Abstract
Neutrophils are important effector cells in innate and acquired immunity, but the magnitude and character of their phagocytic and bactericidal responses depend on cues derived from mediators in the local microenvironment. This study investigated the effect of bovine interleukin-8 (IL-8) and granulocyte colony-stimulating factor (G-CSF) on priming and activation of bovine neutrophils in vitro and in vivo. Neutrophils were isolated from blood and cultured for up to 18 h, with or without cytokines, and then Mannheimia haemolytica-induced oxidative burst and phagocytosis of Staphylococcus aureus were measured by flow cytometry. Neither IL-8 nor G-CSF directly triggered an oxidative burst, but incubation with these cytokines for 18 h primed neutrophils for a greater oxidative burst triggered by M. haemolytica and for enhanced uptake of S. aureus. The maximal response was observed when neutrophils were incubated with both cytokines together, at concentrations of 200 ng/ml for G-CSF and 400 ng/ml for IL-8. The IL-8-induced priming effect was reduced by treatment with a neutralizing antibody to IL-8, and was not attributed to endotoxin contamination. Instillation of IL-8 into the lung using a bronchoscope induced neutrophil recruitment within 18 h. Neutrophils from IL-8-treated lung showed dose-dependent enhancement of the oxidative burst triggered by M. haemolytica. Histologically, neutrophils filled alveoli and bronchioles, and scattered macrophages contained neutrophils with morphological features of apoptosis. Thus, prolonged in vitro or in vivo exposure to IL-8 and/or G-CSF enhances the subsequent oxidative burst and phagocytic responses of bovine neutrophils.
A critical role for impaired pulmonary defenses in the pathogenesis of shipping fever pneumonia in beef calves is suggested by the close association of this common and economically important disease with the stresses of weaning and transport (28) and the fact that bacterial pneumonia is caused by pathogens that are carried by many healthy calves (13). Neutrophils are important effector cells in both innate and acquired immunity. These leukocytes have surface receptors for complement and other innate defense proteins, as well as Fc receptors that allow efficient recognition and phagocytosis of antibody-opsonized bacteria. Neutrophils are beneficial in most bacterial infections, using hypohalous acids, superoxide anion, antibacterial granule proteins, and proteases to kill and degrade bacteria (27, 36). Nevertheless, these same products of neutrophils have the capacity to injure lung tissue, and neutrophil-derived cytokines are able to amplify the inflammatory response, further reducing alveolar ventilation and gas exchange in the inflamed lung. Whether neutrophil responses are beneficial or harmful in calves with pulmonary bacterial infection remains controversial (2, 30, 35) and probably depends on the magnitude of bacterial challenge, the immune status of the host, and specific virulence factors of the bacterial pathogen (17, 32).
Neutrophils do not respond to bacteria in a stereotyped manner. Rather, the magnitude and the character of their phagocytic and bactericidal responses depend on cues derived from soluble or matrix-bound mediators in the local microenvironment. A variety of stimuli induce an oxidative burst in neutrophils in vitro, including phagocytosis of opsonized particles, the complement-derived anaphylatoxin C5a, the leukotoxin of Mannheimia haemolytica, and phorbol myristate acetate. Many other stimuli do not directly induce an oxidative burst but enhance or “prime” neutrophils for greater responses to agonists. Mediators that prime neutrophils include platelet-activating factor, leukotriene B4, chemokines such as interleukin-8 (IL-8), tumor necrosis factor alpha (TNF-α), and lipopolysaccharide (14, 20, 21, 26, 31, 36). A recent report of a child with recurrent bacterial infections associated with a defect in lipopolysaccharide- and cytokine-induced priming of neutrophils implies that this phenomenon of priming is critical to effective neutrophil function (12).
Bovine IL-8 is chemotactic for neutrophils in vivo and in vitro and is responsible for 15 to 60% of the neutrophil chemotactic activity of bronchoalveolar lavage fluid in calves with pneumonic pasteurellosis (4-6). The effects of IL-8 on other neutrophil functions is less well characterized. Short-term (30-min) treatment of bovine neutrophils with human IL-8 induced secretion of secondary granules and increased expression of CD11b but did not elicit primary granule release or oxidative burst (14, 21). Previous studies have reported that IL-8 primes neutrophils for greater chemiluminescent responses to opsonized zymosan but have not fully characterized this effect (21).
Bovine granulocyte colony stimulating factor (G-CSF) induces bone marrow neutrophil responses following systemic administration to calves. Daily administration of G-CSF induces a rapid mature neutrophilia within 24 h of injection that increases in magnitude in a dose-dependent manner during treatment. Immature neutrophils appear in blood on days 2 to 3 or days 3 to 12, depending on the dose of G-CSF administered. In the marrow, synchronous myeloid hyperplasia develops within 9 days of treatment (8, 9, 18). Exposure of bovine neutrophils to G-CSF causes increased cell surface expression of CD11a, CD11b, and CD18 and reduced expression of L-selectin (15). Treatment of human neutrophils with G-CSF increases C3bi receptor expression, enhances phagocytosis and bacterial killing of Staphylococcus aureus, and primes for but does not induce an oxidative burst (16, 29, 38). Previous studies indicate that neutrophils from preparturient cows treated with G-CSF have enhanced phagocytosis of S. aureus and cytotoxicity; reduced chemotactic function, iodination, and bactericidal activity; but no significant alterations in the oxidative burst (18). The in vitro effects of G-CSF on antibacterial functions of bovine neutrophils have not been evaluated.
The purpose of this study was to evaluate the effect of bovine IL-8 and G-CSF on priming and activation of bovine neutrophils and to investigate whether these cytokines have a synergistic effect on neutrophils. We hypothesized, based on similar studies using human neutrophils (38), that G-CSF would prime neutrophils whereas treatment of neutrophils with both IL-8 and G-CSF would lead to neutrophil activation.
