Abstract
The ST6Gal-I sialyltransferase produces Siglec ligands for the B-cell-specific CD22 lectin and sustains humoral immune responses. Using multiple experimental approaches to elucidate the mechanisms involved, we report that ST6Gal-I deficiency induces immunoglobulin M (IgM) antigen receptor endocytosis in the absence of immune stimulation. This coincides with increased antigen receptor colocalization with CD22 in both clathrin-deficient and clathrin-enriched membrane microdomains concurrent with diminished tyrosine phosphorylation of Igα/β, Syk, and phospholipase C-γ2 upon immune activation. Codeficiency with CD22 restores IgM antigen receptor half-life at the cell surface in addition to reversing alterations in membrane trafficking and immune signaling. Diminished immune responses due to ST6Gal-I deficiency further correlate with constitutive recruitment of Shp-1 to CD22 in unstimulated B cells independent of Lyn tyrosine kinase activity and prevent autoimmune disease pathogenesis in the Lyn-deficient model of systemic lupus erythematosus, resulting in a significant extension of life span. Protein glycosylation by ST6Gal-I restricts access of antigen receptors and Shp-1 to CD22 and operates by a CD22-dependent mechanism that decreases the basal rate of IgM antigen receptor endocytosis in altering the threshold of B-cell immune activation.
Cells of the mammalian immune system undergo selective changes in protein glycosylation during differentiation, immune activation, and autoimmune disease (2, 22, 23). A subset of Golgi glycosyltransferases involved in these posttranslational glycan modifications are termed sialyltransferases and produce sialic acid linkages on glycoproteins. Sialyltransferases vary in their substrate specificities and expression profiles and may thereby participate uniquely or collaboratively in different physiologic processes (20, 42, 45). For example, sialic acids contribute to the glycan ligands of the selectins that modulate leukocyte trafficking (31, 37). A subset of sialyltransferases are involved in selectin ligand formation, and each may uniquely modulate leukocyte and endothelial physiology (12). A distinct sialic acid linkage formed by the ST3Gal-I sialyltransferase does not participate in construction of selectin ligands but instead inhibits caspase activation mechanisms associated with CD8+ T-cell apoptosis (34). In contrast, the ST6Gal-I sialyltransferase promotes B-cell immune activation responses and serum immunoglobulin (Ig) expression. Loss of ST6Gal-I function does not grossly alter B-cell development or trafficking but attenuates IgM antigen receptor (B-cell receptor [BCR]) activation, depresses Ca2+ mobilization, decreases mature B-cell proliferation, and results in low antibody titer induction upon immunization (16). How protein glycosylation by ST6Gal-I maintains B-cell immune responses is not resolved but may involve the functions of endogenous lectins that bind to glycan ligands produced by ST6Gal-I.
Mammalian immune receptors of the Siglec family are transmembrane lectins that exhibit extracellular binding specificities for sialic acid linkages and often encode cytoplasmic immunoreceptor tyrosine inhibitory motif (ITIM) domains (8). These hallmarks of Siglec structure suggest that binding to cell surface glycan ligands may in some contexts modify intracellular signal transduction pathways involving protein tyrosine phosphorylation. The B-cell-specific CD22 (Siglec-2) glycoprotein contains cytoplasmic domain ITIMs that are tyrosine phosphorylated upon activation and which recruit the Shp-1 tyrosine phosphatase to dampen immune signaling (7, 9, 11, 30, 43). The extracellular domain of CD22 bears Siglec ligand binding activity toward α2-6-linked sialic acid to underlying β1-4-linked galactose (33, 39). CD22 Siglec ligands are produced by ST6Gal-I in the Golgi apparatus and are expressed on the B-cell surface, where they typically occupy (or “mask”) cell surface CD22 Siglec binding activity (5, 16, 36). The masking of CD22 Siglec binding activity is reduced among postactivated splenic and bone marrow B cells, and thus ST6Gal-I function may be regulated in some contexts (5, 10, 13, 36). These findings infer a role for ST6Gal-I in modulating immune function by constructing Siglec ligands for CD22. Indeed, previous studies have shown that mutant CD22 molecules bearing mutations in the Siglec binding domain impart altered B-cell activation responses in vitro and in vivo (18, 19, 32). More recently, increased colocalization of cell surface IgM with CD22 and clathrin domains was observed among mice lacking ST6Gal-I, while restoration of normal IgM colocalization frequency with clathrin occurred coincident with elevated B-cell immune activation responses in the absence of both CD22 and ST6Gal-I (6).
