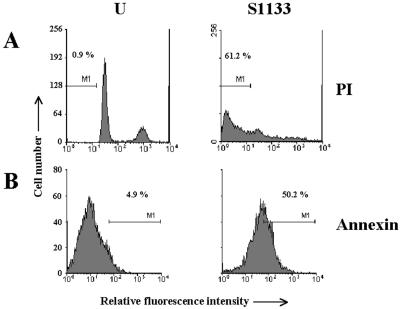
We next quantified the extent of apoptosis in infected cells by flow cytometry, with propidium iodide and fluorescein-conjugated annexin V as fluorochromes. DNA staining with propidium iodide showed that while only 1% of the uninfected cells displayed a sub-G0/G1 DNA content, this fraction was increased up to 60% in avian reovirus-infected cells (Fig. 2A). A similar increase was also detected by cytometric analysis of the redistribution of phosphatidylserine to the outer layer of cell membranes with fluorescein-conjugated annexin V (Fig. 2B).
FIG. 2.
Cytometric analysis. CEF cells were mock infected (U) or infected at 50 PFU/cell with the avian reovirus S1133 (S1133). Cells were harvested at 20 h postinfection, permeabilized, and subsequently stained with either propidium iodide (PI) or annexin V-FITC conjugate (annexin). One million cells were then analyzed by flow cytometry, and cell number was plotted against fluorescence. M1 indicates the percentage of apoptotic cells.
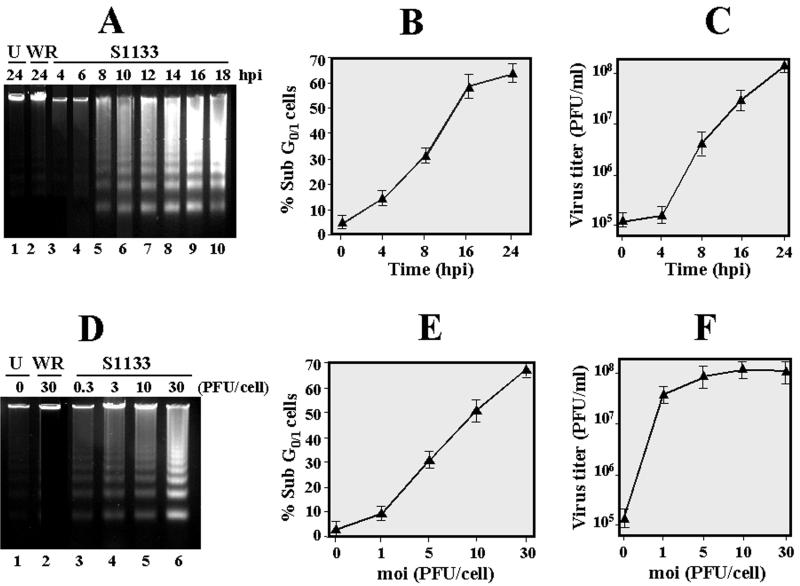
Apoptosis is a virus-induced event that is triggered at an early infection stage. In order to demonstrate the connection between viral infection and induction of apoptosis, we next compared the effects of time and multiplicity of infection on both apoptosis induction and viral growth. When CEF monolayers were infected with a viral multiplicity of 20 PFU/cell, oligonucleosomal DNA laddering could be detected as soon as 8 h postinfection, and the intensity of the ladder bands increased with time of infection, reaching a plateau at 16 h postinfection (Fig. 3A). Similar results were obtained when the extent of apoptosis was quantified by flow cytometric analysis of propidium iodide-stained cells; in this case, an increase in the percentage of sub-G0/G1 cells was already evident at 4 h postinfection, and the fraction of low-DNA-containing cells increased almost linearly until it reached a plateau at 16 h postinfection (Fig. 3B). In contrast, the production of infectious virus progeny increased almost linearly from 8 to 24 h postinfection, and no increase in infectious progeny virus production was observed at 4 h postinfection (Fig. 3C).
FIG. 3.
Analysis of apoptosis dependence and viral growth on time and multiplicity of infection. (A to C) Mock-infected cells (U) and cells infected with 20 PFU of either vaccinia virus (WR) or the avian reovirus S1133 (S1133) per cell were incubated for the indicated times. Cells were then either subjected to flow cytometric analysis after propidium iodide staining (B) or lysed for subsequent electrophoretic DNA analysis (A) and determination of virus titers (C). (D to F) Similar analyses were performed with cells infected for 12 h with the indicated S1133 multiplicities. The values shown in panels B, C, E, and F are means of four different experiments, and error bars indicate standard deviations of the means.
Next we examined the dependence of apoptosis and viral growth on the multiplicity of infection. When cells were infected with a multiplicity as low as 3 PFU/cell, DNA laddering was detected as early as 12 h postinfection, and its intensity increased with increasing multiplicity of infection (Fig. 3D). A linear increase in the extent of apoptosis with inocula from 1 to 30 PFU/cell was also observed by flow cytometric analysis of propidium iodide-stained cells (Fig. 3E). On the contrary, no significant change in the production of infectious progeny virus was observed in the inoculum multiplicity range between 1 and 30 PFU/cell (Fig. 3F), suggesting that the number of viral particles but not their replicative capacity is important for avian reovirus to elicit apoptosis. Furthermore, these results, and the fact that DNA laddering was not detected in either uninfected cells or vaccinia virus-infected cells, strongly suggest that avian reovirus infection is the direct trigger of apoptosis in infected cells.
