Abstract
HIV-1 infection in the brain induces neuronal apoptosis leading to HIV-associated dementia. To explore the underlying mechanism, we developed a murine model by using human peripheral blood mononuclear cell (PBMC)–transplanted nonobese diabetic (NOD)–severe combined immunodeficiency (SCID) (hu-PBMC-NOD-SCID) mice. Administration of lipopolysaccharide (LPS) to HIV-1-infected hu-PBMC-NOD-SCID mice induced infiltration of HIV-1-infected human cells into the perivascular region of the brain and neuronal apoptosis was found in macrophage (M)-tropic but not T cell (T)-tropic HIV-1-infected brains. The apoptotic neurons were frequently colocalized with the HIV-1-infected macrophages that expressed tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL). Administration of a neutralizing antibody against human TRAIL but not human TNF-α or Fas ligand (FasL) blocked the neuronal apoptosis in the HIV-1-infected brain. These results strongly suggest a critical contribution of TRAIL expressed on HIV-1-infected macrophages to neuronal apoptosis.
HIV-1 infection in the brain induces a neuropathology, termed HIV encephalopathy, that is characterized by astrocytosis, microglial nodules, cell infiltration with macrophages, decreased synaptic density, and selective neuronal loss (1). These neurodegenerative changes result in cognitive and motor dysfunction, designated HIV-associated dementia. The neuronal damage is caused by apoptosis, as demonstrated histologically in brains from HIV encephalopathy patients (2) and experimentally by in vitro infection with HIV-1 (3). HIV-1 infects macrophages and microglia but not neurons in the infected brain, suggesting an indirect mechanism for HIV-1-induced neuronal apoptosis (4). A critical contribution of neurotoxic substances released from the infected and/or activated macrophages/microglia, including excitatory amino acids, IL-1β, and tumor necrosis factor (TNF)-α, to neuronal apoptosis has been suggested through in vitro studies (5, 6). However, the precise mechanism for HIV-induced neuronal apoptosis in vivo has not been elucidated yet because of the lack of an appropriate animal model. Although the neuropathogenesis of simian immunodeficiency virus (SIV) or chimeric simian/HIV (SHIV) in a monkey model has been reported, the molecular mechanism leading to neuronal apoptosis in vivo has not been clarified (7, 8). We developed an HIV-1-susceptible mouse model by using human peripheral blood mononuclear cell (PBMC)–transplanted nonobese diabetic (NOD)–severe combined immunodeficiency (SCID) (hu-PBMC-NOD-SCID) mice (9). When infected with either macrophage (M)-tropic or T cell (T)-tropic HIV-1, a systemic infection with high levels of viremia, which led to a progressive CD4+ T cell loss, was reproducibly established in these mice. Using this mouse model, we recently demonstrated a critical contribution of TRAIL to the depletion of HIV-1-uninfected bystander CD4+ T cells in lymphoid organs that is largely responsible for the pathogenesis of AIDS (10). In the present study, we developed a mouse model that HIV-1 infection extended to central nervous system (CNS). Intraperitoneal administration of LPS to the M-tropic HIV-1-infected hu-PBMC-NOD-SCID mice induced a neuropathology that resembled part of the HIV encephalopathy of human patients, including infiltration of HIV-infected macrophages, astrocytosis, and neuronal apoptosis in the brain. Using this model, we demonstrated a critical contribution of TRAIL to the HIV-1-induced neuronal apoptosis.
Materials and Methods
Reconstitution and HIV-1 Infection of hu-PBMC-NOD-SCID Mice.
NOD (NOD/Shi) scid/scid (NOD-SCID) mice were used at 6–8 weeks of age. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of the participating institutions. Reconstitution with human PBMCs and infection with HIV-1 were performed as described (9). Briefly, human PBMCs were isolated from a healthy HIV-1-seronegative donor and injected i.p. Five days later, mice were inoculated i.p. with 1,000 ID50 of JRFL HIV-1 isolate (11), 100,000 ID50 of NL4-3 (12), or recombinant HIV-1 expressing green fluorescent protein (GFP). The T-tropic HIV-1 expressing GFP (NL-EGFP) was constructed from NL4-3 by inserting an enhanced GFP (EGFP) gene and an internal ribosome entry site (IRES) sequence between gp41 and the nef sequence by PCR-based subcloning. The ATG codon of EGFP was placed 2 bp downstream of gp41 termination codon and nef expression was rescued from insertion of the IRES sequence. The M-tropic HIV-1 expressing GFP (NL-CSFV3-EGFP) was constructed by replacing the V3 sequence in the NL-EGFP with the V3 sequence from JRCSF (11). One hundred micrograms of LPS (Sigma) was injected i.p. 7 days after HIV-1 inoculation. Three or 21 days later, the brains were fixed in 4% periodate-lysine-paraformaldehyde (PLP) fixative. The brains were cut into four serial coronal sections at the level of corpus callosum, hippocampus, and cerebellum. Then they were subjected to immunohistological analyses.
