Abstract
The G protein-coupled receptors S1P2/Edg5 and S1P3/Edg3 both mediate sphingosine-1-phosphate (S1P) stimulation of Rho, yet S1P2 but not S1P3 mediates downregulation of Rac activation, membrane ruffling, and cell migration in response to chemoattractants. Specific inhibition of endogenous Gα12 and Gα13, but not of Gαq, by expression of respective C-terminal peptides abolished S1P2-mediated inhibition of Rac, membrane ruffling, and migration, as well as stimulation of Rho and stress fiber formation. Fusion receptors comprising S1P2 and either Gα12 or Gα13, but not Gαq, mediated S1P stimulation of Rho and also inhibition of Rac and migration. Overexpression of Gαi, by contrast, specifically antagonized S1P2-mediated inhibition of Rac and migration. The S1P2 actions were mimicked by expression of V14Rho and were abolished by C3 toxin and N19Rho, but not Rho kinase inhibitors. In contrast to S1P2, S1P3 mediated S1P-directed, pertussis toxin-sensitive chemotaxis and Rac activation despite concurrent stimulation of Rho via G12/13. Upon inactivation of Gi by pertussis toxin, S1P3 mediated inhibition of Rac and migration just like S1P2. These results indicate that integration of counteracting signals from the Gi- and the G12/13-Rho pathways directs either positive or negative regulation of Rac, and thus cell migration, upon activation of a single S1P receptor isoform.
Regulation of cell migration is critical in such diverse biological processes as organogenesis, neuronal axon pathfinding, wound healing, inflammatory responses, vascular remodeling, and tumor cell dissemination (21). Extracellular cues called attractants and repellants, which are either soluble or membrane bound, instruct cells to advance and to retreat, respectively (36, 40). A number of chemokines, growth factors, cytokines, and other inflammatory mediators have been shown to stimulate directed cell migration, whereas a much more limited number of biological mediators have been shown to inhibit cell motility in a manner dependent on their concentration gradients. The latter include metastin (28), Slit, semaphorins, ephrins (44), and a lipid mediator, sphingosine 1-phosphate (S1P) (42). S1P is a bioactive lysophospholipid that exerts a wide variety of biological activities, most of which are mediated via Edg family G protein-coupled receptors (GPCRs), including S1P1/Edg1, S1P2/Edg5/AGR16/H218, and S1P3/Edg3 (7, 16, 39, 43). S1P has been demonstrated to be quite unique as an extracellular regulator of motility in that it exerts either stimulatory or inhibitory actions on cell motility (42). These bimodal actions are apparently cell type specific; thus, S1P stimulates chemotaxis in vascular endothelial cells (22) and embryonic fibroblasts (24), whereas it inhibits cell migration in vascular smooth muscle cells (3, 33) and melanoma cells (34). We recently showed that this bimodal regulation by S1P is based upon a diversity of S1P receptor isotypes, which mediate either stimulatory or inhibitory regulation for cell migration (31, 42). Thus, we found that S1P2 acts as a repellant receptor to mediate inhibition of chemotaxis toward attractants, whereas S1P1 and S1P3 act as attractant receptors to mediate migration directed toward S1P. Elimination of the S1P receptor gene in mice (24) and development of a drug to target S1P receptors (4, 25) have revealed that S1P is involved in regulation of cell migration in vivo, thus contributing to morphogenesis and regulation of lymphocyte homing.
Small GTPases of the Rho family, primarily Rac, Cdc42, and Rho, are well-known regulators of actin organization and myosin motor function and thereby of cell motility (10, 14, 47). These Rho GTPases show distinct activities on actin cytoskeletons: Rho mediates stress fiber formation and focal adhesion, while Rac and Cdc42 direct peripheral actin assembly that results in formation of lamellipodia and filopodia, respectively. Despite limitation of our understanding of intracellular signaling from the membrane to the cytoskeleton, a model has emerged from the observations in a variety of cell types that attractive extracellular cues activate Rac or Cdc42, while repulsive cues inhibit Rac or Cdc42 and stimulate Rho (9, 38, 42, 48). In fact, the repellant receptor S1P2 negatively regulates cellular Rac activity through mechanisms involving stimulation of a GTPase-activating protein (GAP) for Rac (31). In contrast, the attractant receptors S1P1 and S1P3 mediate activation of Rac via Gi (22, 31, 32). Neither of these S1P receptors affects Cdc42 activity under our experimental conditions. Interestingly, the repellant receptor S1P2 and the attractant receptor S1P3 similarly mediate stimulation of cellular RhoA activity, most likely via G12/13. Expression of N17Rac, but not N19RhoA or C3 toxin treatment, inhibited cell migration, indicating an essential role of Rac in cell migration (31, 33).
In the present study we explored the mechanisms by which S1P2 receptor activation leads to Rac inhibition. The results of the present study demonstrate for the first time that inhibitory regulation of Rac by the GPCR is mediated via G12/13 and Rho, through a downstream signaling mechanism not involving Rho kinase/ROCK/ROK. Our data also show that Gi exerts a stimulatory regulation for Rac which antagonizes and completely reverses G12/13-mediated inhibitory regulation of Rac. Indeed, we found that the attractant receptor S1P3 was converted to a repellant receptor upon pertussis toxin (PTX) treatment. Thus, these results indicate that integration of signals from Gi and G12/13 determines cellular Rac activity, which directs migration toward or away from a GPCR agonist.
MATERIALS AND METHODS
Materials.
S1P and 1-monooleoyl lysophosphatidic acid (LPA) were purchased from Biomol (Plymouth Meeting, Pa.) and Avanti (Birmingham, Ala.), respectively. They were dissolved, aliquoted, and stored as described previously (29). Recombinant human insulin-like growth factor I was purchased from R&D Systems. A rabbit polyclonal anti-Gαq/11 and a mouse monoclonal anti-Rac antibody were purchased from Upstate Biotechnology. Rabbit polyclonal antibodies against Gαs/olf (C-18), Gαi3 (C-10), anti-Gα12 (S-20), Gα13 (A-20), and GRK2 (H-222) and a mouse monoclonal anti-RhoA antibody were bought from Santa Cruz Biotechnology. A rabbit polyclonal anti-Gα13 antibody (371778) was bought from Calbiochem. A mouse monoclonal anti-ERK1/2 antibody (clone 03-6600) was obtained from Zymed Laboratories Inc. An anti-FLAG M2 antibody and tetramethyl rhodamine isocyanate (TRITC)-labeled phalloidin were obtained from Sigma. PTX was bought from List Biological Laboratories. AlCl3 and NaF were obtained from Wako Pure Chemicals (Osaka, Japan) and were added to media at a molar ratio of 1:4 (AlCl3 to NaF) to generate AlF4−. Y-27632 and HA-1077 were supplied by Mitsubishi Pharma (Wako, Japan) and Asahi Chemical Industry (Fuji, Japan), respectively. Botulinum C3 toxin, the glutathione S-transferase (GST)-human PAK1 (amino acids 75 to 131) fusion protein, GST-mouse rhotekin (amino acids 7 to 89), and a mouse monoclonal anti-myc antibody (9E10) were prepared as described previously (41).
Plasmids, adenoviruses, and transfections.
pME18S-myc-N19RhoA, pME18S-myc-V14RhoA, pGEX-2T-rhotekin, pGEX-2T-PAK, and an adenovirus encoding myc-N19RhoA were described previously (31, 33, 35). cDNAs encoding full-length mouse Gαs, Gαi2, Gα12, Gα13, and Gαq, and the C-terminal peptide of human β-adrenergic receptor kinase (βARK-CT; βARK residues 495 to 689), were obtained by reverse transcription-PCR (RT-PCR) from total mouse brain RNA and human brain RNA (Sawady Technology, Tokyo, Japan), respectively. PCR-based methods were used to generate the cDNAs encoding myc-tagged C-terminal regions of Gαs (residues 319 to 377), Gα12 (residues 326 to 379), Gα13 (residues 321 to 377), and Gαq (residues 306 to 359), which were designated Gαs-CT, Gα12-CT, Gα13-CT, and Gαq-CT; S1P2 with a FLAG-epitope at its N terminus (FLAG-S1P2); and fusion receptors S1P2-Gα12, S1P2-Gα13, and S1P2-Gαq, which have full-length Gα12, Gα13, and Gαq, respectively, fused to the C terminus of S1P2. The cDNAs of full-length Gα proteins, their C termini, and the FLAG-S1P2 and S1P2-Gα fusion receptors were ligated onto the mammalian expression vector pCAGGS (a gift from M. Miyazaki, Osaka University Medical School) to generate pCAGGS-Gαi2, pCAGGS-Gα12, pCAGGS-Gα13, pCAGGS-Gαq, pCAGGS-Gαs-CT, pCAGGS-Gα12-CT, pCAGGS-Gα13-CT, pCAGGS-Gαq-CT, pCAGGS-FLAG-S1P2, pCAGGS-S1P2-Gα12, pCAGGS-S1P2-Gα13, and pCAGGS-S1P2-Gαq, respectively. βARK-CT cDNA was ligated onto the mammalian expression vector pME18S (a gift from K. Maruyama, Tokyo Medical and Dental University) to generate pME18S-βARK-CT. Replication-deficient adenoviruses encoding Gα12-CT, Gα13-CT, and Gαq-CT were generated and amplified as described previously (8). pCAGGS-LacZ and an adenovirus encoding LacZ were kindly donated by I. Saito (Institute of Medical Sciences, University of Tokyo).