We have further investigated the mechanisms and glycoprotein specificity by which ST6Gal-I modulates B-cell immune function among mice lacking combinations of ST6Gal-I, CD22, and the Lyn protein tyrosine kinase. Our findings are consistent with those recently published (6) and further show that ST6Gal-I function normally restricts Shp-1 recruitment to CD22 independent of Lyn activity. We also find that ST6Gal-I deficiency selectively increases the rate of IgM BCR and CD22 endocytosis, reducing the cell surface BCR half-life on unstimulated B cells by a CD22-dependent mechanism, and further attenuates autoimmune disease pathogenesis, yielding a significant increase in the life span of Lyn-deficient mice.
MATERIALS AND METHODS
Mice, cells, and antibodies.
ST6Gal-I-deficient mice (16) and sex-matched wild-type littermates were used to harvest splenic and lymph node B lymphocytes. The ST6Gal-I mutation was bred for more than 10 generations into the C57BL/6 background prior to experimentation. Mice bearing null mutations in CD22 and the Lyn-encoded tyrosine kinase were previously described (3, 25). MD4 transgenic mice (14) were kindly provided by R. Rickert (Burnham Institute, San Diego, CA) with permission from C. C. Goodnow (Australian National University, Canberra, Australia). These mice were bred to generate littermates with and without ST6Gal-I function. CD22 or Lyn mutant mice were bred with ST6Gal-I-null mates to produce compound heterozygous offspring used for generating littermates with the indicated genotypes in experimentation. B lymphocytes were enriched to >90% from spleen and lymph nodes using a negative sorting strategy with biotin-conjugated anti-Thy-1.2, CD43 (clone S7), Ter-119, NK-1.1, Gr-1, and Mac-1 (PharMingen) antibodies and streptavidin-conjugated magnetic beads (Dynal, Great Neck, NY).
Protein half-lives and endocytosis.
Isolated B cells were incubated with EZ-Link Sulfo-NHS-biotin (Pierce) for 30 min at 4°C to label cell surface proteins. Following two washes in ice-cold phosphate-buffered saline (PBS) with 100 mM glycine to quench excess biotin, 1 × 106 aliquots of cells were incubated at 37°C in RPMI 1640 containing 0.1 mM 2-mercaptoethanol, 10% fetal calf serum (FCS), and 2 mM l-glutamine. Cells were harvested at indicated times and lysed at 4°C in lysis buffer containing 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholate, 1% NP-40, and protease inhibitor cocktail and then sonicated for two 30-s pulses. Cells were incubated for the indicated times in the absence or presence of methyl-β-cyclodextrin (10 mM) as described previously (41). Lysates were incubated with monoavidin-agarose (Pierce) for 4 h. at 4°C. Precipitates were washed in lysis buffer, and biotinylated proteins were eluted by boiling in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. Total B-cell membrane proteins comprising 1 × 106 cell equivalents were loaded in each lane of 5 to 15% gradient SDS-PAGE gels, and the resulting gel blots were probed with antibodies including horseradish peroxidase (HRP)-conjugated anti-IgM and goat antibodies recognizing CD22 (clone Y19), Shp-1 (clone C19), and CD45 (clone M20), followed by HRP-conjugated anti-goat IgG (Santa Cruz). Blots were visualized by enhanced chemiluminescence (Amersham Biosciences), and the signals were quantified (UVP; BioImaging Systems).
Colocalization measurements.
Isolated B cells (1 × 106 cells/ml) maintained at 4°C in RPMI 1640 with 2% FCS were treated with 2% paraformaldehyde prior to antibody labeling. Identical results were obtained when cells were instead maintained at 4°C and incubated with antibodies prior to treatment with 2% paraformaldehyde. No effect of cell isolation was observed as B cells among total splenocytes and lymph node cells treated as described above gave identical results. The antibodies used included CD22-fluorescein isothiocyanate (FITC), CD45-FITC (Pharmingen), anti-IgM-Texas red (Jackson, West Grove, PA), and CTB(GM1)-FITC (Sigma) with treatment for 30 min. Following washing, cells were rendered permeable by treatment with CytoFix/CytoPerm (BD Biosciences) for 20 min before incubation with antibody to clathrin (clone X22; Affinity BioReagents) followed by aminomethylcuoumarin acetate (AMCA)-conjugated secondary antibody (Jackson, West Grove, PA). Cells were allowed to settle on glass slides and mounted with antifade aqueous mount medium (BioMedia Corp., CA). Deconvolution images were obtained using a DeltaVision Restoration microscope system (Applied Precision Inc., Issaquah, WA) and analyzed using DeltaVision SoftWorx (version 2.50) in cell sections of 0.2 μm. Quantification of fluorescent signals and their localization were accomplished for each 0.2-μm section in the linear range of the digital camera by object-based analysis algorithms using MetaMorph software (Universal Imaging Corporation, Downington, PA). Maximum projection views of cells are shown which combine all 0.2-μm images (z-stacks). A range of fluorescent signal exclusion thresholds was also applied in these analyses to validate the default value otherwise input by the software and as used for visualization.
