Abstract
As a response to high light, plants have evolved non-photochemical quenching (NPQ), mechanisms that lead to the dissipation of excess absorbed light energy as heat, thereby minimizing the formation of dangerous oxygen radicals. One component of NPQ is pH dependent and involves the formation of zeaxanthin from violaxanthin. The enzyme responsible for the conversion of violaxanthin to zeaxanthin is violaxanthin de-epoxidase, which is located in the thylakoid lumen, is activated by low pH, and has been shown to use ascorbate (vitamin C) as its reductant in vitro. To investigate the effect of low ascorbate levels on NPQ in vivo, we measured the induction of NPQ in a vitamin C-deficient mutant of Arabidopsis, vtc2-2. During exposure to high light (1,500 μmol photons m−2 s−1), vtc2-2 plants initially grown in low light (150 μmol photons m−2 s−1) showed lower NPQ than the wild type, but the same quantum efficiency of photosystem II. Crosses between vtc2-2 and Arabidopsis ecotype Columbia established that the ascorbate deficiency cosegregated with the NPQ phenotype. The conversion of violaxanthin to zeaxanthin induced by high light was slower in vtc2-2, and this conversion showed saturation below the wild-type level. Both the NPQ and the pigment phenotype of the mutant could be rescued by feeding ascorbate to leaves, establishing a direct link between ascorbate, zeaxanthin, and NPQ. These experiments suggest that ascorbate availability can limit violaxanthin de-epoxidase activity in vivo, leading to a lower NPQ. The results also demonstrate the interconnectedness of NPQ and antioxidants, both important protection mechanisms in plants.
Excess absorbed light energy can lead to the formation of dangerous oxygen radicals, a problem that plants minimize by dissipating the excess energy as heat. This thermal dissipation process is called non-photochemical quenching of chlorophyll fluorescence (NPQ). NPQ consists of three mechanisms, one of which is energy dependent, rapidly induced, and rapidly reversible. This energy-dependent quenching is called qE. For the establishment of qE in high light, a proton gradient is required. This gradient causes the protonation of some photosystem II (PS II) proteins and activates the xanthophyll cycle. Both of these elements, the protonation of proteins and the xanthophyll cycle, are required for a maximum qE (for review, see Müller et al., 2001).
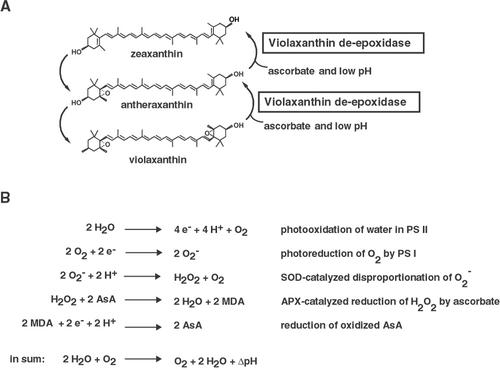
The xanthophyll cycle (Fig. 1A) consists of the de-epoxidation of violaxanthin first to antheraxanthin and then to zeaxanthin by VDE. Zeaxanthin and antheraxanthin are the two xanthophyll pigments required for maximum qE (Gilmore and Yamamoto, 1993). In low light, antheraxanthin and zeaxanthin are slowly epoxidized to violaxanthin by the enzyme zeaxanthin epoxidase. The VDE enzyme was first partially purified from spinach (Spinacia oleracea; Hager and Perz, 1970) and lettuce (Lactuca sativa; Yamamoto and Higashi, 1978) and the gene has since been cloned from lettuce, Arabidopsis, and tobacco (Nicotiana tabacum; Bugos and Yamamoto, 1996; Bugos et al., 1998). VDE is localized to the thylakoid lumen and is activated by a pH below 6.5 with a maximum activity at about pH 5.0 in vitro (Hager, 1969; Hager and Holocher, 1994). Also, it has been shown in vitro that VDE requires ascorbate as reductant (Hager, 1969). An Arabidopsis mutant that is lacking VDE activity has been isolated by a screen for plants exhibiting lower NPQ (Niyogi et al., 1998). This VDE mutant, npq1-2, shows a 70% reduction in NPQ compared with the wild type. A reduction of the NPQ level is also observed in tobacco antisense VDE plants (Chang et al., 2000).
Figure 1.