MATERIALS AND METHODS
Reagents.
All media and supplements were obtained from Canadian Life Technologies (Burlington, Ontario, Canada), unless otherwise specified. Culture medium (cRPMI) consisted of RPMI 1640 containing 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (100 IU/ml), and streptomycin (100 μg/ml). Recombinant bovine G-CSF was a generous gift from Amgen, Inc. (Thousand Oaks, Calif.). Recombinant bovine IL-8 was produced as previously described (4). 2,7-Dichlorodihydrofluorecein diacetate (H2DCFDA) and calcein AM were purchased from Molecular Probes Inc. (Eugene, Oreg.), and fluorescein isothiocyanate, isomer I (FITC), and HEPES were purchased from Sigma Chemical Co. (St. Louis, Mo.). RPMI, Hanks buffered salt solution, brain heart infusion (BHI) broth, and phosphate-buffered saline (PBS) were prepared in-house, according to standard protocols.
FITC-labeled S. aureus was prepared by inoculating BHI broth with a pure isolate of S. aureus and incubating for 24 h at 37°C. Bacteria were heat killed in a 65°C water bath for 90 min, washed twice in PBS, resuspended in 1.0 M bicarbonate buffer (pH 9.5) containing 0.04% FITC, and incubated for 2 h at 37°C in the dark. Bacteria were washed twice in PBS, resuspended to 4 × 108 cells/ml in PBS, and stored at −20°C in the dark.
Opsonized, heat-killed M. haemolytica was prepared by inoculating BHI broth with a pure isolate of M. haemolytica and incubating for 24 h at 37°C. Bacteria were heat killed in a 58°C water bath for 60 min and washed twice in PBS, and the concentration was adjusted to 2 × 109 CFU/ml in PBS, as estimated by absorbance at 525 nm. Killed M. haemolytica cells were opsonized by adding equal volumes of bacterial suspension to 10% adult bovine serum in PBS. After incubating for 30 min at 37°C, bacteria were washed twice in PBS, resuspended to 109 CFU/ml in RPMI with 10% FBS and 1 mM HEPES, and stored at −20°C.
Neutrophil function assays.
Bovine peripheral blood leukocytes were isolated from clinically normal, 2 to 4 month old, Holstein calves as previously described (4). Neutrophil assays were performed using aseptic conditions. Over 95% of the isolated cells were granulocytes, of which 87 to 98% were neutrophils depending on the number of eosinophils in whole blood. Cell viability exceeded 95% based on Trypan blue staining.
Oxidative burst in neutrophils was measured using flow cytometry to detect oxidation of the fluorochrome H2DCFDA, based on a modification of a previously described protocol (3, 10a, 34). Neutrophils, at a concentration of 107 cells/ml, were incubated with cytokines at 37°C in cRPMI, and 10 mM H2DCFDA was added for the final 30 min of incubation. The cells were then added to either medium (RMPI containing 10% FBS and 1 mM HEPES) alone or medium containing 107 CFU/ml of heat-killed, opsonized M. haemolytica for a further 30 min at 37°C to stimulate superoxide release. Samples were placed in an ice bath, and mean fluorescence of the neutrophil population was measured within 20 min by flow cytometry (FACScan; Becton-Dickinson, Mountain View, Calif.). Samples in which neutrophils and bacteria were held on ice prior to and after mixing served as the negative control. Fluorescence was measured on 10,000 cells within the gated neutrophil population, and the difference between the mean fluorescence of the test and that of chilled samples was calculated.
The phagocytosis of FITC-labeled S. aureus was measured using flow cytometry. Neutrophils, at a concentration of 107 cells/ml in cRMPI, were incubated for 18 h with cytokines and then transferred to 5-ml culture tubes containing an equal volume of 108 FITC-labeled S. aureus/ml. Following a 30-min incubation in a 37°C shaking water bath, tubes were placed on ice, and chilled PBS containing 2.5 × 10−3 M EDTA and 0.5% Trypan blue was added to arrest phagocytosis and quench extracellular fluorescence. Samples in which neutrophils and bacteria were held on ice prior to and after mixing served as the negative control. Test specificity was confirmed in preliminary studies, using samples treated with cytochalasin D to inhibit phagocytosis. Internalization of fluorescent bacteria was analyzed by flow cytometry as described above.
The concentration of endotoxin in the cytokine preparations was measured using a kit (E-toxate; Sigma). To confirm that these levels of endotoxin did not cause priming of neutrophils, E. coli endotoxin was used in place of cytokines in the oxidative burst assay. Neutrophils were incubated in triplicate with endotoxin at concentrations of 0, 0.008, 0.08, 0.8, and 8.0 endotoxin units (EU)/ml for 18 h at 37°C and then stimulated with opsonized M. haemolytica, and the oxidative burst was measured as described above.
A mouse IgG2a monoclonal antibody against ovine IL-8 (clone 8 M6; Serotec, Raleigh, N.C.), which has previously been shown to inhibit IL-8-induced chemotaxis of bovine neutrophils (4, 5), was used to confirm the specificity of the priming effect of IL-8 on neutrophils. Neutrophils were treated in duplicate for 18 h with IL-8 (400 ng/ml), with or without the addition of a 2- or 20-μg/ml concentration of anti-IL-8 antibody (8 M6) or an irrelevant antibody (mouse IgG1 anti-CD18, clone MHM23; Dako Diagnostics, Mississauga, Ontario, Canada). After incubation, oxidative burst in response to killed, opsonized M. haemolytica was measured as described above.