Protein phosphotyrosine and coprecipitation.
Isolated B lymphocytes (1 × 106) were suspended in 50 μl of RPMI 1640 with 5% FCS and 10 mM HEPES. Cells were warmed to 37°C before stimulation with 1.2 μg/ml of goat F(ab′)2 anti-mouse IgM. At the indicated times, 450 μl of lysis buffer was added (with substitution of Triton X-100 for NP-40). Antibodies to Igβ and CD22, as well as Lyn, Syk, phospholipase C-γ2 (PLC-γ2), Shp-1, or Vav (10 μl) (Santa Cruz Biotechnology) were added to the lysates with 25 μl of protein A-Sepharose. The lysates were incubated for 4 h and washed three times in lysis buffer. Immune complexes were subjected to SDS-PAGE on a 5 to 15% gradient gel after elution in sample buffer. Gels were transferred to nitrocellulose, and tyrosine phosphorylation was detected by blotting with monoclonal antibody 4G10 (Santa Cruz Biotechnology). Blots were then stripped and reprobed with antibodies to Lyn, Syk, PLC-γ2, Shp-1, Igα, Igβ, and CD22.
BrdU pulse-chase and flow cytometry.
Mice were given drinking water ad libitum containing 0.8 mg bromodeoxyuridine (BrdU)/ml for 3 weeks (pulse) and returned to water lacking BrdU (chase) for 2 weeks. Animals were sacrificed at specified times, and lymphocytes were isolated and analyzed for BrdU incorporation. Single-cell suspensions from spleen, lymph node, or bone marrow were harvested and subjected to red blood cell lysis with NH4Cl. Cell surface binding of anti-CD4-phycoerythrin, CD8-allophycocyanin (APC), and B220-Cychrome antibodies (PharMingen) was carried out in 100 μl of fluorescence-activated cell sorter (FACS) buffer (2% FCS in PBS) with 1 × 106 cells on ice for 10 min. Cells were then resuspended in 0.5 ml of ice-cold PBS, and 1.2 ml ice-cold 95% ethanol was added dropwise under gentle mixing to fix the cells. After 30 min of incubation, cells were centrifuged and washed with 2 ml of PBS and then incubated in of 1 ml of PBS, 1% paraformaldehyde, and 0.01% Tween 20 for 30 min at 22°C. Cells were again centrifuged and resuspended in 1 ml of 0.15 M NaCl, 5 mM MgCl2, 10 μM HCl, and 50 Kunitz units/ml of DNase-1 for 10 min. at 22°C. After centrifugation, cells were washed with 2 ml of PBS, incubated in 10 μl of FITC-conjugated anti-BrdU antibody (Becton Dickinson) for 30 min, and then again sedimented, washed in 2 ml PBS, and finally resuspended in 0.5 ml FACS buffer for fluorescence-activated cell sorting analyses, performed on a FACScalibur flow cytometer using CellQuest software (Becton Dickinson). Flow cytometry using lectin conjugates was accomplished as described previously (16).
Calcium mobilization and protein phosphotyrosine analyses.
Isolated B cells were measured for calcium flux following stimulation by Indo-1 loading followed by FACS analysis, as previously described (16). Radiolabeled thymidine incorporation was used to measure B-cell proliferation responses to goat F(ab′)2 anti-mouse IgM (Jackson) as described previously (16).
Histology.
Examinations of frozen embedded kidney, liver, or spleen (O.C.T. medium; Sakura Finetek, Torrance, CA) were performed on 5-μm sections fixed in formalin and rehydrated in PBS. Sections were incubated with anti-IgM-rhodamine or anti-IgG-FITC (Jackson ImmunoResearch Labs, West Grove, PA). Washed and stained sections were mounted in aqueous gel mount (Biomeda Corp., Foster City, CA) and observed by fluorescent microscopy at a magnification of ×200 (Zeiss, Göttingen, Germany).
Lyn-deficient autoimmune disease.
Antinuclear antibody (ANA) detection and serum antibody titers to double-stranded DNA (dsDNA), histones, Sm antigen, and total kidney protein were assayed as previously described (4).
Statistical analysis.
Analyses of variance and Student's t test were used as described in the figure legends to compare the groups shown in each experiment. Results are expressed as means and standard errors of the mean unless otherwise indicated.
RESULTS
Glycoprotein-selective acceleration of IgM antigen receptor and CD22 endocytosis in ST6Gal-I deficiency.