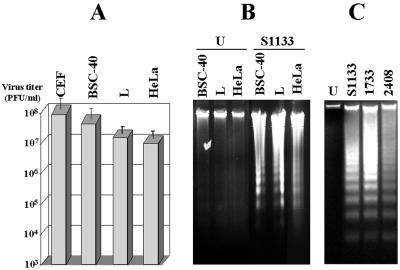
Apoptosis induction is not unique to the S1133 strain of avian reovirus or to primary cultures of CEF cells. Different cells from a single host individual respond differently to a given viral infection (19, 27, 28, 39, 52). Equally, different virus strains may cause different cytopathic effects in a given cell type (31, 49, 62, 64). To assess whether the avian reovirus is able to induce apoptosis in cells other than CEF, internucleosomal DNA cleavage was analyzed in mammalian BSC-40, L, and HeLa cells infected with avian reovirus S1133. The virus was able to replicate (Fig. 4A) and to cause apoptosis (Fig. 4B) in all three S1133-infected mammalian cells examined.
FIG. 4.
Infections of different cell lines with different avian reovirus strains. (A) The cell lines indicated at the top of the figure were infected at 20 PFU/cell with the avian reovirus S1133. At 20 h postinfection, cells were lysed, and the virus concentration of the cell extracts was determined by plaque assay on fresh CEF monolayers. The values shown are means of three independent experiments, and error bars indicate standard deviations of the means. (B) The cell lines indicated at the top of the figure were mock infected (U) or infected with 20 PFU of the avian reovirus S1133 (S1133) per cell. At 20 h postinfection, DNA was extracted and analyzed by electrophoresis. (C) Electrophoretic analysis of DNA extracted from CEF that were either mock infected (U) or infected with 20 PFU of the avian reovirus isolates per cell as indicated at the top of the figure.
Next, we investigated the potential of three different avian reovirus isolates to induce apoptosis. DNA laddering was evident in CEF infected with each of the three viruses (Fig. 4C). Similar results were obtained with BSC40, HeLa, and L cells (data not shown).
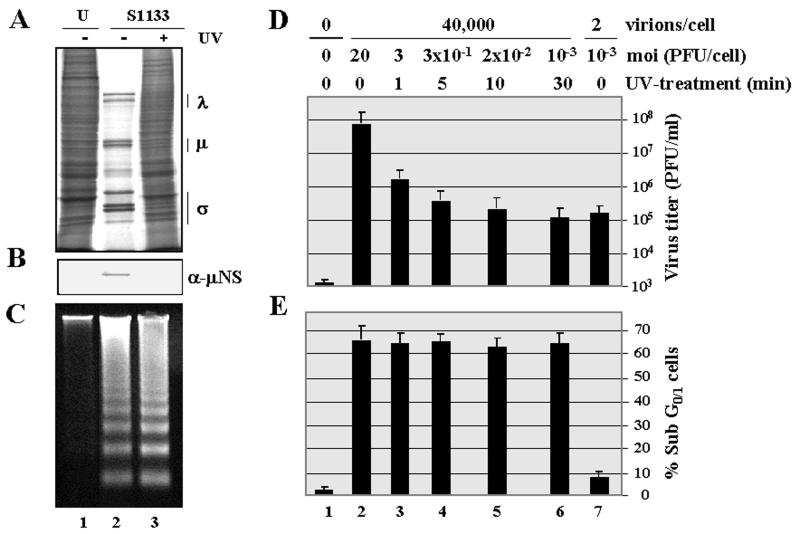
Cell death activation in infected cells occurs in the absence of viral gene expression. In order to determine whether viral gene expression is required for apoptosis induction, the effect of UV treatment on the capacity of avian reovirions to induce apoptosis was first investigated. When purified avian reovirions were subjected to a 30-min UV treatment, the capacity of the virus to synthesize viral proteins was greatly reduced, as demonstrated by metabolic labeling with [35S]methionine and by immunoblot analysis of intracellular levels of the nonstructural μNS protein (Fig. 5A and B, compare lanes 2 and 3). This treatment also caused a drastic reduction in the intracellular production of infectious virus particles (Fig. 5D, compare lanes 2 and 6). In contrast, UV treatment did not affect the apoptosis-inducing ability of the virus, as revealed by DNA fragmentation (Fig. 5C) and flow cytometric analysis (Fig. 5E, compare lanes 2 and 6). Furthermore, similar kinetics of apoptosis induction were obtained by infection of CEF with avian reovirions, whether before or after UV irradiation (data not shown). These results strongly suggest that viral mRNA synthesis and subsequent steps in viral replication are not needed for apoptosis induction in avian reovirus-infected cells.
FIG. 5.
