Abstract
During drought, the plant hormone abscisic acid (ABA) triggers stomatal closure, thus reducing water loss. Using infrared thermography, we isolated two allelic Arabidopsis mutants (ost1-1 and ost1-2) impaired in the ability to limit their transpiration upon drought. These recessive ost1 mutations disrupted ABA induction of stomatal closure as well as ABA inhibition of light-induced stomatal opening. By contrast, the ost1 mutations did not affect stomatal regulation by light or CO2, suggesting that OST1 is involved specifically in ABA signaling. The OST1 gene was isolated by positional cloning and was found to be expressed in stomatal guard cells and vascular tissue. In-gel assays indicated that OST1 is an ABA-activated protein kinase related to the Vicia faba ABA-activated protein kinase (AAPK). Reactive oxygen species (ROS) were shown recently to be an essential intermediate in guard cell ABA signaling. ABA-induced ROS production was disrupted in ost1 guard cells, whereas applied H2O2 or calcium elicited the same degree of stomatal closure in ost1 as in the wild type. These results suggest that OST1 acts in the interval between ABA perception and ROS production. The relative positions of ost1 and the other ABA-insensitive mutations in the ABA signaling network (abi1-1, abi2-1, and gca2) are discussed.
INTRODUCTION
Stomatal pores located in the plant epidermis control the uptake of CO2 for photosynthesis and the loss of water through transpiration. The closing and opening of the pore result from the osmotic shrinking and swelling, respectively, of the two surrounding guard cells. These guard cells integrate a variety of environmental and endogenous signals to tightly regulate the stomatal aperture (Willmer and Fricker, 1996). In response to drought, plants synthesize the hormone abscisic acid (ABA), which triggers stomatal closure, thus reducing transpirational water loss. ABA acts directly on guard cells and induces stomatal closure via the efflux of potassium and anions from guard cells and the removal of organic osmolytes (MacRobbie, 1998; Schroeder et al., 2001).
Pharmacological and cell biological studies have led to major advances in our understanding of the mechanisms of ABA signal transduction in stomatal guard cells (Blatt, 2000; Assmann and Wang, 2001; Schroeder et al., 2001). In particular, substantial evidence indicates that the ABA regulation of plasma membrane ion channels is mediated by increases in cytosolic Ca2+. ABA increases cytosolic Ca2+ by inducing both Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space. Additional second messengers that contribute to ABA-induced stomatal closure include increases in cytoplasmic pH and production of phosphatidic acid.
More recently, a number of mutations that affect ABA signaling in Arabidopsis guard cells were characterized. The type-2C protein phosphatases ABI1 and ABI2 were identified initially on the basis of the ABA-insensitive abi1-1 and abi2-1 dominant mutations (Koornneef et al., 1984; Leung et al., 1994, 1997; Meyer et al., 1994). However, subsequent characterization of loss-of-function alleles indicated that ABI1 and ABI2 are negative regulators of ABA action (Gosti et al., 1999; Merlot et al., 2001). Recessive mutations leading to ABA-supersensitive stomatal closing identified additional negative regulators of ABA signaling in guard cells. These include the farnesyltransferase β-subunit ERA1 (Cutler et al., 1996; Pei et al., 1998), the mRNA cap binding protein ABH1 (Hugouvieux et al., 2001), and the Sm-like snRNP protein SAD1 (Xiong et al., 2001).
Conversely, the recessive gca2 mutation inhibits ABA-induced stomatal closure, indicating that GCA2 is a positive regulator of ABA signaling (Pei et al., 2000). The GCA2 gene has not been cloned. Similarly, disruption of the GPA1 gene, which encodes a heterotrimeric GTP binding protein α-subunit, impairs the ABA inhibition of stomatal opening (Wang et al., 2001). Finally, dominant mutations in the syntaxin-related protein Nt-SYR1 from tobacco (Leyman et al., 1999), the AAPK protein kinase from Vicia faba (Li et al., 2000), and the small GTPase AtRAC1 from Arabidopsis (Lemichez et al., 2001) inhibit ABA-induced stomatal closure. The relative positions of these various signaling elements are not firmly established, and our knowledge of the ABA signaling cascades remains fragmentary. Nevertheless, the emerging consensus is that the ABA regulation of stomatal aperture is mediated by a ramified network made up of different signaling modules (Hetherington, 2001; Schroeder et al., 2001).
To gain further insights into ABA signaling in guard cells, we developed a novel genetic screen for Arabidopsis mutants with altered stomatal responses to drought. Transpiration causes leaf cooling because evaporation of water is associated with heat loss. Leaf surface temperature can be measured continuously and nondestructively using infrared thermography, which provides a convenient indicator of the transpiration of individual plants (Raskin and Ladyman, 1988; Jones, 1999). We used infrared thermal imaging to screen for Arabidopsis mutants that displayed a reduced ability to close their stomata in response to drought stress and hence that appeared colder than the wild type (Merlot et al., 2002). Two of the mutants recovered correspond to a novel locus designated Open Stomata1 (OST1).
We report here the characterization of these recessive ost1 mutants and of the corresponding gene. Stomata of the ost1 mutants were insensitive to ABA but were responsive to light and CO2. The OST1 gene was isolated and shown to encode an ABA-activated protein kinase related to the V. faba AAPK. We investigated the place of OST1 in the guard cell ABA signaling network. Our results suggest that OST1 acts in the interval between ABA perception and the production of reactive oxygen species (ROS). We propose a model of this ABA signaling pathway that integrates the present data on ost1 and previous results on other ABA-insensitive mutants.