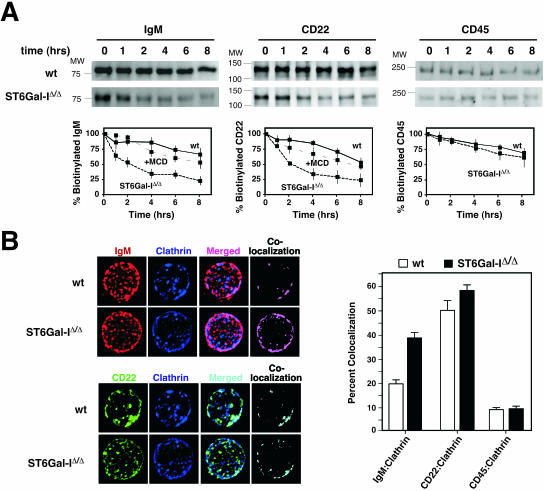
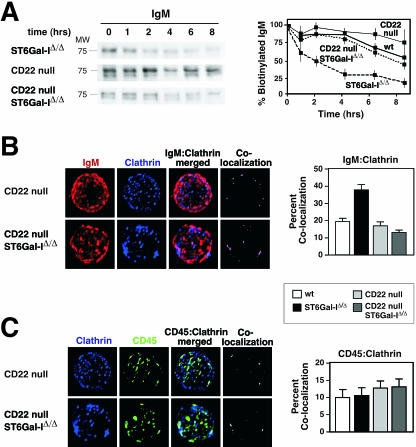
B cells lacking ST6Gal-I exhibit reductions in total expression of IgM and CD22 that appear to reflect decreased levels at the cell surface (16). Splenic and lymph node B cells were placed under cell culture conditions following cell surface biotinylation to determine the cell surface half-lives of various glycoproteins. Normally, the half-lives of IgM, CD22, and CD45 on the wild-type unstimulated B-cell surface were 8 h or longer. In contrast, the cell surface half-lives of IgM and CD22 were reduced to 2 h and the rate of degradation was significantly increased in the absence of ST6Gal-I (Fig. 1A). No effect of ST6Gal-I deficiency on CD45 cell surface half-life was observed. Treatment of ST6Gal-I-deficient B-cell cultures with the cholesterol-depleting endocytosis inhibitor methyl-β-cyclodextrin significantly elevated IgM and CD22 half-lives at the cell surface to almost wild-type values. These findings indicate that ST6Gal-I deficiency selectively increases the basal rate of endocytosis and degradation involving B-cell surface glycoproteins IgM and CD22.
FIG. 1.
ST6Gal-I deficiency increases IgM and CD22 endocytosis. (A) Isolated splenic and lymph node B cells were biotinylated and cultured for the indicated number of hours in the absence of immune stimulation. Biotinylated cell surface proteins were isolated and analyzed by antibody blotting to detect IgM, CD22, and CD45. Measurements (plotted) revealed glycoprotein-selective decreases in IgM and CD22 cell surface half-lives from ∼8 h among wild-type (wt) B cells to 2 h in the absence of ST6Gal-I. No effect was seen on the rate of CD45 turnover. Treatment of ST6Gal-I-deficient B-cell cultures with the endocytosis inhibitor methyl-β-cyclodextrin (MCD) increased IgM and CD22 cell surface half-lives to ∼8 h. Positions of molecular weight standards are denoted. (B) Isolated splenic and lymph node B cells were analyzed by fluorescent deconvolution microscopy. ST6Gal-I deficiency significantly increased colocalization of IgM with clathrin (P < 0.001). No significant change in colocalization of CD22 or CD45 with clathrin was observed. These data were derived from 20 individual B cells representing three separate littermate pairs 6 to 8 weeks of age.
IgM endocytosis occurs by two pathways involving lipid rafts and clathrin-coated pits, although the major route appears to be mediated by clathrin and represents the predominant mechanism for antigen uptake and processing (35, 41). We chose fluorescent deconvolution microscopy to quantitatively investigate whether ST6Gal-I deficiency selectively altered glycoprotein colocalization among lipid raft and clathrin-rich domains at the cell surface of intact resting B cells. In the absence of ST6Gal-I, we observed a twofold increase in the colocalization of IgM with clathrin encompassing 40% of cell surface IgM, as well as a comparably high degree of CD22 colocalization with clathrin, consistent with recent findings (Fig. 1B) (6). Moreover, an increase in IgM-clathrin colocalization among GM1-positive membrane domains was also observed implying the fusion of GM1-clathrin microdomains prior to endocytosis (see Fig. S1A in the supplemental material) (6). In addition, we observed that colocalization with clathrin was not altered among other glycoprotein substrates of ST6Gal-I, including CD45 (Fig. 1B). ST6Gal-I deficiency therefore induces a glycoprotein-selective increase in IgM colocalization with clathrin domains coincident with a decrease in cell surface IgM half-life among resting B cells due to an increase in the basal rate of IgM endocytosis. Normally, IgM endocytosis is induced following BCR stimulation and correlates with attenuated immune signaling (41). We analyzed IgM immune activation responses among ST6Gal-I-deficient B cells with emphasis on detecting membrane-proximal positive and negative signaling components.
