Abstract
The translation initiation factors (IFs) IF1/eIF1A and IF2/eIF5B have been conserved throughout all kingdoms. Although the central roles of the bacterial factors IF1 and IF2 were established long ago, the importance of their eukaryotic homologs, eukaryotic IFs (eIFs) eIF1A and eIF5B, has only recently become evident. The translation machinery in eukaryotes is more complex and accordingly, eIF1A and eIF5B seem to have acquired a number of new functions while also retaining many of the roles of bacterial IF1 and IF2. IF1 and IF2 have been shown to interact on the ribosome but no binding has been detected for the free factors. In contrast, yeast eIF1A and eIF5B have been reported to interact in the absence of ribosomes. Here, we have identified the binding interface between human eIF1A and the C-terminal domain of eIF5B by using solution NMR. That interaction interface involves the C termini of the two proteins, which are not present in bacterial IF1 and IF2. The interaction is, therefore, unique to eukaryotes. A structural model for the interaction of eIF1A and eIF5B in the context of the ribosome is presented. We propose that eIF1A and eIF5B simultaneously interact at two sites that are >50 Å apart: through their C termini as reported here, and through an interface previously identified in bacterial IF1 and IF2. The binding between the C termini of eIF1A and eIF5B has implications for eukaryote-specific mechanisms of recruitment and release of translation IFs from the ribosome.
Translation is one of the central processes in the cell and is highly conserved throughout all kingdoms. The conservation is most obvious for the ribosome and for the translation elongation factors and not as clear for translation initiation. The mechanism of selection of the initiation codon, as well as the complexity of the initiation machinery, is very different between bacteria and eukaryotes (reviewed in ref. 1). However, in recent years, it was established that two of the three bacterial initiation factors (IFs), IF1 and IF2, have eukaryotic homologs, the eukaryotic IFs (eIFs) eIF1A and eIF5B, respectively. Furthermore, there is accumulating evidence that IF1/eIF1A and IF2/eIF5B may have some similar functions throughout evolution. All of these data suggest that the complex translation initiation machinery in eukaryotes did not replace but rather was built on the much simpler prokaryotic system (2, 3).
IF1 has been shown to bind to the A-site of the 30S subunit (4). It has been proposed to help direct the formylmethionyl-initiator tRNA (fMet-tRNAf) to the P-site of the ribosome and to stabilize the binding of mRNA and IF2 to the 30S ribosomal subunit (4–6). Its structure consists of an oligonucleotide/oligosaccharide binding (OB) fold (7). eIF1A, the eukaryotic homolog of IF1, has a homologous OB fold in its N-terminal part but contains an additional subdomain C terminal to the OB fold, consisting of two helices and two strands (8). Both proteins seem to bind RNA with the same surfaces on the OB fold but eIF1A also uses its new C-terminal subdomain for RNA binding. In addition, eIF1A has long unstructured N- and C-terminal tails that are highly charged. The C-terminal tail is primarily negatively charged but contains a few hydrophobic residues at the very end that were proposed to be involved in protein–protein interactions (8). eIF1A has been shown to promote 43S complex formation by stabilizing binding of the ternary complex eIF2-GTP-methionyl-initiator tRNA (Met-tRNAi) to the 40S subunit (9). Together with eIF1, eIF1A is required for proper formation of the 48S complexes (10). In addition to these eukaryote-specific functions, eIF1A stabilizes binding of mRNA to the 40S ribosomal subunit (10) and has been proposed to bind to the ribosomal A-site similarly to IF1 (3, 8, 11). Mutations of residues on the RNA-binding surface of eIF1A cause defects in proper 43S and 48S preinitiation complex formation (8).
The x-ray structure of the archaeal homolog of IF2/eIF5B has recently been determined. The archaeal protein consists of four domains, conserved among all kingdoms,§ forming an extended, chalice-like structure (12). Most nonthermophilic bacteria and the eukaryotes have an additional not well-conserved N-terminal region, which is positively charged and is thought to stabilize binding of IF2/eIF5B to the small ribosomal subunit (6). Both IF2 and eIF5B have been found to promote subunit joining. Their subsequent release from the ribosome occurs on GTP hydrolysis, stimulated by the ribosome (1, 13). IF2 has an additional function: recruitment of the fMet-tRNAf to the 30S subunit (1). The CTD of IF2 (IF2-CTD) is necessary and sufficient for fMet-tRNAf binding. IF2 binds fMet-tRNAf with ≈1 μM affinity and contacts both fMet and the acceptor end of the tRNA (14, 15). In eukaryotes, eIF2 but not eIF5B is responsible for bringing the initiator tRNA to the small ribosomal subunit (16). No Met-tRNAi binding has been reported for eIF5B, although in vivo data in yeast support a role for eIF5B in Met-tRNAi binding to the ribosome (17) (see Discussion). IF2 has been cross-linked to IF1 on the ribosome (18) but no binding has been reported off the ribosome, and it was proposed that domain II of IF2§ interacts with IF1 (6). Surprisingly, it was recently reported that yeast eIF1A and eIF5B bind to each other even off the ribosome, and the interaction was mapped not to domain II, but to domain IV of eIF5B (eIF5B-CTD), which is located >50 Å away from domain II (19). So far, it has not yet been determined which surface of eIF5B-CTD binds to eIF1A nor which region of eIF1A is responsible for the interaction.