Effect of UV treatment on avian reovirus replication and apoptosis induction. Monolayers of CEF were mock infected (U) or infected with purified avian S1133 reovirions (S1133) either before (−UV) or after (+UV) treatment with UV light. For the experiments shown in panels A to D, the virus input contained approximately 40,000 particles/cell, which corresponds to a multiplicity of infection of 20 PFU/cell in the sample of untreated virus and to a multiplicity of 10−3 PFU/cell in the sample of UV-treated virus. (A) At 16 h postinfection, cells were labeled with [35S]methionine-cysteine, and 1 h later cytoplasmic extracts were prepared and analyzed by SDS-PAGE. Positions of the three size classes of avian reovirus polypeptides are shown on the right. (B) Proteins from extracts of nonradiolabeled cells were analyzed by Western blotting with polyclonal antibodies raised against the nonstructural protein μNS. (C) At 18 h postinfection, cellular DNA was extracted and analyzed by electrophoresis. (D and E) Cells were mock infected (lane 1) or infected with purified avian reovirions for 18 h, either before (lanes 2 and 7) or after UV treatment, with the number of viral particles and multiplicities of infection (moi) indicated on the top. (D) Cytoplasmic extracts were then prepared, and their virus concentration was determined on fresh monolayers of CEF. (E) Cells were then analyzed by flow cytometry after propidium iodide staining. Values in panels D and E are means of three independent experiments, and error bars indicate standard deviations of the means.
However, since the possibility still existed that gene expression by a small subset of reovirions not inactivated by the UV treatment could be the trigger for apoptosis induction, control experiments were subsequently performed. First, cells were infected with samples of purified reovirions that had been UV irradiated for various times. A comparative analysis of both virus production and the extent of apoptosis revealed that while the UV treatment caused a progressive and drastic reduction in viral replication (Fig. 5D, compare lanes 2 to 6), it did not diminish the extent of apoptosis (Fig. 5E, compare lanes 2 to 6). Furthermore, when infection was performed with two virus samples of similar infectivity (Fig. 5D, compare lanes 6 and 7) but markedly different reovirion content (40,000 versus 2 reovirions/cell), apoptosis was only triggered by the sample containing the large number of viral particles (Fig. 5E, compare lanes 6 and 7). These results demonstrate that the residual infectivity remaining in the sample of UV-treated virus is not sufficient to trigger apoptosis and suggest that a threshold number of input virus particles is required for apoptosis induction by avian reovirus. Taken together, our data strongly suggest that intracellular viral gene expression is dispensable for apoptosis induction in avian reovirus-infected cells.
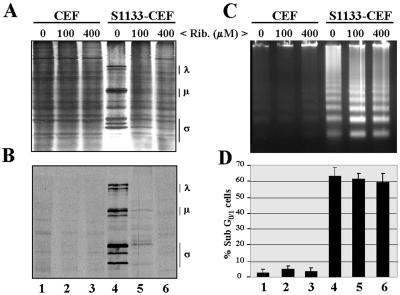
To confirm this suggestion, we next investigated the effect of ribavirin on apoptosis induction in avian reovirus-infected cells. Ribavirin is a guanosine nucleoside analog that has been reported to inhibit mammalian reovirus transcription and replication without affecting the expression of cellular genes or the capacity of mammalian reovirus to induce apoptosis (12, 53). Similar results showing that ribavirin likewise inhibits avian reovirus gene expression have been reported recently (7).
In order to determine the appropriate ribavirin concentration to be used in our experiments and to test its toxicity for CEF, we performed initial trials with uninfected cells. The presence of ribavirin in the culture medium of uninfected cells at concentrations up to 400 μM did not promote apoptosis (Fig. 6C and D, lanes 1 to 3) and did not affect either the viability of the cells for at least 3 days (data not shown) or their protein synthesis ability, as analyzed 16 h after addition of the inhibitor (Fig. 6A, lanes 1 to 3). In the experiments with infected cells, we found that whereas ribavirin did not prevent intracellular uncoating of parental reovirions (Fig. 7C, lane 4), it strongly and specifically inhibited viral but not cellular protein synthesis in a dose-dependent manner, as demonstrated by SDS-PAGE analysis of radiolabeled extracts from infected cells before and after immunoprecipitation with antireovirus antiserum (Fig. 6A and B, lanes 4 to 6).
FIG. 6.
Effect of ribavirin on protein synthesis and apoptosis induction. Monolayers of either mock-infected CEF (U) or CEF infected with 20 PFU of avian reovirus S1133 (S1133) per cell were treated, from the onset of the infection, with the concentrations of ribavirin (Rib.) indicated at the top of the figure. (A and B) At 16 h postinfection, cells were labeled with [35S]methionine-cysteine, and 1 h later cytoplasmic extracts were prepared and analyzed by SDS-PAGE and autoradiography, either before (A) or after (B) immunoprecipitation with anti-S1133 rabbit antiserum. Positions of the three size classes of avian reovirus polypeptides are shown on the right. (C) At 18 h postinfection, cellular DNA was extracted and analyzed by electrophoresis. (D) At 18 h postinfection, cells were analyzed by flow cytometry after propidium iodide staining. The values shown are the means of three independent experiments, and error bars indicate standard deviations of the means.
FIG. 7.