ST6Gal-I promotes protein tyrosine phosphorylation in immune signaling.
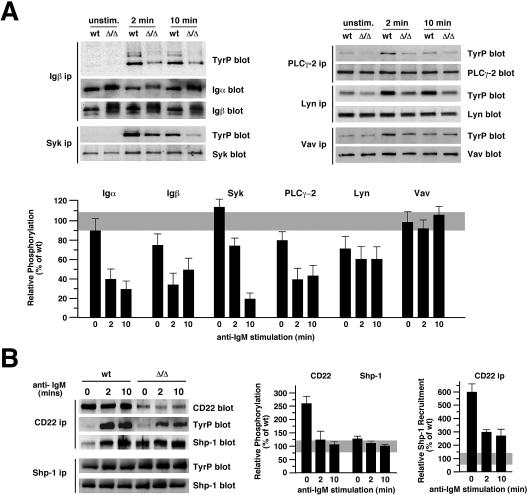
Levels of protein expression and phosphotyrosine accumulation before and after IgM cross-linking were measured among immune signal transducers, including Igα/β heterodimers, the Lyn and Syk tyrosine kinases, PLC-γ2, and the Vav guanine nucleotide exchange factor. These proteins were found at normal abundance among B cells lacking ST6Gal-I, while some migrated at slightly different molecular weights consistent with altered glycosylation. Measurements of tyrosine phosphorylation indicated, in contrast, that only 20 to 40% of normal levels were induced among positive signal transducers Igα/β, Syk, and PLC-γ2 in ST6Gal-I deficiency (Fig. 2A). The Lyn tyrosine kinase, in comparison, which has roles in both positive and negative immune signaling, acquired 60% of normal phosphotyrosine induction, while no change in Vav phosphorylation was observed when corrected for protein level. These findings show that ST6Gal-I deficiency results in a selective and significant decrease in phosphotyrosine accumulation on key positive immune signal transduction mediators in response to B-cell activation.
FIG. 2.
ST6Gal-I deficiency depresses B-cell immune signal transduction coincident with inducing Shp-1 recruitment to CD22. (A) Isolated B cells were immune stimulated with 1.2 μg/ml of anti-IgM antibody for the indicated times followed by immunoprecipitation (ip) of proteins analyzed for expression level and phosphotyrosine (TyrP) accumulation by 4G10 antibody. Absence of ST6Gal-I did not significantly alter protein expression levels but significantly diminished phosphotyrosine accumulation among Igα/β, Syk, PLC-γ2 and Lyn. Results are representative of six separate experiments with 6- to 8-week-old littermates. unstim., unstimulated. (B) ST6Gal-I deficiency increased CD22 tyrosine phosphorylation and induced Shp-1 recruitment to CD22 prior to immune activation (left panel). A 2.5-fold increase in CD22 tyrosine phosphorylation was observed in ST6Gal-I deficiency prior to immune activation (middle panel). The level of Shp-1 recruitment to CD22 was elevated 6-fold prior to immune activation and remained 2.5-fold increased subsequently when compared with the levels of CD22 expression (right panel). Relative phosphorylation was calculated as ratios of phosphotyrosine to the level of protein abundance in ST6Gal-I deficiency and graphed as a percentage of wild-type (wt) measurements (gray).
ST6Gal-I inhibits Shp-1 recruitment to CD22 prior to antigen receptor activation.
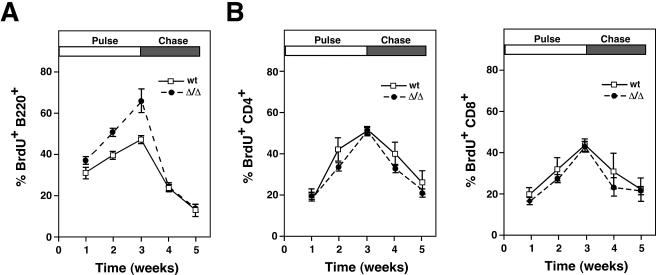
The proportion of CD22 bearing phosphotyrosine was increased 2.5-fold in ST6Gal-I deficiency among unstimulated splenic and lymph node B cells, although on a cell equivalent basis, no significant increase was measured due to the reduction in CD22 levels. Remarkably, ST6Gal-I deficiency induced a sixfold increase in the ratio of Shp-1 recruitment to CD22, resulting in a cellular level of CD22-Shp-1 complexes normally detected only after IgM stimulation of wild-type B cells (Fig. 2B). Following ST6Gal-I-deficient B-cell activation, Shp-1 recruitment to CD22 remained elevated when normalized to CD22 antigen levels. These findings reveal that absence of ST6Gal-I induces the recruitment of Shp-1 to CD22 prior to BCR stimulation coincident with decreased phosphotyrosine immune signaling upon activation. Deficits in BCR signaling can result in reduced B-cell life span (44). Consistent with this finding and the reduction in BCR signaling in ST6Gal-I deficiency, we observed a significant increase in the rate of B-cell production and turnover among ST6Gal-I-deficient mice, while T cells were unaffected (Fig. 3). These observations suggest that reduced B-cell half-life and immune signaling in ST6Gal-I deficiency may result from the presence of CD22-Shp-1 complexes that are in proximity with the IgM BCR prior to activation.
