Abstract
The early steps of the intracellular trafficking of human papillomavirus type 16 (HPV-16), -31, and -58 pseudovirions were studied by investigating the effects of drugs acting at defined points of endocytosis pathways on virus-like particle-mediated pseudoinfection by overexpression of a dominant-negative form of the Eps15 protein to inhibit clathrin-mediated endocytosis and by electron microscopy. The results obtained suggested the involvement of clathrin-mediated endocytosis in HPV-16 and HPV-58 entry and caveola-mediated endocytosis in HPV-31 entry.
As a consequence of interactions with their receptors, viruses can follow a variety of pathways for entry into cells. Among nonenveloped DNA viruses, adenovirus (16), adeno-associated virus (2), and human polyomavirus JC (23) enter cells by means of clathrin-coated endocytic vesicles whereas simian virus 40 (SV40) and mouse polyomavirus are taken up into cells via caveolae (1, 21, 22, 24).
Papillomaviruses are small nonenveloped tumorigenic DNA viruses that infect the basal cells of the epithelium. Papillomavirions contain two proteins, L1 and L2, which encapsidate a closed, circular, double-stranded DNA of about 8 kbp. The viral capsid of 50 to 55 nm contains 72 pentamers of L1, and expression of the major capsid protein L1 results in the self-assembly of virus-like particles (VLPs) which have similar characteristics to the virions. Attempts to efficiently grow human papillomaviruses (HPVs) in cell culture have to date been unsuccessful, and consequently, surrogate systems using HPV pseudovirions have been developed to test for viral infectivity (4, 13, 25, 26, 29, 30).
It has previously been reported that in order to infect target cells, papillomaviruses bind to specific cell surface receptors (19, 31), penetrate the plasma membrane by an unknown mechanism, and target their genomes to the cell nucleus (18, 20, 33). There is a growing body of evidence that heparan sulfate proteoglycans act as primary receptors for papillomaviruses and mediate viral attachment (5, 10, 12). Heparan sulfate proteoglycans interact with the carboxyl terminus of the HPV L1 protein (12). Although heparan sulfate proteoglycans are widely distributed on the surfaces of many cell types, they may not be sufficient to allow efficient HPV entry. Evander et al. (9) and McMillan et al. (17) have already shown that alpha-6 integrin is used by HPV type 6 (HPV-6) as a receptor for HPV entry into host cells.
Electron microscopy examination has shown that bovine papillomavirus type 1 (BPV-1) virions are seen in the lipid layer-surrounded vesicles (33), suggesting that caveolae are involved in BPV-1 internalization and that they are associated with microtubules in the cytoplasm (15). In addition, uptake of HPV-33 by small and smooth endocytic vesicles (27, 31) suggests that this virus is internalized by caveolae. However, it was reported recently that this virus entry is not inhibited by nystatin, suggesting that it does not require a caveola-dependent pathway (27). The papillomaviruses then penetrate the nuclear membrane to replicate in the nucleus. It has been shown that disassembly of HPV-11, -16, and -45 capsids into capsomers is required for nuclear import (18, 20). However, the mechanisms by which HPVs are taken up into cells and travel to the nucleus are still poorly understood.
In order to elucidate the entry of HPV pseudovirions into cells, we investigated the effects of drugs acting at defined points in the endocytosis of virions on VLP-mediated pseudoinfection by using transgene expression as an end point for efficient transduction. Using the same reporter plasmid, this method provided a direct evaluation and comparison of the transgene expression of HPV-16, -31, and -58 pseudovirions under the same conditions. In addition, the early events of VLP internalization were studied by electron microscopy to show the localization of VLPs within the cell. We also investigated whether any differences between HPV types could be revealed.