RESULTS
Isolation of ost1 Mutants
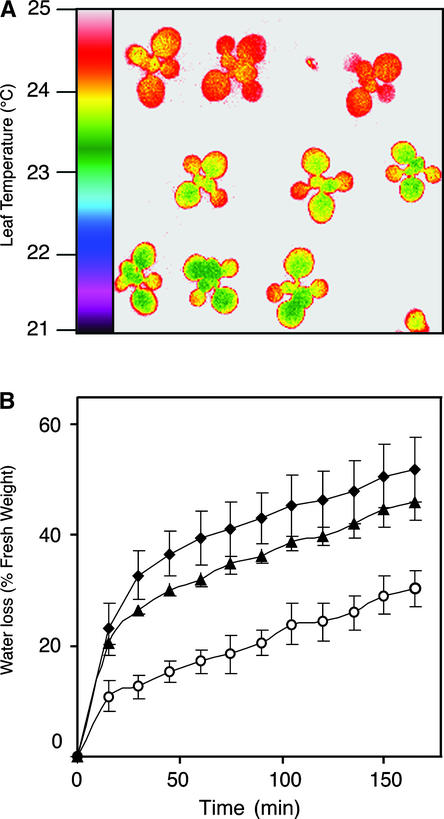
The ost1 mutants were identified in a screen based on thermal imaging of drought-stressed plants (Merlot et al., 2002). Plantlets derived from an ethyl methanesulfonate–mutagenized population in the Arabidopsis accession Landsberg erecta (Ler) were subjected to drought stress. These plantlets then were analyzed by infrared thermography, and the individuals that displayed a lower leaf temperature than that of the wild type were selected. Among several mutants recovered during this screen, two corresponded to independent recessive alleles of a novel locus that was called OST1. Upon drought, the leaf temperature of ost1-1 and ost1-2 plantlets was ∼1°C lower than that of wild-type plantlets (Figure 1A). This temperature difference reflected the inability of ost1 leaves to limit their transpirational water loss in response to water stress (Figure 1B).
Figure 1.
ost1 Mutants Are Defective in the Regulation of Transpiration upon Water Stress.
(A) False-color infrared image of drought-stressed plantlets. Twelve-day-old Ler wild-type (top row), ost1-1 (middle row), and ost1-2 (bottom row) plantlets were subjected to drought for 4 days. As calculated from the quantification of infrared images, the leaf temperatures were 24.7 ± 0.18°C for the wild type, 23.66 ± 0.24°C for ost1-1, and 23.21 ± 0.21°C for ost1-2 (means ± sd of measurements on ∼5000 square pixels).
(B) Kinetics of water loss from detached leaves of the wild type (open circles), ost1-1 (closed diamonds), and ost1-2 (closed triangles). Water loss is expressed as the percentage of initial fresh weight. Values are means ± sd of four samples of three leaves each.
ost1 Mutations Impair the ABA Regulation of Stomatal Aperture
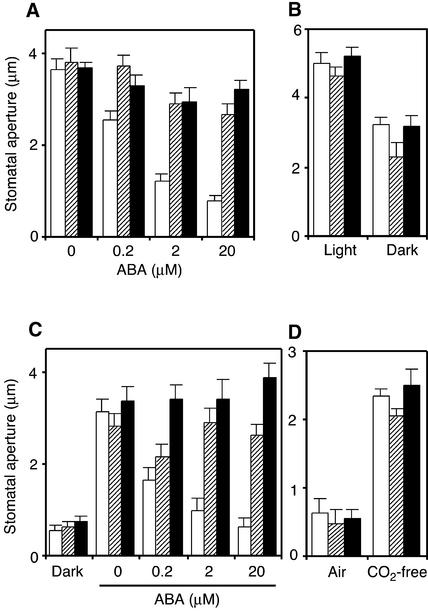
The responsiveness of ost1 stomata to various stimuli was analyzed in vitro using isolated epidermal peels. Preopened ost1 stomata failed to close in response to ABA (Figure 2A), which is consistent with the inability of ost1 plants to reduce water loss upon drought. By contrast, ost1 stomata closed to the same extent as the wild type in response to darkness (Figure 2B). Similarly, the ost1 mutations had no effect on the ability of preclosed stomata to open in response to light (Figure 2C) or CO2-free air (Figure 2D). However, the ost1 mutants were impaired in the ABA inhibition of light-induced stomatal opening (Figure 2C). These data indicate that the ost1 mutations do not cause a general defect in stomatal functioning but specifically disrupt ABA signaling in guard cells.
Figure 2.
ost1 Mutations Impair the ABA Induction of Stomatal Closing and the ABA Inhibition of Stomatal Opening.
Stomatal apertures were measured on epidermal peels of the wild type (white columns), ost1-1 (hatched columns), and ost1-2 (black columns). Values are means ± se (n = 60).
(A) ABA-induced stomatal closing. Stomata were preopened in the light for 3 h and then incubated in the indicated concentrations of ABA for 3 h in the light.
(B) Darkness-induced stomatal closing. Stomata were preopened in the light for 2.5 h and then incubated in darkness for 2.5 h.
(C) ABA inhibition of light-induced stomatal opening. Stomata were preclosed in darkness and then incubated for 2.5 h in the light at the indicated concentrations of ABA.
(D) Induction of stomatal opening by CO2-free air. Stomata were preclosed in darkness in normal air and then incubated for 3 h in CO2-free air in darkness.
When grown under well-watered conditions, the ost1 plants did not display any visible phenotypic alteration. Moreover, the ost1 mutations did not affect the degree of seed dormancy or the sensitivity of seed germination to inhibition by exogenous ABA (data not shown), indicating that OST1 does not control ABA responsiveness in seeds.