The phagocytosis of FITC-labeled S. aureus was measured using flow cytometry. Neutrophils, at a concentration of 107 cells/ml in cRMPI, were incubated for 18 h with cytokines and then transferred to 5-ml culture tubes containing an equal volume of 108 FITC-labeled S. aureus/ml. Following a 30-min incubation in a 37°C shaking water bath, tubes were placed on ice, and chilled PBS containing 2.5 × 10−3 M EDTA and 0.5% Trypan Blue was added to arrest phagocytosis and quench extracellular fluorescence. Samples in which neutrophils and bacteria were held on ice prior to and after mixing served as the negative control. Test specificity was confirmed in preliminary studies, using samples treated with cytochalasin D to inhibit phagocytosis. Internalization of fluorescent bacteria was analyzed by flow cytometry as described above.
Intrapulmonary IL-8 testing.
The in vivo effect of IL-8 was examined in a clinically healthy, hematologically normal, 4-month-old, male Holstein calf. The calf was treated with flufenicol (20 mg/kg of body weight) every two days for three treatments to eliminate subclinical bronchopneumonia, and the drug was withdrawn for 6 days prior to IL-8 administration. The calf was sedated with xylazine (0.15 mg/kg) and butorphanol (0.06 mg/kg). A bronchoscope was wedged into a bronchus, and 10 ml of IL-8 or PBS was slowly instilled. Six distinct bronchi were infused: two with PBS alone, one with 3 μg of IL-8, two with 30 μg of IL-8, and one with 300 μg of IL-8. The calf was euthanized 18 h later, and the same bronchi were lavaged with PBS. Six sections of each area of lung supplied by these bronchi were fixed in formalin; histologic sections were processed routinely, stained with hematoxylin and eosin, and examined microscopically. The total number of cells retrieved by lavage of each site was determined using a Coulter counter, and differential counts were performed on Wright's-stained cytocentrifuge preparations. Cells were washed twice in PBS, and the oxidative burst assay was performed as described above.
Statistical analysis.
Data are presented as mean ± standard error of the mean. Data were analyzed using the Student's t test. When experiments were repeated using neutrophils from different calves on different days, data were analyzed using repeated-measures analysis of variance (ANOVA). If the ANOVA test was significant at P < 0.05, then individual comparisons were evaluated using Tukey's multiple-comparison test. For evaluation of the dose-dependent effect for both cytokines in combination, the overall effect of each cytokine was analyzed by two-way ANOVA, followed by Student's t tests to estimate the significance of individual differences.
RESULTS
Effect of IL-8 and G-CSF on oxidative burst of bovine neutrophils.
To determine the effect of IL-8 and G-CSF on bovine neutrophil function, we examined whether treatment of isolated peripheral blood neutrophils for 18 h with these cytokines would alter their response to opsonized, heat-killed M. haemolytica bacteria. In a preliminary experiment, the neutrophil oxidative burst induced by M. haemolytica was significantly enhanced in a dose-dependent manner by pretreatment with IL-8 at a concentration of 400 or 4,000 ng/ml or with G-CSF at a concentration of 200 or 2,000 ng/ml (P < 0.005). Treatment with both IL-8 and G-CSF enhanced oxidative burst to M. haemolytica above that observed with either cytokine alone, with G-CSF (200 ng/ml) and IL-8 (400 ng/ml), resulting in maximal responsiveness. Incubation of neutrophils with either IL-8 or G-CSF, in the absence of M. haemolytica, did not induce an oxidative burst at the concentrations examined.
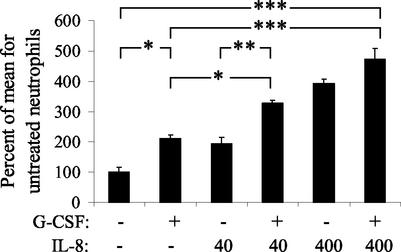
To confirm this priming effect, neutrophils isolated from five different calves on different days were pretreated with IL-8 (0, 4.0, or 400 ng/ml) and/or G-CSF (0 or 200 ng/ml), and this was followed by stimulation with M. haemolytica. The priming effect of 400-ng/ml IL-8 was significant (P < 0.001). G-CSF also significantly (P < 0.05) increased the responsiveness of neutrophils, but to a lesser degree than IL-8. Combined treatment with IL-8 (400 ng/ml) and G-CSF (200 ng/ml) induced maximal priming of neutrophils (Fig. 1).
FIG. 1.
IL-8 and G-CSF prime bovine neutrophils for an enhanced oxidative burst triggered by M. haemolytica. Neutrophils were incubated for 18 h with IL-8 (40 or 400 ng/ml) and/or G-CSF (200 ng/ml). Both IL-8 and G-CSF significantly enhance the subsequent oxidative burst response to M. haemolytica, and combined treatment with IL-8 and G-CSF significantly augmented the response compared to treatment with G-CSF alone. The data represent five replicates, using neutrophils isolated from different calves on different days. The level of significance is based on repeated-measures ANOVA (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The potential role of endotoxin as a contributor to this priming effect was examined. The endotoxin concentrations were low (0.19 EU per μg of IL-8) in the IL-8 preparation, and undetectable in the G-CSF. Thus, the neutrophil samples incubated with the concentration of IL-8 that gave the maximal effect (400 ng/ml) contained 0.078 EU of endotoxin/ml. Nevertheless, treatment of neutrophils for 18 h with 0.008, 0.08, 0.8, or 8.0 EU of endotoxin/ml did not enhance the subsequent oxidative burst induced by killed, opsonized M. haemolytica.