The cells were infected with adenoviruses at a multiplicity of infection of 200 by incubating cells with an adenovirus-containing medium for 1 h, which conferred successful gene transduction in nearly 100% of cells. After recovery in growth medium for 24 h, the cells were serum deprived for 24 h before experiments.
Transient transfection with expression plasmid vectors was carried out by using LipofectAMINE (Invitrogen) 48 h before each experiment. To study the migration of transiently transfected cells, the cells were cotransfected with either one of the Gα-CT expression plasmids, the βARK-CT expression plasmid, or the empty vector and pCAGGS-LacZ as a transfection marker (31) for 3 h. In some experiments in which the actin cytoskeleton was evaluated (Fig. 2C), the green fluorescent protein (GFP) expression vector pEGFP-C1 (Clontech) was employed as a transfection marker. After recovery in growth medium for 21 h, the cells were serum deprived for 24 h.
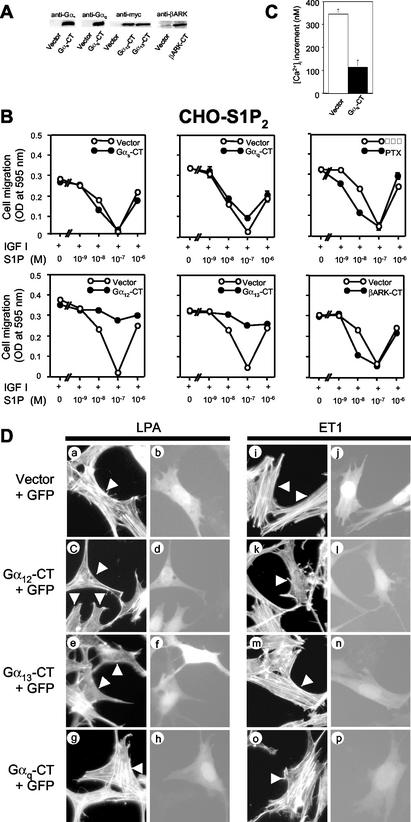
FIG. 2.
Selective blockade of G12 and G13, but not Gs, Gi, or Gq, relieves S1P inhibition of migration in S1P2 receptor-expressing cells. (A) Western blot analysis showing expression of the Gα C-terminal peptides and βARK-CT. CHO-S1P2 cells were transfected with either expression vectors for the Gα-CTs and βARK-CT or an empty vector and were subjected to Western blot analysis using respective, specific antibodies and an anti-myc tag antibody. (B) Expression of G12-CT and G13-CT treatment relieves S1P inhibition of IGF I-directed chemotaxis.CHO-S1P2 cells were either cotransfected with LacZ and one of the expression vectors for Gαs-CT, Gαq-CT, Gα12-CT, Gα13-CT, and βARK-CT at a weight ratio of 1:2.5 or pretreated with PTX (200 ng/ml) as described in Materials and Methods. Transwell migration of transfected CHO-S1P2 cells was determined in the presence of IGF I (100 ng/ml) and various concentrations of S1P in the lower chamber. (C) Expression of Gαq-CT inhibits the S1P-induced [Ca2+]i increase in S1P2-overexpressing CHO cells. CHO-S1P2 cells stably expressing Gαq-CT and CHO-S1P2 cells expressing the vector control were stimulated by S1P at 10−8 M, and the peak increment in the [Ca2+]i was determined. (D) Inhibition of LPA- and ET1-induced stress fiber formation by expression of Gα12-CT or Gα13-CT. Swiss 3T3 cells were cotransfected with pEGFP-C1 and either an expression vector for Gα12-CT or Gα13-CT or an empty vector at a weight ratio of 1:10. Cells were stimulated with LPA or ET1 at 10−7 M for 10 min. F-actin was visualized with TRITC-labeled phalloidin. Arrowheads indicate transfected cells identified with GFP fluorescence.
To establish CHO cells that stably express S1P2-Gα fusion receptors, cells were cotransfected with pCAGGS-S1P2-Gα and the neomycin resistance gene expression vector pKM3 (27) and were selected in the presence of 0.7 mg of G418 (Nacalai, Kyoto, Japan)/ml. To establish CHO-S1P2 cells that stably express full-length Gα protein and Gαq-CT, CHO-S1P2 cells were cotransfected with either pCAGGS-Gα, pCAGGS-Gαq-CT, or the Zeocin resistance gene expression vector pCMV/Zeo (Invitrogen) and were selected in the presence of 50 μg of Zeocin (Invitrogen)/ml and 0.7 mg of G418/ml. Cloned cells were isolated and tested for expression of transduced genes.
In the experiments using CHO-S1P2 cells that express N19Rho or V14Rho, CHO-S1P2 cells were cotransfected with either pME18S-myc-N19RhoA or pME18S-myc-V14RhoA and pCMV/Zeo and were selected in the presence of 50 μg of Zeocin/ml. The Zeocin-resistant cell populations were employed in these experiments.
Cells.
CHO-K1 (CHO) cells, Swiss 3T3 cells, and COS7 cells were grown in Ham's F-12 (CHO) or Dulbecco's modified Eagle medium (3T3 and COS7) supplemented with 10% fetal bovine serum (Equitech-Bio, Ingram, Tex.), 100 U of penicillin/ml, and 100 μg of streptomycin/ml (Wako Pure Chemicals). CHO cells that stably overexpress either S1P1, S1P2, or S1P3, i.e., CHO-S1P1, CHO-S1P2, and CHO-S1P3 cells, respectively, have been described previously (13, 29, 30) and were maintained in the presence of 0.7 mg of G418/ml. Cells were treated with C3 toxin (10 μg/ml) in F-12 medium containing 10% fetal bovine serum for 48 h and then in serum-free F-12 medium for a further 24 h. PTX (200 ng/ml) treatment was carried out by incubating cells in serum-free F-12 medium containing PTX for 24 h before experiments.
Transwell migration assay.
Chemotactic migration of cells was measured in a modified Boyden chamber (Neuroprobe) using polycarbonate filters with 8-μm pores as described in detail previously (31, 33). In migration assays using transiently transfected cell populations, the migratory cells attached to the lower side of the membrane were subjected to stainining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside as a substrate. The number of migratory cells staining positive with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside was determined by using a microplate reader as described above.
Determination of the activities of Rho and Rac.
Pulldown assay methods to determine GTP-bound active forms of Rho and Rac have been described in detail previously (31, 33, 35). Briefly, cell extracts were incubated with the GST-rhotekin Rho-binding domain (for determination of Rho activity) or the GST-PAK CRIB domain (for determination of Rac activity) immobilized to glutathione-Sepharose 4B beads (Pharmacia Amersham Biotech) at 4°C for 45 min, followed by three washes. Bound Rho and Rac proteins were quantitatively detected by Western blotting using specific, monoclonal antibodies against RhoA and Rac.
Western blotting, [Ca2+]i measurement, and fluorescence microscopy.
Western blotting was performed as described previously (29). The band shift of activated p42 and p44 extracellular signal-regulated kinase (ERK) was detected by Western blot analysis of total cell lysates with a mouse monoclonal anti-ERK antibody (8). Intracellular free Ca2+ concentration ([Ca2+]i) was measured as described previously (29) in Fura-2-loaded cells with a CAF-110 spectrofluorimeter (Japan Spectroscopy, Inc., Tokyo, Japan) with excitation at 340 and 380 nm and emission at 500 nm.
To evaluate actin cytoskeletons, cells were transfected as indicated 48 h before experiments and were serum starved for 24 h. After treatment with receptor agonists and/or Rho kinase inhibitors for indicated times, the cells were fixed in 3.7% formaldehyde in phosphate-buffered saline and processed as described previously (41). F-actin was visualized with TRITC-labeled phalloidin under an inverted fluorescence microscope IX70 (Olympus, Tokyo, Japan).