Map-Based Cloning of the OST1 Gene
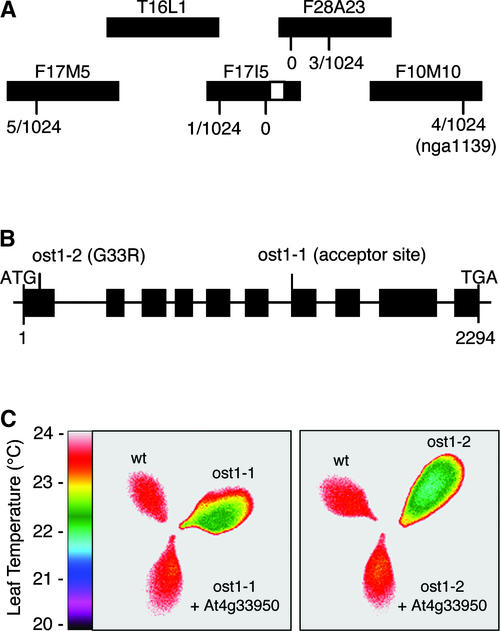
To genetically map the OST1 gene, homozygous ost1-2 mutant plants in the Ler accession were crossed to wild-type plants in the Columbia accession. The resulting F1 plants were allowed to self. From the segregating F2 population, ost1 mutants were identified by thermal imaging as colder individuals under drought conditions. A mapping survey of polymorphism markers distributed throughout the five Arabidopsis chromosomes placed the OST1 locus on chromosome 4, between markers AG and nga1139. New simple sequence length polymorphism markers in this region were designed using the Arabidopsis polymorphism database generated by Cereon Genomics (Cambridge, MA; http://www.arabidopsis.org/cereon/). Mapping of 1024 chromosomes narrowed OST1 to the 119-kb region between markers CER449763 on BAC clone F17I5 and CER451680 on BAC clone F28A23 (Figure 3A).
Figure 3.
Map-Based Cloning of the OST1 Gene.
(A) Genetic mapping of 1024 chromosomes localized OST1 (white rectangle) to the 119-kb region between markers CER449763 on BAC F17I5 and CER451680 on BAC F28A23.
(B) OST1 consists of 10 exons (rectangles). ost1-1 is a G-to-A change at nucleotide 1486 that disrupts the acceptor splice site of intron 6. ost1-2 is a G-to-A change at nucleotide 97 that converts Gly-33 to Arg.
(C) False-color infrared image of detached leaves from the Ler wild type (wt), ost1-1 and ost1-2 mutants, and ost1-1 and ost1-2 mutants transformed with the wild-type At4g33950 gene. Functional complementation was observed in eight independent transgenic lines.
We examined the sequence of this genomic region for genes predicted to encode signal transduction elements. Among them, the protein kinase gene At4g33950 on BAC clone F17I5 was considered a likely candidate. This gene was found by sequence analysis to be mutant in both ost1-1 and ost1-2 (Figure 3B). Compared with wild-type Ler, ost1-1 contains a G-to-A change 1486 bp downstream of the predicted translation initiation codon that disrupts the acceptor splice site of intron 6. ost1-2 contains a G-to-A change at nucleotide 97, which converts Gly-33 to Arg. Finally, transformation with a 4.5-kb genomic fragment encompassing At4g33950 restored the wild-type phenotype in both ost1-1 and ost1-2 (Figure 3C). These data demonstrate that At4g33950 corresponds to OST1.
OST1 cDNA was obtained by reverse transcriptase–PCR (RT- PCR) and sequenced. Comparison of the genomic and cDNA sequences showed that OST1 consists of 10 exons (Figure 3B). The wild-type OST1 sequence has been deposited in GenBank.
OST1 Kinase-Like Proteins
OST1 encodes a 362–amino acid protein with a predicted molecular mass of 41 kD. The N-terminal region of the OST1 protein (amino acids 1 to 297) presents all of the conserved features of the catalytic domains of protein Ser/Thr kinases (Figure 4A). In particular, the ost1-2 mutation affects an invariant residue required for ATP binding and thus is predicted to abolish OST1 kinase activity (Hanks and Quinn, 1991).
Figure 4.
Structure of the OST1 Protein.
(A) Alignment of the predicted amino acid sequences of the 10 members of the Arabidopsis OSKL family, V. faba AAPK, and barley PKABA. Residues conserved between OST1 and at least one other sequence are shaded black. Conserved subdomains of the protein kinase family are indicated by roman numerals. Positions of the ost1 mutations are shown. Dashed lines represent spaces that were introduced to maximize alignment. Arabidopsis Genome Initiative identification numbers of the OSKL family sequences are as follows: OST1 (At4g33950), OSKL2 (At5g66880), OSKL3 (At3g50500), OSKL4 (At1g78290), OSKL5 (At4g40010), OSKL6 (At1g60940), OSKL7 (At1g10940), OSKL8 (At5g08590), OSKL9 (At5g63650), and OSKL10 (At2g23030).
(B) Relation tree of OSKL proteins from Arabidopsis and other plant species.
Database searches revealed that OST1 shares between 67 and 80% amino acid identity with nine other predicted protein kinases from Arabidopsis. We named the members of this kinase subfamily OSKL for OST1 kinase-like proteins (Figure 4A). All of these protein sequences but OSKL5 and OSKL10 are supported by full-length cDNA sequences present in the databases. OSKL7 and OSKL8 were previously designated ASK1 and ASK2, respectively, by Park et al. (1993). OSKL2 and OSKL3 were previously designated ATHPROKINB and ATHPROKINA, respectively. The OSKL proteins contain an N-terminal kinase domain similar to that of SNF1/AMPK (Hardie et al., 1998) and a novel C-terminal domain that presumably is regulatory. This group of plant protein kinases also has been designated subfamily 2 of SNF1-related protein kinases (SnRK2) by Hardie (1999). To date, no biological function has been assigned to any of the Arabidopsis OSKL2 to OSKL10 genes.
OST1 also is similar to kinases from various other plant species, including the V. faba AAPK (Li et al., 2000) and barley PKABA (Gomez-Cadenas et al., 1999) proteins, which have been proposed to act in ABA signaling (Figure 4). In particular, the AAPK protein kinase has been shown by in-gel assays to be activated by ABA in V. faba guard cell protoplasts (Li and Assmann, 1996; Mori and Muto, 1997).