Here we sought to identify the mutual binding interface of human eIF1A and eIF5B. With NMR, we have mapped the region of eIF1A, responsible for eIF5B binding, to the extreme C terminus of eIF1A. Likewise, we find that this segment of eIF1A binds to eIF5B in a narrow groove between two helices in domain IV, near the C terminus. A short C-terminal eIF1A peptide is necessary and sufficient for the interaction with eIF5B in solution. We propose a model for the interactions of eIF1A and eIF5B on the ribosome. The model offers an explanation for the apparent discrepancy between the eIF1A-eIF5B binding interface in eukaryotes and the interaction of their bacterial homologs, IF1 and IF2. The implications and predictions of the model are discussed.
Materials and Methods
Cloning, Protein Expression, and Purification.
Production of human eIF1A, with an N-terminal His tag (8) and the C-terminal 70-kDa fragment of human eIF5B, amino acids 587-1220 (eIF5B587–1220) (13), have been described. The CTD of human eIF5B, amino acids 1076–1220 (eIF5B-CTD), corresponding to the shortest fragment of yeast eIF5B reported to bind to eIF1A in a yeast two-hybrid system (19), was subcloned into Gateway destination vectors (Invitrogen) for expression with N-terminal His and GST tags, according to the manufacturer's instructions. eIF5B-CTD was also transferred into a gateway-compatible version of a protein G fusion vector (20). The 6-kDa protein G (domain GB1) served as a solubility enhancing tag (20).
The His- and GST-tagged eIF5B-CTD proteins were bound to His-tag affinity and glutathione resins (CLONTECH), respectively, and the tags were cleaved with factor Xa (Novagen) on the resin in buffer A0.15 (10 mM sodium phosphate, pH 7.0/7 mM BME/0.01% NaN3/1 mM CaCl2/0.15 M NaCl). For biochemical studies, GST-tagged eIF5B-CTD was eluted from the resin with 10 mM glutathione.
The protein G fusion of eIF5B-CTD (G-eIF5B-CTD) was purified by ion exchange chromatography on a Superflow Q column in buffer B0.05 [10 mM sodium phosphate, pH 6.6/1 mM DTT/0.01% NaN3/1 mM EDTA/0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF)/0.05 M NaCl]. The flow-through from the Q column (G-eIF5B-CTD did not bind to the Q resin) was loaded onto a Superflow SP column in 25 mM NaCl, washed with the same buffer, and eluted with 100 mM NaCl. All proteins were >95% pure, as estimated from Coomassie-stained SDS/PAGE gels.
Modeling, Docking, and Analysis of Structures.
The structure of human eIF5B-CTD was modeled by using the x-ray structure of the archaeal eIF5B homolog (12) as a template. Modeling was performed interactively with swissmodel (21). The model for the eIF5B-CTD structure was evaluated by using NMR data, as described in the next section. molmol (22) was used for structure analysis, manual docking, and the generation of figures. Structure-based sequence alignment between IF1 and eIF1A was obtained from the dali/fssp server (23).
Expression of Isotope-Labeled Proteins for NMR.
The bacterial cultures were grown on minimal medium as described (24). [13C]glucose and 15NH4Cl (Cambridge Isotope Laboratories, Cambridge, MA) were used for 13C- and 15N-isotope labeling, respectively. 15N-/13C-ILV-methyl-labeled eIF1A was obtained by using [γ-13C]-α-ketobutyrate and [γ,γ′-13C2]-α-ketoisovalerate as described (25). [γ-13C]-α-ketobutyrate and [γ,γ′-13C2]-α-ketoisovalerate we synthesized in the lab by V. Guelev (J. D. Gross, V. Guelev, and G.W., unpublished data). The above labeling scheme produced uniformly 15N-labeled eIF1A protein, with specific 13C labeling of both methyl groups of leucine and valine and the δ-methyl group of isoleucine.
NMR Assignments.
The NMR solution structure of human eIF1A was determined in our lab (8) and the nearly complete resonance assignments for eIF1A were already available.
The backbone resonance assignments and partial side-chain assignments for eIF5B-CTD were obtained by using standard NMR triple-resonance (HNCA/HNCOCA) experiments on a uniformly 15N-/13C-labeled protein sample and 15N-edited NOESY-heteronuclear single quantum correlation (HSQC) and total correlation spectroscopy (TOCSY)-HSQC experiments on uniformly 15N-labeled samples. The initial experiments were performed on untagged eIF5B-CTD protein samples in buffer B0.5 (same as buffer B0.05 above, but with 0.5 M NaCl) at 25°C at protein concentrations of 0.2–1 mM. Because of problems with protein precipitation at concentrations ≥0.2 mM at 25°C, further experiments were performed by using G-eIF5B-CTD in the above buffer, at pH 6.6 or 7.5. Ninety-five percent of the backbone resonances were assigned. The triple-resonance spectra were collected on a Bruker Avance 500-MHz spectrometer, with a cryoprobe. The NOESY and TOSCY spectra were collected on a Bruker Avance 600-MHz or a Varian Inova 750-MHz spectrometer. nmrpipe (26) or Felix (Molecular Simulations) was used for processing, and xeasy (27) was used for analysis of the spectra.
NMR Titration Experiments.