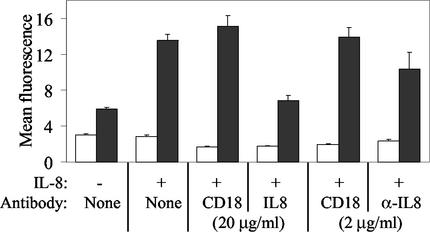
To further confirm that the priming effect was specific for IL-8, neutrophils were incubated with IL-8 and either a neutralizing anti-IL-8 antibody or an irrelevant antibody. Treatment with the anti-IL-8 antibody at 20 and 2.0 μg/ml reduced the priming effect by 54.9 and 25.5% compared to treatment with the irrelevant anti-CD18 antibody and by 49.6 and 23.4% compared to no antibody treatment (Fig. 2). This effect of treatment with anti-IL-8 antibody (20 μg/ml) was significant at P < 0.025.
FIG. 2.
IL-8-induced priming of bovine neutrophils is reduced by a neutralizing anti-IL-8 antibody. In non-IL-8-treated neutrophils, M. haemolytica treatment (black bars) induces an enhanced oxidative burst compared to neutrophils treated with medium alone (white bars). All other bars depict results in neutrophils treated for 18 h with IL-8 (400 ng/ml). Preincubation of IL-8 with a 2- or 20-μg/ml concentration of the IL-8-neutralizing antibody 8 M6 (IL-8) reduced the priming effect of IL-8 in a dose-dependent manner, whereas pretreatment with an irrelevant antibody to CD18 (CD18) did not significantly reduce IL-8-induced enhancement of the M. haemolytica-triggered oxidative burst.
Effect of IL-8 and G-CSF on phagocytosis by bovine neutrophils.
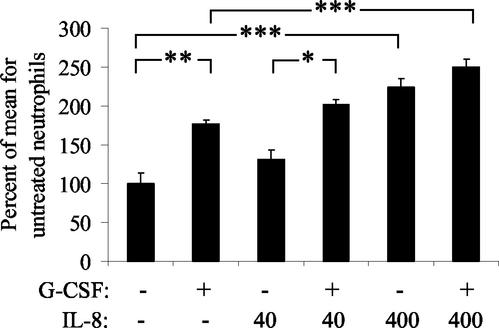
The effect of IL-8 and G-CSF on phagocytosis was examined using the cytokine concentrations found to be effective for priming for an oxidative burst. Treatment of bovine neutrophils for 18 h with IL-8 (400 ng/ml) or G-CSF (200 ng/ml) significantly enhanced phagocytosis of FITC-labeled S. aureus, compared to untreated neutrophils (P < 0.001 and P < 0.01, respectively). Maximal enhancement of phagocytosis was induced by combined treatment with IL-8 and G-CSF (Fig. 3).
FIG. 3.
IL-8 and G-CSF augment phagocytosis of S. aureus by bovine neutrophils. Neutrophils were treated for 18 h with IL-8 at a concentration of 40 or 400 ng/ml and/or G-CSF (200 ng/ml) and then exposed to FITC-labeled S. aureus for 30 min. Treatment with IL-8 (400 ng/ml) and/or G-CSF (200 ng/ml) resulted in dose-dependent enhancement of phagocytosis, and treatment with both cytokines together induced the maximal enhancement of phagocytosis. The data represent five replicates using neutrophils isolated from different calves on different days. The level of significance is based on repeated-measures ANOVA (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Time course of IL-8- and G-CSF-induced priming of bovine neutrophils.
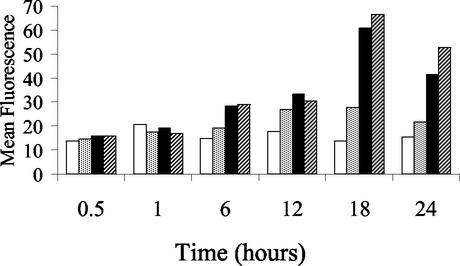
The time course of neutrophil priming by IL-8 and G-CSF was examined by incubating neutrophils with cytokines for 0.5, 1, 6, 12, 18, or 24 h, then measuring oxidative burst in response to opsonized, heat-killed M. haemolytica. A priming effect was observed at 6 and 12 h, was maximal at 18 h, and declined by 24 h (Fig. 4). Incubation with IL-8 and G-CSF, alone or in combination, for 5, 15, or 30 min did not affect subsequent phagocytosis of FITC-labeled S. aureus.
FIG. 4.
Timing of G-CSF- and IL-8-induced priming of bovine neutrophils. Neutrophils were cultured for 24 h, and cytokines were added at specified times prior to measuring the M. haemolytica-induced oxidative burst response. The data indicate neutrophils cultured in medium alone (white bars), G-CSF (200 ng/ml) (grey bars), IL-8 (400 ng/ml) (black bars), or G-CSF and IL-8 combined (hatched bars). Neutrophil priming was maximal at 18 h of cytokine exposure.
Neutrophil responses following intrapulmonary instillation of IL-8.
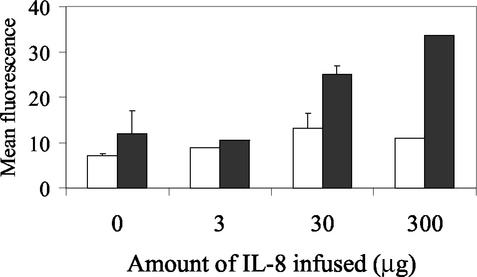
The efficacy of IL-8 as an in vivo neutrophil chemoattractant and activator in the lung was evaluated by instilling IL-8 into the lungs of a normal calf. IL-8 induced a dose-dependent increase in total cell number and percentage of neutrophils in the bronchoalveolar lavage fluid, with 0, 3.0, 30, and 300 μg of IL-8 resulting in neutrophil counts of 0.6, 26.0, 38.0, and 325.5 × 109 cells/liter in bronchoalveolar lavage (BAL) fluid, respectively. The percentage of lavaged macrophages declined in lavage fluid from IL-8-treated areas of lung, and lymphocyte and eosinophil numbers remained low in all treatment conditions. To determine if IL-8 affected neutrophil activation in vivo, oxidative burst stimulated by killed, opsonized M. haemolytica was measured in cells recovered by lavage. Neutrophils from IL-8-treated regions of lung showed a dose-dependent increase in oxidative burst in response to M. haemolytica (Fig. 5).