Statistics.
Values are presented as means ± standard errors of three or more determinations and are representative of at least two independent experiments with similar results.
RESULTS
AlF4− mimics S1P2 actions in inhibiting Rac and migration in IGF I-stimulated cells.
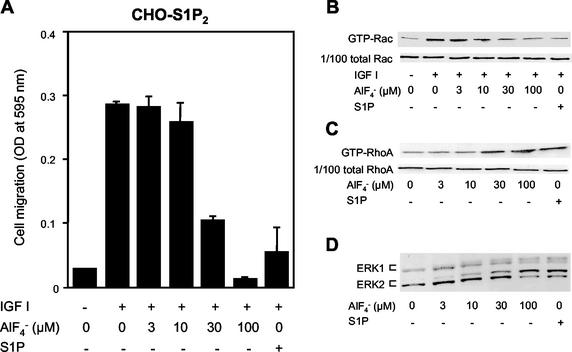
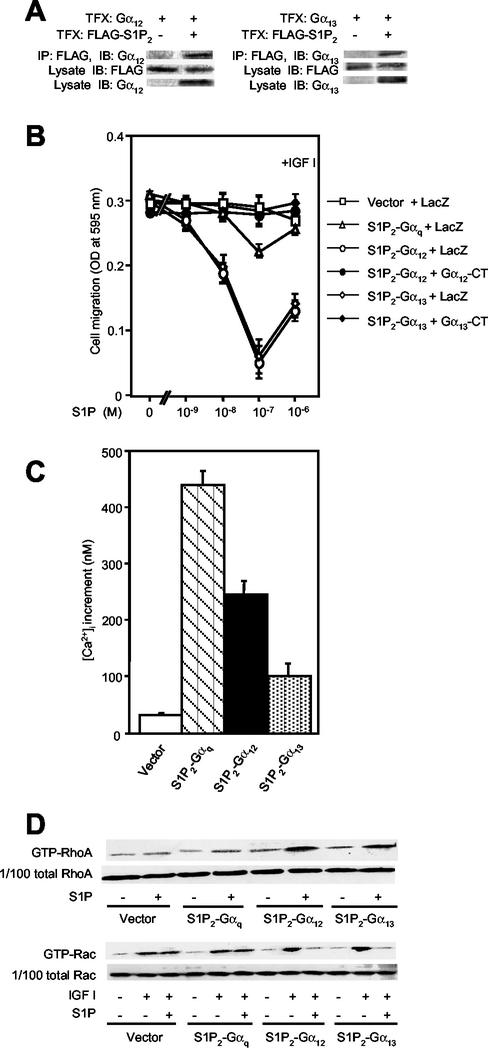
Most of the actions of GPCRs are mediated through heterotrimeric G protein coupling. However, recent studies provide evidence that mechanisms independent of heterotrimeric G protein coupling mediate certain actions of GPCRs, which include regulation of a Na+-H+ exchanger and a Ca2+ channel, as well as activation of STAT (5). To understand in more depth the molecular mechanisms underlying GPCR-mediated inhibition of cell migration, we first investigated whether S1P2-mediated Rac inhibition occurs through heterotrimeric G protein coupling, and, if so, which heterotrimeric G protein mediates Rac inhibition. In CHO cells expressing the S1P2 receptor (CHO-S1P2), but not in naive CHO cells or CHO cells expressing S1P1 or S1P3, S1P dose-dependently and potently inhibited IGF I-directed chemotaxis, which is a Rac-dependent process (31). Direct stimulation of the heterotrimeric G proteins with AlF4− (17) dose-dependently inhibited chemotaxis of CHO-S1P2 cells toward IGF I, like S1P stimulation of the S1P2 receptor (Fig. 1A). AlF4− also mimicked the action of S1P2 in inhibiting IGF I-induced increases in cellular amounts of a GTP-bound, active form of Rac (GTP-Rac) (Fig. 1B) and in stimulating RhoA (Fig. 1C), ERK (Fig. 1D), and Ca2+ mobilization (data not shown). These observations strongly suggest that the heterotrimeric G protein(s) mediated inhibition of Rac and cell migration as well as the other actions of S1P2.
FIG. 1.
AlF4− mimicks S1P actions in inhibiting Rac and migration and stimulating Rho and ERK in S1P2 receptor-expressing cells. (A) AlF4− and S1P inhibit IGF I-directed chemotaxis. Transwell migration of CHO-S1P2 cells toward IGF I (100 ng/ml) was determined in the presence or absence of various concentrations of AlF4− and 10−7 M S1P in the lower chamber. (B) AlF4− and S1P inhibit IGF I-induced Rac stimulation. CHO-S1P2 cells were treated with various concentrations of AlF4− or 10−7 M S1P for 10 min and then stimulated with IGF I (100 ng/ml) for 1 min. Cells were then subjected to a pulldown assay for GTP-Rac as described in Materials and Methods. GTP-Rac bound to the GST-PAK1 CRIB domain immobilized onto Sepharose beads was analyzed by Western blotting using an anti-Rac antibody (top), and 1/100 of total Rac present in the cell lysate is also shown to confirm loading of equal amounts of proteins (bottom). (C) AlF4− and S1P stimulate Rho. CHO-S1P2 cells were stimulated with various concentrations of AlF4− or 10−7 M S1P for 3 min. Cells were then subjected to a pulldown assay for GTP-RhoA as described in Materials and Methods. (D) AlF4− and S1P stimulate ERK1 and -2. CHO-S1P2 cells were stimulated with various concentrations of AlF4− or 10−7 M S1P for 5 min. ERK activation was determined by band shift analysis using Western blotting.
Endogenous G12 and G13 couple S1P2 to inhibition of cell migration.
To examine which member of the heterotrimeric G proteins is responsible for mediating suppression of cell migration, we determined the effects of specific inhibition of receptor-G protein coupling by either transient expression of C-terminal peptides (1, 11) of heterotrimeric G protein α subunits (Gα-CTs) or pretreatment with PTX. Shown in Fig. 2A are Western blot analyses of the expression of Gαs-CT, Gαq-CT, Gα12-CT, and Gα13-CT. CHO-S1P2 cells were cotransfected with one of these peptides and β-galactosidase (LacZ) and subjected to a migration assay (31). As in naive CHO-S1P2 cells (31), S1P inhibited IGF I-directed chemotaxis in vector-transfected CHO-S1P2 cells with a bell-shaped dose-response curve and maximal inhibition at 10−7 M (Fig. 2B).Neither expression of any of these C-terminal peptides nor pretreatment with PTX affected chemotaxis toward IGF I in the absence of S1P (Fig. 2B). Expression of Gα12-CT or Gα13-CT, but not Gαs-CT or Gαq-CT, specifically abolished the S1P inhibition of IGF I-directed chemotaxis. We confirmed that expression of Gαq-CT effectively inhibited the S1P-induced increase in [Ca2+]i, a Gq-mediated response, compared to that with the vector control in S1P-expressing cells (Fig. 2C). Inhibition of Gi/o by PTX pretreatment, and expression of βARK-CT, which acts as a scavenger for βγ subunits (20), to a lesser extent, rather potentiated S1P inhibition of IGF I-directed chemotaxis at lower S1P concentrations. PTX pretreatment or the expression of βARK-CT substantially attenuated S1P-induced ERK activation (data not shown), confirming the effectiveness of PTX and βARK at inhibiting Gi. These observations are consistent with the notion that endogenously expressed G12 and G13 are responsible for mediating a signal that leads to inhibition of migration upon S1P stimulation of the S1P2 receptor.
It has been reported for Swiss 3T3 fibroblasts (12) that endothelin-1 (ET1) and LPA induce stress fiber formation through G12 and G13, respectively. By employing Swiss 3T3 cells and these GPCR agonists, we determined the specificity of the inhibitory actions of Gα12-CT and Gα13-CT. We observed that expression of Gα13-CT abolished LPA-induced stress fiber formation (Fig. 2Da, b, e, and f) but not ET1-induced stress fiber formation (Fig. 2Di, j, m, and n), while expression of Gα12-CT abolished both ET1- and LPA-induced stress fiber formation (Fig. 2Dc, d, k, and l). The results indicate that the Gα13-CT peptide acts as a selective inhibitor for G13 whereas the Gα12-CT peptide acts as an inhibitor for both G12 and G13. Together with the observation using βARK-CT (Fig. 2B), we conclude that the α subunits, but not the βγ dimer, of G13 or both G12 and G13 mediate inhibition of migration.
Endogenous G12 and G13 couple S1P2 to inhibition of Rac and stimulation of Rho.