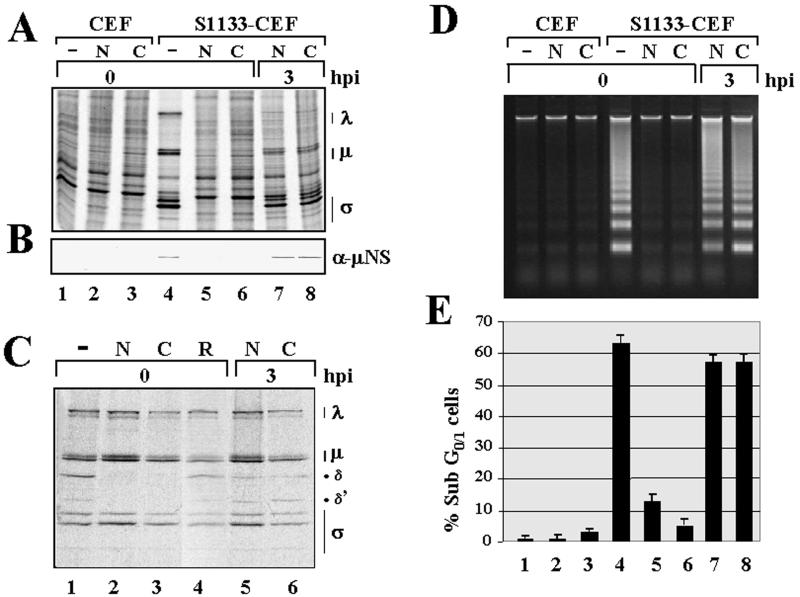
Effect of viral inhibitors on protein synthesis, virus uncoating, and apoptosis induction. (A, B, D, and E) Monolayers of either mock-infected CEF (U) or CEF infected with 20 PFU of avian reovirus S1133 (S1133) per cell were treated at the times indicated with 10 mM ammonium chloride (N) or 100 μM chloroquine (C) or untreated (−). (A) At 16 h postinfection (hpi), cells were labeled for 1 h with [35S]methionine-cysteine, and cytoplasmic extracts were prepared and analyzed by SDS-PAGE and autoradiography. (B) Cytoplasmic extracts of nonradiolabeled cells were analyzed by Western blotting with anti-μNS polyclonal antibodies. (D) Cellular DNA was isolated at 17 h postinfection and analyzed by electrophoresis. (E) Cells were analyzed by flow cytometry after propidium iodide staining. The values shown are means of three independent experiments, and error bars indicate standard deviations of the means. (C) Monolayers of CEF infected with 35S-labeled purified reovirions were treated at the times indicated with the two viral inhibitors and with 200 μM ribavirin (R), and at 5 h postinfection cytoplasmic extracts were prepared and analyzed by SDS-PAGE and autoradiography. Positions of the three size classes of avian reovirus polypeptides are indicated at the right of panels A and C.
Interestingly, while viral protein synthesis was completely abolished in cells treated with 400 μM ribavirin, the ability of avian reovirus to trigger apoptosis was not affected by ribavirin treatment (Fig. 6C and D, compare lanes 4 with lanes 6). Taken together, these results indicate that viral transcription and subsequent steps in viral replication are not required for apoptosis induction in avian reovirus-infected cells.
Intracellular viral uncoating is required for apoptosis induction in avian reovirus-infected cells. To identify the particular stage of the virus life cycle at which apoptosis is triggered in avian reovirus-infected cells and to assess whether apoptosis is induced by the virus from within or from without the host cell, we next investigated whether viral uncoating is required for apoptosis induction in S1133-infected CEF. Avian reovirus uncoating, which takes place within intracellular vacuoles, is largely dependent on the endosome-mediated proteolytic processing of the major outer capsid protein μB/μBC into two specific products, δ and δ′ (17) (Fig. 7C). This processing and virus replication are both blocked by specific inhibitors of the activity of the vacuolar proton-ATPase, such as bafilomycin A1 (our unpublished results), and by different lysosomotropic agents (17) (Fig. 7C), indicating both that avian reovirus penetrates cells via the receptor-mediated endocytosis route and that endosome acidification is necessary for viral uncoating and viral replication.
To assess the dependence of apoptosis on viral uncoating, we first tested the activity of several viral inhibitors in order to select those that do not trigger apoptosis in uninfected cells but block intracellular viral uncoating in infected cells (10, 43). Inhibitors such as bafilomycin A1 and concanamycin were discarded because of their proapoptotic activity in uninfected CEF (data not shown). Ammonium chloride and chloroquine, two weakly basic amines that act as lysosomotropic agents by neutralizing vacuolar acidification (56), were selected because they effectively blocked virus uncoating in S1133-infected CEF (Fig. 7C) without provoking apoptosis in uninfected cells (Fig. 7D and E, compare lane 1 with lanes 2 and 3) or affecting the synthesis of cellular proteins in uninfected or infected cells (Fig. 7A, compare lane 1 with lanes 2, 3, 5, and 6). However, when added to cells at the onset of the infection, the two inhibitors were very effective in blocking (i) viral protein synthesis (Fig. 7A and B, compare lane 4 with lanes 5 and 6); (ii) the intracellular uncoating of reovirions (Fig. 7C, compare lane 1 with lanes 2 and 3); (iii) viral replication (17) (our unpublished data); and (iv) the ability of the avian reovirus to induce apoptosis (Fig. 7D and E, compare lane 4 with lanes 5 and 6). Furthermore, the ability of the inhibitors to block these processes was lost when they were added to cells at 3 h postinfection (lanes 7 and 8 in Fig. 7A, B, D, and E; lanes 5 and 6 in Fig. 7C), suggesting that they block an early stage of the viral replication cycle. Similar results were obtained when avian reovirus-infected cells were cultivated in the presence of E64, a protease inhibitor that has been shown to specifically block the uncoating of mammalian reovirus in infected cells (2, 3) (data not shown). Taken together, these results demonstrate that virus uncoating is necessary for apoptosis induction in avian reovirus-infected cells.