A, The xanthophyll cycle consists of the conversion of violaxanthin to antheraxanthin and zeaxanthin in high light. Ascorbate and a low pH are the requirements for the violaxanthin de-epoxidase (VDE), the enzyme catalyzing the conversion. In low light, zeaxanthin and antheraxanthin are epoxidized to violaxanthin. B, Mehler-peroxidase reaction or water-water cycle. The pseudocyclic electron transport from water to water generates a proton gradient. APX, Ascorbate peroxidase; AsA, ascorbate; MDA, monodehydroascorbate; SOD, superoxide dismutase. B, Based on Asada (1999).
Ascorbate also has another important photoprotective function because of its antioxidant capacity. Ascorbate is one of the two major soluble antioxidants in chloroplasts (Foyer and Harbinson, 1994) and is thought to reduce oxidized alpha-tocopherol, a major lipid-soluble antioxidant (Beyer, 1994). Ascorbate is a cofactor for the antioxidant enzyme-catalyzed reduction of reactive oxygen species produced by photosystem I (PS I), in the so-called Mehler-peroxidase reaction or water-water cycle (Asada, 1999, Fig. 1B). The Mehler-peroxidase reaction consists of the Mehler reaction, which is the photoreduction of oxygen by PS I to a superoxide anion radical, followed by the dismutation of this superoxide anion radical by superoxide dismutase to hydrogen peroxide and oxygen. Hydrogen peroxide then is reduced by ascorbate peroxidase (APX) to water, followed by the regeneration of ascorbate by direct reduction of monodehydroascorbate by PS I or by the NADPH-dependent monodehydroascorbate reductase. The Mehler-peroxidase reaction results in electron flow from PS II to PS I with no net oxygen evolution. The proton gradient generated by this “pseudocyclic” electron flow has been shown to be important for zeaxanthin formation and qE in intact chloroplasts under conditions in which CO2 fixation was limiting (Neubauer and Yamamoto, 1992). On the other hand, the Mehler-peroxidase reaction is also competing with VDE for ascorbate. It has been shown that the Mehler-peroxidase reaction can limit qE by decreasing ascorbate availability for the VDE reaction in isolated thylakoids (Neubauer and Yamamoto, 1994).
We used a vitamin C-deficient Arabidopsis mutant, called vtc2-2, hereafter called vtc2, to determine how ascorbate availability affects NPQ in vivo. This mutant was isolated previously by a screen for ozone sensitive mutants (Conklin et al., 2000; P.L. Conklin and R.L. Last, unpublished data). We observed a decrease in the extent of NPQ in this mutant, and similar experiments with vtc2 and other vtc mutants have also shown a lowering of NPQ (Noctor et al., 2000; Smirnoff, 2000). Whereas these previous studies did not investigate the basis of the effect on NPQ, here we present detailed evidence that the lowered NPQ in vtc2 is because of ascorbate limitation of VDE activity in vivo. We show that the ascorbate and NPQ phenotypes of vtc2 cosegregate genetically, and that both the de-epoxidation and NPQ defects in the mutant can be rescued by ascorbate feeding.
RESULTS
vtc2 Has Less Ascorbate Than Arabidopsis Ecotype Columbia (Col-0)
Under short-day conditions, the vtc2 mutant had only 25% ± 9% of the wild-type ascorbate level (Table I). In previous measurements (mature leaves from 6-week-old, long day-grown vtc2 plants), vtc2 had about 10% of wild-type ascorbate levels (Conklin et al., 2000). The F1 generation of a cross between the vtc2 mutant and Col-0 showed the same level of ascorbate as Col-0. Therefore, the vtc2-2 allele is recessive for ascorbate deficiency as has been shown previously (Conklin et al., 2000).
Table I.
Total ascorbate content of mature leaves
| Genotype | Ascorbate |
|---|---|
| μmol (g fresh wt)−1 | |
| VTC2/VTC2 | 7.26 ± 0.31 |
| vtc2/vtc2 | 1.83 ± 0.65 |
| vtc2/VTC2 | 7.18 ± 0.89 |
Data shown are the averages of three measurements with sds.
