Abstract
Apicomplexan parasites critically depend on a unique form of gliding motility to colonize their hosts and to invade cells. Gliding requires different stage and species-specific transmembrane adhesins, which interact with an intracellular motor complex shared across parasite stages and species. How gliding is regulated by extracellular factors and intracellular signalling mechanisms is largely unknown, but current evidence suggests an important role for cytosolic calcium as a second messenger. Studying a Plasmodium berghei gene deletion mutant, we here provide evidence that a calcium-dependent protein kinase, CDPK3, has an important function in regulating motility of the ookinete in the mosquito midgut. We show that a cdpk3– parasite clone produces morphologically normal ookinetes, which fail to engage the midgut epithelium, due to a marked reduction in their ability to glide productively, resulting in marked reduction in malaria transmission to the mosquito. The mutant was successfully complemented with an episomally maintained cdpk3 gene, restoring mosquito transmission to wild-type level. cdpk3– ookinetes maintain their full genetic differentiation potential when microinjected into the mosquito haemocoel and cdpk3– sporozoites produced in this way are motile and infectious, suggesting an ookinete-limited essential function for CDPK3.
Introduction
To complete their life cycle, malaria parasites require three invasive ‘zoite’-stages. (i) Merozoites invade erythrocytes in the bloodstream of the vertebrate host. (ii) Ookinetes are the motile zygotes that form in the blood meal of the mosquito vector, penetrate the peritrophic matrix and the mosquito midgut epithelium and then replicate as an oocyst on the gut wall. (iii) Sporozoites are highly motile parasite stages that emerge from the cyst and penetrate cells of the mosquito salivary gland and various tissues of the vertebrate host, before invading and differentiating inside parenchymal cells of the liver. Malarial invasive life cycle stages differ in morphology, size, target tissues and modes and speed of movement, but the molecular mechanisms driving motility and invasion are nevertheless thought to be very similar (Baum et al., 2006).
Much of our molecular understanding of apicomplexan gliding motility comes from studies on tachyzoites of Toxoplasma gondii and on malaria sporozoites [recently reviewed by Kappe et al. (2004) and Keeley and Soldati (2004)]. Motility and invasion require the regulated apical release of adhesive proteins or protein complexes from secretory organelles, such as micronemes, rhoptries and dense granules, which typically form part of the apical complex that characterizes all apicomplexan invasive stages. The actomyosin-based motor complex that powers motility is located between the parasite’s plasma membrane and the underlying inner membrane complex (IMC). The bridge between the motor and the extracellular substrate is provided by a family of micronemal adhesins typified by the thrombospondin-related anonymous protein (TRAP) of the sporozoite. Its translocation to the posterior end of the parasite generates the force for gliding motility and invasion (Sultan et al., 1997). In malaria parasites, each invasive stage uses a different member of the TRAP family, probably reflecting the diverse extracellular substrates and host tissues, with which the malarial life cycle stages interact. In ookinetes the circumsporozoite/TRAP-related protein (CTRP) fulfils this function in the mosquito midgut (Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000), while erythrocyte invasion is thought to involve the merozoite TRAP homologue, MTRAP (Baum et al., 2006).
In marked contrast to the surface adhesins, intracellular components of the motor complex are thought to be highly conserved across all invasive life cycle stages and apicomplexan parasite species (Baum et al., 2006). The conserved cytoplasmic tails of all TRAP-family adhesins engage the motor indirectly, by binding tetramers of the glycolytic enzyme fructose-1,6-bisphosphate aldolase, which in turn is capable of nucleating short actin filaments (Buscaglia et al., 2003; Jewett and Sibley, 2003). The force for parasite gliding and invasion is generated by the dynamic interaction of these actin filaments with a class XIV myosin, MyoA, which is essential for gliding (Meissner et al., 2002). MyoA is anchored to the IMC by a MyoA tail-interacting protein (MTIP; Bergman et al., 2003), which has properties of a myosin light chain (Herm-Gotz et al., 2002), and by two glideosome-associated proteins, GAP45 and GAP50 (Gaskins et al., 2004; Baum et al., 2006).
Studies on T. gondii tachyzoites point towards an important role of calcium as a second messenger regulating gliding. Microneme secretion, motility and invasion occur independently of extracellular or host cell calcium levels, but require the release of calcium from intracellular stores, most likely the endoplasmic reticulum (Carruthers and Sibley, 1999; Lovett et al., 2002). Artificially raising cytosolic calcium levels by pharmacological agents, such as calcium ionophores, is sufficient to trigger microneme release in T. gondii tachyzoites (Carruthers and Sibley, 1999) and Plasmodium falciparum sporozoites (Gantt et al., 2000). Studying individual gliding tachyzoites, Lovett and Sibley (2003) observed rapid increases in cytosolic calcium accompanying or immediately preceding bursts of motility in vitro, illustrating the key role calcium plays as a second messenger.