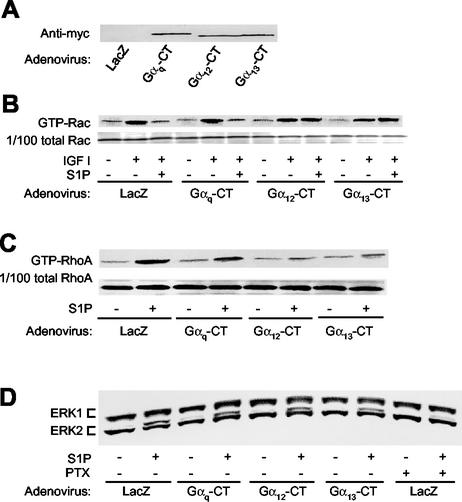
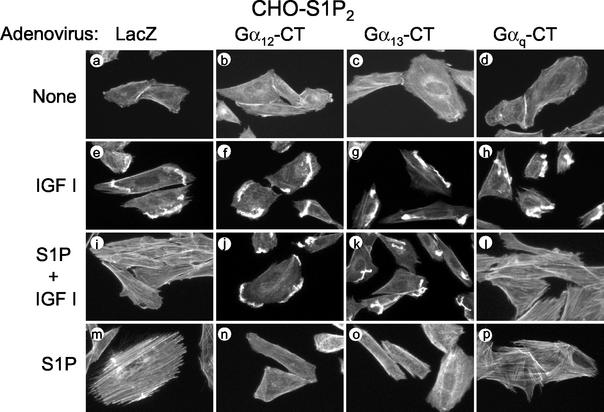
We next expressed myc-tagged Gα12-CT, Gα13-CT, and Gαq-CT, and LacZ as a control, by adenovirus-mediated gene transduction in CHO-S1P2 cells, and we determined the effects of their expression on the activities of RhoA and Rac (Fig. 3) and also on the actin cytoskeleton (Fig. 4). Expression of the inhibitor proteins was confirmed by Western blot analysis (Fig. 3A). Expression of any of these C-terminal peptides did not affect IGF I-induced Rac activation (Fig. 3B) or membrane ruffling (Fig. 4) in the absence of S1P, nor did it affect S1P stimulation of ERK (Fig. 3D), indicating that their expression did not compromise cellular activity in a nonspecific manner. Interestingly, however, expression of either Gα12-CT or Gα13-CT, but not Gαq-CT or LacZ, abolished S1P inhibition of Rac activation in response to IGF I (Fig. 3B). Expression of either Gα12-CT or Gα13-CT, but not Gαq-CT, also greatly inhibited S1P-induced RhoA stimulation compared to LacZ transfection (Fig. 3C). Consistent with the effects on Rho and Rac, expression of Gα12-CT or Gα13-CT, but not of Gαq-CT or LacZ, abolished S1P suppression of peripheral actin filament assembly in response to IGF I, as well as S1P stimulation of stress fiber formation (Fig. 4). These results are consistent with the observations on cell migration and indicate that G12 and G13 are responsible for mediating suppressive effects of S1P on cellular Rac activity, membrane ruffling, and cell migration in CHO-S1P2 cells.
FIG. 3.
Adenovirus-mediated expression of Gα12-CT and Gα13-CT abolishes S1P inhibition of Rac and stimulation of Rho in S1P2 receptor-expressing cells. (A) Western blot analysis of expression of the Gα C-terminal peptides. CHO-S1P2 cells were infected with adenoviruses encoding myc-tagged Gαq-CT, Gα12-CT, Gα13-CT, and LacZ 48 h before experiments and were subjected to Western blot analysis using an anti-myc tag antibody. (B and C) Expression of Gα12-CT and Gα13-CT, but not Gαq-CT abolishes S1P inhibition of IGF I-induced Rac stimulation and S1P stimulation of Rho. CHO-S1P2 cells that had been were infected with the adenoviruses were stimulated with IGF I (100 ng/ml) for 1 min in the presence of S1P (10−7 M) (for the Rac assay) or with S1P (10−7 M) for 3 min (for the Rho assay). For the Rac assay S1P was added 10 min before addition of IGF I. Cells were then subjected to a pulldown assay for GTP-Rac or GTP-RhoA. (D) Inhibition of S1P-induced ERK stimulation by PTX, but not by expression of Gq-CT, G12-CT, or G13-CT. CHO-S1P2 cells were either infected with the adenoviruses as described above or pretreated with PTX as for Fig. 2B. Cells were then stimulated with S1P (10−7 M) for 5 min and subjected to band shift analysis.
FIG. 4.
Expression of Gα12-CT and Gα13-CT, but not Gαq-CT, abolishes S1P inhibition of IGF I-induced membrane ruffling and also S1P stimulation of stress fiber formation in S1P2 receptor-expressing cells. CHO-S1P2 cells were infected with adenoviruses encoding Gα12-CT, Gα13-CT, Gαq-CT, or LacZ as for Fig. 3A. Cells were stimulated with IGF I (100 ng/ml) and/or S1P (10−7 M) for 30 min. Cells were fixed, permeabilized, and stained with TRITC-labeled phalloidin for F-actin.
S1P2-Gα12 and S1P2-Gα13 fusion receptors, but not S1P2-Gαq, mediate inhibition of Rac and migration.
Consistent with the observations showing functional coupling of S1P2 to G12 and G13 (Fig. 2 to 4), we observed coimmunoprecipitation of S1P2 and either G12 or G13 from the cells coexpressing these molecules (Fig. 5A). We further studied and compared the effects of S1P on IGF I-directed migration in CHO cells that stably expressed fusion receptors designated S1P2-Gα12, S1P2-Gα13, and S1P2-Gαq, which have either of the full-length Gα subunit sequences fused to the C terminus of S1P2 (37). In CHO cells expressing either the S1P2-Gα12 or the S1P2-Gα13 fusion receptor, S1P potently inhibited chemotaxis toward IGF I (Fig. 5B). Adenovirus-mediated expression of Gα12-CT or Gα13-CT completely abolished S1P inhibition of IGF I-directed chemotaxis in cells expressing the respective fusion receptor S1P2-Gα12 or S1P2-Gα13. Cells expressing S1P2-Gαq showed a prominent increase in the [Ca2+]i in response to S1P (Fig. 5C); however, they responded to S1P with only a marginal inhibition of cell migration (Fig. 5B). In vector-transfected cells and cells expressing either of the three fusion receptors, IGF I similarly stimulated Rac activity (Fig. 5D). In cells expressing either S1P2-Gα12 or S1P2-Gα13, but not vector-transfected cells or cells expressing S1P2-Gαq, S1P induced a nearly complete inhibition of Rac activation in response to IGF I, as in CHO-S1P2 cells (Fig. 5D). As expected, cells expressing S1P2-Gα12 or S1P2-Gα13, but not vector-transfected cells or cells expressing S1P2-Gαq, showed strong activation of RhoA in response to S1P (Fig. 5D). These observations provided further evidence that G12/13 coupled with S1P2 for inhibition of Rac and migration.
FIG. 5.
The fusion receptors S1P2-Gα12 and S1P2-Gα13, but not S1P2-Gαq, mediate inhibition of Rac and migration. (A) Coimmunoprecipitation of S1P2 and either G12 or G13. COS7 cells were transiently cotransfected with pCAGGS-FLAG-S1P2 and either pCAGGS-Gα12 or pCAGGS-Gα13, and FLAG-tagged S1P2 was immunoprecipitated with an anti-FLAG (M2) antibody. The anti-FLAG immunoprecipitates were analyzed by Western blotting using anti-G12 or anti-G13 antibodies. Portions of cell lysates were analyzed by Western blotting using anti-FLAG, anti-G12, and anti-G13 antibodies. TFX, transfection; IP, immunoprecipitation; IB, immunoblotting. (B) S1P inhibits IGF I-directed chemotaxis in CHO cells expressing S1P2-Gα12 and S1P2-Gα13, but not S1P2-Gαq or vector, which is sensitive to expression of Gα12-CT and Gα13-CT inhibitor peptides. CHO cells stably expressing S1P2-Gα12, S1P2-Gα13, or S1P2-Gαq were infected with adenoviruses encoding Gα12-CT, Gα13-CT, or LacZ and then subjected to transwell migration as for Fig. 2B. (C) S1P-induced[Ca2+]i response in CHO cells expressing the fusion receptors. Cells were stimulated with S1P (10−7 M), and the peak increments of the [Ca2+]i responses were determined. (D) S1P inhibits IGF I-induced Rac stimulation and stimulates Rho in CHO cells expressing S1P2-Gα12 and S1P2-Gα13, but not S1P2-Gαq or vector. Cells were stimulated as described in the legend for Fig. 3B and C and were subjected to a pulldown assay for GTP-Rac and GTP-Rho.