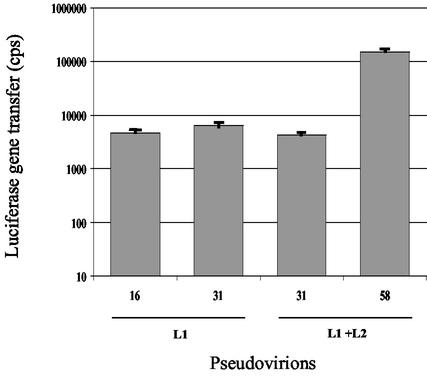
HPV-16 and -31 VLPs composed of L1 protein were produced in Sf21 insect cells by use of recombinant baculoviruses encoding the HPV-16 and -31 L1 open reading frames, respectively, and purified according to previously described procedures (4, 5, 7). VLPs composed of L1 and L2 proteins were also produced for HPV-31 and -58 (6). For the generation of pseudovirions, 5 μg of VLPs and 0.5 μg of a linearized pCMV-Luc plasmid (7.1 kbp) coding for luciferase under the cytomegalovirus promoter (Ozyme) were mixed and incubated for 30 min at room temperature before pseudoinfection of COS-7 cells seeded in 96-well plates according to a recently described procedure called direct interaction (4-6). Luciferase expression was measured by a luminescence assay (Luciferase reporter gene assay with constant light signal; Roche Molecular Biochemical, Meylan, France), and the results were expressed as counts per second (cps) per well. Pseudovirions appeared as empty particles on electron micrographs and we previously showed that the VLPs bound to the end of the linearized DNA molecule (4), suggesting that the DNA plasmid could be partially wrapped around the particles. Pseudoinfection with HPV-16 L1, HPV-31 L1, HPV-31 L1+L2, and HPV-58 L1+L2 VLPs induced luciferase expression levels (means ± standard deviations of three assays performed in duplicate) of 4,663 ± 256, 6,357 ± 1,771, 4,255 ± 295, and 148,614 ± 24,835 cps, respectively (Fig. 1).
FIG. 1.
Gene transfer of HPV-16 and -31 L1 pseudovirions and HPV-31 and -58 L1-plus-L2 pseudovirions in COS-7 cells (results obtained using 5 μg of VLPs per well).
To investigate whether HPV-16, -31, and -58 enter the host cell via clathrin-coated or caveola vesicles, COS-7 cells were preincubated with chlorpromazine (12.5 μg/ml), a clathrin-dependent pathway inhibitor, for 45 min or with nystatin (12.5 μg/ml), which disrupts the cholesterol-enriched caveola-containing membrane microdomain, for 1 h and then were incubated with VLP-DNA complexes. Inhibition of gene transfer efficiency was studied 48 h later. Clathrin-dependent entry can be inhibited with chlorpromazine, which has been shown to cause the disappearance of clathrin-coated pits from the plasma membrane, thus inhibiting initiation of the clathrin-dependent endocytic pathway. Cell survival was assessed by direct examination of the cells and by the methylthiazoletetrazolium method according to the procedure described by Kichler et al. (14). No cell toxicity was observed at the concentrations used in these assays.
Inhibitory effects of chlorpromazine were not observed on gene transfer, as measured by the luciferase expression level, for HPV-31 pseudovirions (Table 1), but 84 and 70% inhibition was observed for HPV-16 and HPV-58 pseudovirions, respectively. In contrast, HPV-31 pseudovirions were inhibited by nystatin, with dramatically decreased gene transfer (96%). In comparison, no effect of nystatin was observed on gene transfer mediated by HPV-16 and HPV-58 pseudovirions (Table 1). In order to investigate if the minor capsid protein L2 influenced the cell entry pathway, we compared inhibition of gene transfer by nystatin and chlorpromazine on HPV-31 pseudovirions composed of L1 or L1 plus L2 proteins. The results indicated that the presence of L2 did not influence the cell entry pathway of the pseudovirions. The entry of HPV VLPs into the host cell via caveolae or clathrin-coated vesicles was also investigated in 293T cells. Very low levels of gene transfer were observed with HPV-16 pseudovirions (104 ± 60 cps), but luciferase activities of 4,558 ± 394 and 2,039 ± 633 cps were observed for HPV-31 and HPV-58 pseudovirions, respectively. Inhibition of gene transfer for these two pseudovirions was investigated with inhibitors of clathrin-coated vesicles (chlorpromazine) and caveola vesicles (nystatin). HPV-58 gene transfer entry was inhibited by chlorpromazine (85 ± 13%) but not by nystatin (0%), and HPV-31 gene transfer was inhibited by nystatin (91 ± 9%) but not by chlorpromazine (5 ± 2%), confirming the results observed with COS-7 cells. In order to show that chlorpromazine and nystatin affect virus entry and not later events, the two drugs were added 20 and 60 min after pseudoinfection with HPV-16 and HPV-31 L1 pseudovirions. The results indicated that no inhibition of gene transfer in COS-7 cells occurred when the drugs were added 1 h after transfection and a limited effect was observed when the drugs were added 20 min after transfection (20 and 16% with HPV-16 and HPV-31, respectively). In addition, fluorescence microscopy showed that chlorpromazine (but not nystatin) inhibited the endocytosis of Texas Red-conjugated transferrin, a well-known marker of clathrin-mediated endocytosis.
TABLE 1.