vtc2 Has Less NPQ Than Col-0
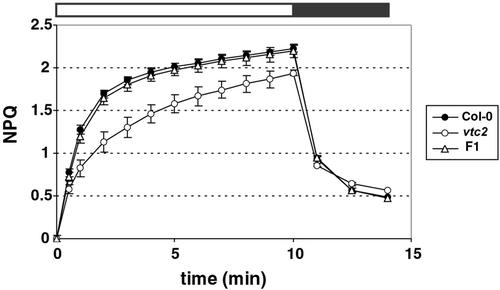
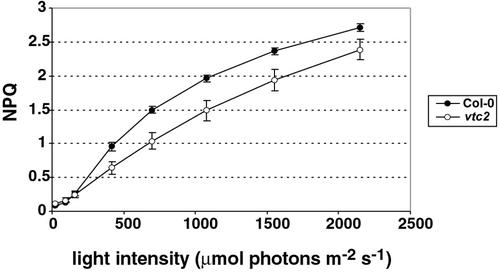
When illuminated with 1,500 μmol photons m−2 s−1, Col-0 showed a rapid establishment of NPQ to a value of 2.2 within 10 min, whereas the mutant showed a slower rate of NPQ induction. Even after 10 min, vtc2 had an NPQ value of less than 2.0 (Fig. 2). In addition, when measuring NPQ at different light intensities (from 25–2,150 μmol photons m−2 s−1), vtc2 consistently had lower NPQ than Col-0 at light intensities above that of the original growth conditions (Fig. 3). The difference in NPQ level did not seem to change with increasing light intensities, but rather stayed constant.
Figure 2.
NPQ phenotype of homozygous and heterozygous vtc2 mutants. NPQ was measured during 10 min of illumination with 1,500 μmol photons m−2 s−1 followed by 4 min of darkness. Data shown are averages of eight measurements with ses.
Figure 3.
Light response curves for NPQ. Data shown are the averages of six measurements with ses.
vtc2 Is an npq Mutant
The vtc2 mutation is the cause of the lowered NPQ. The vtc2 mutant was crossed to Col-0, and the ascorbate and NPQ phenotypes were measured in the resulting F1 and F2 generations. The F1 generation of this cross had wild-type NPQ levels (Fig. 2), showing that the vtc2-2 allele is also recessive for the NPQ phenotype. When screening F2 plants for ascorbate and NPQ deficiency, the ascorbate and NPQ phenotypes cosegregated (Table II). Although there was a continuum in NPQ levels reached after 4 min in high light in both the wild type and the mutant, no ascorbate-deficient plant showed NPQ above 1.6, and no plant with wild-type ascorbate level showed NPQ below 1.6. Furthermore, when averaging the NPQ values obtained from all plants with wild-type ascorbate levels and those from ascorbate-deficient plants, there was a significant difference between the two groups, with an average NPQ of 2.0 ± 0.2 for the wild-type ascorbate level plants versus an average NPQ of 1.3 ± 0.2 for the ascorbate-deficient plants. Out of 44 F2 plants, nine were identified as homozygous vtc2/vtc2 mutants, which is not significantly different from the expected number (11, or 25%) for a single recessive nuclear mutation.
Table II.
Cosegregation of ascorbate and NPQ phenotypes
| Ascorbate Level | NPQ > 1.6 | NPQ < 1.6 |
|---|---|---|
| Wild type | 35 | 0 |
| Ascorbate deficient | 0 | 9 |
Two-week-old plants were screened with the nitroblue tetrazolium squish assay for ascorbate and then screened two weeks later for NPQ.
The Conversion of Violaxanthin to Zeaxanthin Is Slower in vtc2 Than in Col-0
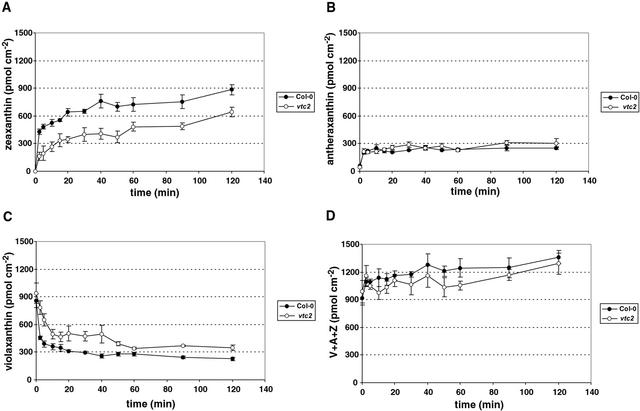
The conversion of violaxanthin to zeaxanthin was slower in the mutant and did not reach the same saturation level as in the wild type (Fig. 4, A and C). In Col-0, 73% of violaxanthin was converted to zeaxanthin after 2 h, whereas in the mutant, only 63% of violaxanthin was de-epoxidized. In the first 2.5 min, 47% of the total violaxanthin was de-epoxidized in Col-0, whereas only 17% of the total violaxanthin in vtc2 was converted to zeaxanthin, showing the much faster rate of conversion in the wild type compared with the mutant. The antheraxanthin levels in both Col-0 and vtc2 increased in the first 2.5 min and then stabilized at the same steady-state value (Fig. 4B), showing that this intermediate pigment did not accumulate in the mutant. The zeaxanthin level in the mutant was substantially lower than in the wild type (Fig. 4A). The wild type had 884 ± 57 pmol zeaxanthin cm−2, and vtc2 had only 643 ± 55 pmol cm−2. Thus, the mutant had about 30% less zeaxanthin than the wild type even after 2 h in high light. Comparing the first 2.5 min, the initial rate of zeaxanthin synthesis in the mutant was only 40% of the wild-type rate. The total xanthophyll pool consisting of violaxanthin, zeaxanthin, and antheraxanthin was similar in wild type and vtc2 before and also during the treatment (Fig. 4D), as were the chlorophyll levels (data not shown).