Rho mediates inhibition of cellular Rac activity and migration through a mechanism not involving Rho kinase.
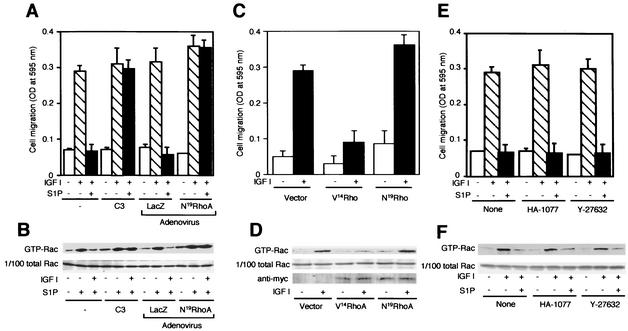
We tested the possibility that Rho, which is an effector of G12/13, was involved in the signaling pathway leading to suppression of Rac activity. Pretreatment of S1P2-expressing cells with botulinum C3 toxin, which inactivates Rho, did not affect migration in response to IGF I; however, it completely abolished the S1P2-mediated inhibition of IGF I-directed migration (Fig. 6A). Similarly, adenovirus-mediated expression of N19RhoA did not affect chemotaxis toward IGF I but totally abolished S1P inhibition of IGF I-directed migration (Fig. 6A). Consistent with these observations, either C3 treatment or N19RhoA expression abolished S1P inhibition of IGF I-induced Rac stimulation, while these treatments did not affect Rac activation in response to IGF I alone (Fig. 6B). On the other hand, we observed that cells that stably expressed a myc-tagged constitutively active RhoA mutant, myc-V14RhoA, showed reductions in both chemotaxis and Rac activation in response to IGF I, compared to vector-control cells (Fig. 6C and 6D).
FIG. 6.
Rho, but not Rho kinase, mediates S1P inhibition of Rac activity and migration in S1P2-expressing cells. (A and B) C3 pretreatment and N19Rho expression abolish S1P inhibition of IGF I-stimulated chemotaxis and Rac activity. CHO-S1P2 cells were either infected with adenoviruses encoding N19Rho or LacZ as for Fig. 3A, pretreated with C3 toxin (10 μg/ml) as described in Materials and Methods, or not pretreated. Transwell migration was determined in Fig. 2B. For the GTP-Rac pulldown assay, the cells were stimulated as for Fig. 3B. (C and D) Expression of V14Rho inhibits IGF I-stimulated chemotaxis and Rac activation. CHO-S1P2 cells were cotransfected with either myc-tagged N19Rho, myc-tagged V14Rho, or an empty vector and pCMV/Zeo, and they were selected in the presence of Zeocin. Zeocin-resistant cell populations, which expressed either of the myc-tagged Rho mutants, were employed in these experiments. Transwell migration was determined in the presence or absence of IGF I (100 ng/ml) in the lower chamber. For the GTP-Rac pulldown assay, cells were stimulated with IGF I (100 ng/ml) for 1 min. Expression of myc-tagged N19Rho and V14Rho proteins in the cell lysate are shown in the bottom gel of panel D. (E and F) Rho kinase inhibitors fail to prevent S1P inhibition of IGF I-stimulated chemotaxis and Rac activation. CHO-S1P2 cells were pretreated or not with HA-1077 (20 μM) or Y-27632 (10 μM) for 30 min before migration and Rac assays. Transwell migration was determined in the presence or absence of IGF I (100 ng/ml) and S1P (10−7 M) in the lower chamber. HA-1077 (20 μM) or Y-27632 (10 μM) was present in both the upper and lower chambers, where indicated. For the Rac assay, cells were stimulated with IGF I (100 ng/ml) for 1 min, with or without a 10-min pretreatment with S1P (10−7 M) and/or HA-1077 (20 μM) or Y-27632 (10 μM).
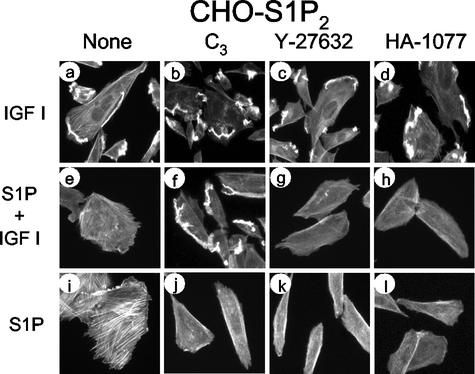
We next studied whether Rho kinase, which is one of the direct effectors of Rho (23, 46), was involved in S1P2-mediated suppression of Rac activity and migration. It was previously reported that a Rho kinase inhibitor abolished V14Rho-induced inhibition of Rac activity (50). HA-1077 and Y-27638, which are two structurally unrelated Rho kinase inhibitors (26, 49), effectively inhibited S1P-induced stress fiber formation in CHO-S1P2 cells (Fig. 7); however, they were totally ineffective in preventing the S1P2-mediated inhibition of IGF I-directed cell migration (Fig. 6E), Rac activation (Fig. 6F), or membrane ruffling (Fig. 7). In contrast, C3 toxin abolished the S1P2-mediated inhibition of IGF I-induced membrane ruffling (Fig. 7f) as well as induction of stress fiber formation (Fig. 7j). S1P2 mediates actin cytoskeletal changes in two ways through Rho: it inhibits membrane ruffling in IGF I-stimulated cells, and it stimulates stress fiber formation. These observations clearly indicate that inhibition of membrane ruffle formation requires a Rho-dependent signal other than the Rho kinase pathway, whereas induction of stress fiber formation requires Rho kinase.
FIG. 7.
Rho kinase inhibitors do not affect S1P inhibition of IGF I-induced membrane ruffling but do abolish S1P-induced stress fiber formation. CHO-S1P2 cells were pretreated either with C3 toxin, as described in Materials and Methods, or with HA-1077 (20 μM) or Y-27632 (10 μM) for 30 min. Cells were then stimulated with IGF I (100 ng/ml) and/or S1P (10−7 M) for 30 min. Cells were stained with TRITC-labeled phalloidin for F-actin. Note that C3, but not HA-1077 or Y-27632, abolishes S1P inhibition of membrane ruffling in response to IGF I, although the Rho kinase inhibitors effectively suppress S1P-induced stress fiber formation.
Overexpression of Gαi counteracts AlF4−- and S1P-induced inhibition of Rac and migration.
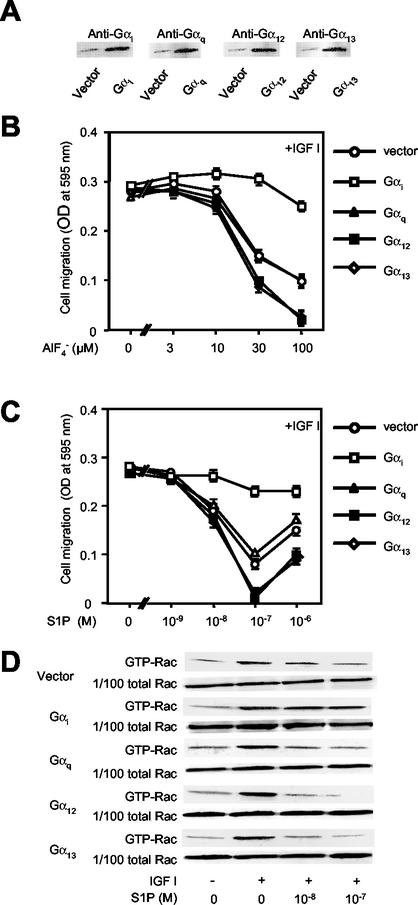
S1P2 couples not only to G12/13 but also to Gi (13, 30, 49). PTX pretreatment of CHO-S1P2cells potentiated S1P inhibition of chemotaxis (Fig. 1B), suggesting that Gi conveyed a signal which counteracted inhibition of migration. To study in more depth the role of Gαi in the regulation of Rac and cell migration by the S1P receptor, we evaluated the effects of overexpression of Gαi2, which is an endogenously expressed Gαi isoform in CHO cells, on AlF4−- and S1P-induced inhibition of Rac and migration in CHO-S1P2 cells (Fig. 8). In cells overexpressing Gαi2, S1P by itself stimulated chemotaxis and Rac moderately, unlike the situation in the vector control cells (data not shown). Overexpression of Gαi2 nearly completely abolished AlF4−- and S1P-induced inhibition of chemotaxis (Fig. 8B and C). The results contrast sharply with those obtained with overexpression of either Gα12or Gα13, which potentiated AlF4−- and S1P-induced inhibition of IGF I-directed chemotaxis (Fig. 8B and C). Overexpression of Gαq did not affect inhibition by AlF4− or S1P. In agreement with these observations on migration, overexpression of Gαi2 nearly abolished S1P inhibition of IGF I-induced stimulation of Rac, whereas overexpression of Gα12 and Gα13 potentiated S1P inhibition of IGF I stimulation of Rac compared to that in vector control cells (Fig. 8D). Expression of Gαq did not affect S1P inhibition of Rac activity. Overexpression of these Gα subunits did not affect chemotaxis or Rac activation in response to IGF I alone (Fig. 8C and D). Thus, Gi generates a stimulatory signal for Rac and consequently migration to antagonize G12/13-mediated inhibition of Rac and migration in S1P receptor signaling.