FIG. 5.
IL-8 infusion into the lung induces neutrophil recruitment and priming in vivo. Neutrophils were harvested from BAL fluid 18 h after infusion of 0 to 300 μg of IL-8, and oxidative burst was measured in untreated neutrophils (white bars) or following stimulation with M. haemolytica (black bars). Neutrophils isolated from sites of infusion of 30 and 300 μg of IL-8 had greater M. haemolytica-induced oxidative burst than those isolated from sites infused with PBS or 3 μg of IL-8, suggesting that IL-8 primes neutrophils in vivo as well as in vitro.
In histologic sections of lung from areas infused with PBS, a few alveoli contained low numbers of neutrophils (up to six cells per 50-μm-diameter alveolus) and rare bronchioles contained a few neutrophils in the lumen. Sections from areas infused with 3 μg of IL-8 appeared histologically similar to the PBS-treated control areas. In sections from areas infused with 30 μg of IL-8, scattered clusters of alveoli contained 5 to 20 neutrophils per 50-μm-diameter alveolus.
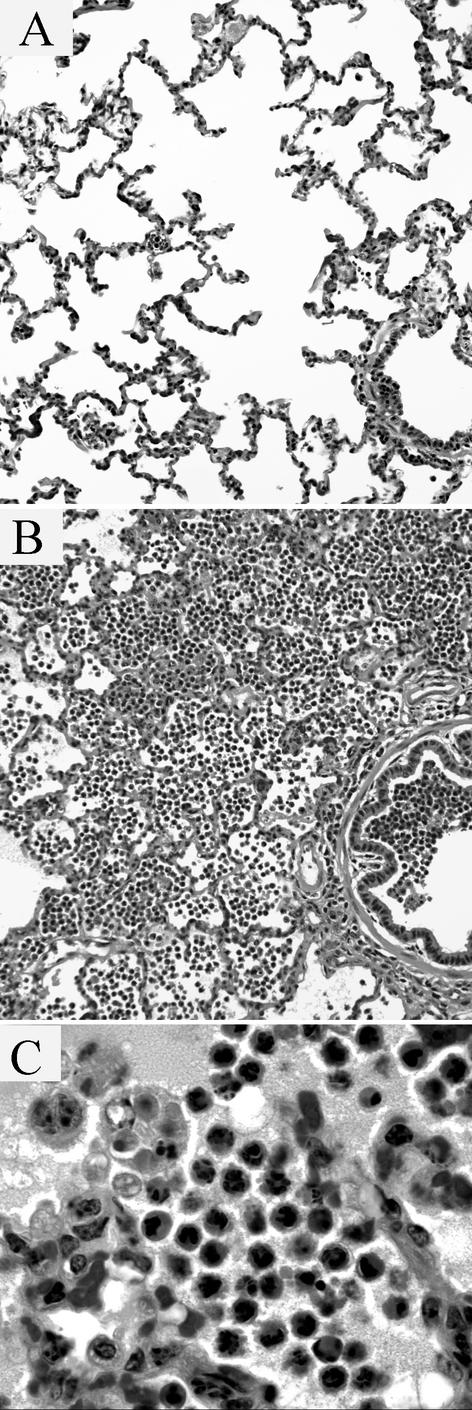
Histologic lesions were more obvious in areas treated with 300 μg IL-8 (Fig. 6A and B). In these sections, lesions followed a lobular pattern and were most intense in the centrilobular region of the lobule (adjacent to bronchioles). Alveoli were filled with numerous neutrophils, up to 60 per 50-μm-diameter alveolus. Most neutrophils were nonnecrotic and had a ruffled cell border, eosinophilic cytoplasm, and a multilobed nucleus. Less than 1% of the neutrophils had rounded nuclei with condensed chromatin, suggestive of apoptosis. Many macrophages contained apoptotic bodies, probably derived from neutrophils (Fig. 6C). These appeared as eosinophilic 4- to 10-μm-diameter globules in the cytoplasm. Some apoptotic bodies contained round 5- to 8-μm-diameter, basophilic aggregates of chromatin, but never contained intact multilobed nuclei. These macrophages had moderately foamy cytoplasm, and were occasionally multinucleated. Bronchioles contained exudate similar to that in the alveoli, and neutrophils were present in the bronchiolar epithelium and peribronchiolar connective tissue. Neutrophil margination in vessels was not apparent. In one section, interlobular septa contained many neutrophils in the lymphatics and the septal connective tissue.
FIG. 6.
Histologic lesions in bovine lung infused with PBS or IL-8. (A and B) In contrast to the sites of PBS infusion (A), infusion of 300 μg of IL-8 (B) induced neutrophil recruitment into alveoli and, to a lesser extent, into bronchioles. (C) Scattered macrophages within the alveoli contain remnants of neutrophils that have condensed eosinophilic cytoplasm and homogeneous dark-staining chromatin, compatible with uptake of apoptotic neutrophils by alveolar macrophages.
DISCUSSION
The purpose of the study was to evaluate the effects of bovine IL-8 and bovine G-CSF, alone and in combination, on priming and activation of bovine neutrophils. These results form the basis for further investigations of the phenomenon of neutrophil priming and activation in response to cytokines. Treatment of bovine neutrophils for 18 h with IL-8 or G-CSF did not directly induce an oxidative burst response. These findings are in agreement with previous reports on the effects of IL-8, where treatment of bovine neutrophils for 30 min with human IL-8 induced chemotaxis and secondary granule secretion but not oxidative burst or primary granule release (11, 14, 22). Similarly, previous studies did not detect significant differences in oxidative burst between G-CSF-treated and untreated cows (18).