Expression Pattern of OST1
Using RT-PCR analysis, the OST1 mRNA was detected in leaves, flowers, stems, and roots (Figure 5A). The specificity of the oligonucleotides used for RT-PCR was tested on OSKL2 and OSKL3 cDNAs (Figure 5B). Characterization of transgenic plants harboring a transcriptional fusion between the β-glucuronidase (GUS) reporter gene and the OST1 promoter (pOST1::GUS) further indicated that in leaves, OST1 expression is confined to guard cells and the vascular system (Figures 5C and 5D). In agreement with this observation, the OST1 mRNA was detected by RT-PCR in guard cell protoplasts but not in mesophyll cell protoplasts (Figure 5A). pOST1::GUS expression also was detected in the guard cells present in stems and flower sepals (data not shown) and in the root vascular system (Figure 5E).
Figure 5.
Expression Pattern of OST1.
(A) RT-PCR analysis of OST1 mRNA expression in guard cell protoplasts (GCP), mesophyll cell protoplasts (MCP), leaf (L), flower (F), stem (S), and root (R) from 4-week-old plants and aerial parts of 2-week-old seedlings grown in vitro that were treated for 1 h with 10 μM ABA (+) or solvent control (−). EF1-α was used as an internal standard for cDNA amounts.
(B) PCR amplification of OST1 (lane 1), OSKL2 (lane 2), and OSKL3 (lane 3) cDNAs with oligonucleotides specific for OST1 (A), OSKL2 (B), and OSKL3 (C).
(C) Expression of pOST1::GUS in leaf tissue.
(D) Expression of pOST1::GUS in leaf guard cells.
(E) Expression of pOST1::GUS in root tissue.
ABA treatment had no detectable effect on OST1 mRNA abundance (Figure 5A) or on the level of pOST1::GUS activity (data not shown), indicating that OST1 expression is not regulated by ABA.
OST1 Kinase Is Activated by ABA
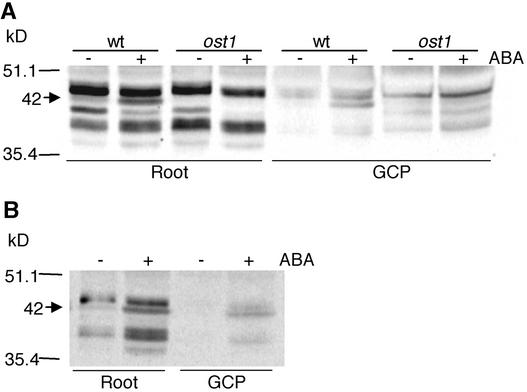
A recombinant OST1 protein was produced in Escherichia coli and migrated at the expected molecular mass upon gel electrophoresis. However, this protein displayed no detectable kinase activity in vitro (data not shown), suggesting that OST1 might be active only upon stimulation of the ABA signaling cascade in planta. A similar result in vitro also was observed in V. faba AAPK (Li et al., 2000). Based on OST1 expression patterns, protein extracts were prepared from Arabidopsis root and guard cell protoplasts and were analyzed by in-gel protein kinase assays. In both roots and guard cell protoplasts from wild-type Ler plants, a 42-kD band with protein kinase activity was enhanced markedly in response to ABA (Figure 6A). This band also was observed when calcium was replaced by EGTA in the assay (Figure 6B). The apparent molecular mass of this kinase closely correlates with the theoretical molecular mass of OST1. Furthermore, the ABA-activated 42-kD band was absent from extracts of the ost1-2 mutant (Figure 6A). These data indicate that, like the V. faba AAPK, OST1 is an ABA-activated Ca2+-independent protein kinase (Li et al., 2000). Furthermore, we observed a band with an apparent molecular mass of <42 kD in root extracts, which seems to be downregulated by ABA and calcium dependent. Although this band seems not to have a direct correlation with the ost1 phenotype, it needs further investigation.
Figure 6.
OST1 Is an ABA-Activated Protein Kinase.
(A) Protein kinase activities of root and guard cell protoplasts (GCP) from the Ler wild type (wt) and ost1-2 mutant were analyzed by an in-gel assay using Histone III-S as the substrate. Root extracts were obtained from 2-week-old seedlings treated for 3 h with 10 μM ABA (+) or solvent control (−). Guard cell protoplasts were treated for 20 min with 30 μM ABA (+) or solvent control (−). Sizes of molecular mass markers are shown at left. The arrow indicates the position of the ABA-activated 42-kD kinase.
(B) Roots and guard cell protoplast extracts from the Ler wild type were analyzed by replacing CaCl2 with EGTA in the kinase buffer.
OST1 Acts Upstream of ROS Production
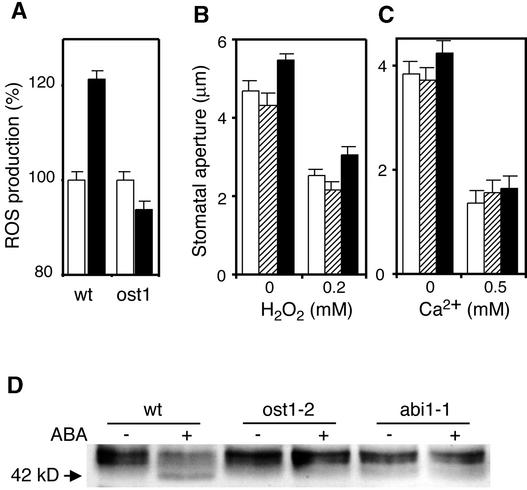
ROS emerged recently as an essential second messenger in ABA signaling (Guan et al., 2000; Pei et al., 2000; Zhang et al., 2001). In particular, ABA-induced stomatal closure involves the production of ROS that activate plasma membrane Ca2+ channels that mediate Ca2+ influx (Pei et al., 2000; Murata et al., 2001). ROS levels were analyzed in guard cells using the fluorescent dye 2′,7′-dichlorofluorescein diacetate. In agreement with previous studies (Pei et al., 2000; Murata et al., 2001), the relative fluorescence emission increased after treatment of wild-type guard cells with ABA (Figure 7A). By contrast, ABA did not increase ROS levels in ost1 guard cells (Figure 7A).