Unlabeled eIF5B-CTD or eIF5B587–1220 protein was added in small aliquots to a sample of 15N-/13C-ILV-methyl-labeled eIF1A. 15N-HSQC and 13C-HSQC spectra were collected at each step, and chemical-shift changes in the spectra on transition of eIF1A from free to bound state were followed until saturation or until the solubility limit was reached. The observed chemical-shift changes were mapped to the corresponding residues on the structure of eIF1A to identify the eIF5B-interacting region. The combined chemical-shift changes (Δσ) were calculated by adding the chemical-shift changes in the 1H (ΔσH) and 15N (ΔσN) dimensions (after dividing ΔσN by 5, to compensate for its greater magnitude compared with ΔσH). Values of Δσ > 0.1 ppm were classified as big, and Δσ < 0.1 ppm were classified as small. Similarly, unlabeled eIF1A or a synthetic peptide, corresponding to the C-terminal 14 or 7 residues of eIF1A (synthesized in the Tufts University Core Facility, Boston), was added to 15N-labeled eIF5B-CTD or G-eIF5B-CTD and 15N-HSQC spectra were collected. The protein samples were in the above NMR buffer B, pH 6.6 or pH 7.5, containing either 0.5 or 0.2 M NaCl. The dissociation constants (KD) of the binding were estimated from the NMR titration curves by plotting the magnitude of the chemical-shift changes as a function of protein concentration.
Analysis of the eIF5B-CTD Model and the Orientation Between eIF1A and eIF5B in the Complex.
Sequential and medium-range NOE peaks from 15N-edited NOESY-HSQC spectra of eIF5B-CTD, together with chemical shift data, were used to obtain information about the secondary structure elements in the eIF5B-CTD. The NMR data were used to evaluate the homology model for the eIF5B-CTD structure.
15N-edited NOESY-HSQC spectra of eIF5B-CTD, in complex with unlabeled eIF1A, and 15N- and 13C-edited NOESY-HSQC spectra of eIF1A, in complex with unlabeled eIF5B-CTD, were used to identify intermolecular NOE peaks between eIF1A and eIF5B and thus obtain information about the orientation between the two proteins in the complex. The data were also analyzed for any indications of conformational changes in the proteins.
Results and Discussion
Human eIF5B Binds to a Short C-Terminal Region of eIF1A.
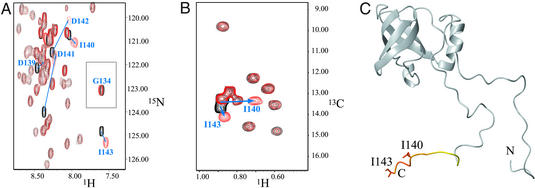
We used NMR chemical-shift mapping to identify the region of eIF1A interacting with eIF5B, taking advantage of the available structure and nearly complete NMR assignments of eIF1A (8). Addition of unlabeled eIF5B-CTD to a sample of 15N-/13C-ILV-methyl-labeled eIF1A led to chemical-shift changes in the 15N-HSQC spectra for only the extreme C terminus of eIF1A (Fig. 1 A and C). The effects were most pronounced for the last six residues, amino acids 138–143 (EDIDDI), and the corresponding peaks were significantly broadened. The NH peaks of Asp-141 and Asp-142 were broadened most and also had the greatest chemical-shift changes (Δσ > 0.5 ppm). Only marginal effects were observed for amino acids 134–137. Accordingly, the 13C-HSQC spectra of 15N-/13C-ILV-methyl-labeled eIF1A showed chemical-shift changes of only the two peaks, corresponding to the δ-methyl groups of Ile-140 and Ile-143 (Fig. 1B). There was no effect on the next most proximal [13C]methyl-containing residue, Ile-133.
Figure 1.
Binding of human eIF5B-CTD to 15N-/13C-ILV-methyl-labeled human eIF1A. (A) Segment of the 15N-HSQC spectrum of eIF1A, in the presence (red) and absence (black) of unlabeled eIF5B-CTD. The segment contains all peaks, whose positions were affected. The initial and final positions of the peaks corresponding to the NH groups of residues 139–143 are connected by arrows. Gly-134 (the most N-terminal residue to show any effect) is also shown. (B) 13C-HSQC spectrum of 13C-ILV-methyl-labeled eIF1A, in the presence (red) and absence (black) of unlabeled eIF5B-CTD. The peaks corresponding to the δ-methyl groups of Ile-140 and Ile-143 are indicated as in A. (C) Mapping the effects of eIF5B-CTD binding on the structure of eIF1A (8). Residues affected by eIF5B-CTD binding are colored from yellow (small chemical-shift changes) to orange (big changes). The side chains of Ile-140 and Ile-143 are shown.
The binding was relatively weak and in fast exchange on the NMR time scale: the peaks gradually move from the positions, corresponding to the free eIF1A, to the positions corresponding to the complex, with increasing concentration of unlabeled eIF5B-CTD. The KD of binding was estimated from the titration curve to be in the order of 10 μM, but could not be precisely determined, because of partial aggregation of eIF5B-CTD in the course of the titration. The binding was not significantly affected by the presence or absence of an N-terminal tag in eIF5B-CTD, by pH (6.6 vs. 7.5) or by salt concentration (200 vs. 500 mM). The latter finding indicated a significant hydrophobic contribution to the interaction. The observed weak binding is consistent with the published data for yeast eIF1A and eIF5B, where binding was observed at high micromolar to millimolar protein concentrations (19). Consistently, no binding between human eIF1A and eIF5B-CTD or eIF5B587–1220 (corresponding to the full-length archaeal eIF5B) was detected in GST pull-down experiments at low micromolar concentrations (data not shown).