In contrast to the inability of IL-8 and G-CSF to directly induce an oxidative burst, these cytokines each primed bovine neutrophils to augment the oxidative burst response following exposure to serum-opsonized M. haemolytica. The priming effect of IL-8 was dose dependent, and maximal at 400 and 4,000 ng/ml, the highest doses tested. G-CSF primed neutrophils at concentrations of 200 and 2,000 ng/ml. The greatest priming effect was apparent when neutrophils were coincubated with both cytokines, in which case the maximum effects were induced by IL-8 at a concentration of 400 or 4,000 ng/ml and G-CSF at a concentration of 200 ng/ml. To confirm these findings, the experiments were repeated using neutrophils isolated from five different calves, and similar findings were obtained for all calves. Endotoxin contamination, at the levels present in the recombinant IL-8 preparation, did not prime neutrophils for greater responses to M. haemolytica. Addition of a monoclonal antibody to ovine IL-8—which has been previously shown to neutralize the in vitro neutrophil chemotactic activity of bovine IL-8 (4)—reduced the priming effect by 55%, confirming that IL-8 was specifically responsible for enhancing the subsequent response to M. haemolytica.
Significant IL-8-induced priming of neutrophils was not detected when neutrophils were exposed to IL-8 for 30 or 60 min, but was striking after 18 h of incubation. In contrast, exposure of human neutrophils to IL-8 for as little as 5 min enhanced the subsequent oxidative burst response to FMLP and IL-8 (10, 38). The delayed effect found in this study suggests that IL-8-induced priming of neutrophil oxidative burst is a consequence of induced gene expression, rather than a sequel to rapid intracellular signaling mechanisms.
Proximal signaling pathways differ between the major groups of agonists—cytokines, platelet-activating factor, and opsonized particles (37). The pathways of IL-8-stimulated signal transduction have been recently reviewed (23, 39). IL-8 receptors signal through heterotrimeric G proteins to activate phospholipase C, stimulating production of diacyglycerol and inositol triphosphate (IP3). Rapid IP3-mediated release of calcium from the endoplasmic reticulum and more-prolonged influx from the extracellular environment induce many of the downstream effects of IL-8 stimulation of neutrophils. However, alternative intracellular calcium-dependent signaling pathways—other than the phospholipase Cb/IP3 pathway—exist in human neutrophils. These include G protein-mediated activation of sphingosine kinase, with sphingosine-1-phosphate-induced calcium mobilization, and a third postulated pathway involves cADP ribose as a trigger for ryanodine receptor-mediated calcium release (23, 39). Nevertheless, the mechanisms whereby these proximal signaling pathways lead to enhanced responses to other agonists have not been defined.
The phenomenon of neutrophil priming represents a dose-dependent constellation of responses to a wide variety of agonists. The major priming agonists that have been shown to act on bovine neutrophils include IL-8, TNF-α, platelet-activating factor, leukotriene B4, and lipopolysaccharide. Priming agonists do not fully activate neutrophils; that is, they do not induce an oxidative burst or secretion of primary granules. In general, neutrophils exposed to these priming agonists in vitro undergo the following pattern of response: altered expression of surface receptors, secretion of secondary granules, reduced chemotaxis, shape change, and enhanced responsiveness to other agonists (36). Surface receptor alterations in G-CSF-treated bovine neutrophils include reduced expression of l-selectin and increased expression of the integrins CD11a, CD11b, CD11c, and CD18 (15). In addition, reduced expression of the C5a receptor CD88 (1) and polarized cell surface distribution of the P-selectin receptor sialyl Lewis X antigen (24) have been documented in cytokine-primed human neutrophils. Stimulation of bovine neutrophils with IL-8 induces shape change, characterized by elongation of the cell and formation of lamellipodia (4), and secretion of secondary granules, detected by release of alkaline phosphatase into the culture medium (14).
Neutrophil responses to agonists are not standardized; rather, the character and the magnitude of the response is dependent on the dose of the agonist, the presence of prior or concurrent stimuli from other agonists, and other contextual signals from the tissue microenvironment including the tissue matrix, serum, oxygen tension, and temperature (36). Thus, neutrophil activation in vivo at sites of inflammation may not necessarily follow the patterns predicted by in vitro experiments. Because the functional changes in IL-8-elicited bovine neutrophils have not been previously evaluated, we evaluated the effect of IL-8 administration in vivo to verify its neutrophil chemotactic and priming activities in the bovine lung. Intrabronchial perfusion of 3.0 to 300 μg of IL-8 elicited a dose-dependent increase in the number of neutrophils in BAL fluid. Histologic evaluation confirmed that IL-8 recruits neutrophils into alveoli and bronchioles.
The in vitro phenomenon of priming also occurred in vivo, in neutrophils that were recruited to the lung by bronchoscope-delivered IL-8. This in vivo effect represents more than simple IL-8-exposure, as these neutrophils would also have experienced stimulation of adhesion receptors; chemotaxis through the endothelium, basement membrane, and type I pneumocyte; and exposure to surfactant lipids and proteins during the process of recruitment from the blood to the pulmonary alveolus.