Figure 4.
Changes in xanthophyll pigments during illumination. A, Zeaxanthin. B, Antheraxanthin. C, Violaxanthin. D, Xanthophyll cycle pool. Data shown are averages of five measurements with ses.
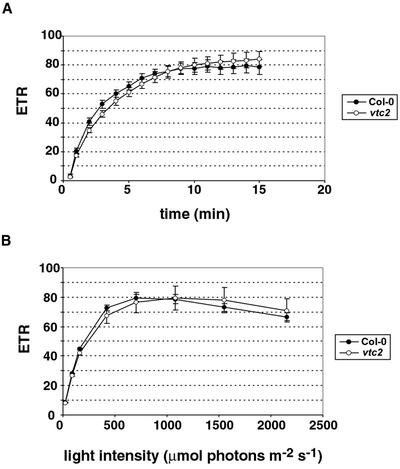
Col-0 and vtc2 Have the Same Electron Transport Rate (ETR)
Ascorbate is both a reductant for VDE, and it also plays an important role in the Mehler-peroxidase pathway that might be involved in generating a sufficient pH gradient for activation of VDE. Because of this dual function of ascorbate, we compared the ETR in wild type and the mutant. If ascorbate was limiting for the Mehler-peroxidase pathway, a decrease in ETR in the mutant compared with the wild type could be expected. Even though the NPQ levels were quite different in Col-0 and vtc2, the ETRs were the same except for the first 3 min in which vtc2 had a slightly lower rate (Fig. 5A). The ETR increased in both Col-0 and vtc2 at the beginning of the illumination (during the induction of photosynthesis) and reached saturation after 10 min of high light. Also, vtc2 and Col-0 had the same ETR when comparing different light intensities (Fig. 5B). Both genotypes showed a maximum ETR at light intensities around 1,000 μmol photons m−2 s−1, followed by a decrease in the rate at higher intensities.
Figure 5.
ETR in vtc2. A, ETR versus illumination time. B, Light response curve for ETR. Data shown are averages of 10 (A) or six (B) measurements with ses.
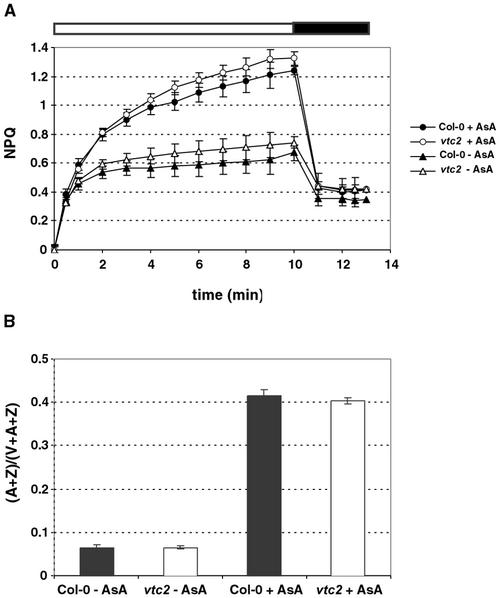
Ascorbate Feeding Restores NPQ and Violaxanthin De-Epoxidation in Isolated Thylakoids and Detached Leaves
Thylakoids were isolated with buffer that did not contain any ascorbate. Ascorbate is typically added to buffers to stabilize thylakoids because the naturally occurring ascorbate in the thylakoids generally is lost during the isolation. This was apparent when measuring NPQ in thylakoids isolated without addition of ascorbate, in which NPQ only reached a value of approximately 0.7 in both Col-0 and vtc2 (Fig. 6A). After adding ascorbate, both genotypes showed a 2-fold increase in NPQ. A corresponding increase was seen in the de-epoxidation state (Fig. 6B) of Col-0 and vtc2 thylakoids. The thylakoids had equally low de-epoxidation states when no ascorbate was present, but each showed a very large increase in de-epoxidation state when ascorbate was added. Again, in either case there was never a difference in the levels of NPQ or de-epoxidation state between Col-0 or vtc2, arguing that there are no inherent differences between Col-0 and vtc2 thylakoids except for the ascorbate level.