Inhibition of gene transfer by HPV-16, HPV-31, and HPV-58 pseudovirions in COS-7 cells by use of inhibitors of cell trafficking
| Inhibitor | % Inhibition of luciferase gene transfer (mean ± SD)
|
|||
|---|---|---|---|---|
| HPV-16 (L1) | HPV-31 (L1) | HPV-31 (L1 + L2) | HPV-58 (L1 + L2) | |
| Chlorpromazine | 84 ± 0.4 | 2.8 ± 0.1 | 0 ± 0 | 70 ± 10 |
| Nystatin | 0 ± 0.5 | 96 ± 1 | 71 ± 6 | 0 ± 0 |
A dominant-negative mutant of Eps15 (epidermal growth factor receptor pathway substrate clone 15), a constituent of plasma membrane clathrin-coated pits, was also used to block clathrin-mediated uptake. Lipofectamine transfection of COS-7 cells with the GFP-EΔ95/295 plasmid coding for this dominant-negative mutant (3), used according to the protocol of Benmerah et al. (3), decreased the gene transfer of HPV-16 pseudovirions by 82 ± 4%, whereas inhibition of only 15 ± 3% was observed with HPV-31.
Cell entry of HPV-16 and -31 VLPs was also investigated by electron microscopy. For this purpose, COS-7 cells were grown on 75-cm2 plastic culture flasks to 80% confluency. After being scraped off and rinsed in Dulbecco's modified Eagle's medium (DMEM) at 4°C, the cells were treated with HPV pseudovirions in DMEM for 1 h at 4°C. Cells were then washed with DMEM at 37°C and incubated for 10 min, 20 min, and 1 h at 37°C. At 0, 10, 20, and 60 min, cells were washed in phosphate-buffered saline, fixed, and embedded in Epon resin according to a previously reported procedure (7). Ultrathin sections were collected on copper grids, stained with 1% uranyl acetate and 1% lead citrate in distilled water, and then observed at ×10,000 to ×50,000 nominal magnification using a JEOL 1010 electron microscope.
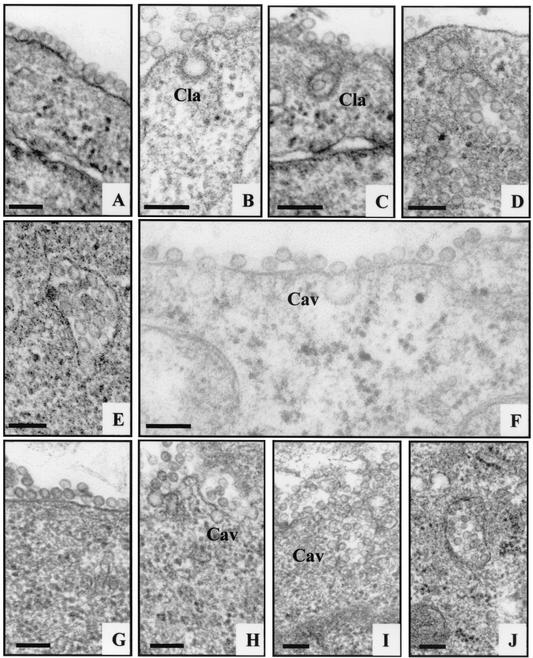
The results obtained (Fig. 2) confirmed that HPV-16 pseudovirions enter the cell by endocytosis through clathrin-coated pits. Electron micrographs indicated that the surfaces of COS-7 cells are rich in clathrin-coated pits. As observed for BPV-1 (33), attachment of HPV-16 and HPV-31 VLPs at 4°C demonstrated that viruses were able to attach to but not penetrate the cell under low-temperature conditions. Shifting to 37°C resulted in the appearance of HPV-16 pseudovirions in a vesicular structure with characteristics of clathrin-coated vesicles (Fig. 2B and C). The plasma membrane appeared to surround the VLPs, thus forming an endocytic vesicle. These vesicles enlarged with time, and after 1 h at 37°C the virions remained clustered in large vesicles resembling endosomes of 250 to 500 nm in diameter (Fig. 2D and E). At that time, most vesicles were smooth rather than coated. Electron micrographs of thin sections of cells after 10 min of incubation at 37°C in the presence of 0.5 μg of HPV-31 VLPs showed the presence of noncoated flask-shaped invaginations of the plasma membrane which had the characteristics of caveola structures (1, 22) (Fig. 2F). Using 5 μg of VLPs, the viral capsid became associated with caveola vesicles as early as 10 min after binding (Fig. 2H). With time, these vesicles enlarged, with a bunch-of-grapes shape which is characteristic of caveola vesicles (Fig. 2I). After 1 h, large vesicles (250 to 500 nm) containing large quantities of VLPs could be seen, similar in shape and characteristics to those observed with HPV-16-containing vesicles (Fig. 2J).