In the previous experiments we used only the eIF5B-CTD. To test whether more N-terminal regions of eIF5B also contact eIF1A, we repeated the NMR titration experiment with eIF5B587–1220. No significant chemical-shift changes in additional regions of eIF1A were observed (data not shown). It was not possible to complete the NMR titration experiment to saturation because of limitations of eIF5B587–1220 solubility and, therefore, we may not have been able to detect binding that was very weak and/or caused only small chemical-shift changes on eIF1A.
Building and NMR Validation of a Homology Model of Human eIF5B-CTD.
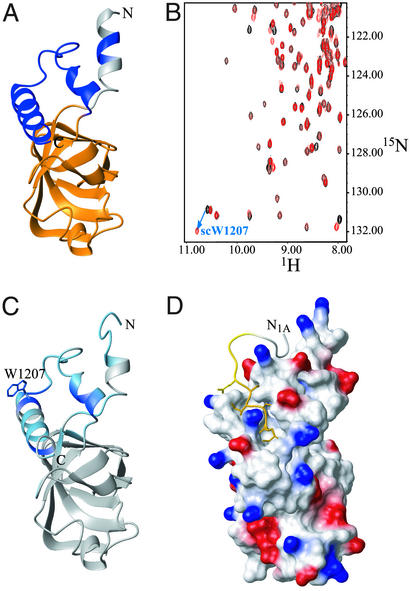
To identify the surface of eIF5B-CTD contacting eIF1A, we needed to know the NMR backbone resonance assignments and the structure of eIF5B-CTD. Backbone and partial side-chain assignments for human eIF5B-CTD were obtained as described in Materials and Methods. The high degree of sequence conservation (19% identity and 46% similarity) between the human and archaeal proteins in that region allowed us to model the structure of eIF5B-CTD by using the crystal structure of aIF5B as a template (Fig. 2A). It was found that both the position and boundaries of most secondary structure elements in the model were consistent with the NMR data (15N-NOESY-HSQC and chemical shifts) except in some of the connecting loops, where minor discrepancies were observed or the conformation was difficult to evaluate. The only major discrepancy was that the first seven residues were predicted to be helical in the model [and are helical in archaeal eIF5B (12)] but were unstructured in our construct. These residues are not part of the eIF5B-CTD and may require the rest of the protein for folding. This finding indicated that, as predicted from sequence homology, the structures of the CTDs of the human and archaeal eIF5B homologs are very similar.
Figure 2.
Binding of human eIF1A to 15N-labeled human eIF5B-CTD. (A) Homology-based model of the structure of human eIF5B-CTD. The model was created as described in Materials and Methods, using the x-ray structure of the archaeal IF2/eIF5B homolog (12) as a template. Residues belonging to the helix connecting eIF5B-CTD to domain III are colored in gray; the eIF5B-CTD is colored in orange; and segments present in the archaeal and eukaryotic IF2/eIF5B homologs, but not in bacteria (12), are colored in dark blue. NMR data indicated that the first seven residues were not helical (as in the model) but unstructured, and this is reflected in C and D. (B) 15N-HSQC spectrum of protein G-tagged eIF5B-CTD, in the presence (red) and absence (black) of an unlabeled C-terminal eIF1A peptide FDDIGDDDEDIDDI. As an example, the initial and final positions of the side-chain NH peak of Trp-1207 (which had the greatest chemical-shift change) are connected by an arrow. (C) Mapping the effects of eIF1A binding on the structure of eIF5B-CTD. Residues affected by eIF1A binding are colored from light blue (small chemical-shift changes) to dark blue (big changes). The side chain of Trp-1207 is shown. Big changes (Δσ > 0.1 ppm) were observed for residues 1188, 1191, 1197, 1203, 1207, and 1216. Smaller changes (Δσ < 0.1 ppm) were observed for residues 1076–1082, 1088–1090, 1092, 1118, 1186, 1189, 1192, 1194, 1195, 1198–1202, 1204, 1209–1215, and 1220. (D) Interaction interface between human eIF1A and eIF5B-CTD. eIF5B-CTD, in the same orientation as in C, is in surface representation and colored by electrostatic potential. Positive charges are in blue and negative charges are in red. The C-terminal 14 residues of eIF1A are displayed but only the last five residues were used in the docking, because the rest of the C terminus of eIF1A remains unstructured in the complex. The coloring of eIF1A is as in Fig. 1C. The side chains of amino acids 138–143 are displayed.
Human eIF1A Binds to a Hydrophobic Groove in the CTD of eIF5B.