Protein kinases are emerging as important downstream effectors of the calcium signal(s) regulating gliding (Dobrowolski et al., 1997; Carruthers et al., 1999; Wiersma et al., 2004). Candidates include members of the family of calcium-dependent protein kinases (CDPK), one of which, TgCDPK2, was identified as a prominent target for a kinase inhibitor, KT5926 (IC50 c. 100 nM), that blocks tachyzoite motility in T. gondii (Kieschnick et al., 2001). Members of the CDPK family have been identified in plants, green algae, ciliates and apicomplexan parasites (see Harper and Harmon, 2005 for review). They characteristically combine within the same protein a serine/threonine protein kinase domain with a regulatory carboxy-terminal calmodulin-like domain composed of four calcium-binding EF hands. The P. falciparum genome encodes five typical members of the CDPK family but none of these have so far been implicated in regulating gliding motility. CDPK1 is secreted from asexual blood stage parasites by an unusual, acylation-dependent mechanism (Moskes et al., 2004), but its molecular function remains unknown. Another family member, CDPK4, plays a key role in cell cycle regulation during male gamete formation (Billker et al., 2004). CDPK3 was also speculated to have a function in gametocytes, based on the transcriptional upregulation of the cdpk3 gene in sexual stages of P. falciparum (Li et al., 2000). However, most recently a Plasmodium berghei mutant with a disrupted cdpk3 gene has provided strong evidence for an essential function of CDPK3 in the ookinete (Ishino et al., 2006). The authors of that study conclude CDPK3 has no fundamental function in gliding, but defining a novel transition point in the midgut invasion process, they suggest that CDPK3 is required for ookinetes to traverse a gel-like layer surrounding the blood meal of Anopheles stephensi mosquitoes, in which cdpk3– ookinetes migrating out of the blood meal were concluded to become trapped.
Studying a similar cdpk3 gene knock-out mutant in P. berghei we here confirm an important function of CDPK3 in malaria transmission. Importantly, and in marked contrast to the previous study, we show that cdpk3– ookinetes are severely compromised in productive gliding motility in vitro. The crucial role of CDPK3 in regulating gliding motility provides a satisfactory explanation for the strongly reduced transmission of the cdpk3– mutant. We argue that the developmental defect of cdpk3– ookinetes can be understood within the traditional model of mosquito midgut invasion by the ookinete, and that no revision of this model is thus required.
Results
Ookinetes need CDPK3 to penetrate the mosquito midgut epithelium
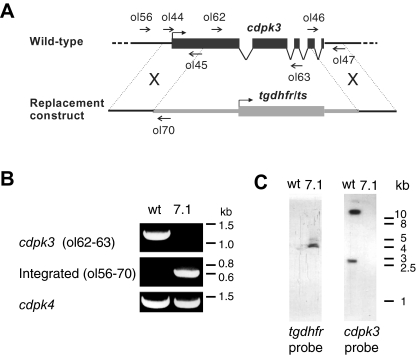
We generated a cdpk3 gene knock-out clone in P. berghei by replacing the protein-coding regions corresponding to amino acids 181–543 with a resistance marker (Fig. 1A). Integration of the targeting construct and deletion of the cdpk3 gene was verified by diagnostic PCR and Southern blot analysis (Fig 1B and C). cdpk3– parasites had no detectable defect in asexual erythrocytic development in mice and normal numbers of male and female gametocytes were formed (data not shown). When cultured in vitro, cdpk3– gametocytes differentiated into gametes, fertilized, and the zygotes developed into morphologically mature ookinetes. Conversion rates from female gametes to ookinetes were equally high in wild-type (80.3 ± 7.8%, n = 3) and cdpk3– parasites (76.0 ± 5.6%, n = 3), showing that CDPK3 has no essential function up to the stage of ookinete formation. However, when A. stephensi mosquitoes were allowed to feed on mice carrying cdpk3– gametocytes, the number of oocysts present on the midgut epithelium 10 days later was reduced by about 98% compared with wild type (Table 1), a marked decrease that is in good agreement with the 99% blockade observed by Ishino et al. (2006) with a separately derived cdpk3– parasite. We conclude that CDPK3 is important in ookinetes to give rise to oocyst infections. To test this hypothesis, equal numbers of wild-type or cdpk3– ookinetes from in vitro cultures were offered to mosquitoes in membrane feeders and oocyst numbers counted 10 days later. Infectivity of cdpk3– ookinetes was reduced by 99% (Table 1, Experiments 8 and 9). When we re-introduced the pbcdpk3 gene into the knock-out clone on an episomally maintained plasmid (Fig. 2A), ookinetes expressed a CDPK3 protein with a carboxy-terminal epitope tag (Fig. 2B), and their infectivity was restored to wild-type levels (Fig. 2C). These results confirm that a CDPK3-dependent defect at or after the ookinete stage is responsible for the dramatic reduction in oocyst numbers.
Fig. 1. Targeted disruption of the cdpk3 gene.
A. Illustration of the cdpk3 gene locus and the disruption construct used.
B. Diagnostic PCR showing absence of a cdpk3-specific product and presence of an integration-specific product in cdpk3– clone 7.1.