Administration of mAbs.
One milligram of anti-human TRAIL mAb (clone RIK-2, IgG; ref. 13), anti-human Fas ligand (FasL) mAb (clone NOK-1, IgG; ref. 14), anti-human TNF-α mAb (clone 28401.111, Genzyme/Techne, Minneapolis), or a control mouse IgG (Inter-Cell Technologies, Hopewell, NJ) was administered i.p. at the time of the LPS injection. The mice were killed 3 days later, and the brains were subjected to histological analyses.
Immunohistological Staining.
Abs against the following molecules were used for PLP fixed section as primary Abs: human CD3 (rabbit polyclonal IgG, Dako), human CD68 (clone PGM1, mouse monoclonal IgG, Dako), glial fibrillary acidic protein (GFAP) (rabbit polyclonal IgG, Dako), HIV-1 gag p24 (clone Kal-1, mouse monoclonal IgG, Dako), and mouse F4/80 (clone F4/80, rat monoclonal IgG, Serotec). Detection of the immunohistological staining was performed as described (10). Nonimmunized rabbit, mouse, and rat Ig (Dako) were used as negative controls. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) staining of frozen sections was carried out using an indirect method as described (10). Absence of either digoxigenin-labeled dUTP or TdT served as a negative control. DNase-treated sections served as positive controls.
Frozen sections were incubated with Abs against human CD68 (clone PGM1), human TRAIL (clone RIK-2), IL-1β (rabbit polyclonal IgG, Endogen, Woburn, MA), human TNF-α (rabbit polyclonal IgG, Endogen), human FasL (rabbit polyclonal IgG, Santa Cruz Biotechnology), HIV-1 gag p24 (goat polyclonal IgG, ViroStat, Portland, ME), or the active form of caspase-3 (rabbit polyclonal IgG, R & D Systems). The sections were then incubated with FITC-conjugated secondary Abs as described (10). Dual-color staining, including TUNEL, was performed FluoroNissl Green (FNG, FMP) (15), NeuroTrace blue fluorescent Nissl stain (FNB, FMP), or anti-microtubule-associated protein (MAP)-2 mAb (clone HM-2, mouse monoclonal IgG, Sigma). HIV gag p24 (Kal-1, Dako) and human CD68 (PGM1, Dako) were also used. Secondary Abs were used as described (10). Triple-color analysis for GFP, CD68, and TRAIL, TNF-α, or FasL was also performed as described (10). An absence of primary Ab was used as a negative control. Sections were covered with Vector Shield mounting medium (Vector Laboratories) and observed under a Zeiss LSM 310 confocal laser-scanning microscope.
Serial coronal sections (at least three slices) were scored for the immunostained cells by a single investigator in a blinded manner. All over the sections were evaluated in each sample. Apoptotic cells were also identified using active form of caspase-3 staining and hematoxylin/eosin (H&E) staining. Cells undergoing apoptosis were identified by characteristic morphology including nuclear fragmentation and cell shrinkage with condensed nuclei on H&E-stained sections. In dual-color detection of GFP and human CD3 or CD68, the percentage of double-positive cells was calculated. In dual-color staining of CD68 or CD3 and TNF-α, IL-1β, TRAIL, or FasL, the percentage of double-positive cells in CD68+ or CD3+ cells was calculated.
Statistics.
Data are presented as the mean ± SD of slices. Welch's t test was used to compare the mean values between two groups. P values <0.05 were regarded as statistically significant.
Results
Development and Characterization of an HIV-CNS Infection Model.
It has been proposed that HIV infection in the brain is initiated by the migration of M-tropic HIV-infected macrophages (16). Thus, we first examined the migration of HIV-infected human PBMCs into the brain of hu-PBMC-NOD-SCID mice after infection with a M-tropic JRFL or a T-tropic NL4-3 virus. Low levels of human CD3+ T cell migration [24.4 ± 14.4 cells per slice (cps) in JRFL-infected brains and 30.0 ± 19.7 cps in NL4-3-infected brains] and, less frequently, HIV gag p24+ cell migration (10.2 ± 7.69 cps in JRFL-infected brains and 7.25 ± 4.57 cps in NL4-3-infected brains), but not human CD68+ macrophages, were found in the meninx and around the vessel of the brain 10 days after infection (Table 1). Because we observed similar levels of CD3+ T cell migration in mice 28 days after infection (data not shown) and in brains from uninfected mice (21.6 ± 12.3 cps, Table 1), these data appear to represent the baseline distribution of T cells and macrophages in hu-PBMC-NOD-SCID mice. We observed no apparent neuropathology excepting sparse astrocytosis in the brains of infected mice (data not shown).
Table 1.