DISCUSSION
Apoptosis is a common pathway of virus-induced cell death, and elimination of infected cells by apoptosis is considered an important process for controlling virus infection in multicellular organisms (reviewed in reference 48). In this report we show that avian reovirus-infected cells but not uninfected cells or vaccinia virus-infected cells display morphological and biochemical changes consistently associated with apoptosis and currently used to identify apoptotic cells. The close correlation observed between the degree of apoptosis and both multiplicity and time of infection clearly demonstrates that apoptosis is directly induced by the avian reovirus infection. Furthermore, the proapoptotic activity of avian reoviruses appears to be a general feature of these viruses, since it is not specific for a particular virus isolate or to the infection of a specific type of cell (e.g., avian cells or primary cultured cells). Avian reoviruses should therefore be included in the already long list of apoptosis-inducing viruses.
Having demonstrated that avian reovirus induces apoptosis, the main thrust of the present study was to assess the stage of the virus life cycle at which apoptosis is triggered. Our findings that an increase in the amount of sub-G0/G1 cells is already evident at 4 h postinfection and that lysosomotropic agents that block virus uncoating and virus-induced apoptosis, when present from the onset of the infection, lose their blocking capacity when added at 3 h postinfection indicate that apoptosis is triggered during an early stage of the virus replication cycle. On the other hand, the fact that apoptosis is similarly induced by infection with UV-irradiated reovirions strongly suggests that viral gene expression is dispensable for apoptosis induction by avian reovirus. However, since it can be argued that the UV treatment of reovirions is not fully effective in completely blocking viral mRNA synthesis, we performed control experiments to test the possibility that apoptosis may be triggered by gene expression from the small subset of viral particles that resisted inactivation by these treatments. This possibility is very unlikely, since increasing the period of exposure of purified reovirions to UV drastically reduced their infectivity but did not alter their ability to cause apoptosis, and since apoptosis was not triggered by infection with a sample of nonirradiated virus with an identical multiplicity of infection but many fewer viral particles than an apoptosis-inducing sample of UV-treated virus.
Furthermore, the capacity of avian reovirus to induce apoptosis was not diminished by the presence of the viral RNA synthesis inhibitor ribavirin, in spite of the fact that this inhibitor was very effective in inhibiting viral protein synthesis (Fig. 6B) (7). A similar effect of ribavirin on apoptosis induction by mammalian reovirus was reported after submission of this paper (12). Taken together, these results demonstrate both that low levels of viral gene expression are not sufficient to induce apoptosis and that viral transcription and subsequent steps in viral replication are dispensable for apoptosis induction by avian reovirus. Our findings also indicate that a threshold number of input avian reovirions, whether replication competent or replication incompetent, is necessary to trigger apoptosis. A similar nondependence of apoptosis on viral gene expression has also been reported for cells infected with other viruses, including Sindbis virus (36), vaccinia virus (52), bovine herpesvirus 1 (28), avian leukosis virus (8), and mammalian reoviruses (12, 47, 64).
Since blockage of viral mRNA synthesis was also shown to abolish the virus-induced shutoff of cellular protein synthesis and the intracellular production of viral proteins, our results further suggest that viral mRNAs, nonstructural viral proteins, and virus-induced shutoff are all dispensable for apoptosis induction in avian reovirus-infected cells. We also observed that neither apoptosis induction nor the cytopathic effects induced by avian reovirus infection are prevented by inhibition of viral gene expression. Conversely, all treatments that blocked apoptosis induction in infected cells also abolished the cytopathic effects of the infection. These findings strongly suggest that the cytopathic effect that follows avian reovirus infection is mediated by the induction of apoptosis.
The nondependence of apoptosis on viral gene expression indicates that apoptosis is induced by parental virus particles at a stage prior to virus transcription. Parental virus particles could trigger apoptosis during their extracellular interaction with components of the cell membrane, during their cell entry or after their intraendosomal conversion to subviral particles upon virus uncoating. In this report we present evidence that inhibition of intraendosomal virus uncoating abolishes the ability of the avian reovirus to induce apoptosis, indicating both that virion disassembly is required to trigger apoptosis and that apoptosis is induced during a postattachment step. A report that appeared after submission of this paper revealed that intraendosomal virus uncoating is also required for mammalian reovirus to elicit apoptosis (12). Thus, it appears that virus disassembly is a general requirement for apoptosis induction by members of the Orthoreovirus genus.
Since inhibition of intracellular virus uncoating should not prevent the extracellular attachment or internalization of the virus (6, 16, 26), our results demonstrate that virus attachment and virus uptake are not sufficient to induce apoptosis in avian reovirus-infected cells. However, we do not rule out the possibility that receptor engagement by avian reovirus may play a role in apoptosis, although this possibility is currently difficult to test, since nothing is known about the nature or identity of the avian reovirus cell receptors. In this regard, it has recently been shown that mammalian reovirus binding to the cell surface receptor junction adhesion molecule is necessary to activate NF-κB and to induce apoptosis (4) and that the extent of apoptosis is enhanced by virus binding to cell surface sialic acid (13), suggesting that mammalian reovirus binding to the cell surface activates cellular signaling pathways that lead to apoptosis induction.