FIG. 8.
Overexpression of Gαi counteracts AlF4−- and S1P-induced inhibition of Rac and migration in S1P2 receptor-expressing cells. (A) Western blot analysis of expression of full-length Gα proteins. CHO-S1P2 cells stably expressing either Gαi2, Gαq, Gα12, Gα13, or an empty vector were subjected to Western blot analysis using respective, specific anti-Gα antibodies described in Materials and Methods. (B and C) Overexpression of Gαi markedly attenuates AlF4−- and S1P-induced inhibition of IGF I-directed chemotaxis, but overexpression of Gα12 or Gα13 enhances such inhibition. Transwell migration of the CHO-S1P2 cells that stably express one of these Gα subunits or the empty vector was determined as for Fig. 1A and 2B. (D) Overexpression of Gαi markedly attenuates S1P inhibition of IGF I-induced Rac stimulation. CHO-S1P2 cells that stably express one of the Gα subunits or an empty vector were stimulated as for Fig. 3B and then subjected to a pulldown assay for GTP-Rac.
S1P3 mediates S1P inhibition, rather than stimulation, of Rac and cell migration upon Gi inactivation.
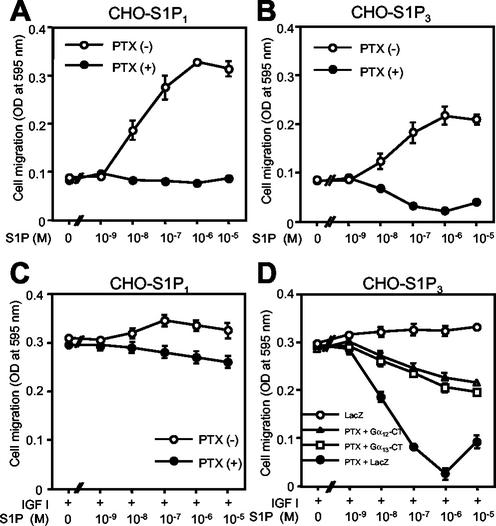
As we reported previously (31), S1P by itself directed chemotaxis in both S1P1- and S1P3-expressing CHO cells (CHO-S1P1 and CHO-S1P3 cells, respectively) (Fig. 9A and B). This contrasts with CHO-S1P2 cells, in which S1P by itself does not stimulate chemotaxis (31). PTX pretreatment of both CHO-S1P1 and CHO-S1P3 cells totally inhibited migration toward S1P, indicating that S1P-directed chemotaxis is Gi/o dependent (Fig. 9A and B). When CHO-S1P1 cells were stimulated with a combination of S1P and IGF I, they showed a chemotactic response slightly greater than that to stimulation with IGF I alone (Fig. 9C). PTX pretreatment did not affect CHO-S1P1 cell migration stimulated by IGF I alone, and it slightly reduced cell migration stimulated by the combination of S1P and IGF I. In CHO-S1P3 cells, the combination of S1P and IGF I induced a stimulation of chemotaxis slightly larger than that with IGF I alone, as in CHO-S1P1 cells (Fig. 9D). PTX did not affect the response to IGF I alone in this cell type, either. In contrast to CHO-S1P1 cells, however, inactivation of Gi with PTX in CHO-S1P3 cells dramatically changed the responses to S1P: the cells showed inhibition, rather than stimulation, of IGF I-directed chemotaxis in response to S1P, with a nearly complete inhibition at 10−6 M (Fig. 9D). Moreover, adenovirus-mediated expression of either Gα12-CT or Gα13-CT abolished S1P inhibition of IGF I-directed chemotaxis in PTX-treated CHO-S1P3 cells (Fig. 9D).
FIG. 9.
PTX pretreatment abolishes S1P stimulation of migration in S1P1- and S1P3-expressing cells and uncovers S1P3-mediated, G12/13-dependent inhibition of IGF I-directed chemotaxis. (A and B) PTX pretreatment abolishes chemotaxis toward S1P in CHO-S1P1 and CHO-S1P3 cells. Cells were pretreated or not with PTX as described in Materials and Methods and were then subjected to a transwell migration assay in the presence of various concentrations of S1P in the lower chamber. (C and D) PTX pretreatment unveils S1P-induced, G12/13-dependent inhibition of IGF I-directed chemotaxis in CHO-S1P3 cells, but not in CHO-S1P1 cells. CHO-S1P3 cells were infected with adenoviruses encoding Gα12-CT, Gα13-CT, and LacZ and were then pretreated or not with PTX, as for Fig. 3D. Cells were then subjected to a transwell migration assay as for Fig. 2B.
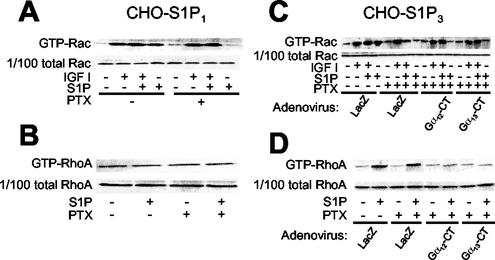
In CHO-S1P1 cells S1P by itself stimulated Rac, which was totally inhibited by PTX (Fig. 10A). IGF I also stimulated Rac, but in a PTX-insensitive manner. The combination of S1P and IGF I induced a slightly but consistently greater response in Rac activation compared to that with stimulation by either alone. In CHO-S1P3 cells S1P by itself stimulated Rac in a PTX-sensitive manner (Fig. 10C), which was similar to the findings for CHO-S1P1 cells. In contrast to the situation in PTX-pretreated CHO-S1P1 cells, however, in PTX-pretreated CHO-S1P3 cells S1P markedly inhibited IGF I-stimulation of Rac (Fig. 10C), which was consistent with the S1P inhibition of IGF I-directed chemotaxis. Expression of either Gα12-CT or Gα13-CT abolished the S1P inhibition of Rac in PTX-pretreated, IGF I-stimulated CHO-S1P3 cells (Fig. 10C), just as with cell migration (Fig. 9D). In CHO-S1P3 cells, S1P induced stimulation of RhoA via G12/13 irrespective of PTX pretreatment (Fig. 10D). S1P did not change the level of GTP-RhoA in CHO-S1P1 cells (Fig. 10B). Thus, inactivation of Gi in CHO-S1P3 cells converts S1P-induced stimulation of Rac and migration to inhibition of Rac and migration. These observations indicate that the S1P receptor isoforms S1P1, S1P2, and S1P3 exert distinct regulatory actions on Rac and cell migration through differential coupling to G12/13 and Gi, which convey signals to inhibit and stimulate Rac and migration, respectively.
FIG. 10.
PTX pretreatment abolishes S1P stimulation of Rac in S1P1- and S1P3-expressing cells and uncovers S1P3-mediated, G12/13-dependent inhibition of IGF I-induced Rac stimulation. (A and B) Effects of PTX pretreatment on Rac and Rho activities in CHO-S1P1 cells. Cells were pretreated or not pretreated with PTX, stimulated with IGF I and/or S1P, and subjected to a pulldown assay for GTP-Rac and GTP-Rho, as described in the legends for Fig. 3B, C, and D. (C and D) Effects of PTX treatment and expression of Gα12-CT and Gα13-CT on Rac and Rho activities in CHO-S1P3 cells. Cells were infected with adenoviruses encoding Gα12-CT, Gα13-CT, and LacZ and then pretreated or not with PTX, as for Fig. 3D. Cells were stimulated in the same way as for panels A and B and were subjected to a pulldown assay for GTP-Rac and GTP-Rho.