Figure 7.
OST1 Acts Upstream of ROS Production.
(A) ABA-induced ROS production. Changes in ROS levels were analyzed by measuring 2′,7′-dichlorofluorescein diacetate fluorescence levels in guard cells of the Ler wild type (wt) and ost1-2 after treatment with 50 μM ABA (black columns) or solvent control (white columns). Values are means ± se (n = 58).
(B) H2O2-induced stomatal closing. Stomatal apertures were measured on epidermal peels of the wild type (white columns), ost1-1 (hatched columns), and ost1-2 (black columns). Stomata were preopened in the light for 2 h in the presence of 0.1 mM EGTA and then incubated for 2 h in 0.2 mM CaCl2, 0.1 mM EGTA, and the indicated concentrations of H2O2 (Pei et al., 2000). Values are means ± se (n = 60).
(C) Extracellular Ca2+–induced stomatal closing. Stomatal apertures were measured on epidermal peels of the wild type (white columns), ost1-1 (hatched columns), and ost1-2 (black columns). Stomata were preopened in the light for 2 h and then incubated in the indicated concentrations of extracellular Ca2+ for 2 h. Values are means ± se (n = 60).
(D) OST1 kinase activity. In-gel kinase assay of extracts from roots of the Ler wild type, ost1-2, and abi1-1 treated for 3 h with 10 μM ABA (+) or solvent control (−). The arrow indicates the position of the ABA-activated 42-kD kinase.
To investigate the physiological relevance of this impairment of ABA-induced ROS production in ost1, we determined whether this mutation could be bypassed by an experimental increase of H2O2. As shown in Figure 7B, externally applied H2O2 elicited the same degree of stomatal closure in ost1 as in the wild type. Likewise, increased extracellular calcium induced similar stomatal closing in the wild type and in ost1 (Figure 7C). Similar results have been obtained for the inhibition of stomatal opening mediated by H2O2 and calcium (data not shown). These data support the idea that the ost1 mutation disrupts ABA signaling upstream of ROS production.
Like ost1, the ABA-insensitive abi1-1 mutation impairs ABA-induced ROS production and stomatal closure (Murata et al., 2001). In-gel kinase assays showed that ABA activation of the 42-kD OST1 kinase was inhibited in abi1-1 (Figure 7D). These data indicate that OST1 kinase acts downstream of ABI1 in the signaling cascade linking ABA to ROS production.
DISCUSSION
Our characterization of ost1 recessive mutants provides genetic evidence that the OST1 protein kinase is an essential element of the ABA signaling pathway that mediates stomatal regulation in response to drought. The role of OST1 in guard cells is consistent with pharmacological studies showing that phosphorylation events are central positive regulators in ABA-induced stomatal closure (MacRobbie, 1998; Schroeder et al., 2001).
Arabidopsis OST1 shares 79% amino acid identity with V. faba AAPK (Li et al., 2000). AAPK, also called ABR kinase by Mori and Muto (1997), is activated by ABA (Li and Assmann, 1996; Mori and Muto, 1997). AAPK activity was detected in guard cell protoplasts but not in leaf epidermal or mesophyll cell protoplasts (Li and Assmann, 1996; Mori and Muto, 1997). Transient transformation of V. faba guard cells with a dominant-negative form of AAPK impaired ABA-induced stomatal closing (Li et al., 2000). All of these data are consistent with the present results on OST1. It is unknown whether any OSKL other than OST1 is expressed in Arabidopsis guard cells. However, ost1 guard cells are essentially insensitive to ABA, suggesting that no other OSKLs have an overlapping role with OST1 in stomatal regulation. Hence, Arabidopsis OST1 and V. faba AAPK most likely are true functional orthologs.
The recessive ost1 mutations and the dominant-negative form of AAPK (Li et al., 2000) have largely concordant effects on stomatal regulation in Arabidopsis and V. faba, respectively. In both cases, ABA-induced stomatal closure was impaired, whereas closure elicited by darkness and opening elicited by light were unaffected. Similarly, ost1 mutations had no effect on stomatal opening in response to CO2-free air, and dominant AAPK had no effect on stomatal closure induced by increased CO2. Surprisingly, however, the dominant form of AAPK had no effect on the ABA inhibition of light-induced stomatal opening (Li et al., 2000), whereas this ABA response clearly was abolished in the ost1 mutants. It is unclear to what extent these conflicting observations may result from differences between the experimental strategies used (loss-of-function ost1 mutations versus overexpression of a dominant form of AAPK) or from differences between the ABA signaling networks in Arabidopsis and V. faba guard cells. In any case, although the results with the dominant AAPK suggested that ABA promotion of stomatal closure and ABA inhibition of stomatal opening may use different signaling cascades (Li et al., 2000), OST1 clearly is required for both of these ABA responses.
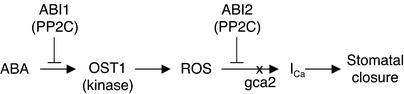
Like ost1, the abi1-1, abi2-1, and gca2 mutations impair ABA-induced stomatal closure in Arabidopsis. As illustrated in Figure 8, the three latter mutations were shown recently to affect a signaling cascade that involves ABA-induced ROS production and ROS activation of plasma membrane Ca2+ channels that mediate Ca2+ influx (Pei et al., 2000; Murata et al., 2001). Our results suggest that this same ABA signaling pathway is inhibited by ost1 mutation. ABA-induced ROS production was not affected in abi2-1 and gca2, but the stomata of these two mutants failed to close in response to externally applied H2O2 (Pei et al., 2000; Murata et al., 2001). These data suggest that ABI2 and GCA2 act downstream of ROS production. By contrast, both ost1 and abi1-1 impaired ABA-induced ROS production without affecting the ability of stomata to close in response to H2O2 or extracellular calcium (Allen et al., 1999; Murata et al., 2001). These results indicate that ABI1 and OST1 act upstream of ROS production. Finally, ABA activation of the OST1 kinase was impaired in abi1-1, suggesting that OST1 acts between ABI1 and ROS production. These various sets of observations are summarized in the minimal model shown in Figure 8.