The dependence of apoptosis on virus disassembly but not on virus gene expression suggests that the apoptotic program is triggered by avian reovirus products that are generated by the proteolytic and conformational changes that reovirions undergo within acidic endosomes upon uncoating and not by their parental reovirions. These products could activate the apoptotic program by at least two different mechanisms. First, apoptosis could be directly induced by viral proteins, either those that are dissociated from the virus during entry or those that remain attached to subviral particles; the most likely such candidates are the cleaved products of μBC, δ, and δ′ and those internal reovirion proteins that become exposed after virus uncoating. In this regard, specific viral proteins from different viruses, including adenovirus (15, 58), baculovirus (50), birnavirus (22), chicken anemia virus (14), flavivirus (11), hepadnavirus (40), herpesvirus (42), parvovirus (59), polyomavirus (69), porcine respiratory syndrome virus (61), retrovirus (5, 57), and togavirus (18), have been shown to induce apoptosis in infected and/or transfected cells.
Alternatively, virus uncoating could promote activation of the intracellular cell death program indirectly, by altering the integrity of the endosomal membrane and thus facilitating the translocation of endosomal enzymes to the cytosol and/or by reducing the cytosolic pH. Uncontrolled leakage of endosomal and lysosomal proteases to the cytosol was suggested to be associated with several forms of apoptosis (9, 63), and it has been reported that cathepsins B and D have proapoptotic activity (35, 66). It was further suggested that the release of lysosomal proteases to the cytosol may cause apoptosis directly by procaspase activation and/or indirectly by mitochondrial attack with concomitant discharge of proapoptotic factors (41). On the other hand, an uncoating-mediated alteration of the endosomal membrane could also lead to cytosol acidification, and this has been shown to promote cytochrome c-mediated activation of caspases and apoptosis (46).
To gather evidence to discriminate between these possibilities, we tried to generate infectious subvirion particles (ISVPs) with the ability to replicate in cells treated with lysosomotropic agents; such particles have been successfully generated in vitro by controlled proteolytic digestion of mammalian reovirions (23, 60), and it has recently been reported that infection of HeLa cells with mammalian reovirus ISVPs induces apoptosis (12). Assuming that these in vitro-generated ISVPs enter cells by directly crossing the cytoplasmic membrane and not by the endocytic route, one would expect apoptosis to occur in ISVP-infected cells treated with lysosomotropic agents only if apoptosis is directly induced by structural components of ISVPs, not if it is triggered by ISVP-induced leakiness of the endosomal membrane. Absence of apoptosis under these conditions would suggest that alteration of the endosomal membrane is required for apoptosis induction. Unfortunately, and in spite of the many different proteolytic treatments of purified reovirions tried, we have not yet been able to generate such endosome acidification-independent infectious ISVPs because the digested particles tend to aggregate and become noninfectious.
Many questions regarding avian reovirus-induced apoptosis remain unanswered. What is the trigger that activates apoptosis in infected cells? Is apoptosis beneficial or detrimental for virus replication and/or for virus dissemination to neighboring cells? What cellular factors are involved in avian reovirus-induced apoptosis? What is the pathway of avian reovirus-induced apoptosis? These questions will be addressed in future work in our laboratory.
Acknowledgments
We are grateful to Laboratorios Intervet (Salamanca, Spain) for providing the specific-pathogen-free embryonated eggs and avian reovirus isolates 1733 and 2408. We thank Chema Diaz and Lois Hermo for providing technical support and assistance. We also thank Terence Dermody for critical reading of the manuscript.
This work was partially financed by Spanish Ministry of Science and Technology grant PB97-0523.
REFERENCES
- 1.Arends, M. J., R. G. Morris, and A. H. Willey. 1990. Apoptosis: the role of the endonuclease. Am. J. Pathol. 136:593-608. [PMC free article] [PubMed] [Google Scholar]
- 2.Baer, G. S., and T. S. Dermody. 1997. Mutations in reovirus outer-capsid protein σ3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 71:4921-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, A. J., A. A. Kembhavi, M. A. Brown, H. Kirschke, C. G. Knight, M. Tamai, and K. Hanada. 1982. l-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H, and L. Biochem. J. 201:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 5.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basak, S., and H. Turner. 1992. Infectious entry pathway for canine parvovirus. Virology 186:368-376. [DOI] [PubMed] [Google Scholar]
- 7.Bodelón, G., L. Labrada, J. Martínez-Costas, and J. Benavente. 2002. Modification of late membrane permeability in avian reovirus-infected cells. Viroporin activity of the S1-encoded nonstructural p10 protein. J. Biol. Chem., 277:17789-17796. [DOI] [PubMed]