FIG. 3.
Lymphocyte production and life span kinetics in ST6Gal-I deficiency. (A) Increased rate of production (pulse) and decreased half-life (chase) were noted among B cells by in vivo BrdU-labeling studies. (B) CD4+ and CD8+ T cells retained a normal rate of production and unaltered half-life in ST6Gal-I deficiency. Six mice of the indicated genotypes and 6 to 10 weeks of age were analyzed at each time point. wt, wild type.
Colocalization of IgM with CD22 is increased by ST6Gal-I deficiency.
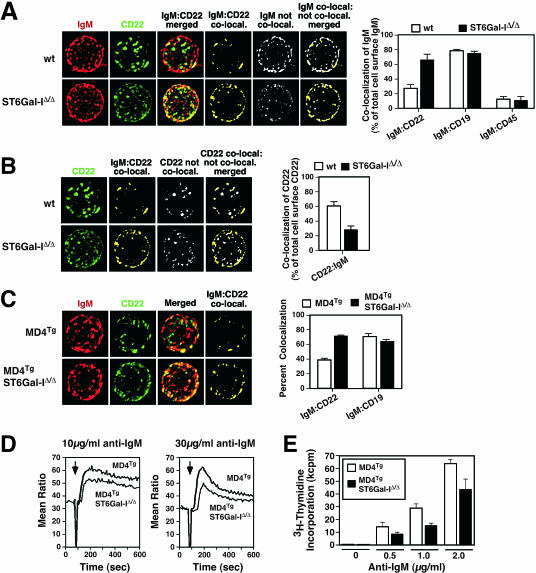
Cell surface IgM localization by fluorescent deconvolution microscopy revealed that 30% of the total cell surface IgM signal was normally colocalized with CD22 among isolated wild-type unstimulated splenic or lymph node B cells. A 2.5-fold increase in IgM colocalization with CD22 occurred in the absence of ST6Gal-I encompassing 75% of the total cell surface IgM signal (Fig. 4A), consistent with recent observations that measured fluorescent signals present on a fraction of the total B-cell surface (6). In addition, our studies indicated no significant changes in the colocalization of IgM with CD19 or CD45. We further observed a significant degree of CD22 coclustering detected among the total cell surface of wild-type B cells, unlike ST6Gal-I-deficient B cells that exhibited a more disperse expression pattern (Fig. 4A). This dispersion of cell surface CD22 correlated with a decrease in the amount of total cell surface CD22 colocalized with IgM in the absence of ST6Gal-I, implying a shift from homotypic to heterotypic CD22 interactions (Fig. 4B). In findings similar to results recently published, increased IgM-CD22 colocalization was also observed among clathrin domains in ST6Gal-I deficiency (see Fig. S1B in the supplemental material) (6). Parallel analyses of developmental and immune activation markers in our studies indicated comparable B-cell populations among wild-type and ST6Gal-I-deficient mice, including marginal zone B cells, and cocapping involving IgM and CD22 proceeded normally at 37°C in the absence of ST6Gal-I (see Fig. S2 in the supplemental material) (reference 16; data not shown).
FIG. 4.