To identify the binding site of eIF1A on eIF5B, we performed NMR chemical-shift mapping experiments. Addition of unlabeled eIF1A to an 15N-labeled sample of eIF5B-CTD caused chemical-shift changes in a number of peaks corresponding mainly to residues in the C-terminal helical subdomain of eIF5B-CTD [helices H13 and H14 in the archaeal eIF5B structure (12)], including the solvent-exposed side-chain of Trp-1207, which showed the most significant effect: Δσ > 0.4 ppm (Fig. 2C). Chemical-shift changes were also observed for residues in helix H12, which packs against H13. Because only the C terminus of eIF1A was affected by eIF5B-CTD binding (Fig. 1), we repeated the NMR titration with a synthetic peptide, corresponding to the C-terminal 14 residues of eIF1A (Fig. 2B). The C-terminal 14-residue eIF1A peptide, as well as an even shorter seven-residue C-terminal peptide, had similar affinity and produced essentially the same effects on the 15N-HSQC spectrum of 15N-labeled eIF5B-CTD as full-length eIF1A (data not shown), confirming that the C terminus of eIF1A was necessary and sufficient for the interaction of eIF1A with eIF5B-CTD.
We collected 15N-edited NOESY-HSQC spectra of eIF5B-CTD in the presence of the unlabeled eIF1A peptide, as well as 15N-edited NOESY-HSQC and 13C-edited NOESY-HSQC spectra of eIF1A in the presence of unlabeled eIF5B-CTD and identified several intermolecular NOE peaks. We were able to unambiguously assign one pair of NOE peaks, between the δ-methyl group of Ile-140 from eIF1A and the side chain NH of Trp-1207 from eIF5B-CTD. Fig. 2D represents the most likely orientation between the C termini of eIF1A and eIF5B in the complex. It shows the eIF1A peptide lying along a narrow groove on the surface of the helical subdomain of eIF5B-CTD. The docking involved only the last five residues of eIF1A, because residues N-terminal to Asp-139 remained unstructured after binding eIF5B.
The Interaction Between eIF1A and the eIF5B-CTD Is Unique to Eukaryotes.
The results presented here map the interaction interface between human eIF1A and eIF5B in solution to the C terminus of eIF1A and the C-terminal helical subdomain of eIF5B-CTD (Fig. 2D). Interestingly, neither of these two segments is present in bacteria: the long, unstructured C-terminal tail of eIF1A is found only in eukaryotes, and the C-terminal helical subdomain of eIF5B-CTD first appears in archaea (Fig. 3A). Therefore, this interaction is unique to eukaryotes. The bacterial homologs IF1 and IF2 have been cross-linked to each other only on the ribosome (18), but not in their free form. More importantly, the interaction was proposed to involve the region of IF2, corresponding to domain II of eIF5B (6). Thus, this interaction would be distinct from that of the human proteins we have analyzed here. Two alternative explanations for these findings can be proposed and are discussed in the next section: (i) the eukaryotic proteins have somehow lost the initial interaction and replaced it with a new one or (ii) the new interaction interface in eukaryotes is in addition to, and not instead of, the original interaction in bacteria.
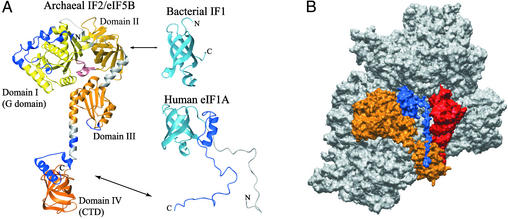
Figure 3.
Model for the interactions of IF1/eIF1A and IF2/eIF5B on the ribosome. (A) Conservation of IF1/eIF1A and IF2/eIF5B throughout evolution. The structures of bacterial IF1 (7), human eIF1A (8), and the archaeal IF2/eIF5B homolog (12) are shown. The individual domains of eIF5B are painted from yellow (domain I) to dark orange (domain IV) and the connecting segments between the domains are in gray. Segments in eIF1A and eIF5B painted in blue, as well as the N-terminal segment of eIF1A (painted in gray), are not present in bacteria. The arrows indicate the interactions between IF1 and IF2 (Upper) and eIF1A and eIF5B (Lower). (B) Surface representation of the proposed orientation of IF1/eIF1A and IF2/eIF5B on the ribosome. The structure of eIF5B (painted in orange) was docked on the x-ray structure of the 70S bacterial ribosome (28), by orienting the G domain (domain I) toward the L7/L12 stalk on the large ribosomal subunit (not shown); the CTD (domain IV) toward the acceptor end of the P-site tRNA (red); and domain II toward the A-site of the small subunit (painted in gray). The structure of human eIF1A (blue) was aligned to the structure of IF1, and this alignment was used to replace IF1 with eIF1A in the IF1 cocrystal structure with the 30S ribosomal subunit (4). The location of the positively charged N-terminal segment of eIF1A (not shown) is not known. The unstructured C-terminal tail of eIF1A was extended and directed along the A-site groove in the ribosome toward the CTD of eIF5B. The C terminus of eIF1A was able to reach several angstroms beyond its binding site on eIF5B-CTD.
IF1/eIF1A and IF2/eIF5B, respectively, share a high degree of homology and the structures of IF1 and the OB-fold portion of eIF1A are very similar. Although the structure of only the CTD of IF2 is known, the high degree of sequence conservation of IF2/eIF5B suggests that IF2/eIF5B proteins from all kingdoms have very similar structures. The insertions in archaea and eukaryotes, compared with eubacteria, do not seem to alter the overall structure and dimensions of the protein (Fig. 3A). Remarkably, no significant gaps/insertions are found between archaea and eukaryotes over the entire length of the archaeal IF2/eIF5B homolog (12).