C. Southern blot analysis of EcoRI/HindIII-digested genomic DNA, showing presence of the tgdhfr/ts resistance gene in clone 7.1 and two bands diagnostic of the intact cdpk3 locus in wild type. The presence of two bands is due to HindIII restriction sites within the cdpk3 gene.
Table 1.
Oocyst counts from A. stephensi mosquitoes fed either directly on infected mice (experiments 1–7) or on membrane feeders containing uninfected mouse blood mixed with different numbers of ookinetes from cultures (experiments 8 and 9).
| Clone | Oocysts per mosquito a | Prevalence b | nc | |
|---|---|---|---|---|
| Experiments 1–7 (fed on infected mice) | Wild type | 375.41 | 97.14 (3.80) | 7 |
| cdpk3– | 8.25 | 77.81 (21.99) | 8 | |
| Experiment 8 (fed on 800 ookinetes per µl) | Wild type | 38.96 | 100.00 | 1 |
| cdpk3– | 0.15 | 15.00 | 1 | |
| Experiment 9 (fed on 2200 ookinetes per µl) | Wild type | 148.36 | 100.00 | 1 |
| cdpk3– | 0.07 | 10.00 | 1 |
Geometric mean oocyst numbers per mosquito are given
Prevalence of infections is given as the percentage of mosquitoes infected (standard deviation)
n gives the number of independent infectious feeds. For each feed 20–30 mosquitoes were dissected
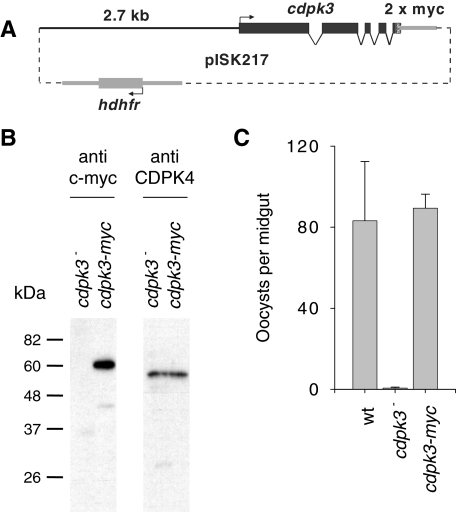
Fig. 2. Functional complementation of the cdpk3– mutant.
A. Schematic illustration of the complementation plasmid, showing the pbcdpk3 gene fused to an N-terminal double c-myc epitope tag under the control of 2.7 kb upstream sequence and a generic 3′UTR from the pbdhfr/ts gene.
B. Western blot analysis of protein extracts from 105 ookinetes of the cdpk3– clone 7.1 and a CDPK3 myc-complemented parasite population. The blot was first probed with the anti-myc mouse monoclonal antibody 9E10 (Sigma, UK). As a loading control the membrane was then stripped and re-probed with an rabbit polyclonal serum raised against T. gondii CDPK2 (Kieschnick et al., 2001) that recognizes CDPK4 in P. berghei (Billker et al., 2004).
C. Oocyst numbers counted 10 days after mosquitoes were allowed to feed on blood containing 800 ookinetes per µl. Averages oocyst numbers and standard deviations from two experiments using independent ookinetes culture are shown. Twenty-five midguts were dissected in each replicate experiment.
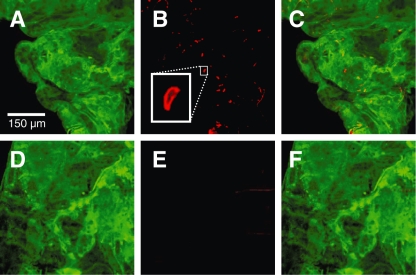
To test whether cdpk3– ookinetes retained the genetic potential to undergo ookinete-oocyst development we microinjected equal numbers of in vitro cultured wild-type and cdpk3– ookinetes into the haemocoel of A. stephensi. When given direct access to the mosquito in this way, both wild-type and cdpk3– ookinetes matured ectopically into oocysts, gave rise to heavy salivary gland infections within 21 days, and produced salivary gland sporozoites that were infectious to mice (Table 2). We conclude that cdpk3– ookinetes retain their full developmental potential but have a specific defect in overcoming the physical barrier posed by the midgut epithelium. We next asked whether cdpk3– ookinetes could bind and invade midgut epithelial cells. Equal numbers of wild-type and cdpk3– ookinetes (800 per µl) were fed to A. stephensi in a membrane feeding apparatus. Midguts were dissected within 2–4 h and processed for immunostaining to visualize ookinetes associated with the gut epithelium (Fig. 3). On average, 34.9 wild-type ookinetes (±14.3; n = 15 midguts) were associated with the midgut cells. In marked contrast, only 1.1 cdpk3– ookinetes (±0.9; n = 14 midguts) had accessed the gut.
Table 2.