HIV-CNS infection and apoptosis in HIV-infected hu-PBMC-NOD-SCID mice
| PBMC | Virus | LPS | n | Brain
|
Peritoneal space, HLA+ (×104 cells)§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ | CD68+ | p24+* | TUNEL+ | cFNG+TUNEL+ | AC† | MN‡ | |||||
| + | JRFL | − | 5 | 24.4 ± 14.4¶ | NDe | 10.2 ± 7.69¶ | 11.4 ± 5.37¶ | NDe | ± | − | 213.2 ± 159.7¶ |
| + | None | − | 5 | 21.6 ± 12.3 | NDe | NT | 7.50 ± 1.48 | NDe | ± | − | 462.9 ± 77.3 |
| − | None | − | 4 | NT | NT | NT | NDe | NDe | − | − | NT |
| + | JRFL | + | 12 | 91.3 ± 34.4¶ | 21.3 ± 5.31 | 58.4 ± 21.5¶ | 78.8 ± 21.5¶ | 13.1 ± 4.47 | + | + | 47.1 ± 37.2¶ |
| + | None | + | 6 | 37.0 ± 27.4 | 13.0 ± 4.98 | NT | 25.0 ± 10.8 | NDe | + | + | 157.7 ± 98.4 |
| − | None | + | 5 | NT | NT | NT | NDe | NDe | + | + | NT |
| + | NL4-3 | − | 4 | 30.0 ± 19.7 | NDe | 7.25 ± 4.57 | 10.0 ± 4.24 | NDe | ± | − | 125.2 ± 69.6 |
| + | NL4-3 | + | 4 | 36.0 ± 12.7 | 13.0 ± 4.16 | 26.8 ± 7.46 | 33.3 ± 9.36 | NDe | + | + | 18.2 ± 15.6 |
| + | NL-CSFV3-EGFP | + | 5 | 74.8 ± 63.2 | 18.8 ± 10.4 | 45.4 ± 32.6 | 56.4 ± 8.32 | 10.8 ± 4.97 | + | + | 77.3 ± 67.4 |
| + | NL-EGFP | + | 5 | 33.0 ± 20.7 | 10.2 ± 2.39 | 22.2 ± 7.95 | 31.4 ± 9.86 | NDe | + | + | 65.8 ± 43.9 |
hu-PBMC-NOD-SCID mice were infected with the indicated strains of HIV-1, and LPS was given 7 days after infection. Three days later, mice were sacrificed and brains were subjected to immunohistological staining. Numbers of human CD3+, human CD68+, HIV gag p24+, TUNEL+, and TUNEL+ cytoplasmic FluoroNissl Green (cFNG)+ cells per slice are indicated as the mean ± SD. Four to 12 mice in each group. NDe, not detected; NT, not tested.
Number of p24+ cells was almost similar to that of GFP+ cells.
AC, astrocytosis; +, significant proliferation of sharp GFAP+ cells; ±, slight proliferation of GFAP+ cells; −, no proliferation.
MN, microglial nodule.
Numbers of HLA-ABC+ cells in mouse peritoneal lavage.
P < 0.05 compared by Welch's t test between untreated and LPS-treated mice with JRFL infection.
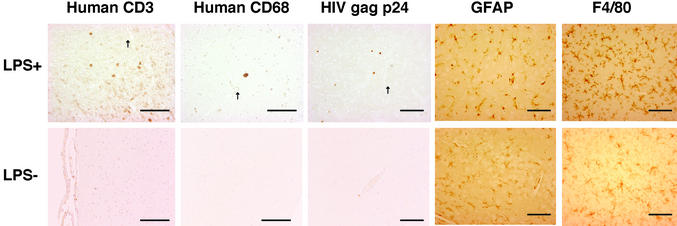
To facilitate the migration of HIV-infected macrophages into the brain, we administered a sublethal dose of LPS (100 μg) i.p. to the JRFL- or NL4-3-infected hu-PBMC-NOD-SCID mice 7 days after infection, because LPS has been reported to enhance the transendothelial migration of macrophages and T cells into the brain through its activation (17). A marked increase in human CD68+ macrophages (21.3 ± 5.3 cps in JRFL-infected brains and 13.0 ± 4.16 cps in NL4-3-infected brains) and HIV gag p24+ cells (58.4 ± 21.5 cps in JRFL-infected brains and 26.8 ± 7.46 cps in NL4-3-infected brains), as well as human CD3+ T cells (91.3 ± 34.4 cps in JRFL-infected brains and 36.0 ± 12.7 cps in NL4-3-infected brains), was found in the brain parenchyma of closed regions to vessels (Fig. 1, arrows) 3 days after LPS treatment (10 days after infection; Fig. 1, Table 1) and 21 days after LPS treatment (data not shown), as compared with the LPS-untreated mice (P < 0.05). In contrast, the number of human cells [human leukocyte antigen (HLA)-ABC+ (HLA+)] recovered from mouse peritoneal lavages decreased after LPS treatment (213.2 ± 159.7 × 104 cells without LPS treatment versus 47.1 ± 37.2 × 104 cells with LPS treatment in JRFL-infected mice; Table 1, P < 0.05). These infiltrated cells were mainly localized in the perivascular region of cortex, basal ganglia, hippocampus, and cerebellum. We also found an increased number of GFAP+ astrocytes (astrocytosis) and clustering of F4/80+ microglia (microglial nodule) 3 days after LPS treatment in both JRFL- and NL4-3-infected hu-PBMC-NOD-SCID mice, as well as HIV-uninfected hu-PBMC-NOD-SCID and untransplanted NOD-SCID mice, compared with mice that were not treated with LPS (Fig. 1, Table 1), indicating that these features were independent of HIV-1 infection.