Histologic evaluation of the IL-8-perfused lung revealed evidence of neutrophil apoptosis and uptake by macrophages. This is consistent with previous findings after intradermal injection of IL-8 (4). Neutrophils undergo spontaneous apoptosis after 24 to 48 h of in vitro culture, but apoptosis can be delayed by treatment of the neutrophils with pro-inflammatory cytokines such as IL-1, gamma interferon, granulocyte-monocyte colony stimulating factor, C5a, or, in some studies, tumor necrosis factor alpha or lipopolysaccharide (7, 19, 25, 33). Together, these findings suggest that IL-8-recruited neutrophils have a short life span in the lung and are cleared by apoptosis within 18 h, whereas neutrophil life span may be prolonged if these cells are costimulated by the other proinflammatory mediators expected to be present during an inflammatory response to bacterial infection.
These findings establish that in vitro exposure of bovine neutrophils for 18 h to the cytokines IL-8 and G-CSF does not directly induce neutrophil activation but does prime neutrophils for greater responses to bacterial pathogens. This priming effect also occurs in vivo, and neutrophils that are recruited to the lung in response to IL-8 spontaneously develop morphological features of apoptosis in the absence of other stimuli.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Agriculture and Food Beef Research Program.
We thank Amgen, Inc., for their generous donation of bovine G-CSF.
Editor: J. D. Clements
REFERENCES
- 1.Binder, R., A. Kress, G. Kan, K. Herrmann, and M. Kirschfink. 1999. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol. Immunol. 36:885-892. [DOI] [PubMed] [Google Scholar]
- 2.Breider, M. A., R. D. Walker, F. M. Hopkins, T. W. Schultz, and T. L. Bowersock. 1988. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 52:205-209. [PMC free article] [PubMed] [Google Scholar]
- 3.Casado, J. A., J. Merino, J. Cid, M. L. Subira, and A. Sanchez-Ibarrola. 1993. Simultaneous evaluation of phagocytosis and Fc gamma R-mediated oxidative burst in human monocytes by a simple flow cytometry method. J. Immunol. Methods 159:173-176. [DOI] [PubMed] [Google Scholar]
- 4.Caswell, J. L., D. M. Middleton, and J. R. Gordon. 1999. Production and functional characterization of recombinant bovine interleukin-8 as a specific neutrophil activator and chemoattractant. Vet. Immunol. Immunopathol. 67:327-340. [DOI] [PubMed] [Google Scholar]
- 5.Caswell, J. L., D. M. Middleton, and J. R. Gordon. 2001. The importance of interleukin-8 as a neutrophil chemoattractant in the lungs of cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 65:229-232. [PMC free article] [PubMed] [Google Scholar]
- 6.Caswell, J. L., D. M. Middleton, S. D. Sorden, and J. R. Gordon. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124-131. [DOI] [PubMed] [Google Scholar]
- 7.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 8.Cullor, J. S., N. Fairley, W. L. Smith, S. L. Wood, J. D. Dellinger, M. S. Inokuma, and L. M. Souza. 1990. Hemogram changes in lactating dairy cows given human recombinant granulocyte colony stimulating factor (r-MethuG-CSF). Vet. Pathol. 27:311-316. [DOI] [PubMed] [Google Scholar]
- 9.Cullor, J. S., W. Smith, J. G. Zinkl, J. D. Dellinger, and T. Boone. 1992. Hematologic and bone marrow changes after short- and long-term administration of two recombinant bovine granulocyte colony-stimulating factors. Vet. Pathol. 29:521-527. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, R. H., M. J. Finnen, M. E. Hill, and J. M. Lackie. 1992. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology 75:157-163. [PMC free article] [PubMed] [Google Scholar]
- 10a.du Manoir, J. M., B. N. Albright, G. Stevenson, S. H. Thompson, G. B. Mitchell, M. E. Clark, and J. L. Caswell. 2002. Variability of neutrophil and pulmonary alveolar macrophage function in swine. Vet. Immunol. Immunopathol. 89:175-186. [DOI] [PubMed] [Google Scholar]
- 11.Elbim, C., S. Bailly, S. Chollet-Martin, J. Hakim, and M. A. Gougerot-Pocidalo. 1994. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect. Immun. 62:2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbim, C., P. Rajagopalan-Levasseur, S. Chollet-Martin, J. L. Gaillard, M. Fay, J. Hakim, A. Fischer, J. L. Casanova, and M. A. Gougerot-Pocidalo. 1999. Defective priming of the phagocyte oxidative burst in a child with recurrent intracellular infections. Microbes Infect. 1:581-587. [DOI] [PubMed] [Google Scholar]
- 13.Frank, G. H., Briggs, R. E., and Debey, B. M. 1993. Pasteurellosis in production animals. Aust. Ctr. Int. Agric. Res. Proc. 43:83-88. [Google Scholar]
- 14.Galligan, C. L., and B. L. Coomber. 2000. Effects of human IL-8 isoforms on bovine neutrophil function in vitro. Vet. Immunol. Immunopathol. 74:71-85. [DOI] [PubMed] [Google Scholar]
- 15.Heidari, M., J. A. Harp, and M. E. Kehrli, Jr. 2001. Expression, purification, and in vitro biological activities of recombinant bovine granulocyte-colony stimulating factor. Vet. Immunol Immunopathol. 81:45-57. [DOI] [PubMed] [Google Scholar]
- 16.Kapp, A., and G. Zeck-Kapp. 1990. Activation of the oxidative metabolism in human polymorphonuclear neutrophilic granulocytes: the role of immuno-modulating cytokines. J. Investig. Dermatol. 95:94S-99S. [DOI] [PubMed]