Salivary gland sporozoites in A. stephensi that were either fed on or injected with in vitro cultured ookinetes
| Infectious to mice b | ||||
|---|---|---|---|---|
| Clone | Sporozoites in glands a | By bite | By injection | |
| Fed on 800 ookinetes per µl | Wild type | 19 800 (±4 900) | 2/2 | n.d. |
| cdpk3– | 0 (±0) | 0/2 | n.d. | |
| Injected with 600 ookinetes | Wild type | 13 000 (±3 200) | 2/2 | n.d. |
| cdpk3– | 4 800 (±200) | 2/2 | 4/4 | |
Arithmetic mean sporozoites per mosquito are given (± standard deviation from repeat experiments with different ookinete cultures). For each data point 18–30 pairs of glands were examined
Infectivity to mice was determined by allowing mosquitoes to feed on naïve mice or by injection of isolated sporozoites into a tail vein. Whenever sporozoites were observed in the glands, these were highly infectious, resulting in blood infections from day 4 post infection. Data are given as mice positive/mice infected; n.d., not done
Fig. 3.
cdpk3– ookinetes fail to associate with the mosquito midgut epithelium. Representative midgut epithelial sheets are shown that were dissected 2 h after mosquitoes had fed on equal numbers of cultured wild-type (A–C) or cdpk3– (D–F) ookinetes. Midguts were fixed, permeabilized and immunostained with an antibody against A. stephensi annexin to visualized the epithelium (panels A and D), and against the ookinete surface antigen P28 (panels B and E). All images are stacks of confocal sections. A higher magnification inset in (B) shows an ookinete associated with the midgut epithelium.
CDPK3 is required for productive gliding motility of ookinetes
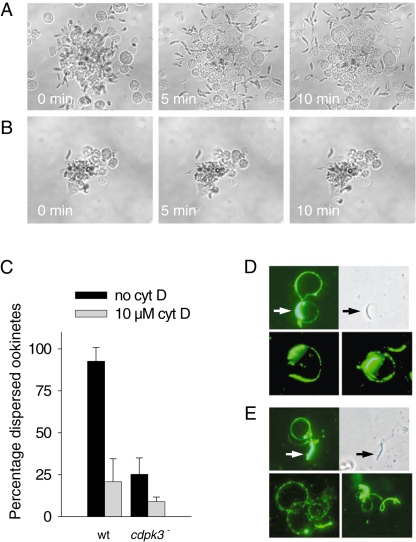
Recognizing that the reduced infectiousness of cdpk3– ookinetes could result from a fundamental defect in motility, we compared the ability of wild-type and cdpk3– ookinetes to glide on glass slides in vitro. We exploited the fact that P. berghei ookinetes purified from cultures are usually found in aggregates. We find that their dispersal relies on gliding motility and is sensitive to the anti-cytoskeletal agent cytochalasin D. When cocultured with insect cells, aggregates of wild-type ookinetes dispersed rapidly (Fig. 4A and Movie S1). In marked contrast, cdpk3– ookinetes showed a strongly reduced ability to glide productively and aggregates thus failed to disperse (Fig. 4B and Movie S2). Motility of cdpk3– ookinetes was abnormal, showing frequent flexing, bending, twirling and pendular motions, but only rare bouts of translocation over short distances (Movie S2). These observations were confirmed in a quantitative analysis, in which the proportion of cdpk3– ookinetes that dispersed from aggregates was scored and compared with wild type, either in the absence or in the presence of cytochalasin D (Fig. 4C). Prompted by the reported conservation among the motor components across invasive parasites stages (Baum et al., 2006), we asked whether sporozoite motility was also CDPK3-dependent. Twenty to 25% of wild-type and cdpk3– sporozoites obtained from ookinete-injected mosquitoes produced typical circular motility traces on glass slides (Fig. 4E) that were indistinguishable from wild type in shape and size (Fig. 4D). Motility traces were of similar length, corresponding to on average 1.7 ± 0.5 (n = 23) complete revolutions in wild type and 1.9 ± 0.8 (n = 23) in the cdpk3– sporozoites. Motility in cdpk3– sporozoites was consistent with their normal infectivity for mice. Together with the absence of a cdpk3– phenotype in asexual parasite development, these data suggest a key role for CDPK3 in regulating productive gliding motility specifically at the ookinete stage.
Fig. 4. Motility of wild-type and cdpk3– ookinetes and sporozoites.
A. Wild-type ookinetes purified from in vitro cultures disperse from aggregates after being incubated with insect cells.
B. cdpk3– ookinetes fail to show productive gliding motility. Each sequence of images is representative of at least three independent experiments with ookinetes cultured from different infected mice.
C. Quantification of ookinete dispersal. The proportion of dispersed ookinetes was determined after 24 h of culture in the absence and presence of cytochalasin D. Arithmetic means ± standard deviations from three independent experiments are shown.