Figure 1.
Migration of human PBMCs after LPS treatment and neuropathology in the brain of HIV-1-infected hu-PBMC-NOD-SCID mice. hu-PBMC-NOD-SCID mice were infected with JRFL and LPS was administered or not 7 days after infection. Three days later, the brains were subjected to immunohistochemical staining. Single staining for the indicated markers of brains from infected mice with or without LPS treatment is shown. Arrows indicate vessels. One representative mouse in Table 1 is shown. (Scale bars, 100 μm.)
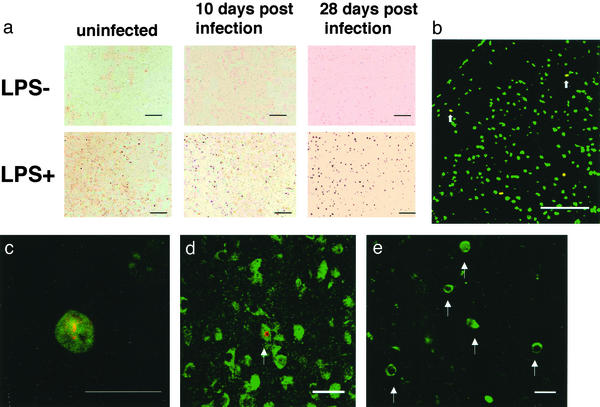
Neuronal apoptosis is a hallmark of HIV encephalopathy leading to neuronal dysfunction (6). We examined whether our mouse model had such a feature of the disease. After LPS treatment, histological examination clearly demonstrated apoptosis in the brain. We initially performed TUNEL staining for apoptotic cells. The LPS treatment markedly increased the number of TUNEL+ apoptotic cells (78.8 ± 21.5 cps in JRFL-infected brains and 33.3 ± 9.36 cps in NL4-3-infected brains) in cortex, basal ganglia, hippocampus, and cerebellum of HIV-1 infected hu-PBMC-NOD-SCID mice (Table 1) 3 days after LPS treatment (10 days after infection), compared with the LPS-untreated mice (11.4 ± 5.37 cps in JRFL-infected brains and 10.0 ± 4.24 cps in NL4-3-infected brains; Fig. 2a, Table 1). A similar level of increase was also observed 21 days after LPS treatment (28 days after infection; Fig. 2a). To identify apoptotic neurons, we next performed FNG staining to label Nissl bodies in the cytoplasm as a neuron-specific marker, along with TUNEL staining (Fig. 2b). The FNG stains nucleus of all cells and only cytoplasmic Nissl bodies of neurons. Notably, 10–20% of the TUNEL+ cells were cytoplasmic FNG+ (cFNG+) neurons (Fig. 2 b and c), and we observed these only after LPS treatment and only in the JRFL-infected mice (13.1 ± 4.47 cps in JRFL-infected brains, Table 1), not NL4-3-infected mice (Table 1). We further verified the identity of the apoptotic neurons by dual-color staining for TUNEL and another neuron-specific marker, anti-microtubule-associated protein (MAP)-2 (Fig. 2d). We also confirmed the apoptosis event in the brain of HIV-1-infected mice by immunostaining using an Ab specific for active form of caspase-3 (Fig. 2e). In addition, we found a modest increase in apoptotic cells (25.0 ± 10.8 cps) in the brain of HIV-uninfected hu-PBMC-NOD-SCID mice at 3 days after LPS treatment (Fig. 2a, Table 1), but these cells did not include cFNG+ neurons (Table 1) and disappeared at 21 days after LPS treatment (data not shown).
Figure 2.
Apoptosis in the brains of M-tropic HIV-1JRFL-infected hu-PBMC-NOD-SCID mice after LPS treatment. hu-PBMC-NOD-SCID mice were infected with JRFL, and LPS was administered or not 7 days after infection. The brains were subjected to histological staining 3 or 21 days later. (a) TUNEL staining of brains from uninfected or infected mice 3 or 21 days later with or without LPS treatment. (Scale bar, 100 μm.) (b–e) Apoptotic neurons in the brain of infected mice 3 days after LPS treatment. (b) FNG (green)/TUNEL (red) staining (low magnification). Arrows indicate cFNG+ TUNEL+ apoptotic neurons. (Scale bar, 100 μm.) (c) FNG (green)/TUNEL (red) staining (high magnification) shows a cFNG+ TUNEL+ neuron. (Scale bar, 20 μm.) (d) MAP-2 (green)/TUNEL (red) staining shows a green cytoplasm and a red nucleus. (Scale bar, 20 μm.) (e) Active form of caspase-3 (green) staining. Arrows indicate active form of caspase-3+ cells. (Scale bar, 20 μm.)