FIG. 2.
HPV-16 and HPV-31 cell internalization. HPV-16 VLPs (A to E) and HPV-31 VLPs (F to J) are shown. The electron micrographs show cells after incubation with VLPs at 4°C (A and G) and at 37°C for 10 min (B, F, and H), 20 min (C and I), and 1 h (D, E, and J). Bars, 150 nm. Cav, caveolae; Cla, clathrin-coated vesicles.
These electron microscopy findings support the results obtained by inhibition of gene transfer with cell traffic inhibitors and suggest the involvement of clathrin-mediated endocytosis in HPV-16 entry and caveola-mediated endocytosis for HPV-31.
Nonenveloped DNA viruses are typically internalized by clathrin-coated vesicles. The results obtained in the present study suggest that this is the case for HPV-16 and HPV-58. The caveola-mediated endocytosis pathway is reported to be part of the entry process for an increasing number of naked and enveloped viruses such as SV40, mouse polyomavirus, respiratory syncytial virus, and filoviruses (1, 8, 24, 32). We showed here that HPV-31 cell entry appears to involve caveolae since HPV-31 pseudovirions are sensitive to a cholesterol inhibitor (nystatin) and are present in smooth endocytic vesicles, as observed by electron microscopy. While supporting the hypothesis that HPV-31 enters by caveolae, the drug inhibition study cannot rule out the possibility that the drug inhibits entry independently of the caveola-dependent pathway. Inhibition of the formation of clathrin-coated vesicles by expression of a dominant-negative mutant of Eps15, a principal component of clathrin-coated vesicles, confirmed that, in contrast to HPV-16 and HPV-58, HPV-31 did not enter cells via clathrin-coated vesicles.
A comparison of the structure of HPV virions and VLPs obtained by cryoelectron microscopy and reconstructions of images indicates that they are structurally very similar (11). However, differences in cell trafficking between HPV virions and artificial pseudovirions could be hypothesized due to the absence of the minor L2 protein in the pseudovirions used for the study. The lack of L2 protein could be responsible for differences in molecular interactions with the cell components and in particle surface conformation. The presence or absence of L2 protein did not affect the entry of HPV-31. This is in agreement with results for HPV-11 obtained by Zhou et al. (33), who demonstrated that the L2 minor protein had no crucial role in the uptake and early steps of cell entry. The results obtained were also similar to those reported for mouse polyomavirus by Richterova et al. (24), who did not observe any differences in cell trafficking between VP1 VLPs and infectious virions. In addition, we cannot rule out the possibility of differences in the molecular interactions driving cell entry between pseudovirions in which the DNA plasmid is situated outside the VLPs and HPV virions in which the DNA genome is packaged inside the capsids. However, all three HPV pseudovirions investigated were generated by the same procedure (4), by use of the same DNA plasmid, suggesting that the differences in cell entry we observed are more probably due to differences in L1 proteins than to the presence of plasmid DNA on the surfaces of the pseudovirions. It is surprising that very closely related viruses such as HPV-16, -31, and -58 use different endocytosis pathways. However, SV40 and JC virus, two closely related members of the polyomavirus family, use caveola- and clathrin-mediated uptake pathways, respectively (1, 23, 28). It is possible that the cell surface receptor interacting with the virus largely determines its endocytosis route. It could thus be hypothesized that HPV-31 uses a different coreceptor from that used by HPV-16 and -58.
Acknowledgments
We thank A. Dautry-Varsat for her kind gift of the GFP-EΔ95/295 plasmid and helpful discussion, B. Arbeille for assistance in interpreting the electron microscopy findings, and C. Mougin for helpful discussions.