D and E. Motility trails of wild-type (D) and cdpk3– sporozoites (E) obtained from salivary glands of ookinete-injected mosquitoes. Trails were visualized using a monoclonal antibody directed against the circumsporozoite protein, which labels both the sporozoites (arrows) and the trails of surface proteins shed by gliding sporozoites. The upper row of each panel shows corresponding immunofluorescence and phase contrast images of the same sporozoites. The bottom row of each panel illustrates additional representative circumsporozoite trails.
Discussion
Due to their stage-specific expression patterns, the plant-like CDPK of apicomplexan parasites have the potential to translate the ubiquitous second messenger calcium into different, stage-specific cellular responses in the parasite. We have previously identified a specific essential role for CDPK4 in the initiation of cell cycle events in male gametocytes of P. berghei (Billker et al., 2004). Our current analysis of a cdpk3– deletion mutant shows that this protein kinase has no essential function during asexual and sexual intraerythrocytic parasite development and that fertilization and the differentiation of the zygote into morphologically normal ookinetes do not rely on CDPK3. Furthermore, when ookinetes are given direct access to the mosquito haemocoel, CDPK3 is not required for their further differentiation into oocyst and infectious sporozoites. However, our analysis concurs with recent work studying a similar mutant (Ishino et al., 2006) in concluding that cdpk3– ookinetes are severely affected in their ability to establish an infection in the mosquito, because they fail to access the midgut epithelium. Our functional analysis is supported by a complementation experiment, which shows that re-introducing a cdpk3 allele into the cdpk3– mutant on an episomally maintained plasmid restores infectivity.
Using video microscopy and a quantitative ookinete spreading assay, we show here for the first time that cdpk3– ookinetes have a severely reduced ability to move productively on glass slides and to disperse from aggregates in vitro. This motility defect is sufficient to explain the reduced ability of cdpk3– ookinetes to engage and invade the mosquito midgut epithelium. Importantly, cdpk3– ookinetes are still capable of some bending, twirling and pendular movements and limited translocation, which may account for their residual infectivity of 1–2% of wild type. How CDPK3 regulates ookinete gliding remains to be defined. Gliding in other apicomplexan zoites can be regulated through the apical secretion of microneme proteins that form the bridge between the extracellular substrate and the intracellular motor. Microneme secretion is calcium-dependent in many apicomplexan zoites, including malarial sporozoites (Gantt et al., 2000) and in T. gondii tachyzoites it is a precondition for motility and host cell invasion (Carruthers et al., 1999). Our unpublished observations suggest ookinete motility is stimulated in the presence of insect cells and – consistent with the regulatory function of CDPK3 – is inhibited by a chelator of cytosolic calcium. However, whether the secretion of the microneme adhesin CTRP is controlled by cytosolic calcium levels and CDPK3 is unknown. Looking at two micronemal proteins of the ookinete, SOAP and CTRP, we have been unable with currently available reagents to establish a suitable microneme secretion assay to examine this possibility.
Recent in vivo imaging studies showed that ookinetes and sporozoites not only initiate and terminate gliding, but display twirling and bending actions, circular and straight-segment movements, and shape changes, in addition to invasive motility (Frischknecht et al., 2004; Vlachou et al., 2004; Amino et al., 2006). Furthermore, oocyst-derived sporozoites exhibit chemotaxis towards a heat-stable factor from mosquito salivary glands (Akaki and Dvorak, 2005) and it is tempting to speculate that ookinetes may be able to similarly direct their motility towards the midgut epithelium. This surprisingly large behavioural repertoire probably requires other layers of regulation in addition to microneme secretion. Potential phosphorylation-dependent control points include (i) the nucleation, stabilization or destabilizing of actin filaments by aldolase or other actin-binding molecules, such as toxofilin, which is subject to dynamic phosphorylation in T. gondii (Delorme et al., 2003), or (ii) the activity of myosin, which may involve the myosin light chain-like MTIP protein (Bergman et al., 2003). It will be interesting to examine whether molecular components of the motor complex are substrates for CDPK3. The ookinete-specific essential function for CDPK3 is unexpected in view of the universal role of calcium in zoite motility and considering that all invasive stages of Plasmodium are thought to use the same molecular motor (Baum et al., 2006). We speculate that in merozoites and sporozoites other members of the CDPK family may have functions similar to CDPK3 in the ookinete.
Our quantitative assay showing that productive gliding in cdpk3– ookinetes is fundamentally disturbed is in marked disagreement with a recent report investigating ookinete gliding in a similar mutant, but on a gel-like substrate (Matrigel™) that contains mouse basement membrane proteins (Ishino et al., 2006). Gliding of cdpk3– ookinetes on the gel surface is reported (but not quantified) as being normal and is contrasted with the failure of cdpk3– ookinetes to penetrate the gel matrix. Analysing mosquito blood meals 20 h after an infectious feed by transmission electron microscopy, Ishino et al. (2006) further show that cdpk3– ookinetes accumulate in an electron-lucent layer surrounding the blood meal. Likening this layer to the Matrigel™ used in vitro, a novel, CDPK3-dependent transition point in the midgut invasion process, is proposed, at which ookinetes would have to switch from CDPK3-independent gliding through the blood meal to a different, CDPK3-dependent mode of motility to penetrate the layer surrounding the blood meal, before eventually gaining access to the epithelium. In an attempt to reconcile our data with the previous report, we performed a quantitative estimate of ookinete gliding in the two movies published by Ishino et al. (2006). Wild-type ookinetes migrating in Matrigel™ cover a distance corresponding to on average 5.61 times their own length (standard deviation = 1.25, n = 16 ookinetes) in 45 min. During the same period cdpk3– ookinetes on the gel surface, while clearly showing some movement, progress by only 0.83 times their own length (standard deviation = 1.33, n = 20 ookinetes), and many ookinetes not included in our estimate fail to disperse from an aggregate on the gel surface, just as we have observed with our cdpk3 KO clone.