These results indicated that LPS treatment of M-tropic JRFL-infected hu-PBMC-NOD-SCID mice induced some neuropathological changes resembling HIV encephalopathy, especially including infiltration of HIV-infected macrophages and neuronal apoptosis in the brain. In contrast, LPS treatment of T-tropic NL4-3-infected hu-PBMC-NOD-SCID mice induced infiltration of only HIV-infected T cells and apoptosis in nonneuronal cells (Table 1), human T cells, and mouse cells including astrocytes, microglias, and endothelial cells (data not shown). These results indicated that the HIV-infected T cells in the brain were not competent to induce neuronal apoptosis, suggesting a critical role of HIV-infected macrophages. Although this model has limitations, it is possible to examine the mechanism of neuronal damage through invading HIV- 1-infected macrophages.
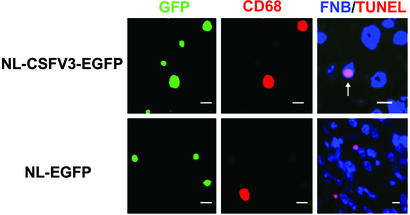
To confirm the requirement of HIV-1-infected macrophage in brain for inducing the neuronal apoptosis, we used recombinant M- (NL-CSFV3-EGFP) and T-tropic (NL-EGFP) HIV-1 expressing GFP as a marker for replicative infection. These recombinant viruses are identical except for the V3 sequence of env that determines the tropism. We infected hu-PBMC-NOD-SCID mice with these viruses and administered LPS. A significant level of systemic infection was established, as estimated by HIV gag p24 plasma levels (data not shown). Both 40–50% of CD68+ macrophages and 10–20% of CD68− CD3+ T cells in the brain were infected (GFP+) by M-tropic NL-CSFV3-EGFP, whereas 10–20% of CD68− CD3+ T cells were infected by NL-EGFP in the brain (Fig. 3). We could not find infected (GFP+) CD68+ macrophages in the brain of NL-EGFP-infected mice. Importantly, apoptotic neurons exhibiting a blue cytoplasm with a pink nucleus, observed by dual-color detection with NeuroTrace blue fluorescent Nissl stain (FNB, blue) and TUNEL (red), were frequently found (10.8 ± 4.97 cps) in the M-tropic virus-infected brain, but not in the T-tropic virus-infected brain (Fig. 3, Table 1). These results further substantiated that the HIV-infected macrophages in the brain were necessary for the induction of neuronal apoptosis.
Figure 3.
Association of HIV-1-infected macrophages with neuronal apoptosis. hu-PBMC-NOD-SCID mice were infected with M-tropic NL-CSFV3-EGFP or T-tropic NL-EGFP, and LPS was administered or not 7 days after infection. Brains were retrieved 10 days after infection and subjected to histological analysis for GFP (green) and CD68 (red), or FNB (blue) and TUNEL (red). In FNB/TUNEL staining, cytoplasmic FNB+ (cFNB+) TUNEL+ apoptotic neurons have a blue cytoplasm and a pink nucleus, which is merged with blue and red, as indicated by the arrow. (Scale bars, 10 μm.)
Critical Contribution of TRAIL to Neuronal Apoptosis.
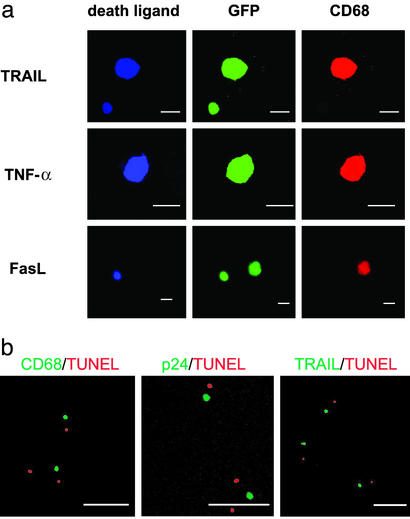
We next explored the molecular mechanism for the HIV-mediated neuronal apoptosis in our model. The expression of TNF-α, IL-1β, TRAIL, and FasL was examined, because these molecules were reportedly expressed in HIV-1-infected macrophages (6, 18, 19). Dual-color staining demonstrated that ≈40–60% of the infiltrating human CD68+ macrophages expressed TNF-α, IL-1β, and/or TRAIL, but not FasL (data not shown). In contrast, in the HIV-1-uninfected brain, ≈40–50% of the infiltrating human macrophages expressed TNF-α and/or IL-1β, but not TRAIL, after LPS treatment (data not shown). Thus, the expression of TRAIL on infiltrating human macrophages was found to be preferentially augmented in the infected brains after LPS treatment. Furthermore, triple-color analysis after infection with NL-CSFV3-EGFP indicated that TRAIL was expressed in ≈50% of HIV-infected CD68+ macrophages and 20% of HIV-infected T cells, whereas TNF-α was expressed in ≈80% of HIV-infected CD68+ macrophages and FasL was expressed ≈30% of the infected T cells but not in the infected CD68+ macrophages (Fig. 4a). Dual-color staining for apoptotic cells by TUNEL and for human CD68, HIV gag p24, or human TRAIL showed that some apoptotic cells were predominantly apposed to HIV-infected macrophages expressing TRAIL but not to HIV-1-infected T cells (Fig. 4b). These results suggested that the occurrence of neuronal apoptosis might be associated with the expression of TRAIL in HIV-infected macrophages.