Increased IgM colocalization with CD22, reduced Ca2+ mobilization, and attenuated proliferation in ST6Gal-I-deficient mouse B cells expressing either endogenous or MD4 transgenic (MD4Tg) IgM BCRs. (A) Intact resting B cells from the spleen or lymph nodes (spleen shown) were analyzed by fluorescent deconvolution microscopy to measure the degree of IgM and CD22 colocalization (co-local.) involving total cell surface signals. Representative deconvolution analysis of a single B cell is shown. Images are normalized for fluorescence intensity and shown as maximum projections that include all z-stacks. Cell surface IgM colocalization with cell surface CD22 was substantially increased from 30% of total IgM among wild-type (wt) B cells to 75% of total IgM in ST6Gal-I-deficient B cells (P < 0.001). Note also the reduction in total IgM signal that is not colocalized with CD22 in the absence of ST6Gal-I (white). No change in IgM colocalization occurred with either CD19 or CD45. (B) Increased cell surface dispersion of CD22 occurs in ST6Gal-I deficiency (green), identical to the corresponding panel in A. Measurements of cell surface CD22 colocalized with IgM (yellow; also identical to corresponding panel in A) compared with CD22 that was not colocalized with IgM (white) revealed that more CD22 was not colocalized in ST6Gal-I deficiency, as presented by the ratio of these measurements in the histogram to the right. (C) Increased colocalization of IgM with CD22, but not CD19, was further observed in MD4Tg mice lacking ST6Gal-I. (D) B lymphocytes from MD4Tg mice with and without ST6Gal-I were analyzed for Ca2+ mobilization in response to IgM cross-linking (arrow) at 10 μg/ml or 30 μg/ml. ST6Gal-I-deficient B cells expressing MD4Tg exhibited a reduction in the amount of cytosolic Ca2+ mobilization expressed as a ratio of bound to unbound Indo-I. (E) During the last 16 h of a 64-h assay period, MD4Tg-expressing B cells stimulated with anti-IgM at the indicated concentrations underwent reduced proliferation in the absence of ST6Gal-I. Proliferation is shown as triplicate measurements indicating mean ± standard error. Measurements in panels A to C were derived from 24 to 48 resting B cells of each genotype isolated from at least three separate donors.
Increased colocalization of IgM and CD22 on mature B cells in ST6Gal-I deficiency might reflect altered B-cell development due to decreased antigen receptor signaling. To address this possibility, ST6Gal-I deficiency was bred into the MD4 transgenic BCR background. The MD4 transgene encodes a functional IgM BCR specific for hen egg lysozyme and inhibits endogenous BCR expression, resulting in a homogenous population of mature B cells expressing the MD4 BCR (14). Among such adult mice and their naïve peripheral B cells that developed in the absence of hen egg lysozyme, IgM-CD22 colocalization was nevertheless significantly increased in the absence of ST6Gal-I (Fig. 4C). Moreover, immune activation responses were decreased in measurements of Ca2+ mobilization (Fig. 4D) and proliferation (Fig. 4E). These findings together show that ST6Gal-I deficiency among naïve mature peripheral MD4 transgenic B cells increases IgM-CD22 colocalization and depresses immune activation responses similar to ST6Gal-I-deficient B cells expressing an endogenously derived and diverse BCR repertoire. In both wild-type and MD4 transgenic BCR backgrounds, we noted that cell surface IgM colocalized with CD22 is only partially further colocalized with clathrin. Therefore, we investigated whether CD22 may be involved in controlling IgM trafficking in ST6Gal-I deficiency.
CD22 is required to accelerate IgM endocytosis in ST6Gal-I deficiency.
The half-life of cell surface IgM was further investigated among mice lacking CD22 (25). CD22 deficiency alone moderately increased the half-life of cell surface IgM among naïve resting B cells compared with wild-type B cells, while the absence of both CD22 and ST6Gal-I reduced IgM endocytosis and restored IgM cell surface half-life to normal (Fig. 5A). Consistent with recent observations, the absence of CD22 further reduced IgM-clathrin colocalization in ST6Gal-I deficiency to levels observed among wild-type B cells (Fig. 5B) (6). In addition, CD45 half-life at the cell surface and colocalization with clathrin were unaffected by loss of CD22 and ST6Gal-I (Fig. 5C) (data not shown). These findings reveal that the elevated level of cell surface IgM-clathrin colocalization and the glycoprotein-selective increase in the rate of IgM endocytosis which are provoked by ST6Gal-I deficiency both require CD22. The loss of CD22 further elevated ST6Gal-I-deficient B-cell immune responses involving Ca2+ mobilization and cell proliferation, consistent with recent findings (6; data not shown). Thus, in ST6Gal-I deficiency, CD22 attenuates immune signal transduction and induces IgM BCR endocytosis coincident with increased IgM-CD22 colocalization and constitutive Shp-1 recruitment to CD22. Uncoupling Shp-1 recruitment to CD22 might therefore elevate BCR immune responses among ST6Gal-I-deficient B cells.
FIG. 5.
CD22 is required to increase IgM endocytosis and degradation in ST6Gal-I deficiency. (A) The diminished cell surface half-life of IgM in the absence of ST6Gal-I was restored to normal among ST6Gal-I-deficient B cells further lacking CD22. Results are plotted along with those obtained from the wild-type and ST6Gal-I-deficient B cells presented in Fig. 1. CD22 deficiency slightly decreased the rate of IgM degradation compared with that in wild-type B cells. No change in the half-life of cell surface CD45 was observed. (B) Increased IgM-clathrin colocalization in ST6Gal-I deficiency was decreased to wild-type (wt) levels by further loss of CD22. (C) CD45-clathrin colocalization was not altered by ST6Gal-I deficiency (n = 20; P < 0.001).
Lyn restricts Shp-1 recruitment to CD22 by an ST6Gal-I-dependent mechanism.