As we show here that cdpk3– ookinetes have a general motility defect, it is no longer necessary to assume CDPK3 has a more specific function for ookinetes when they penetrate the digested periphery of the blood meal. It is well established that haemolysis and digestion proceed centripetally from the periphery of the blood mass inwards (Clements, 1992) and immotile objects resistant to digestive enzymes, such as cdpk3– ookinetes, must therefore accumulate in the narrow lysis zone that forms between the undigested blood and the midgut epithelium. Finally, our membrane feeding assays showing that mature cultured cdpk3– ookinetes still fail to infect when brought in immediate intimate contact with the midgut epithelium, rule out any specific function for CDPK3 at the hypothetical transition point between the undigested erythrocyte mass and the peripheral layer as suggested by Ishino et al. (2006). We conclude that a fundamental motility defect is entirely sufficient to explain the phenotype of cdpk3– ookinetes within the traditional model of ookinete interactions with the blood meal. Future studies should focus on dissecting the exact molecular functions of CDPK3 and other CDPK family members to assess the importance of this group of plant-like protein kinases as targets for pharmacological intervention.
Experimental procedures
Parasite maintenance, in vitro culture and transmission to mosquitoes
The P. berghei ANKA wild-type strain 2.34 and transgenic lines were maintained in phenyl hydrazine-treated Theiler’s Original outbred mice and transmitted by A. stephensi, strain SD500, as described previously (Sinden et al., 2002). The course of infections and gametocyte production were monitored on Giemsa-stained blood films. Ookinetes were grown in vitro by culturing of gametocyte-infected mouse blood, quantified and then purified by selective lysis of erythrocytes as described previously (Sinden et al., 2002). Macrogamete-to-ookinete conversion rates were determined in 24 h cultures by live immunofluorescence microscopy with a Cy3-conjugated monoclonal antibody against the macrogamete/zygote/ookinete surface antigen p28, as previously described (Tewari et al., 2005a). For transmission experiments, batches of 50 mosquitoes, which had been starved overnight, were either fed directly on anaesthetized infected mice on day 4 of a blood-induced infection, or were allowed to feed on a membrane feeder apparatus loaded with a suspension of cultured ookinetes in blood from an uninfected mouse. All feeds were done for 20 min at 19°C. Unfed mosquitoes were removed the following day. Oocysts were counted on dissected midguts on day 10 after feeding. Alternatively, adult female mosquitoes were infected by microinjection of 60 nl of cultured ookinetes into the thorax, using a Nanoject II hand-held microinjector (Drummond, USA). Wild-type and cdpk3–ookinetes for injection were cultured in vitro from infected blood, from which leucocytes had been removed (Sinden et al., 2002). Ookinete numbers in 20–24 h cultures were determined in a haemocytometer, ookinete density adjusted to 104 per µl, and cultures back-filled into borosilicate injector needles.
Deletion and complementation of the cdpk3 gene
A targeting vector for pbcdpk3 was constructed in plasmid pBS-DHFR, in which polylinker sites flank a T. gondii dhfr/ts expression cassette conveying resistance to pyrimethamine. A 608 bp fragment comprising 5′ upstream sequence followed by the first 543 bp of exon1 of cdpk3 was PCR amplified from P. berghei genomic DNA and inserted into KpnI and ApaI restriction sites upstream of the dhfr/ts cassette of pBS-DHFR. A 686 bp fragment comprising the last two exons and 3′ flanking region of pbcdpk3 was then inserted downstream of the dhfr/ts cassette. The replacement construct was excised as a KpnI/BamHI fragment and used for the electroporation of cultured P. berghei schizonts as described (Menard and Janse, 1997; Billker et al., 2004). Following dilution cloning of drug-resistant parasites, genotyping of a cdpk3– clone, named 7.1, was carried out by Southern blot analysis and diagnostic PCR across the predicted integration site in principle as described previously for other gene deletions (Billker et al., 2004). A complementation vector was constructed in two steps in plasmid p141, a generic version of the myc-tagging vector p142 described previously (Billker et al., 2004). First a 2.7 kb fragment representing the cdpk3 5′ upstream intergenic region was PCR-amplified from P. berghei genomic DNA using oligonucleotides ol175 (3′-GAGAGGTACCGGGAAAATGAT GTAACTATAAGATG, restriction site underlined) and ol176 (3′-CATGCTAGCTTTTACGTATTAAACTATTTCCAAAAT) and inserted into KpnI abd NheI digested p142, giving rise to p142/11. Next the complete cdpk3 genomic sequence, including all introns, but excluding the TAA stop codon, was amplified from genomic DNA using primers ol177 (3′-CATGCTAGCATGAATCAATTATGTGTAGAAAG) (restriction site underlined) and ol178 (3′-GAGAGGGCCCATACTT TAGTTTCATCATTTCGCAA) and inserted into the NheI and ApaI restriction sites of 142/11, downstream of the putative cdpk3 promoter and 5′UTR sequence, in frame with a double c-myc epitope tag, followed by the stop codon and 0.5 kb of the P. berghei dhfr/ts 3′UTR. The resulting plasmid, p217, was confirmed by sequencing. p217 was introduced into the cdpk3– clone 7.1 by electroporation and maintained as an episome by selection for the hdhfr gene as described previously (Billker et al., 2004).