- 8.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. T. Young. 1996. CAR1, a TNF-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 9.Brunk, U. T., H. Dalen, K. Roberg, and H. B. Hellquist. 1997. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 23:616-626. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco, L. 1995. Entry of animal viruses and macromolecules into cells. FEBS Lett. 350:151-154. [DOI] [PubMed] [Google Scholar]
- 11.Christianne, J. M. B., M. M. Hulst, R. J. M. Moormann, P. A. van Rijn, and J. T. van Oirschot. 1997. Glycoprotein Erns of pestiviruses induces apoptosis in lymphocytes of several species. J. Virol. 71:6692-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly, J. L., and T. S. Dermody. 2002. Virion disassembly is required for apoptosis induction by reovirus. J. Virol. 76:1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly, J. L., E. S. Barton, and T. Dermody. 2001. Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis. J. Virol. 75:4029-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danen-van Oorschot, A. A., A. J. Van der Eb, and M. H. Noteborn. 2000. The chicken anemia virus-derived protein apoptin requires activation of caspase for induction of apoptosis in human tumor cells. J. Virol. 74:7072-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A which is inhibited by E1B. Genes Dev. 7:546-554. [DOI] [PubMed] [Google Scholar]
- 16.Donelli, G., F. Superti, A. Tinari, and M. L. Marziano. 1992. Mechanism of astrovirus entry into Graham 293 cells. J. Med. Virol. 38:271-277. [DOI] [PubMed] [Google Scholar]
- 17.Duncan, R. 1996. The low-pH-dependent entry of avian reovirus is accompanied by two specific cleavages of the major outer capsid protein μ2C. Virology 219:179-189. [DOI] [PubMed] [Google Scholar]
- 18.Duncan, R., A. Esmaili, L. M. Law, S. Bertholet, C. Hough, T. C. Hobman, and H. L. Nakhasi. 2000. Rubella virus capsid protein induces apoptosis in transfected RK13 cells. Virology 275:20-29. [DOI] [PubMed] [Google Scholar]
- 19.Duncan, R., J. Muller, N. Lee, A. Esmaili, and H. O. L. Nakhasi. 1999. Rubella virus-induced apoptosis varies among cell lines and is modulated by Bcl-XL and caspase inhibitors. Virology 255:117-128. [DOI] [PubMed] [Google Scholar]
- 20.Duvall, E., and A. H. Wyllie. 1986. Death and the cell. Immunol. Today 7:115-119. [DOI] [PubMed] [Google Scholar]
- 21.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 22.Fernández-Arias, A., S. Martínez, and J. F. Rodríguez. 1997. The major antigenic protein of infectious bursal disease virus, VP2, is an apoptotic inducer. J. Virol. 71:8014-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García Martínez, C., R. Guinea, J. Benavente, and L. Carrasco. 1996. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J. Virol. 70:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillet, G., and G. Brun. 1996. Viral inhibition of apoptosis. Trends Microbiol. 312:312-317. [DOI] [PubMed] [Google Scholar]
- 25.Grande, A., and J. Benavente. 2000. Optimal conditions for the growth, purification and storage of the avian reovirus S1133. J. Virol. Methods 85:43-54. [DOI] [PubMed] [Google Scholar]
- 26.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 27.Grivel, J. C., N. Malkevitch, and L. Margolis. 2000. Human immunodeficiency virus type 1 induces apoptosis in CD4+ but not in CD8+ T cells in ex vivo-infected human lymphoid tissue. J. Virol. 74:8077-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanon, E., G. Meyer, A. Vanderplasschen, C. Dessy-Doizé, E. Thiry, and P.-P. Pastoret. 1998. Attachment but not penetration of bovine herpesvirus 1 is necessary to induce apoptosis in target cells. J. Virol. 72:7638-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardwick, J. M. 1997. Virus-induced apoptosis. Adv. Pharmacol. 41:295-336. [DOI] [PubMed] [Google Scholar]
- 30.Hardwick, J. M. 1998. Viral interference with apoptosis. Semin. Cell Dev. Biol. 9:339-349. [DOI] [PubMed] [Google Scholar]
- 31.Hechtfischer, A., M. Marschall, A. Helten, C. Boswald, and H. Meier-Ewert. 1997. A highly cytopathogenic influenza C virus variant induces apoptosis in cell culture. J. Gen. Virol. 78:1327-1330. [DOI] [PubMed] [Google Scholar]
- 32.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 33.Houge, G., and S. O. Doskeland. 1996. Divergence towards the end? Cleavage of the divergent domains of ribosomal RNA in apoptosis. Experientia 52:963-967. [DOI] [PubMed] [Google Scholar]
- 34.Houge, G., B. Robaye, T. S. Elkhom, J. Golstein, G. Mellgren, B. T. Gjessten, G. Majno, and I. Joris. 1995. Apoptosis, oncosis, and necrosis. Am. J. Pathol. 146:3-15. [PMC free article] [PubMed] [Google Scholar]
- 35.Isahar, K., Y. Ohsawa, S. Kanamori, M. Shibata, S. Waguri, N. Sato, T. Gotow, T. Watanabe, T. Momoi, K. Urase, E. Kominami, and Y. Uchiyama. 1999. Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases. Neuroscience 91:233-249. [DOI] [PubMed] [Google Scholar]
- 36.Jan, J. T., and D. E. Griffin. 1999. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J. Virol. 73:10296-10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones, R. C. 2000. Avian reovirus infections. Rev. Sci. Tech. Off. Int. Epizoot. 19:614-625. [DOI] [PubMed] [Google Scholar]