Figure 6.
Thylakoid treatment with ascorbate. A, NPQ of isolated wild-type and vtc2 thylakoids with added buffer (−AsA) or with added ascorbate (+AsA) during 10 min of illumination and 3 min of darkness. B, De-epoxidation state of the thylakoids after illumination. Data shown are averages of seven measurements with ses.
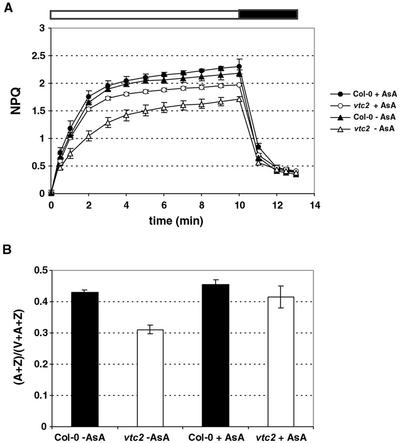
The lower NPQ phenotype could also be partially rescued in detached leaves by feeding with 10 mm ascorbate. Col-0 leaves reached a NPQ level of 2.3 when fed with ascorbate, which was slightly higher than for the control leaves (2.2, Fig. 7A). The leaves of the vtc2 mutants fed with ascorbate, on the other hand, showed a large increase in the level of NPQ compared with those fed with water. Whereas control leaves had a maximum NPQ of 1.7, ascorbate-fed leaves had an NPQ level of 2.0. This rescue effect could also be seen in the de-epoxidation state of leaf discs taken immediately after the end of the NPQ measurement. Whereas the de-epoxidation state of Col-0 leaves only changed slightly, there was a significant increase in the de-epoxidation state in vtc2 to nearly wild-type levels (Fig. 7B).
Figure 7.
Ascorbate feeding to detached leaves. A, NPQ of wild-type and mutant leaves after feeding with ascorbate (+AsA) or water (−AsA) during 10 min of illumination and 3 min of darkness. B, De-epoxidation state after illumination. Data shown are averages of seven measurements with ses.
DISCUSSION
NPQ Deficiency in vtc2 Is Caused by Ascorbate Limitation
Several lines of evidence suggest that the ascorbate content within the chloroplasts of the vtc2 mutant is reduced in comparison with the wild type. First, NPQ and the xanthophyll cycle are thylakoid-localized processes, so the ascorbate content within the chloroplast must be affected. Second, it has been estimated that chloroplasts contain 20% to 40% of the ascorbate found in a mesophyll cell (Foyer et al., 1983). However, if we were to assume that the ascorbate content in chloroplasts was not affected in vtc2, then all other compartments would completely lack ascorbate. Because ascorbate is required among other things for cell cycle function (Smirnoff, 1996), this seems highly unlikely. Therefore, it can be concluded that the ascorbate content of chloroplasts must be affected in the vtc2 mutant.
The vtc2 mutant is not only affected in its ascorbate content (Table I), but also in its ability to perform maximum NPQ (Fig. 2). Using genetic cosegregation analysis, it was established that the inability to perform maximum NPQ is caused by the mutant's deficiency in ascorbate (Table II). In a similar but less extensive experiment, vtc2 and three other vtc mutants all showed less NPQ than the wild type during a very short light treatment (Smirnoff, 2000). None of the mutants were affected in qP. In another experiment, these results were confirmed for vtc1 (Noctor et al., 2000). The fact that all ascorbate-deficient mutants tested had less NPQ indicates that ascorbate is likely to be the cause of the NPQ phenotype.