In view of the high degree of conservation in the ribosomal subunits, as well as IF1/eIF1A and IF2/eIF5B (Fig. 3A) among all kingdoms, it is likely that the binding and orientation of eIF1A and eIF5B on the eukaryotic ribosome would be similar to that of IF1 and IF2 on the bacterial ribosome. If that is the case, domain II of eIF5B and the OB fold of eIF1A would be in proximity on the ribosome, as in bacteria. Further support for the hypothesis that the previously uncharacterized interaction interface is in addition to the one in bacteria, comes from inspection of the available eIF1A sequences from various organisms. All of them have a long negatively charged C-terminal tail and the sequences at the extreme C terminus are conserved. The C-terminal tail of free eIF1A is unstructured (8) and could extend to >70 Å in length. This length is more than sufficient to allow the OB-fold domain of eIF1A (corresponding to bacterial IF1) to bind to domain II of eIF5B, whereas the C terminus could bind to domain IV of eIF5B, >50 Å away from domain II. It is thus possible that in eukaryotes the interaction interface between eIF1A and eIF5B is in addition to that found in bacteria and the two interactions would occur simultaneously.
Model for the Interactions of eIF1A and eIF5B in the Context of the Ribosome.
It is intriguing whether the proposed two-point interaction between eIF1A and eIF5B can be rationalized in the context of the ribosome. There are no direct structural data available about the location of either IF2 or eIF5B on the ribosome but abundant biochemical experiments have been reported to define the approximate position of IF2 (for a review see ref. 6). The G domain (domain I) binds to the GTPase-activating center (the base of the L7/L12 stalk) on the large ribosomal subunit. The CTD (domain IV) binds to the acceptor end of fMet-tRNA. The binding involves the fMet, and the interacting surface of IF2-CTD has been mapped by NMR (15). A number of bases in the 23S rRNA is protected by IF2 in footprinting assays, and IF2 has been cross-linked to IF1 on the ribosome (18). Although the biochemical data do not prove a direct interaction between the two proteins, they indicate that IF1 and IF2 are at least in close proximity on the ribosome. When domains I and IV of IF2/eIF5B are oriented toward the GTPase-activating center on the large ribosomal subunit and the acceptor end of the P-site tRNA, respectively, only domain II can come close to IF1/eIF1A in the A-site. It is therefore likely that cross-linking of IF2 to IF1 (18) involves domain II of IF2, as has been proposed (6). It is not known, however, whether all reported interactions of IF2 occur simultaneously. Furthermore, the observed RNA protection could be caused by direct binding or conformational changes in the RNA. By using the known interactions of bacterial IF2 as a guide, we docked the homologous archaeal IF2/eIF5B structure to the x-ray structure of the ribosome (28). Our model (Fig. 3B) showed that it is possible to place IF2/aIF5B in proximity to all of the above interacting partners simultaneously, except for some of the bases, protected by IF2 (not shown).
IF1 binds to the A-site of the 30S subunit (4). The location of eIF1A on the ribosome has not yet been mapped but the existing data support the idea that it would bind similarly to IF1, because the two proteins are homologous and use similar surfaces for RNA binding (3, 8, 11). We, therefore, aligned the structures of eIF1A and IF1 and replaced IF1 with eIF1A in the cocrystal structure of IF1 with the 30S ribosomal subunit (4). We then extended the unstructured C-terminal tail of eIF1A (amino acids 118–143) and directed it toward the helical subdomain of eIF5B-CTD. The eIF1A C terminus is negatively charged and unlikely to interact with the rRNA, because of electrostatic repulsion. Accordingly, in our model it passes through the groove in the ribosome that forms the A-site for tRNA binding. As can be seen in Fig. 3B, the length of the C-terminal eIF1A tail would be more than sufficient to allow simultaneous binding of eIF1A to domains II and IV (CTD) of eIF5B in the context of the ribosome.
Orientation of IF2/eIF5B on the Ribosome and Binding to the Initiator tRNA.
As discussed by others (3), if IF2 and eIF5B are similarly oriented on the ribosome, the eIF5B-CTD would be at least in proximity to the acceptor end of the Met-tRNAi and could interact with it, like IF2-CTD with fMet-RNAf. In eukaryotes, another protein, eIF2 (homologous to the elongation factor EF1A), is responsible for bringing Met-tRNAi to the 40S ribosomal subunit. Later, after recognition of the initiating AUG codon and hydrolysis of eIF2-bound GTP, eIF2 releases the Met-tRNAi and leaves the preinitiation complex. To date, no binding of eIF5B to Met-tRNAi has been reported. However, in yeast, overexpression of Met-tRNAi partially relieves the slow-growth phenotype of an eIF5B deletion strain, suggesting that eIF5B may (either directly or indirectly) stabilize Met-tRNAi on the ribosome (17). Free fMet has been shown by NMR to bind weakly but specifically to the fMet-tRNAf binding surface of IF2-CTD (15); and we have found by NMR titration weak binding of methionine-ethyl ester (mimicking the glycosidic bond between methionine and the 3′ end of the initiator tRNA) to the corresponding surface of eIF5B-CTD (data not shown). We, therefore, hypothesize that, although eIF5B does not bring Met-tRNAi to the ribosome, it may be involved in stabilizing and/or orienting Met-tRNAi on the 40S subunit, after it has been released from eIF2. It is likely that the binding affinity of eIF5B for Met-tRNAi would be lower than that of eIF2, so that eIF5B does not compete with it for the free Met-tRNAi pool in the cell. The binding of eIF5B and eIF1A to 48S initiation complexes is weak, making it difficult to purify and study such complexes. Binding of eIF5B-GMPPNP to preassembled 80S/Met-tRNAi complexes causes increased sensitivity to RNase T1 cleavage at G18 in the D-stem and at the adjacent G45 in Met-tRNAi, but we were unable to detect significant UV cross-linking of eIF5B to radiolabeled tRNA in 80S/Met-tRNAi and this effect is likely to be indirect (data not shown). The interaction of eIF5B with the 80S ribosome may be different during the initiation process, where the 60S subunit is recruited to a 48S complex already containing eIF5B as well as eIF1A. It is, therefore, hard to speculate whether the above results argue against a direct interaction between eIF5B and Met-tRNA in the context of the 80S ribosome in vivo.