Figure 4.
Association of apoptotic cells with HIV-1-infected macrophages expressing death-inducing molecules. (a) Expression of TRAIL, TNF-α, or FasL on HIV-1-infected macrophage. hu-PBMC-NOD-SCID mice were infected with M-tropic NL-CSFV3-EGFP and LPS was given or not 7 days after infection. Three days later, brain sections were subjected to histological analysis for GFP (green), human CD68 (red), and human TRAIL, TNF-α, or FasL (blue) simultaneously. A representative cell is shown. (Scale bars, 10 μm.) (b) hu-PBMC-NOD-SCID mice were infected with JRFL, and LPS was given 7 days after infection. Three days later, brain sections were subjected to dual-color staining for human CD68, HIV gag p24, or human TRAIL (green) and TUNEL (red). (Scale bars, 100 μm.)
Finally, we examined the contribution of TRAIL, TNF-α, and FasL to the neuronal apoptosis in the brain of JRFL-infected hu-PBMC-NOD-SCID mice by administering neutralizing mAbs against these molecules at the time of LPS treatment. The infiltration of human CD3+ T cells, human CD68+ macrophages, and HIV gag p24+ cells in the brain was not affected by the administration of these mAbs (data not shown). Neuronal apoptosis was significantly inhibited by the anti-human TRAIL mAb (RIK-2; P = 0.0005 by Welch's t test), but not by the anti-human TNF-α mAb (28401.111) or the anti-human FasL mAb (NOK-1), as compared with control mouse IgG (Table 2). These results indicated a critical contribution of human TRAIL to the neuronal apoptosis in the brain of M-tropic HIV-1-infected hu-PBMC-NOD-SCID mice.
Table 2.
Inhibition of apoptosis by anti-human TRAIL mAb, TNF-α mAb, or FasL mAb
| Ab | n | TUNEL+ | TUNEL+ cFNG+ |
|---|---|---|---|
| Human TRAIL | 8 | 35.0 ± 13.2 | 0.38 ± 0.34* |
| Human TNF-α | 5 | 42.8 ± 3.56 | 5.20 ± 2.59 |
| Human FasL | 6 | 68.3 ± 15.3 | 9.17 ± 1.47 |
| Control mouse IgG | 5 | 69.4 ± 39.8 | 8.60 ± 2.07 |
hu-PBMC-NOD-SCID mice were infected with the JRFL strain of HIV-1 and given LPS and the indicated neutralizing mAb 7 days after infection. Three days later, mice were killed and brains were subjected to histological analyses. Data are indicated as the mean ± SD of the number of TUNEL+ cells and TUNEL+ cFNG+ cells per slice from five to eight mice.
P < 0.05 compared with control mouse IgG by Welch's t test.
Discussion
In the present study, we developed a murine model of the HIV-CNS infection. In HIV-infected hu-PBMC-NOD-SCID mice without LPS treatment, no neuropathological changes excepting sparse astrocytosis were found. On the other hand, LPS treatment significantly induced the migration of HIV-infected T cells and macrophages into brain, along with the appearance of intense astrocytosis and microglial nodule. However, the same levels of astrocytosis and microglial nodules were also induced in uninfected mice with LPS treatment, indicating that these pathological changes did not solely result from HIV infection in brain. Although our murine model was contrived to a model of HIV encephalopathy, we cannot fully address the mechanism. Most importantly, however, neuronal apoptosis was specifically induced in infected brain region through migration of HIV-infected macrophages but not T cells after LPS treatment. Thus, we used this model to explore the cellular and molecular mechanism for the HIV-induced neuronal apoptosis in vivo.