Staining of infected midguts
Midguts from fed mosquitoes were dissected at the indicated times in 1.85% paraformaldehyde in phosphate- buffered saline (PBS), pH 7.3. The blood meal was removed and the midgut epithelia were fixed for 1 h in 3.7% paraformaldehyde, washed once in PBS and then incubated for 30 min in PBS, 0.1% TritonX-100, 5% normal goat serum (NGS, Jackson Immunoresearch). Gut epithelial cells were stained with a mouse monoclonal antibody against mosquito annexin (Kotsyfakis et al., 2005), diluted in PBS, 5% NGS, for at least 1 h at room temperature. Three 10 min washes in PBS, 5% NGS, were followed by an incubation with Alexa488-conjugated goat anti-mouse antibody (Molecular Probes) for 1 h. Following another three washes, ookinetes were visualized with a Cy3-conjugated mouse monoclonal antibody 13.1 directed against the ookinete surface protein P28 (Winger et al., 1988). Stained midgut epithelial sheets were mounted in Vectashield (Vector Laboratories) and analysed by confocal microscopy.
Motility assays
Ookinetes purified from in vitro cultures were mixed on a microscope slide with Aedes aegytpi Mos20 cells in M199 medium (Sigma, UK), supplemented with 10% bovine calf serum. Ten microlitres of cell suspension was placed under Vaseline-rimmed coverslips and digital images recorded every 30 s for 10 min. Wild-type and mutant ookinetes were analysed in parallel. To quantify motility, aggregated ookinetes purified from in vitro cultures were seeded in duplicates into cocultures with Mos20 cells in LabTek 8-well chamber slides (Nunc, UK). 10 µM cytochalasin D (Calbiochem, UK) was added to one of the two wells for each sample. After 20–24 h ookinetes were visualized by staining with the Pb70 antibody against an ookinete cytoskeletal protein (Siden-Kiamos et al., 2000). Dispersed ookinetes and those that remained in aggregates were counted separately in randomly selected fields under the microscope. At least 200 ookinetes were counted in each sample. The proportion of all ookinetes that had dispersed is a measure of the motility of the ookinetes, while the sample containing cytochalasin D indicates the random occurrence of single ookinetes, as ookinetes are non-motile under these conditions (I. Siden-Kiamos and C. Louis, unpublished). To examine sporozoite gliding, we visualized motility trails using the 3D11 mouse monoclonal antibody (Yoshida et al., 1980), generously provided by Laurent Renia, against the circumsporozoite protein, using a previously described protocol (Tewari et al., 2005b).
Acknowledgments
This work was supported by an MRC Career Development Award to OB, the EMBO Short-term Fellowship ASTF 296-2003 to ISK, by a Wellcome Trust Studentship awarded to AE, and by European Union funds awarded to OB, RES and CL through the BioMalPar Network of Excellence.
Supplementary material
The following supplementary material is available for this article online:
Movie S1. Motility of wild-type ookinetes; the parasites move out of a central aggregate of ookinetes. Purified wild-type ookinetes were mixed with Mos20 cells under a Vaseline-rimmed coverslip. Images were captured every 30 s for 10 min.
Movie S2. Motility of cdpk3– ookinetes; the ookinetes show some residual motility but the majority of the ookinetes do not display translocational motility and thus are not able to move out of the aggregate. Purified mutant ookinetes were mixed with Mos20 cells under a Vaseline-rimmed coverslip. Images were captured every 30 s for 10 min.