This study was supported by grants from the Association pour la Recherche sur le Cancer (no. 5836) and from the Association “Vaincre la Mucoviscidose” (no. TG105). L.B. was supported by a fellowship from the Région Centre.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps 15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Bousarghin, L., A. Touzé, A. L. Combita, S. El Mehdaoui, P.-Y. Sizaret, M.-M. Bravo, and P. Coursaget. 2002. Detection of neutralizing antibodies against human papillomaviruses (HPV) by inhibition of gene transfer mediated by HPV pseudovirions. J. Clin. Microbiol. 40:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combita, A. L., A. Touzé, L. Bousarghin, P.-Y. Sizaret, N. Munoz, and P. Coursaget. 2001. Gene transfer using human papillomavirus pseudovirions varies according to virus genotype and requires cell surface heparan sulfate. FEMS Microbiol. Lett. 204:183-188. [DOI] [PubMed] [Google Scholar]
- 6.Combita, A. L., A. Touzé, L. Bousarghin, N. D. Christensen, and P. Coursaget. 2002. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J. Virol. 76:6480-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Mehdaoui, S., A. Touzé, S. Laurent, P. Y. Sizaret, D. Rasschaert, and P. Coursaget. 2000. Gene transfer using recombinant rabbit hemorrhagic disease virus capsids with genetically modified DNA encapsidation capacity by addition of packaging sequences from L1 or L2 protein of human papillomavirus type 16. J. Virol. 74:10332-10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evander, M., I. H. Frazer, E. Payne, Y. M. Qi, K. Hengst, and N. A. McMillan. 1997. Identification of the α6 integrin as a candidate receptor for papillomaviruses. J. Virol. 71:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giroglou, T., L. Florin, F. Schafer, R. E. Streeck, and M. Sapp. 2001. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 75:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagensee, M. E., N. H. Olson, T. S. Baker, and D. A. Galloway. 1994. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J. Virol. 68:4503-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce, J. G., J. S. Tung, C. T. Przysieck, J. C. Cook, E. D. Lehman, J. A. Sands, K. U. Jansen, and P. M. Keller. 1999. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 274:5810-5822. [DOI] [PubMed] [Google Scholar]
- 13.Kawana, K., H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1998. In vitro construction of pseudovirions of human papillomavirus type 16: incorporation of plasmid DNA into reassembled L1/L2 capsids. J. Virol. 72:10298-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kichler, A., J. C. Pages, C. Leborgne, S. Druillennec, C. Lenoir, D. Coulaud, E. Delain, E. Le Cam, B. P. Roques, and O. Danos. 2000. Efficient DNA transfection mediated by the C-terminal domain of human immunodeficiency virus type 1 viral protein R. J. Virol. 74:5424-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, W.-J., Y.-M. Qi, K. N. Zhao, Y. H. Liu, X. S. Liu, and I. H. Frazer. 2000. Association of bovine papillomavirus type 1 with microtubules. Virology 282:237-244. [DOI] [PubMed] [Google Scholar]
- 16.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan, N. A., E. Payne, I. H. Frazer, and M. Evander. 1999. Expression of alpha 6 integrin confers papillomavirus binding upon receptor-negative B-cell. Virology 261:271-274. [DOI] [PubMed] [Google Scholar]
- 18.Merle, E., R. C. Rose, L. LeRoux, and J. Moroianu. 1999. Nuclear import of HPV11 L1 capsid protein is mediated by karyopherin alpha2beta1 heterodimers. J. Cell. Biochem. 74:628-637. [PubMed] [Google Scholar]
- 19.Müller, M., L. Gissmann, R. J. Cristiano, X. Y. Sun, I. H. Frazer, A. B. Jenson, A. Alonso, H. Zentgraf, and J. Zhou. 1995. Papillomavirus binding and uptake by cells from different tissues and species. J. Virol. 69:948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson, L. M., R. C. Rose, and J. Moroianu. 2002. Nuclear import strategies of high risk HPV16 L1 major capsid protein. J. Biol. Chem. 277:23958-23964. [DOI] [PubMed] [Google Scholar]
- 21.Norkin, L. C. 1999. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol. Rev. 168:13-22. [DOI] [PubMed] [Google Scholar]
- 22.Parton, R. G., and M. Lindsay. 1999. Exploitation of major histocompatibility complex class I molecules and caveolae by simian virus 40. Immunol. Rev. 168:23-31. [DOI] [PubMed] [Google Scholar]
- 23.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richterova, Z., D. Liebl, M. Horak, Z. Palkova, J. Stokrova, P. Hozak, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi, J. L., L. Gissmann, K. Jansen, and M. Müller. 2000. Assembly of human papillomavirus type 16 in Saccharomyces cerevisiae. Hum. Gene Ther. 20:1165-1176. [DOI] [PubMed] [Google Scholar]
- 27.Selinka, H. C., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 28.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touzé, A., and P. Coursaget. 1998. In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 26:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpers, C., F. Unckell, P. Schirmacher, R. E. Streeck, and M. Sapp. 1995. Binding and internalization of human papillomavirus-like particles by eucaryotic cells. J. Virol. 69:3258-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werling, D., J. C. Hope, P. Chaplin, R. A. Collins, G. Taylor, and C. J. Howard. 1999. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J. Leukoc. Biol. 66:50-58. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, J., L. Gissmann, H. Zentgraf, H. Müller, M. Picken, and M. Müller. 1995. Early phase in the infection of cultured cells with papillomavirus virions. Virology 214:167-176. [DOI] [PubMed] [Google Scholar]