Administration of LPS to the HIV-1-infected mice induced infiltration of the HIV-infected human macrophages and T cells into the perivascular region of the brain tissue. This effect of LPS seemed to be mediated by an increased expression of integrin very late antigen 4 (VLA-4) on human macrophages and T cells and an increased expression of vascular cell adhesion molecule 1 (VCAM-1) on the endothelium in the brain (unpublished observation). It has been reported that human VLA-4 could interact with mouse VCAM-1 (20) and that transendothelial migration of human T cells and macrophages was augmented with LPS treatment (21). Consistent with this idea, an increased expression of VCAM-1 has been implicated in the migration of macrophages into the brain during HIV and simian immunodeficiency virus (SIV) infection (22). LPS is an endotoxin produced by Gram-negative bacteria and has a potent stimulatory effect on endothelial cells and macrophages/microglia (23). It has been proposed that some factor associated with advanced HIV infection in the periphery is an important trigger for the infiltration of HIV-infected macrophages into the brain that leads to neuronal apoptosis (6). In fact, increased expression of human leukocyte antigen (HLA)-DR on human T cells and TRAIL and TNF-α on human macrophages was observed after LPS treatment (data not shown). These changes might be induced by activation of macrophages directly by LPS or indirectly by proinflammatory cytokines, such as TNF-α and IL-1β, secreted from these cells. This result suggests that similar changes in patients with HIV encephalopathy may be also mediated by a similar mechanism.
Using this model, we demonstrated that HIV-1 infection in the brain-infiltrating cells is essential for the induction of neuronal apoptosis. Apoptotic neurons were found only in the perivascular region of brains from M-tropic virus-infected mice after LPS treatment. In our mouse model, a large number of CD3+ T cells was also infiltrated into brain tissue coincidentally with infiltration of macrophages. This is apparently different from the pathological feature of HIV-infected human or SIV-infected monkey brain, and may represent a limitation of our mouse model. However, the level of TRAIL expression on HIV-1-infected macrophages in the brain was higher than that on HIV-1-infected T cells, suggesting that the TRAIL molecules expressed on HIV-1-infected macrophages have a critical role in the development of neuronal apoptosis. This requirement of HIV-infected macrophages for neuronal apoptosis is consistent with previous observations in the specimens from HIV encephalopathy patients (2) and in the brains of SCID mice inoculated intracerebrally with HIV-1-infected human macrophages (24). These findings suggest that macrophage activation by HIV infection itself or by M-tropic viral proteins might induce neurotoxicity. In this respect, it is noteworthy that the infection with a recombinant M-tropic virus (NL-CSFV3-EGFP), but not with a T-tropic virus (NL-EGFP), was neuropathogenic, because these viruses differ only in their V3 sequence of env. This result further substantiates that the ability of HIV-1 to infect macrophages plays a key role in the induction of neuronal apoptosis.
We further explored the molecular mechanism for the HIV-infected cell-mediated neuronal apoptosis by using our model. The brain-infiltrating cells expressed TRAIL in an HIV infection-dependent manner, whereas TNF-α and IL-1β were expressed independent of HIV infection after LPS treatment. TRAIL was preferentially expressed in HIV-infected cells especially macrophages, which were apposed to apoptotic neurons (Fig. 4b). More important, administration of a neutralizing anti-TRAIL mAb markedly inhibited the neuronal apoptosis in the brain (Table 2). In addition, we recently found significant up-regulation of TRAIL expression on macrophages in brain tissues of HIV encephalopathy patients (Y.M., unpublished data). These results revealed a critical role of TRAIL expressed on HIV-infected cells in neuronal apoptosis. TRAIL expression in HIV-infected cells might be induced by HIV-1 tat, as recently demonstrated (18), or by IFN, as previously demonstrated in peripheral blood monocytes in vitro (25). We also confirmed the high level of TRAIL expression on in vitro cultured HIV-1-infected, monocyte-derived macrophages (Y.M., unpublished data). The neurotoxic effect of human TRAIL was further verified against primary cultured neuronal/glial cells from fetal mice in vitro (Y.M., unpublished data). In this system, however, it remains to be determined whether TRAIL can induce apoptosis in neurons directly or whether it might act indirectly to stimulate glial cells to produce neurotoxic substances such as excitatory amino acids. However, it has been demonstrated that TRAIL directly induced apoptosis for neuronal cells in vitro (26). TRAIL-induced apoptosis is controlled by the expression of death-inducing or decoy receptors and intracellular proteins such as c-FLIP (cellular FLICE-inhibitory protein; ref. 27). Furthermore, it has recently been reported that a death-inducing TRAIL receptor was constitutively expressed on neurons (28). Further studies are needed to elucidate the molecular mechanisms for the TRAIL expression in HIV-infected macrophages and the TRAIL-mediated neuronal apoptosis.
In conclusion, this study has shown a critical role of TRAIL expressed on HIV-infected cells for neuronal apoptosis in the CNS of our murine HIV-1-infected model. This result suggests that a similar mechanism may be operative in the pathogenesis of HIV-associated dementia in early stages. We also previously demonstrated a critical contribution of TRAIL to the depletion of HIV-uninfected bystander CD4+ T cells in lymphoid organs, a step leading to AIDS (10). Therefore, TRAIL may be an important target for therapeutic intervention of HIV-associated dementia, as well as AIDS, in HIV-1-infected patients. Further studies in neuropathogenic monkey models are expected to address this possibility.