In a more detailed recent study, the vtc1 mutant showed only a slight decrease in NPQ (Veljovic-Jovanovic et al., 2001). The authors concluded that ascorbate was not limiting for the de-epoxidation of violaxanthin or that ascorbate was preferentially accumulated in the chloroplast. In light of our finding that ascorbate deficiency does limit VDE activity and reduces NPQ in vtc2, it seems likely that the two mutants are differentially affected by the lower ascorbate content. It is still unknown which gene, if biosynthetic or regulatory, is affected in vtc2, whereas the defect in vtc1 is in GDP-Man pyrophosphorylase required for synthesis of ascorbate. It is also possible that the compartmentalization of ascorbate is different in these two mutants.
Lowered De-Epoxidation State and NPQ Can Be Rescued by Feeding with Ascorbate
Another line of evidence for the reduced ascorbate content in the chloroplasts and for this limitation causing the lowered NPQ comes from the results of our measurements of the de-epoxidation state and NPQ in isolated thylakoids. When thylakoids were isolated without ascorbate in the isolation buffer, internal ascorbate was lost, leading to a low de-epoxidation state and NPQ in both the mutant and the wild type. This low-NPQ phenotype is consistent with measurements of NPQ in maize (Zea mays) chloroplasts that had been isolated without ascorbate (Ivanov and Edwards, 2000). After adding the same amount of ascorbate to the isolated thylakoids, both mutant and wild type had higher and similar de-epoxidation states and NPQ levels (Fig. 6). This suggests that the observed difference in whole plants is not caused by an inherent difference between the thylakoids other than the ascorbate content. By feeding ascorbate to detached leaves, we also showed that the difference in de-epoxidation state and NPQ between wild type and mutant could be decreased drastically (Fig. 7), again confirming that ascorbate deficiency is causing the difference in the NPQ phenotype.
Reduced Amount of Ascorbate Likely Has a Direct Effect on VDE
If we consider the ascorbate level in the chloroplast to be affected, then there are two ways in which the reduced ascorbate availability could affect the xanthophyll cycle activity (Fig. 4), the NPQ level (Figs. 2 and 3), and the rate of the VDE enzyme. First, there could be a direct effect on the xanthophyll cycle by reduced availability of reductant for VDE. Second, the Mehler-peroxidase pathway (water-water cycle) could be affected, which would result in a lowered proton gradient across the thylakoid membrane. This in turn might affect the activity of VDE, which has an optimum activity at pH 5 (Hager, 1969). It also could affect the degree of PS II protein protonation, which is also required for qE.
Our results indicate that reduced ascorbate content is affecting the xanthophyll cycle directly. Direct substrate limitation of ascorbate on VDE activity has been shown by stimulating ascorbate oxidation by APX in vitro (Neubauer and Yamamoto, 1994). After adding hydrogen peroxide that is metabolized by APX, VDE activity was transiently inhibited and qP was increased. In addition, the Km (0.36 mm) of APX was found to be lower than the Km (3.1 mm) of VDE (Neubauer and Yamamoto, 1994), making it likely that APX would be a better competitor for ascorbate than VDE. In a more recent study, it has been argued that the Km of VDE, which is strongly pH dependent, could be expressed as a single Km of 0.1 mm for the acid form of ascorbate (Bratt et al., 1995). Even though in this case VDE would have a higher affinity for ascorbate than APX, there is at least 30 times more APX than VDE in thylakoids. It has been estimated that there is one thylakoid-associated APX protein per 0.2 to 0.7 electron transport chains (Miyake and Asada, 1992), whereas there is only one VDE protein per 20 to 100 electron transport chains (Arvidsson et al., 1996). In addition, the different locations of APX and VDE favor APX because APX in the stroma could still take up the available ascorbate before it could reach VDE in the lumen. A possible direct substrate limitation on VDE in vivo even in the wild type could be seen in the feeding experiment. After feeding ascorbate, the NPQ increased from a value of 2.2 (−AsA) to 2.3 (+AsA). This was also accompanied by a small increase in the de-epoxidation ratio (Fig. 7). Similarly, an increase in zeaxanthin content has been reported after infiltrating detached maize leaves with ascorbate (Leipner et al., 2000). Thus, it is conceivable that ascorbate limitation of VDE and NPQ could occur in wild-type plants under certain oxidative stress conditions that affect the redox state of the ascorbate pool, perhaps via APX activity.