DISCUSSION
Multiple classes of cell surface receptors including receptor tyrosine kinases and GPCRs trigger chemotactic behavior of cells (21, 36). In GPCR-activated chemotactic signaling, liberation of βγ subunits from the heterotrimeric Gi, activation of phosphatidylinositol 3-kinases, and stimulation of the small GTPase Rac are considered to constitute the important signaling cascade for stimulating chemotaxis (15). However, much less is known about signaling mechanisms of chemorepellant receptors. We recently identified S1P2 as the first example of a GPCR that mediates negative regulation of Rac, which serves as a signal for inhibiting cell migration directed toward a chemoattractant (31, 33, 42). In the present study, first, we observed that direct activation of heterotrimeric G proteins with AlF4− (17) mimicked all of the known S1P actions in S1P2 receptor-expressing cells (Fig. 1A to D), strongly suggesting that heterotrimeric G protein coupling mediates inhibition of Rac and migration downstream of S1P2 stimulation. By utilizing specific G protein inhibitors (1, 11), we demonstrated that it is the G12/13 family protein that couples S1P2 to inhibition of Rac, cell migration, and membrane ruffling (Fig. 2B, 3, and 4). Consistent with the critical roles of G12/13, the fusion receptors S1P2-Gα12 and S1P2-Gα13, but not S1P2-Gαq, mediated S1P inhibition of Rac and cell migration (Fig. 5), and overexpression of either Gα12 or Gα13, but not Gαq, potentiated the inhibitory effects of S1P (Fig. 8). In addition, the observation that expression of βARK-CT, which sequesters βγ subunits (20), did not abolish S1P inhibition of migration but rather slightly augmented it suggests that Gα12 and Gα13, but not βγ subunits of G12/13, mediate S1P inhibition of migration (Fig. 2B). It is of note that the metastin receptor hTOT7T175 and the thrombin receptor PAR1, which are GPCRs capable of inhibiting cell migration, are also G12/13 coupled (18, 28), although these receptors are not known to be able to inhibit Rac. However, the present study also indicates that GPCRs that couple to G12/13 do not always inhibit Rac or cell migration (see below).
Since Rho is a well-established effector of the G12/13 class of heterotrimeric G proteins (12), we next examined its involvement in the S1P2 signaling leading to Rac inhibition. Inhibition of endogenous Rho activity by C3 toxin treatment or N19RhoA expression abolished G12/13-mediated inhibition of Rac, cell migration, and membrane ruffling (Fig. 6A, 6B, and 7). Conversely, expression of V14Rho mimicked S1P inhibition of Rac and also of migration, like N17Rac expression (Fig. 6C and D). These observations, together with the observation that S1P2 mediates Rho stimulation via G12/13 (Fig. 1 and 3) (31), clearly indicate that Rho mediates Rac inhibition. Although it was previously demonstrated that expression of V14Rho resulted in inhibition of cellular Rac activity (50), this is the first demonstration that receptor activation of the G12/13-Rho signaling pathway mediates Rac inhibition. In certain cell types (6, 19), Gq as well as G12/13 was demonstrated to mediate Rho stimulation. However, in CHO-S1P2 cells, the Gq inhibitor peptide did not affect S1P-induced Rho stimulation (Fig. 3B). Besides the inhibitory action on cellular Rac activity, Rho may also act to inhibit cell motility through its stimulating effects on contractile actin-myosin filaments, which result in the formation of stress fibers and focal adhesions (2, 27), although precise mechanisms relating these structures to inhibition of migration remain to be elucidated.
Rho kinase is a candidate Rho effector that mediates Rac inhibition. Indeed, a previous study showed that expression of V14Rho in PC12 cells inhibited nerve growth factor-induced activation of Rac and that this inhibition was reversed by the Rho kinase inhibitor Y-27632 (50). It was also reported recently that Y-27632 inhibition of Rho kinase unveiled previously unrecognized Rho-dependent activation of Rac via mDia, which is another direct effector of Rho (45). In contrast to these previous reports, however, we did not observe any prevention of S1P2-mediated inhibition of Rac, cell migration, or membrane ruffling by two structurally different Rho kinase inhibitors, Y-27632 and HA-1077, although they effectively inhibited S1P2-mediated, Rho-dependent stress fiber formation (Fig. 6E, 6F, and 7). Our results indicate that a Rho effector other than Rho kinase plays a critical role in S1P2 receptor-mediated Rac inhibition. It is possible that the effects of activation of endogenous Rho by receptor stimulation and V14RhoA expression could be distinct both temporally and spatially, resulting in different effector stimulation. Although the exact explanation for the discrepancy is not known at present, these disparate observations suggest that there exist both Rho kinase-dependent and -independent mechanisms for Rho regulation of Rac activity. The differential effects of C3 toxin and the Rho kinase inhibitors on the actin cytoskeletal changes (Fig. 7) also indicate that the S1P2 receptor stimulates Rho to mediate dissolution of membrane ruffles and induction of stress fiber formation, which are uncoupled processes, through Rho kinase-independent and -dependent pathways, respectively, in CHO cells.
Another point that should be noted in the present results is the counteraction between G12/13 and Gi with regard to Rac regulation. Thus, overexpression of Gαi reversed S1P2-mediated, G12/13-dependent suppression of Rac and migration (Fig. 8C and D), like that of the G12/13 inhibitor peptides. Moreover, we demonstrated in the present study that S1P3, an isoform of S1P2, exerts dual regulation for cellular Rac activity via G12/13 and Gi (Fig. 10C). As we and others (31, 32) reported previously, S1P3 as well as S1P1 mediate Gi-dependent stimulation of Rac activity and migration (Fig. 9 and 10). S1P1 couples exclusively to Gi, whereas S1P3, like S1P2, couples to Gq and G12/13 in addition to Gi (43, 49). Inactivation of Gi by PTX treatment uncovered S1P3-mediated, G12/13-dependent inhibitory regulation for Rac and migration (Fig. 9D and 10C). The S1P2 receptor also couples to Gi to activate ERK (Fig. 3D) (13); however, this coupling appears to be less efficient than those of S1P1 and S1P3, as evidenced by the fact that the dose-response curve for S1P-induced, Gi-dependent ERK activation in CHO-S1P2 cells is shifted at least 1 order rightward from that in S1P1- and S1P3-expressing cells (30). Robust activation by S1P2 of the G12/13-Rho pathway likely masks the Gi-mediated, modest stimulatory effect on Rac, resulting in evident inhibition of cellular Rac activity and migration, even in naive CHO-S1P2 cells (Fig. 1 to 3).
Besides the S1P2 and S1P3 receptors, a number of GPCRs have been shown to couple to Gi, G12/13, and also Gq (18, 28, 39). In certain cell types, Gq as well as G12/13 may transmit a stimulatory signal to Rho (6, 19). It is likely that integration of Gi- and G12/13- or Gq-dependent, positive and negative regulatory signals for cellular Rac activity determines the eventual regulatory activities of the GPCRs with regard to cell migration.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science and Culture of Japan, the Japan Society for the Promotion of Science Research for the Future Program, the Hoh-Ansha Foundation, and the Honjin Foundation.
We thank Nobuko Yamaguchi and Yasuhiro Hiratsuka for preparing the manuscript and for technical assistance, respectively.