It is interesting to note that even if the known interactions of IF2 with the large ribosomal subunit were not used as restraints for docking, the orientation of IF2/eIF5B on the small subunit could not be drastically changed. One could, therefore, suggest that even though the individual interactions involving IF2/eIF5B on the ribosome may or may not occur simultaneously, the general orientation of IF2/eIF5B is likely to be reached after binding at the initiating AUG codon (and in the case of eIF5B, after GTP hydrolysis by eIF2) and may be important for recruitment of the large subunit.
Recruitment and Release of eIF1A and eIF5B from the Initiation Complex.
The relatively weak binding between eIF1A and eIF5B indicates that their complex in solution would not be very stable. It is, however, likely that the interaction between the C termini of eIF1A and eIF5B helps their coordinated recruitment to the 40S ribosomal subunit. The assembly of complexes through multiple weak interactions has certain advantages over a small number of strong interactions. (i) It is easier to achieve high specificity, because the binding of individual components to nonspecific sites would likely be negligible, whereas if the specific interaction is strong, the affinity of the protein for a suboptimal ligand could be significant. (ii) The complex assembly will depend strongly on the presence and concentrations of the individual components, which in turn would allow more efficient coordination and regulation. The need for high specificity, fidelity, and regulation on multiple levels increases dramatically with the complexity of the system. In translation initiation, the trend seems to be adding new steps and components to the existing ones. In this context, a remarkable feature of the binding interface between eIF1A and eIF5B in eukaryotes is that it adds an interaction between old partners, conserved among all kingdoms, and increases the complexity of the system without adding new components.
The fact that virtually all eIF1A homologs have similar long C-terminal tails and that the conserved eIF5B-CTD binding site is at the very C terminus supports the hypothesis that eIF1A does indeed have two eIF5B-binding sites and interacts with eIF5B at the ribosome through both sites simultaneously. It is, however, unlikely that this two-point interaction could occur in solution. (i) Bacterial IF1 and IF2 have been found only to interact on the ribosome, and their interaction may be either too weak to occur in solution or may depend on binding to the ribosome. (ii) Anchoring both ends of the eIF1A C-terminal tail on eIF5B in solution would cause a significant entropy loss by severely restricting the movement of this unstructured segment. The entropy cost would be much smaller on the ribosome, where there is limited free space, and the negatively charged C terminus of eIF1A would experience electrostatic repulsion from the RNA.
Both IF2 and eIF5B, in their GTP-bound form, promote recruitment of the large ribosomal subunit. Their release, after subunit assembly, requires GTP hydrolysis, stimulated by the large ribosomal subunit. It is not known when eIF1A is released from the initiation complex but it is interesting to note that IF1, and likely also eIF1A, are located in the A-site of the ribosome in a cavity that remains unoccupied after assembly of the ribosomal subunits. The model presented here predicts that IF1/eIF1A is sandwiched between the small subunit and IF2/eIF5B. It is, therefore, tempting to speculate that throughout evolution, IF1/eIF1A is released coordinately with its binding partner IF2/eIF5B after ribosomal subunit assembly. The interaction between the C termini of eIF1A and eIF5B also favors the idea that eIF1A dissociates from the ribosome together with eIF5B.
In summary, we have determined the binding interface between human eIF1A and eIF5B in solution and proposed a model for the possible interactions of eIF1A and eIF5B in the context of the ribosome. The validity of our structural model, as well as the predictions stemming from it, await further experimental testing.
Acknowledgments
We thank Vladimir Guelev for providing the precursors for 13C-methyl labeling and for helpful discussions. A.M. was funded by a postdoctoral fellowship from the American Cancer Society. G.W. acknowledges support by National Institutes of Health Grant CA68282 and National Science Foundation Grant MCB9816072. T.V.P. acknowledges support from National Institutes of Health Grant GM63940.
Abbreviations
- IF
initiation factor
- eIF
eukaryotic IF
- fMet-tRNAf
formylmethionyl-initiator tRNA
- Met-tRNAi
methionyl-initiator tRNA
- CTD
C-terminal domain
- OB
oligonucleotide/oligosaccharide binding
- HSQC
heteronuclear single quantum correlation
Footnotes
There are multiple nomenclatures for the domain organization of IF2/eIF5B. Throughout this article, we will use the nomenclature based on the four-domain archaeal eIF5B structure, starting with the G domain (domain I), and the C-terminal domain (CTD) being domain IV. This nomenclature does not take into account the less conserved N-terminal region, which is variable in length, present in most bacteria and eukaryotes, and for which no structural data is available.