The Mehler-peroxidase pathway has been implied to promote electron transport, especially during the first few minutes of photosynthetic induction after a dark-light transition when CO2 assimilation is limited (Schreiber and Neubauer, 1990; Polle, 1996). In vtc2, a small difference in ETR was apparent in the first 3 min of illumination with high light (Fig. 4A), implying there might be a slight limitation of the Mehler-peroxidase reaction that could also have an effect on NPQ during the first few minutes. In favor of this hypothesis, there was no significant difference between the ETRs of the mutant and the wild type after feeding ascorbate (data not shown). We are in the process of isolating APX-deficient mutants so that we can test the influence of the Mehler-peroxidase pathway on NPQ and VDE in more detail.
After the induction period, however, there was very little difference in steady-state ETR between the vtc2 mutant and wild type regardless of whether it was measured at a constant light intensity for 15 min or at different light intensities (Fig. 4). This implies that the Mehler-peroxidase reaction is not significantly affected in vtc2, or that it does not play a major role in electron transport and hence in the establishment of the proton gradient in vivo. The experiments that have shown that this reaction mediates the formation of zeaxanthin and qE have been done in isolated chloroplasts in which carbon fixation had been inhibited (Neubauer and Yamamoto, 1992). In conclusion, it seems most likely that the effect of reduced ascorbate content is as a direct substrate limitation on VDE activity in vivo.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis Col-0 and the vitamin C-deficient mutant, vtc2-2, also Col-0 ecotype, were grown in soil for 6 weeks in controlled growth conditions of 10 h light, 22°C/14 h dark, 18°C, with a light intensity of 150 μmol photons m−2 s−1. The vtc2 mutant was isolated in a screen for ozone-sensitive plants (Conklin et al., 2000; P.L. Conklin and R.L. Last, unpublished data). For the cosegregation analysis, plants were grown for 2 weeks on minimal plant nutrient agar plates (Haughn and Somerville, 1986) and then transferred to soil.
Thylakoid Preparation
Thylakoids for the NPQ measurements were prepared as in Gilmore et al. (1998) except that ascorbate was omitted.
Fluorescence Measurements
Standard modulated chlorophyll fluorescence measurements were done with overnight dark-adapted plants using an FMS2 instrument (Hansatech, King's Lynn, UK). Dark-adapted plants (overnight) or thylakoids were subjected to a saturating light pulse and then illuminated (1,500 μmol photons m−2 s−1) for 10 min followed by a 4-min dark recovery period. For light response curves, plants were illuminated for 5-min periods, each with increasing light intensities. NPQ was calculated as (Fm' − Fm)/ Fm' and ΦPS II as (Fm' − Fs)/ Fm', where Fm' is maximum PS II fluorescence in the light-adapted state, Fm is maximum PS II fluorescence in the dark-adapted state, and Fs is steady-state fluorescence. ETR was calculated as photosynthetically active radiation × 0.5 × 0.84 × ΦPS II. To measure NPQ in isolated thylakoids, the thylakoids were diluted to 50 μm chlorophyll in reaction buffer lacking ascorbate (Gilmore et al., 1998), and either ascorbate (30 mm final concentration) or more buffer was added.
Pigments
Plants were illuminated with high light (1,500 μmol photons m−2 s−1) for several minutes to 2 h; leaf disc samples were taken and immediately frozen in liquid nitrogen. The frozen disc was ground to a fine powder and extracted with 150 μL of 100% (v/v) acetone by vortexing for 1 min. The extract was centrifuged for 20 s, and the supernatant was saved. Another 150 μL of 100% (v/v) acetone was added to the pellet and mixed thoroughly. The extract was centrifuged again, and the supernatants were pooled. Fifteen microliters of the filtered (0.2-μm nylon filter) supernatant was subjected to HPLC and separated on a Spherisorb S5 ODS1 4.6- × 250-mm cartridge column (Waters, Milford, MA) at 30°C. HPLC analysis was performed using a modification of the method of García-Plazaola and Becerril (1999). Pigments were eluted with a linear gradient from 100% (v/v) solvent A (acetonitrile:methanol:0.1 m Tris-HCl, pH 8.0; 84:2:14 [v/v]) to 100% (v/v) solvent B (methanol:ethyl acetate, 68:32 [v/v]) for 15 min, followed by 3 min of solvent B. The solvent flow rate was 1.2 mL min−1. Pigments were detected by A445 with a reference at 550 nm by a diode array detector.