REFERENCES
- 1.Akhter, S. A., L. M. Luttrell, H. A. Rockman, G. Iaccarino, R. J. Lefkowitz, and W. J. Koch. 1998. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280:574-577. [DOI] [PubMed] [Google Scholar]
- 2.Banyard, J., B. Anand-Apte, M. Symons, and B. R. Zetter. 2000. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene 19:580-591. [DOI] [PubMed] [Google Scholar]
- 3.Bornfeldt, K. E., L. M. Graves, E. W. Raines, Y. Igarashi, G. Wayman, S. Yamamura, Y. Yatomi, J. S. Sidhu, E. G. Krebs, S. Hakomori, and R. Ross. 1995. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 130:193-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann, V., M. D. Davis, C. E. Heise, R. Albert, S. Cottens, R. Hof, C. Bruns, E. Prieschl, T. Baumruker, P. Hiestand, C. A. Foster, M. Zollinger, and K. R. Lynch. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453-21457. [DOI] [PubMed] [Google Scholar]
- 5.Brzostowski, J. A., and A. R. Kimmel. 2001. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem. Sci. 26:291-297. [DOI] [PubMed] [Google Scholar]
- 6.Chikumi, H., J. Vazquez-Prado, J. M. Servitja, H. Miyazaki, and J. S. Gutkind. 2002. Potent activation of RhoA by Gαq and Gq-coupled receptors. J. Biol. Chem. 277:27130-27134. [DOI] [PubMed] [Google Scholar]
- 7.Chun, J., E. J. Goetzl, T. Hla, Y. Igarashi, K. R. Lynch, W. Moolenaar, S. Pyne, and G. Tigyi. 2002. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 54:265-269. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi, J., M. Makuuchi, T. Nakaoka, T. Collins, and Y. Takuwa. 1999. Angiotensin II stimulates platelet-derived growth factor-B chain expression in newborn rat vascular smooth muscle cells and neointimal cells through Ras, ERK, and JNK mechanisms. Circ. Res. 85:565-574. [DOI] [PubMed] [Google Scholar]
- 9.Dickson, B. J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11:103-110. [DOI] [PubMed] [Google Scholar]
- 10.Evers, E. E., G. C. M. Zondag, A. Malliri, L. S. Price, J. P. ten Klooster, R. A. van der Kammen, and J. G. Collard. 2000. Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer 36:1269-1274. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist, A., M. Bunemann, A. Li, M. M. Hosey, and H. E. Hamm. 1999. A dominant-negative strategy for studying roles of G proteins in vivo. J. Biol. Chem. 274:6610-6616. [DOI] [PubMed] [Google Scholar]
- 12.Gohla, A., S. Offermanns, T. M. Wilkie, and G. Schultz. 1999. Differential involvement of Gα12 and Gα13 in receptor-mediated stress fiber formation. J. Biol. Chem. 274:17901-17907. [DOI] [PubMed] [Google Scholar]
- 13.Gonda, K., H. Okamoto, N. Takuwa, Y. Yatomi, H. Okazaki, T. Sakurai, S. Kimura, R. Sillard, K. Harii, and Y. Takuwa. 1999. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 337:67-75. [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch, E., V. L. Katanaev, C. Garlanda, O. Azzolino, L. Pirola, L. Silengo, S. Sozzani, A. Mantovani, F. Altruda, and M. P. Wymann. 2000. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287:1049-1053. [DOI] [PubMed] [Google Scholar]
- 16.Hla, T., M. J. Lee, N. Ancellin, J. H. Paik, and M. J. Kluk. 2001. Lysophospholipids—receptor revelations. Science 294:1875-1878. [DOI] [PubMed] [Google Scholar]
- 17.Kahn, R. A. 1991. Fluoride is not an activator of the smaller (20-25 kDa) GTP-binding proteins. J. Biol. Chem. 266:15595-15597. [PubMed] [Google Scholar]
- 18.Kamath, L., A. Meydani, F. Foss, and A. Kuliopulos. 2001. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 61:5933-5940. [PubMed] [Google Scholar]
- 19.Katoh, H., J. Aoki, Y. Yamaguchi, Y. Kitano, A. Ichikawa, and M. Negishi. 1998. Constitutively active Gα12, Gα13, and Gαq induce Rho-dependent neurite retraction through different signaling pathways. J. Biol. Chem. 273:28700-28707. [DOI] [PubMed] [Google Scholar]
- 20.Koch, W. J., B. E. Hawes, J. Inglese, L. M. Luttrell, and R. J. Lefkowitz. 1994. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G β γ-mediated signaling. J. Biol. Chem. 269:6193-6197. [PubMed] [Google Scholar]
- 21.Lauffenburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell 84:359-369. [DOI] [PubMed] [Google Scholar]
- 22.Lee, M. J., S. Thangada, K. P. Claffey, N. Ancellin, C. H. Liu, M. Kluk, M. Volpi, R. I. Shaafi, and T. Hla. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99:301-312. [DOI] [PubMed] [Google Scholar]
- 23.Lim, L., E. Manser, T. Leung, and C. Hall. 1996. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur. J. Biochem. 242:171-185. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., R. Wada, T. Yamashita, Y. Mi, C. X. Deng, J. P. Hobson, H. M. Rosenfeldt, V. E. Nava, S. S. Chae, M. J. Lee, C. H. Liu, T. Hla, S. Spiegel, and R. L. Proia. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 106:951-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandala, S., R. Hajdu, J. Bergstrom, E. Quackenbush, J. Xie, J. Milligan, R. Thornton, G.-J. Shei, D. Card, C. Keohane, M. Rosenbach, J. Hale, C. L. Lynch, K. Rupprecht, W. Parsons, and H. Rosen. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346-349. [DOI] [PubMed] [Google Scholar]
- 26.Nagumo, H., Y. Sasaki, Y. Ono, H. Okamoto, M. Seto, and Y. Takuwa. 2000. Rho kinase inhibitor HA1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. 278:C57-C65. [DOI] [PubMed] [Google Scholar]
- 27.Nobes, C. D., and A. Hall. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144:1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtaki, T., Y. Shintani, S. Honda, H. Matsumoto, A. Hori, K. Kanehashi, Y. Terao, S. Kumano, Y. Takatsu, Y. Masuda, Y. Ishibashi, T. Watanabe, M. Asada, T. Yamada, M. Suenaga, C. Kitada, S. Usuki, T. Kurokawa, H. Onda, O. Nishimura, and M. Fujino. 2001. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613-617. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto, H., N. Takuwa, K. Gonda, H. Okazaki, K. Chang, Y. Yatomi, H. Shigematsu, and Y. Takuwa. 1998. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J. Biol. Chem. 273:27104-27110. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto, H., N. Takuwa, Y. Yatomi, K. Gonda, H. Shigematsu, and Y. Takuwa. 1999. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem. Biophys. Res. Commun. 260:203-208. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, H., N. Takuwa, T. Yokomizo, N. Sugimoto, S. Sakurada, H. Shigematsu, and Y. Takuwa. 2000. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol. Cell. Biol. 20:9247-9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paik, J. H., S. Chae, M. J. Lee, S. Thangada, and T. Hla. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J. Biol. Chem. 276:11830-11837. [DOI] [PubMed]
- 33.Ryu, Y., N. Takuwa, N. Sugimoto, S. Sakurada, S. Usui, H. Okamoto, O. Matsui, and Y. Takuwa. 2002. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ. Res. 90:325-332. [DOI] [PubMed] [Google Scholar]
- 34.Sadahira, Y., F. Ruan, S. Hakomori, and Y. Igarashi. 1992. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc. Natl. Acad. Sci. USA 89:9686-9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurada, S., H. Okamoto, N. Takuwa, N. Sugimoto, and Y. Takuwa. 2001. Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am. J. Physiol. Cell. Physiol. 281:C571-C578. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Madrid, F., and M. A. Del Pozo. 1999. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert, R., K. Wenzel-Seifert, and B. K. Kobilka. 1999. GPCR-Gα fusion proteins: molecular analysis of receptor-G-protein coupling. Trends Pharmacol. Sci. 20:383-389. [DOI] [PubMed] [Google Scholar]
- 38.Shamah, S. M., M. Z. Lin, J. L. Goldberg, S. Estrach, M. Sahin, L. Hu, M. Bazalakova, R. L. Neve, G. Corfas, A. Debant, and M. E. Greenberg. 2001. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105:233-244. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel, S., and S. Milstien. 2000. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta 1484:107-116. [DOI] [PubMed] [Google Scholar]
- 40.Stoker, M., and E. Gherardi. 1991. Regulation of cell movement: the motogenic cytokines. Biochim. Biophys. Acta 1072:81-102. [DOI] [PubMed] [Google Scholar]
- 41.Takuwa, N., Y. Fukui, and Y. Takuwa. 1999. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70S6K-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol. Cell. Biol. 19:1346-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takuwa, Y. 2002. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biophys. Biochim. Acta 1582:112-120. [DOI] [PubMed] [Google Scholar]
- 43.Takuwa, Y., N. Takuwa, and N. Sugimoto. 2002. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J. Biochem. (Tokyo). 131:767-771. [DOI] [PubMed] [Google Scholar]
- 44.Tessier-Lavigne, M., and C. S. Goodman. 1996. The molecular biology of axon guidance. Science 274:1123-1133. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji, T., T. Ishizaki, M. Okamoto, C. Higashida, K. Kimura, T. Furuyashiki, Y. Arakawa, R. B. Birge, T. Nakamoto, H. Hirai, and S. Narumiya. 2002. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J. Cell Biol. 157:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uehata, M., T. Ishizaki, H. Satoh, T. Ono, T. Kawahara, T. Morishita, H. Tamakawa, K. Yamagami, J. Inui, M. Maekawa, and S. Narumiya. 1997. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389:990-994. [DOI] [PubMed] [Google Scholar]
- 47.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 48.Wahl, S., H. Barth, T. Ciossek, K. Aktories, and B. K. Mueller. 2000. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Windh, R. T., M. J. Lee, T. Hla, S. An, A. J. Barr, and D. R. Manning. 1999. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J. Biol. Chem. 274:27351-27358. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi, Y., H. Katoh, H. Yasui, K. Mori, and M. Negishi. 2001. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J. Biol. Chem. 276:18977-18983. [DOI] [PubMed] [Google Scholar]