- 38.Kerr, J. F. R., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, B., O. Kim, J. H. Tai, and C. Chae. 2000. Transmissible gastroenteritis virus induces apoptosis in swine testicular cell lines but not in intestinal enterocytes. J. Comp. Pathol. 123:64-66. [DOI] [PubMed] [Google Scholar]
- 40.Kim, H., H. Lee, and Y. Yun. 1998. X-gene product of hepatitis B virus induces apoptosis in liver cells. J. Biol. Chem. 273:381-385. [DOI] [PubMed] [Google Scholar]
- 41.Li, W., X. Yuan, G. Nordgren, H. Dalen, G. M. Dubowchik, R. A. Firestone, and U. T. Brunk. 2000. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 470:35-39. [DOI] [PubMed] [Google Scholar]
- 42.Lu, J. J.-Y., J.-Y. Chen, T.-Y. Hsu, W. C. Y. Yu, I.-J. Su, and C.-S. Yang. 1996. Induction of apoptosis in epithelial cells by Epstein-Barr virus latent membrane protein 1. J. Gen. Virol. 77:1883-1892. [DOI] [PubMed] [Google Scholar]
- 43.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Costas, J., C. González-López, V. N. Vakharia, and J. Benavente. 2000. Possible involvement of the double-stranded RNA-binding core protein σA in the resistance of avian reovirus to interferon. J. Virol. 74:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez-Costas, J., R. Varela, and J. Benavente. 1995. Endogenous enzymatic activities of the avian reovirus S1133: identification of the viral capping enzyme. Virology 206:1017-1026. [DOI] [PubMed] [Google Scholar]
- 46.Matsuyama, S., J. Llopis, Q. L. Deveraux, R. Y. Tsien, and J. C. Reed. 2000. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2:318-325. [DOI] [PubMed] [Google Scholar]
- 47.Oberhaus, S. M., T. S. Dermody, and K. L. Tyler. 1998. Apoptosis and the cytopathic effect of reoviruses. Curr. Top. Microbiol. Immunol. 233:23-49. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien, V. 1998. Viruses and apoptosis. J. Gen. Virol. 79:1833-1845. [DOI] [PubMed] [Google Scholar]
- 49.Price, G. E., H. Smith, and C. Sweet. 1997. Differential induction of cytotoxicity and apoptosis by influenza virus strains of different virulence. J. Gen. Virol. 78:2821-2829. [DOI] [PubMed] [Google Scholar]
- 50.Prikhod'ko, E. A., and L. K. Miller. 1996. Induction of apoptosis by baculovirus transactivator IE1. J. Virol. 70:7116-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radvi, E. S., and R. M. Welsh. 1995. Apoptosis in viral infections. Adv. Virus Res. 45:1-59. [DOI] [PubMed] [Google Scholar]
- 52.Ramsy-Ewing, A., and B. Moss. 1998. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology 242:138-149. [DOI] [PubMed] [Google Scholar]
- 53.Rankin, J. T., Jr., S. B. Eppes, J. B. Antzak, and W. K. Joklik. 1989. Studies on the mechanism of the antiviral activity of ribavirin against reovirus. Virology 168:147-158. [DOI] [PubMed] [Google Scholar]
- 54.Robertson, M. D., and G. E. Wilcox. 1986. Avian reovirus. Vet. Bull. 56:155-174. [Google Scholar]
- 55.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 56.Seglen, P. O. 1983. Inhibitors of lysosomal function. Methods Enzymol. 96:737-764. [DOI] [PubMed] [Google Scholar]
- 57.Sheila, A. S., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shtrichman, R., and T. Kleinberger. 1988. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J. Virol. 72:2975-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanley, M., N. Yaegashi, K. Tada, N. Tanaka, and K. Sugamura. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J. Virol. 72:3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturzenbecker, L. J., M. Nibert, D. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61:2351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suárez, P., M. Díaz-Guerra, C. Prieto, M. Esteban, J. M. Castro, A. Nieto, and J. Ortín. 1996. Open reading frame 5 of porcine reproductive and respiratory syndrome virus as a cause of virus-induced apoptosis. J. Virol. 70:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thoulouze, M. I., M. Lafage, J. A. Montano-Hirose, and M. Lafon. 1997. Rabies virus infects mouse and human lymphocytes and induces apoptosis. J. Virol. 71:7372-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turk, B., D. Turk, and V. Turk.. 2000. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta 1477:98-111. [DOI] [PubMed] [Google Scholar]
- 64.Tyler, K. L., M. K. T. Squier, S. E. Rodgers, B. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uren, G. A., and D. L. Vaux. 1997. Viral inhibitors of apoptosis. Vitam. Horm. 53:175-193. [DOI] [PubMed] [Google Scholar]
- 66.Vancompernolle, K., F. Van Herreweghe, G. Pynaert, M. Van de Craen, K. De Vos, N. Totty, A. Sterling, W. Fiers, P. Vandenabeele, and J. Grooten. 1998. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 438:150-158. [DOI] [PubMed] [Google Scholar]
- 67.van der Heide, L. 2000. The history of avian reovirus. Avian Dis. 44:638-641. [PubMed] [Google Scholar]
- 68.Varela, R., and J. Benavente. 1994. Protein coding assignment of avian reovirus S1133. J. Virol. 68:6775-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, C. H., Y. L. Chen, Y. P. Tsao, and S. L. Chen. 1999. Simian virus 40 T antigen induces p53-independent apoptosis but does not suppress erbB2/neu gene expression in immortalized human epithelial cells. Cancer Lett. 137:107-115. [DOI] [PubMed] [Google Scholar]