Ascorbate
Total ascorbate was determined by a spectrophotometric method using the UV absorption at 265 nm by reduced ascorbate (Conklin et al., 1996). To test ascorbate deficiency qualitatively, the nitroblue tetrazolium squish test was used as described previously (Conklin et al., 2000).
Ascorbate Feeding
Leaves were detached at the petiole with a razor blade under water to prevent embolism. The detached leaves were placed into 1 mL of 10 mm ascorbic acid or water (control) and incubated for 160 min in the dark.
ACKNOWLEDGMENTS
We thank Talila Golan, Ben Gutman, and Xiao-Ping Li for critical reading of the manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative (grant no. 98–35306–6600) and by the Searle Scholars Program/The Chicago Community Trust.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010924.
LITERATURE CITED
- Arvidsson P-O, Bratt CE, Carlsson M, Akerlund H-E. Purification and identification of the violaxanthin deepoxidase as a 43 kDa protein. Photosynth Res. 1996;49:119–129. doi: 10.1007/BF00117662. [DOI] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J Bioenerg Biomembr. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- Bratt CE, Arvidsson P-O, Carlsson M, Akerlund H-E. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res. 1995;45:169–175. doi: 10.1007/BF00032588. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Hieber AD, Yamamoto HY. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem. 1998;273:15321–15324. doi: 10.1074/jbc.273.25.15321. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Yamamoto HY. Molecular cloning of violaxanthin de-epoxidase from romaine lettuce and expression in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:6320–6325. doi: 10.1073/pnas.93.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-H, Bugos RC, Sun W-H, Yamamoto HY. Antisense suppression of violaxanthin de-epoxidase in tobacco does not affect plant performance in controlled growth conditions. Photosynth Res. 2000;64:95–103. doi: 10.1023/A:1026518524426. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Rowell J, Walker D. Measurement of ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Harbinson J. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 1–42. [Google Scholar]
- García-Plazaola JI, Becerril JM. A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: simultaneous determination of carotenoids and tocopherols. Phytochem Anal. 1999;10:307–313. [Google Scholar]
- Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a fluorescence lifetime distributions and intensity in thylakoids. Biochemistry. 1998;37:13582–13593. doi: 10.1021/bi981384x. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching: evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res. 1993;35:67–78. doi: 10.1007/BF02185412. [DOI] [PubMed] [Google Scholar]
- Hager A. Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin-Zeaxanthin-Umwandlung: Beziehung zur Photophosphorylierung. Planta. 1969;89:224–243. doi: 10.1007/BF00385028. [DOI] [PubMed] [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Hager A, Perz H. Veränderung der Lichtabsorption eines Carotenoids im Enzym (De-Epoxidase)-Substrat (Violaxanthin)-Komplex. Planta. 1970;93:314–322. doi: 10.1007/BF00384105. [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Ivanov B, Edwards G. Influence of ascorbate and the Mehler peroxidase reaction on non-photochemical quenching of chlorophyll fluorescence in maize mesophyll chloroplasts. Planta. 2000;210:765–774. doi: 10.1007/s004250050678. [DOI] [PubMed] [Google Scholar]
- Leipner J, Stamp P, Fracheboud Y. Artificially increased ascorbate content affects zeaxanthin formation but not thermal energy dissipation or degradation of antioxidants during cold-induced photooxidative stress in maize leaves. Planta. 2000;210:964–969. doi: 10.1007/s004250050704. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Müller P, Li X-P, Niyogi KK. Non-photochemical quenching: a response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer C, Yamamoto HY. Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol. 1992;99:1354–1361. doi: 10.1104/pp.99.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer C, Yamamoto HY. Membrane barriers and Mehler-peroxidase reaction limit the ascorbate available for violaxanthin de-epoxidase activity in intact chloroplasts. Photosynth Res. 1994;39:137–147. doi: 10.1007/BF00029381. [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Foyer CH. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos Trans R Soc Lond B. 2000;355:1465–1475. doi: 10.1098/rstb.2000.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A. Mehler reaction: friend or foe in photosynthesis. Bot Acta. 1996;109:84–89. [Google Scholar]
- Schreiber U, Neubauer C. O2-dependent electron flow, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth Res. 1990;25:279–293. doi: 10.1007/BF00033169. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. [Google Scholar]
- Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto HY, Higashi RM. Violaxanthin de-epoxidase: lipid composition and substrate specificity. Arch Biochem Biophys. 1978;190:514–522. doi: 10.1016/0003-9861(78)90305-3. [DOI] [PubMed] [Google Scholar]