Figure 8.
Model for the Position of OST1 in the ABA Signaling Cascade.
This minimal linear model integrates the present results on ost1 mutants with previous data on gca2 (Pei et al., 2000) and abi1 and abi2 mutants (Murata et al., 2001). OST1 is an ABA-activated protein kinase. The protein phosphatases 2C (PP2C) ABI1 and ABI2 are negative regulators of ABA action. The GCA2 gene has not been cloned. The relative order of ABI2 and the gca2 mutation is purely speculative. ICa indicates cytosolic calcium increase.
The dominant abi1-1 and abi2-1 mutations decrease ABA responsiveness. However, characterization of loss-of-function mutant alleles (Gosti et al., 1999; Merlot et al., 2001) and overexpression studies (Sheen, 1998) support the notion that the wild-type ABI1 and ABI2 phosphatases are negative regulators of ABA signaling. By contrast, the OST1 protein kinase is a positive regulator of ABA action. Thus, it is intriguing that the OST1 kinase and ABI1 phosphatase seem to have antagonistic effects on the pathway that mediates the ABA activation of ROS production. Identifying and comparing the respective substrates of OST1 and ABI1 will be an important step in understanding the molecular basis of the genetic interactions summarized in Figure 8. Recently, an AAPK-interacting protein (AKIP1) was identified and shown to be implicated in the ABA regulation of post-transcriptional RNA metabolism (Li et al., 2002). It will be intriguing to determine whether a putative OST1-interacting protein in Arabidopsis plays a similar role to AKIP.
Unlike other guard cell ABA signaling mutants (Koornneef et al., 1984; Cutler et al., 1996; Pei et al., 2000; Hugouvieux et al., 2001; Xiong et al., 2001), ost1 displays a wild-type ABA responsiveness in seeds. This is consistent with the fact that no OST1 expression was detected in wild-type Arabidopsis seeds. However, the OST1 homolog PKABA seems to participate in ABA signaling in barley grain. Unlike OST1, the PKABA mRNA is upregulated by ABA (Gomez-Cadenas et al., 1999). Constitutive expression of PKABA in aleurone cells mimicked the ABA repression of genes such as α-amylase (Gomez-Cadenas et al., 1999, 2001). These data raise the intriguing possibility that OSKLs distinct from OST1 may mediate ABA signaling in Arabidopsis seeds. Potential candidates include OSKL2 and OSKL3, because corresponding cDNAs were isolated from developing seed libraries. It will be interesting to determine which OSKLs contribute to ABA signaling in cell types other than guard cells and whether these OSKLs are involved in similar functional modules despite the diversity of responses elicited by ABA in distinct organs and tissues.
METHODS
Plant Material and Culture Conditions
The ost1 (Merlot et al., 2002) and abi1-1 (Koornneef et al., 1984) mutants are derived from the Arabidopsis thaliana accession Landsberg erecta (Ler). Plants were grown routinely in a greenhouse (22°C with a 16-h photoperiod) on soil irrigated with mineral nutrients. For aseptic growth, seeds were surface-sterilized and plated on a medium containing the inorganic salts of Murashige and Skoog (1962) at half concentrations, 100 mg/L myoinositol, 1 mg/L thiamine-HCl, 0.5 mg/L nicotinic acid, 0.5 mg/L pyridoxine-HCl, 1% Suc, 0.8% agar, and 2.5 mM Mes-KOH, pH 5.7. For abscisic acid (ABA) induction, 2-week-old seedlings were treated with 10 μM ABA (mixed isomers; Sigma) diluted from 10 mM stock solutions prepared in ethanol; equivalent volumes of ethanol were included in the ABA-free controls.
Thermal Imaging
Thermal imaging of drought-stressed plantlets was performed as described previously (Merlot et al., 2002). In brief, plantlets were first grown under well-watered conditions (21°C, 60 to 70% RH, 16-h photoperiod) for approximately 1 week. Drought stress then was initiated by withholding watering and transferring the pots to a drier atmosphere (24°C, 50% RH, 16-h photoperiod). Thermal images were obtained using a Thermacam PM250 infrared camera (Inframetrics, North Billerica, MA). Images were saved on a Personal Computer Memory Card International Association card and were analyzed subsequently on a Macintosh computer using version 1.56 of the public domain image-analysis program NIH Image (http://rsb.info.nih.gov/nih-image/).
Stomatal Aperture Bioassays
Leaves from 4- to 5-week old plants (grown in 8 h of light at 22°C and 16 h of darkness at 20°C; 70% RH) were harvested in darkness at the end of the night. Paradermal sections of abaxial epidermis obtained in dim green light were incubated in 10 mM KCl, 7.5 mM potassium iminodiacetate, and 10 mM Mes-KOH, pH 6.15, at 20°C. In Figures 2 and 7, ABA, Ca2+, EGTA, and H2O2 were added to the solution or CO2-free air was bubbled through the solution. Stomatal apertures were measured with an optical microscope (Optiphot-2; Nikon, Tokyo, Japan) fitted with a camera lucida and a digitizing table (TG 1017; Houston Instrument, Austin, TX) linked to a personal computer.
Protoplast Preparation
Guard cell protoplasts (GCPs) were prepared essentially as reported by Pandey et al. (2002). GCPs then were resuspended twice in 0.35 M mannitol, 10 mM KCl, and 1 mM Mes-NaOH, pH 6.1, and incubated in the same solution with ABA or ethanol solvent control. Then, GCPs were pelleted and stored at −80°C.