- 17.Karzai, W., B. U. von Specht, C. Parent, J. Haberstroh, K. Wollersen, C. Natanson, S. M. Banks, and P. Q. Eichacker. 1999. G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am. J. Respir. Crit. Care Med. 159:1377-1382. [DOI] [PubMed] [Google Scholar]
- 18.Kehrli, M. E., Jr., J. P. Goff, M. G. Stevens, and T. C. Boone. 1991. Effects of granulocyte colony-stimulating factor administration to periparturient cows on neutrophils and bacterial shedding. J. Dairy Sci. 74:2448-2458. [DOI] [PubMed] [Google Scholar]
- 19.Kettritz, R., M. L. Gaido, H. Haller, F. C. Luft, C. J. Jennette, and R. J. Falk. 1998. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int. 53:84-91. [DOI] [PubMed] [Google Scholar]
- 20.Maheswaran, S. K., D. J. Weiss, M. S. Kannan, E. L. Townsend, K. R. Reddy, L. O. Whiteley, and S. Srikumaran. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet. Immunol. Immunopathol. 33:51-68. [DOI] [PubMed] [Google Scholar]
- 21.McClenahan, D. J., J. J. Fagliari, O. A. Evanson, and D. J. Weiss. 2000. Role of platelet-activating factor in alveolar septal injury associated with experimentally induced pneumonic pasteurellosis in calves. Am. J. Vet. Res. 61:248-254. [DOI] [PubMed] [Google Scholar]
- 22.Metzner, B., M. Barbisch, F. Parlow, E. Kownatzki, I. Schraufstatter, and J. Norgauer. 1995. Interleukin-8 and GRO alpha prime human neutrophils for superoxide anion production and induce up-regulation of N-formyl peptide receptors. J. Investig. Dermatol. 104:789-791. [DOI] [PubMed] [Google Scholar]
- 23.Mukaida, N. 2000. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 72:391-398. [PubMed] [Google Scholar]
- 24.Nagata, K., T. Tsuji, K. Matsushima, N. Hanai, and T. Irimura. 2000. Redistribution of selectin counter-ligands induced by cytokines. Int. Immunol. 12:487-492. [DOI] [PubMed] [Google Scholar]
- 25.Perianayagam, M. C., V. S. Balakrishnan, A. J. King, B. J. Pereira, and B. L. Jaber. 2002. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 61:456-463. [DOI] [PubMed] [Google Scholar]
- 26.Rainard, P., C. Riollet, B. Poutrel, and M. J. Paape. 2000. Phagocytosis and killing of Staphylococcus aureus by bovine neutrophils after priming by tumor necrosis factor-alpha and the des-arginine derivative of C5a. Am. J. Vet. Res. 61:951-959. [DOI] [PubMed] [Google Scholar]
- 27.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 28.Ribble, C. S., A. H. Meek, E. D. Janzen, P. T. Guichon, and G. K. Jim. 1995. Effect of time of year, weather, and the pattern of auction market sales on fatal fibrinous pneumonia (shipping fever) in calves in a large feedlot in Alberta (1985-1988). Can. J. Vet. Res. 59:167-172. [PMC free article] [PubMed] [Google Scholar]
- 29.Roilides, E., T. J. Walsh, P. A. Pizzo, and M. Rubin. 1991. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J. Infect. Dis. 163:579-583. [DOI] [PubMed] [Google Scholar]
- 30.Slocombe, R. F., J. Malark, R. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253-2258. [PubMed] [Google Scholar]
- 31.Swain, S. D., P. L. Bunger, K. M. Sipes, L. K. Nelson, K. L. Jutila, S. M. Boylan, and M. T. Quinn. 1998. Platelet-activating factor induces a concentration-dependent spectrum of functional responses in bovine neutrophils. J. Leukoc. Biol. 64:817-827. [DOI] [PubMed] [Google Scholar]
- 32.Terashima, T., M. Kanazawa, K. Sayama, T. Urano, F. Sakamaki, H. Nakamura, Y. Waki, K. Soejima, S. Tasaka, and A. Ishizaka. 1995. Neutrophil-induced lung protection and injury are dependent on the amount of Pseudomonas aeruginosa administered via airways in guinea pigs. Am. J. Respir. Crit. Care Med. 152:2150-2156. [DOI] [PubMed] [Google Scholar]
- 33.Van Oostveldt, K., M. J. Paape, H. Dosogne, and C. Burvenich. 2002. Effect of apoptosis on phagocytosis, respiratory burst and CD18 adhesion receptor expression of bovine neutrophils. Domest. Anim. Endocrinol. 22:37-50. [DOI] [PubMed] [Google Scholar]
- 34.Wan, C. P., E. Myung, and B. H. Lau. 1993. An automated micro-fluorometric assay for monitoring oxidative burst activity of phagocytes. J. Immunol. Methods 159:131-138. [DOI] [PubMed] [Google Scholar]
- 35.Weiss, D. J., M. C. Bauer, L. O. Whiteley, S. K. Maheswaran, and T. R. Ames. 1991. Changes in blood and bronchoalveolar lavage fluid components in calves with experimentally induced pneumonic pasteurellosis. Am. J. Vet. Res. 52:337-344. [PubMed] [Google Scholar]
- 36.Witko-Sarsat, V., P. Rieu, B. Descamps-Latscha, P. Lesavre, and L. Halbwachs-Mecarelli. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Investig. 80:617-653. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe, M. B., J. Xu, P. A. Burke, R. A. Forse, and G. E. Brown. 1999. Priming of the neutrophil respiratory burst is species-dependent and involves MAP kinase activation. Surgery 126:248-254. [PubMed] [Google Scholar]
- 38.Yuo, A., S. Kitagawa, A. Ohsaka, M. Saito, and F. Takaku. 1990. Stimulation and priming of human neutrophils by granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor: qualitative and quantitative differences. Biochem. Biophys. Res. Commun. 171:491-497. [DOI] [PubMed] [Google Scholar]
- 39.Zeilhofer, H. U., and W. Schorr. 2000. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 7:178-182. [DOI] [PubMed] [Google Scholar]