References
- 1.Hershey J W B, Merrick W C. In: Translational Control of Gene Expression. Sonenberg N, Hershey J W B, Mathews M B, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 33–88. [Google Scholar]
- 2.Kyrpides N C, Woese C R. Proc Natl Acad Sci USA. 1998;95:224–228. doi: 10.1073/pnas.95.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roll-Mecak A, Shin B S, Dever T E, Burley S K. Trends Biochem Sci. 2001;26:705–709. doi: 10.1016/s0968-0004(01)02024-2. [DOI] [PubMed] [Google Scholar]
- 4.Carter A P, Clemons W M, Jr, Brodersen D E, Morgan-Warren R J, Hartsch T, Wimberly B T, Ramakrishnan V. Science. 2001;291:498–501. doi: 10.1126/science.1057766. [DOI] [PubMed] [Google Scholar]
- 5.Moazed D, Samaha R R, Gualerzi C, Noller H F. J Mol Biol. 1995;248:207–210. doi: 10.1016/s0022-2836(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 6.Moreno J M, Drskjotersen L, Kristensen J E, Mortensen K K, Sperling-Petersen H U. FEBS Lett. 1999;455:130–134. doi: 10.1016/s0014-5793(99)00858-3. [DOI] [PubMed] [Google Scholar]
- 7.Sette M, van Tilborg P, Spurio R, Kaptein R, Paci M, Gualerzi C O, Boelens R. EMBO J. 1997;16:1436–1443. doi: 10.1093/emboj/16.6.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battiste J L, Pestova T V, Hellen C U, Wagner G. Mol Cell. 2000;5:109–119. doi: 10.1016/s1097-2765(00)80407-4. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri J, Si K, Maitra U. J Biol Chem. 1997;272:7883–7891. doi: 10.1074/jbc.272.12.7883. [DOI] [PubMed] [Google Scholar]
- 10.Pestova T V, Borukhov S I, Hellen C U. Nature. 1998;394:854–859. doi: 10.1038/29703. [DOI] [PubMed] [Google Scholar]
- 11.Pestova T V, Kolupaeva V G, Lomakin I B, Pilipenko E V, Shatsky I N, Agol V I, Hellen C U. Proc Natl Acad Sci USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roll-Mecak A, Cao C, Dever T E, Burley S K. Cell. 2000;103:781–792. doi: 10.1016/s0092-8674(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 13.Pestova T V, Lomakin I B, Lee J H, Choi S K, Dever T E, Hellen C U. Nature. 2000;403:332–335. doi: 10.1038/35002118. [DOI] [PubMed] [Google Scholar]
- 14.Meunier S, Spurio R, Czisch M, Wechselberger R, Guenneugues M, Gualerzi C O, Boelens R. EMBO J. 2000;19:1918–1926. doi: 10.1093/emboj/19.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenneugues M, Caserta E, Brandi L, Spurio R, Meunier S, Pon C L, Boelens R, Gualerzi C O. EMBO J. 2000;19:5233–5240. doi: 10.1093/emboj/19.19.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrick W C. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S K, Lee J H, Zoll W L, Merrick W C, Dever T E. Science. 1998;280:1757–1760. doi: 10.1126/science.280.5370.1757. [DOI] [PubMed] [Google Scholar]
- 18.Boileau G, Butler P, Hershey J W, Traut R R. Biochemistry. 1983;22:3162–3170. doi: 10.1021/bi00282a020. [DOI] [PubMed] [Google Scholar]
- 19.Choi S K, Olsen D S, Roll-Mecak A, Martung A, Remo K L, Burley S K, Hinnebusch A G, Dever T E. Mol Cell Biol. 2000;20:7183– 7191. doi: 10.1128/mcb.20.19.7183-7191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Lugovskoy A A, Wagner G. J Biomol NMR. 2001;20:11–14. doi: 10.1023/a:1011258906244. [DOI] [PubMed] [Google Scholar]
- 21.Guex N, Peitsch M C. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 22.Koradi R, Billeter M, Wuthrich K. J Mol Graphics. 1996;14:29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 23.Holm L, Sander C. Nucleic Acids Res. 1999;27:244–247. doi: 10.1093/nar/27.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber D J, Gittis A G, Mullen G P, Abeygunawardana C, Lattman E E, Mildvan A S. Proteins. 1992;13:275–287. doi: 10.1002/prot.340130402. [DOI] [PubMed] [Google Scholar]
- 25.Medek A, Olejniczak E T, Meadows R P, Fesik S W. J Biomol NMR. 2000;18:229–238. doi: 10.1023/a:1026544801001. [DOI] [PubMed] [Google Scholar]
- 26.Delaglio F, Grzesiek S, Vuister G W, Zhu G, Pfeifer J, Bax A. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 27.Bartels C, Xia T H, Billeter M, Guntert P, Wuthrich K. J Biomol NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 28.Yusupov M M, Yusupova G Z, Baucom A, Lieberman K, Earnest T N, Cate J H, Noller H F. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]