To prepare Arabidopsis mesophyll cell protoplasts (MCPs), rosette leaves from 4-week-old plants were excised and portions of the abaxial epidermis were peeled off. The peeled areas were floated peeled-side down on basic medium containing 0.07% cellulysin, 1.5% cellulase Onozuka RS, 0.02% pectolyase Y23, 0.1% polyvinylpyrrolidone 40, 0.25% BSA, and 0.5 mM ascorbic acid. After 2 h at 28°C with agitation, undigested material was removed by filtration through a 25-μm nylon net, and MCPs were collected by centrifugation at 150g for 20 min. MCPs were washed with fresh basic medium, pelleted again, and stored at −80°C.
The purity of the GCP and MCP preparations was 70 to 90% on a cell number basis.
DNA and RNA Analysis
The OST1 gene was amplified by PCR from genomic DNA using oligonucleotides 5′-CTCTGATGTCTTGGTGTC-3′ and 5′-GTGTACTCA-TTGAGACAA-3′. The ost1 cDNA was obtained by reverse transcription of total RNA using the Titan One Tube RT-PCR System (Roche Applied Science, Indianapolis, IN) and the same oligonucleotides as described above. The amplified DNA was cloned into PCR-TOPO2.1 (Invitrogen, La Jolla, CA).
For reverse transcription PCR (RT-PCR) analysis, total RNA was extracted, treated with DNase, and purified on RNeasy columns (Qiagen, Valencia, CA). Five micrograms of total RNA was reverse transcribed with SuperScript (Gibco BRL, Life Technologies). The cDNAs obtained were diluted 10-fold, and 2 μL was used in 50-μL PCR steps. A 590-bp fragment of OST1 cDNA was amplified with oligonucleotides 5′-CTTCCAGCAACTCATTTCAGGAGT-3′ and 5′-ACA-GTTGCTTCTGCAATGATCTGC-3′. As an internal standard for cDNA amounts, a 700-bp fragment of EF1α cDNA was amplified with oligonucleotides 5′-ATGCCCCAGGACATCGTGATTTCA-3′ and 5′-TTGGCGGCACCCTTAGCTGGATCA-3′. For RT-PCR control, OSKL2 and OSKL3 cDNAs were amplified by PCR from total cDNA with the following oligonucleotides: 5′-CCACAGGTCACTAAGGCATCCTA-3′ and 5′-GTTAAAAAAGGCTTTTTATTAGAGAGCGT-3′ for OSKL2, and 5′-TCATAGATCATTGAGACATCCTA-3′ and 5′-CAGACTTTGTCC-TCAGGAATCTC-3′ for OSKL3. PCR products were confirmed by sequence and used as templates for amplification with OST1 oligonucleotides used for RT-PCR.
Transgenic Plants
Transgenic Arabidopsis plants were generated by Agrobacterium tumefaciens–mediated transformation (Clough and Bent, 1998). For functional complementation of the ost1 mutants, an XbaI-SalI fragment of BAC F17I5 containing At4g33950 (nucleotides 55,462 to 59,974) was subcloned into the T-DNA vector pCAMBIA1390. The pOST1::GUS construct was obtained by amplifying 1.4 kb of the OST1 promoter region from BAC F17I5 using oligonucleotides 5′-GCCGGATCCCTCTAGAAGAGTGTATTGGG-3′ and 5′-GCCGGA-TCCTCTTTCCTCTGTCTCTTTGC-3′ and cloning the amplified DNA into the BamHI site of the T-DNA vector pCAMBIA1381Z. Four independent transgenic lines were analyzed by β-glucuronidase histochemical staining (Blazquez et al., 1997).
In-Gel Kinase Assays
Protein extraction and in-gel protein kinase assays were performed as described (Droillard et al., 2000). Protein extracts (30 μg) were electrophoresed on 10% SDS–polyacrylamide gels embedded with 0.5 mg/mL Histone III-S (Sigma) as a substrate for the kinases. After renaturation of the proteins, kinase activity was assayed in 40 mM Hepes, pH 7.5, 2 mM DTT, 20 mM MgCl2, 0.1 mM orthovanadate, 1 mM CaCl2, and 25 μM γ-33P-ATP. Gels were dried on Whatman 3MM paper and analyzed using a Storm imager (Molecular Dynamics, Sunnyvale, CA). To test the Ca2+ independence of OST1 activity, CaCl2 was replaced by EGTA in the kinase activity buffer.
Reactive Oxygen Species Detection
Reactive oxygen species production in guard cells was analyzed using 2′,7′-dichlorofluorescein diacetate essentially as described (Murata et al., 2001). Epidermal leaf peels were prepared from 5-week- old plants (grown under an 8-h photoperiod) and mounted on a microscope slide with medical adhesive (Hollister, Libertyville, IL). Epidermal tissues were incubated for 3 h in 30 mM KCl and 10 mM Mes-KOH, pH 6.15. A concentration of 30 μM 2′,7′-dichlorofluorescein diacetate was added to the incubation medium. After 20 min, the excess of dye was eliminated by two to three washes with distilled water. Epidermal tissues then were incubated for 20 to 30 min with 50 μM ABA or with an equal volume of ethanol as a control. Guard cells were observed with an epifluorescence microscope equipped with a charge-coupled device camera. Images were captured and the relative fluorescence emission of guard cells was analyzed using Adobe Photoshop 5.0 (Mountain View, CA) (Murata et al., 2001).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Accession Numbers
The GenBank accession numbers for the sequences described in this article are AJ316009 (wild-type OST1), L05562 and L05561 (OSKL2 and OSKL3, previously designated ATHPROKINB and ATHPROKINA), AL031032 (At4g33950), AAF27340 (AAPK), and BAB61735 (PKABA).