Acknowledgments
We thank Profs. K. Sugamura and T. Kitamoto (Tohoku University) for helpful discussions, and T. Ohwada and Y. Numata (Tokyo Medical and Dental University) for technical support and critical advice. This work was supported by grants from the Ministry of Health, Labor, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Y.K. was supported by the Naito Foundation.
Abbreviations
- cps
cells per slice
- EGFP
enhanced GFP
- FNG
FluoroNissl Green
- cFNG+
cytoplasmic FNG+
- FasL
Fas ligand
- LPS
lipopolysaccharide
- M-tropic
macrophage-tropic
- PBMC
peripheral blood mononuclear cell
- hu-PBMC-NOD-SCID
human PBMC–transplanted nonobese diabetic–severe combined immunodeficiency
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- T-tropic
T cell-tropic
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
References
- 1.Budka H, Wiley C A, Kleihues P, Artigas J, Asbury A K, Cho E S, Cornblath D R, Dal Canto M C, DeGirolami U, Dickson D. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 2.Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 3.Lipton S A. Curr Opin Neurol. 1997;10:247–253. doi: 10.1097/00019052-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 5.Lipton S A, Gendelman H E. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 6.Kaul M, Garden G A, Lipton S A. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 7.Prospero-Garcia O, Gold L H, Fox H S, Polis I, Koob G F, Bloom F E, Henriksen S J. Proc Natl Acad Sci USA. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buch S, Pinson D, Hou Y, Adany I, Li Z, Mukherjee S, Jia F, Mackay G, Silverstein P, Kumar A, Narayan O. J Med Primatol. 2000;29:96–106. doi: 10.1034/j.1600-0684.2000.290302.x. [DOI] [PubMed] [Google Scholar]
- 9.Koyanagi Y, Tanaka Y, Kira J, Ito M, Hioki K, Misawa N, Kawano Y, Yamasaki K, Tanaka R, Suzuki Y, et al. J Virol. 1997;71:2417–2424. doi: 10.1128/jvi.71.3.2417-2424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura Y, Misawa N, Maeda N, Inagaki Y, Tanaka Y, Ito M, Kayagaki N, Yamamoto N, Yagita H, Mizusawa H, Koyanagi Y. J Exp Med. 2001;193:651–660. doi: 10.1084/jem.193.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 12.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 14.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn B, Toga A W, Motamed S, Merlic C A. Neurosci Lett. 1995;184:169–172. doi: 10.1016/0304-3940(94)11198-r. [DOI] [PubMed] [Google Scholar]
- 16.Williams K C, Corey S, Westmoreland S V, Pauley D, Knight H, deBakker C, Alvarez X, Lackner A A. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nottet H S, Persidsky Y, Sasseville V G, Nukuna A N, Bock P, Zhai Q H, Sharer L R, McComb R D, Swindells S, Soderland C, Gendelman H E. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 18.Zhang M, Li X, Pang X, Ding L, Wood O, Clouse K, Hewlett I, Dayton A I. J Biomed Sci. 2001;8:290–296. doi: 10.1007/BF02256603. [DOI] [PubMed] [Google Scholar]
- 19.Dockrell D H, Badley A D, Villacian J S, Heppelmann C J, Algeciras A, Ziesmer S, Yagita H, Lynch D H, Roche P C, Leibson P J, Paya C V. J Clin Invest. 1998;101:2394–2405. doi: 10.1172/JCI1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hession C, Moy P, Tizard R, Chisholm P, Williams C, Wysk M, Burkly L, Miyake K, Kincade P, Lobb R. Biochem Biophys Res Commun. 1992;183:163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- 21.Bereta J, Bereta M, Cohen S, Cohen M C. Cell Immunol. 1993;147:313–330. doi: 10.1006/cimm.1993.1072. [DOI] [PubMed] [Google Scholar]
- 22.Sasseville V G, Newman W A, Lackner A A, Smith M O, Lausen N C, Beall D, Ringler D J. Am J Pathol. 1992;141:1021–1030. [PMC free article] [PubMed] [Google Scholar]
- 23.Ng Y K, Ling E A. Neurosci Res. 1997;28:111–118. doi: 10.1016/s0168-0102(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 24.Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet H S, Epstein L, Gelbard H, et al. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith T S, Wiley S R, Kubin M Z, Sedger L M, Maliszewski C R, Fanger N A. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsch R, Bechmann I, Deisz R A, Haas D, Lehmann T N, Wendling U, Zipp F. Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, et al. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 28.Dorr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer P H, Nitsch R, Zipp F. J Neurosci. 2002;22:RC209. doi: 10.1523/JNEUROSCI.22-04-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]