This material is available as part of the online article from http://wwww.blackwell-synergy.com
References
- Akaki M, Dvorak JA. A chemotactic response facilitates mosquito salivary gland infection by malaria sporozoites. J Exp Biol. 2005;208:3211–3218. doi: 10.1242/jeb.01756. [DOI] [PubMed] [Google Scholar]
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria lifecycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–5208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, et al. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci. 2003;116:39–49. doi: 10.1242/jcs.00194. [DOI] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Buscaglia CA, Coppens I, Hol WG, Nussenzweig V. Sites of interaction between aldolase and thrombospondin-related anonymous protein in Plasmodium. Mol Biol Cell. 2003;14:4947–4957. doi: 10.1091/mbc.E03-06-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–235. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. I. London: Chapman & Hall; 1992. [Google Scholar]
- Delorme V, Cayla X, Faure G, Garcia A, Tardieux I. Actin dynamics is controlled by a casein kinase II and phosphatase 2C interplay on Toxoplasma gondii toxofilin. Mol Biol Cell. 2003;14:1900–1912. doi: 10.1091/mbc.E02-08-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens JT, Beetsma AL, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos FC, Sinden RE. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski JM, Carruthers VB, Sibley LD. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S, Olivo-Marin JC, et al. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol. 2004;6:687–694. doi: 10.1111/j.1462-5822.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Gantt S, Persson C, Rose K, Birkett AJ, Abagyan R, Nussenzweig V. Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect Immun. 2000;68:3667–3673. doi: 10.1128/iai.68.6.3667-3673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins E, Gilk S, DeVore N, Mann T, Ward G, Beckers C. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J Cell Biol. 2004;165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- Herm-Gotz A, Weiss S, Stratmann R, Fujita-Becker S, Ruff C, Meyhofer E, et al. Toxoplasma gondii myosin A and its light chain: a fast, single-headed, plus-end-directed motor. EMBO J. 2002;21:2149–2158. doi: 10.1093/emboj/21.9.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Jewett TJ, Sibley LD. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol Cell. 2003;11:885–894. doi: 10.1016/s1097-2765(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Kappe SH, Buscaglia CA, Bergman LW, Coppens I, Nussenzweig V. Apicomplexan gliding motility and host cell invasion: overhauling the motor model. Trends Parasitol. 2004;20:13–16. doi: 10.1016/j.pt.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Vontas J, Siden-Kiamos I, Louis C. The annexin gene family in the malaria mosquito Anopheles gambiae. Insect Mol Biol. 2005;14:555–562. doi: 10.1111/j.1365-2583.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- Li JL, Baker DA, Cox LS. Sexual stage-specific expression of a third calcium-dependent protein kinase from Plasmodium falciparum. Biochim Biophys Acta. 2000;1491:341–349. doi: 10.1016/s0167-4781(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Lovett JL, Sibley LD. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci. 2003;116:3009–3016. doi: 10.1242/jcs.00596. [DOI] [PubMed] [Google Scholar]
- Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem. 2002;277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- Menard R, Janse CJ. Gene targeting in malaria parasites. Methods. 1997;13:148–157. doi: 10.1006/meth.1997.0507. [DOI] [PubMed] [Google Scholar]
- Moskes C, Burghaus PA, Wernli B, Sauder U, Durrenberger M, Kappes B. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Mol Microbiol. 2004;54:676–691. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Vlachou D, Margos G, Beetsma A, Waters AP, Sinden RE, Louis C. Distinct roles for pbs21 and pbs25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J Cell Sci. 2000;113:3419–3426. doi: 10.1242/jcs.113.19.3419. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Butcher G, Beetsma A. Maintenance of the Plasmodium berghei life cycle. In: Doolan DL, editor. Methods in Molecular Medicine, Vol. 72: Malaria Methods and Protocols. Totowa, NJ: Humana; 2002. pp. 25–40. [DOI] [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, et al. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC, Fidock DA. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005a;58:1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- Tewari R, Rathore D, Crisanti A. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell Microbiol. 2005b;7:699–707. doi: 10.1111/j.1462-5822.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP, Kafatos FC. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol. 2004;6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- Wiersma HI, Galuska SE, Tomley FM, Sibley LD, Liberator PA, Donald RG. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int J Parasitol. 2004;34:369–380. doi: 10.1016/j.ijpara.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Winger LA, Tirawanchai CS, Nicholas J, Carter HE, Smith J, Sinden RE. Ookinete antigens of P. berghei. Appearance on the zygote surface of a Mr 21 kD determinant identified by transmission blocking monoclonal antibodies. Parasite Immunol. 1988;10:193–207. doi: 10.1111/j.1365-3024.1988.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980;207:71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Yuda M, Sakaida H, Chinzei Y. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med. 1999;190:1711–1716. doi: 10.1084/jem.190.11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Motility of wild-type ookinetes; the parasites move out of a central aggregate of ookinetes. Purified wild-type ookinetes were mixed with Mos20 cells under a Vaseline-rimmed coverslip. Images were captured every 30 s for 10 min.
Movie S2. Motility of cdpk3– ookinetes; the ookinetes show some residual motility but the majority of the ookinetes do not display translocational motility and thus are not able to move out of the aggregate. Purified mutant ookinetes were mixed with Mos20 cells under a Vaseline-rimmed coverslip. Images were captured every 30 s for 10 min.
This material is available as part of the online article from http://wwww.blackwell-synergy.com