Acknowledgments
We thank Marie-Jo Droillard for advice on in-gel kinase assays, Juliette Leymarie for advice on protoplast preparation, and François Parcy, Helen North, and Jeffrey Leung for discussions and comments on the manuscript. This work was supported by the Centre National de la Recherche Scientifique, a European Molecular Biology Organization long-term fellowship to A.-C.M., a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche to S.M., and an Indo-French Centre for the Promotion of Advanced Research grant to A.V.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.007906.
References
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11, 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M., and Wang, X.-Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241. [DOI] [PubMed] [Google Scholar]
- Blazquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Droillard, M.J., Thibivilliers, S., Cazale, A.C., Barbier-Brygoo, H., and Lauriere, C. (2000). Protein kinases induced by osmotic stresses and elicitor molecules in tobacco cell suspensions: Two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett. 474, 217–222. [DOI] [PubMed] [Google Scholar]
- Gomez-Cadenas, A., Verhey, S.D., Holappa, L.D., Shen, Q., Ho, T.H., and Walker-Simmons, M.K. (1999). An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc. Natl. Acad. Sci. USA 96, 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cadenas, A., Zentella, R., Walker-Simmons, M.K., and Ho, T.H. (2001). Gibberellin/abscisic acid antagonism in barley aleurone cells: Site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 13, 667–679. [PMC free article] [PubMed] [Google Scholar]
- Gosti, F., Beaudoin, N., Serizet, C., Webb, A.A.R., Vartanian, N., and Giraudat, J. (1999). ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11, 1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, L.M., Zhao, J., and Scandalios, J.G. (2000). Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22, 87–95. [DOI] [PubMed] [Google Scholar]
- Hanks, S.K., and Quinn, A.M. (1991). Protein kinase catalytic domain sequence database: Identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200, 38–62. [DOI] [PubMed] [Google Scholar]
- Hardie, D.G. (1999). Plant protein serine/threonine kinases: Classification and functions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 97–131. [DOI] [PubMed] [Google Scholar]
- Hardie, D.G., Carling, D., and Carlson, M. (1998). The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821–855. [DOI] [PubMed] [Google Scholar]
- Hetherington, A.M. (2001). Guard cell signaling. Cell 107, 711–714. [DOI] [PubMed] [Google Scholar]
- Hugouvieux, V., Kwak, J.M., and Schroeder, J.I. (2001). An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106, 477–487. [DOI] [PubMed] [Google Scholar]
- Jones, H.G. (1999). Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 22, 1043–1055. [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383. [Google Scholar]
- Lemichez, E., Wu, Y., Sanchez, J.P., Mettouchi, A., Mathur, J., and Chua, N.H. (2001). Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15, 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., Bouvier-Durand, M., Morris, P.-C., Guerrier, D., Chefdor, F., and Giraudat, J. (1994). Arabidopsis ABA-response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264, 1448–1452. [DOI] [PubMed] [Google Scholar]
- Leung, J., Merlot, S., and Giraudat, J. (1997). The Arabidopsis ABSCISIC ACID-INSENSITIVE 2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman, B., Geelen, D., Quintero, F.J., and Blatt, M.R. (1999). A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283, 537–540. [DOI] [PubMed] [Google Scholar]
- Li, J., and Assmann, S.M. (1996). An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8, 2359–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Kinoshita, T., Pandey, S., Ng, C.K.-Y., Gygi, S.P., Shimazaki, K.-I., and Assmann, S.M. (2002). Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418, 793–797. [DOI] [PubMed] [Google Scholar]
- Li, J., Wang, X.Q., Watson, M.B., and Assmann, S.M. (2000). Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287, 300–303. [DOI] [PubMed] [Google Scholar]
- MacRobbie, E.A. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot, S., Gosti, F., Guerrier, D., Vavasseur, A., and Giraudat, J. (2001). The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 25, 295–303. [DOI] [PubMed] [Google Scholar]
- Merlot, S., Mustilli, A.-C., Genty, B., North, H., Lefebvre, V., Sotta, B., Vavasseur, A., and Giraudat, J. (2002). Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609. [DOI] [PubMed] [Google Scholar]
- Meyer, K., Leube, M.P., and Grill, E. (1994). A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264, 1452–1455. [DOI] [PubMed] [Google Scholar]
- Mori, I.C., and Muto, S. (1997). Abscisic acid activates a 48-kilodalton protein kinase in guard cell protoplasts. Plant Physiol. 113, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 493–497. [Google Scholar]
- Murata, Y., Pei, Z.-M., Mori, I.C., and Schroeder, J.I. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in the abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, S., Wang, X.-Q., Coursol, S.A., and Assmann, S.M. (2002). Preparation and application of Arabidopsis thaliana guard cell protoplasts. New Physiol. 153, 517–526. [DOI] [PubMed] [Google Scholar]
- Park, Y.S., Hong, S.W., Oh, S.A., Kwak, J.M., Lee, H.H., and Nam, H.G. (1993). Two putative protein kinases from Arabidopsis thaliana contain highly acidic domains. Plant Mol. Biol. 22, 615–624. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E., and Schroeder, J. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Raskin, I., and Ladyman, J.A.R. (1988). Isolation and characterization of a barley mutant with abscisic acid-insensitive stomata. Planta 173, 73–78. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Sheen, J. (1998). Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc. Natl. Acad. Sci. USA 95, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q., Ullah, H., Jones, A.M., and Assmann, S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292, 2070–2072. [DOI] [PubMed] [Google Scholar]
- Willmer, C., and Fricker, M. (1996). Stomata, 2nd ed. (London: Chapman & Hall).
- Xiong, L., Gong, Z., Rock, C.D., Subramanian, S., Guo, Y., Xu, W., Galbraith, D., and Zhu, J.K. (2001). Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev. Cell 1, 771–781. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D.W., and Song, C.P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]