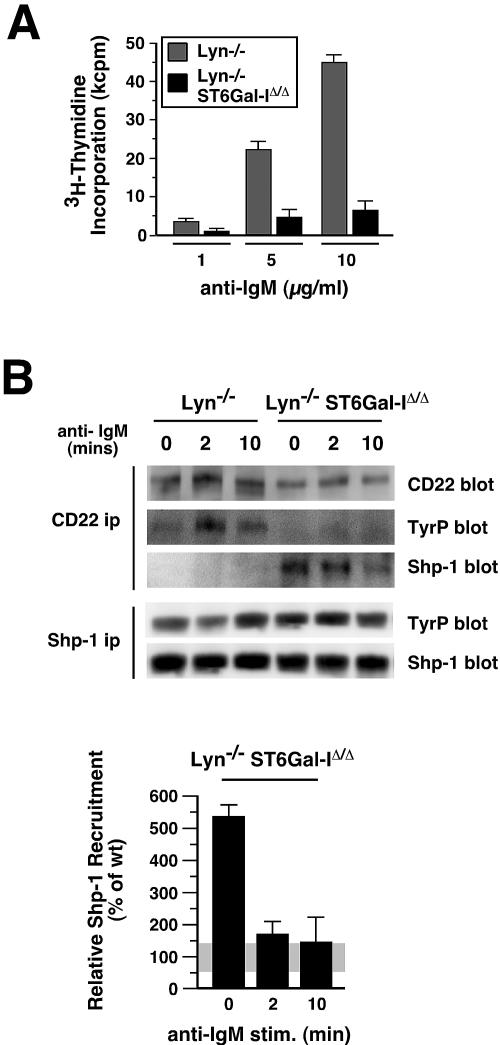
Lyn tyrosine kinase deficiency has been shown to abolish Shp-1 recruitment to CD22 (40). We bred mice lacking ST6Gal-I with Lyn-null mates to investigate whether absence of Shp-1 recruitment to CD22 due to loss of Lyn tyrosine kinase activity would rescue BCR activation deficits caused by ST6Gal-I deficiency. In the absence of both Lyn and ST6Gal-I, splenic and lymph node B cells were, however, significantly less responsive to antigen receptor stimuli than were B cells deficient in Lyn alone (Fig. 6A). The degree of cell proliferation observed by DNA synthesis further correlated with activation marker B7.2 expression among equally viable cells (data not shown). In analyses of CD22 precipitates from Lyn-deficient B cells, Shp-1 recruitment to CD22 was abolished as expected (Fig. 6B). The presence of residual CD22 tyrosine phosphorylation in Lyn deficiency alone has been attributed to the role of the Syk kinase (29). Remarkably however, Shp-1 recruitment to CD22 was restored in Lyn-deficient B cells in the absence of ST6Gal-I (Fig. 6B). The increase in Shp-1 recruitment to CD22 prior to immune stimulation was similar to that observed in ST6Gal-I deficiency alone and yet occurred coincident with a further decrease in CD22 tyrosine phosphorylation to negligible levels by conventional antiphosphotyrosine antibody detection. Interestingly, Shp-1 recruitment to CD22 was not fully sustained following BCR stimulation in Lyn deficiency. These findings indicate that in the absence of immune stimulation Lyn restricts Shp-1 recruitment to CD22 by an ST6Gal-I-dependent mechanism. In ST6Gal-I deficiency, the constitutive recruitment of Shp-1 to CD22 along with increased IgM-CD22 colocalization that are together coincident with elevated BCR endocytosis and depressed B-cell immune signaling may be sufficient to suppress the development of autoimmune disease signs emanating from pathological augmentation of BCR signaling.
FIG. 6.
ST6Gal-I deficiency reduces B-cell activation and promotes Shp-1 recruitment to CD22 in the absence of Lyn. (A) Isolated splenic or lymph node B cells were stimulated by anti-IgM cross-linking at various antibody concentrations and analyzed for cellular proliferation responses by thymidine incorporation. The absence of ST6Gal-I reduced Lyn-deficient B-cell proliferation responses by four- to fivefold. (B) Shp-1 recruitment to CD22 was abolished in B cells lacking Lyn, as expected. In contrast, the additional loss of ST6Gal-I restored Shp-1 recruitment to CD22 and induced CD22-Shp-1 complexes prior to immune stimulation (upper panel). Absence of both Lyn and ST6Gal-I reduced CD22 tyrosine phosphorylation (TyrP) to background levels and did not alter Shp-1 expression or tyrosine phosphorylation. The ratio of Shp-1 recruitment to the endogenous level of CD22 was elevated in the absence of both Lyn and ST6Gal-I, similar to ST6Gal-I deficiency alone (Fig. 2B), as calculated in comparison to measurements among wild-type B cells (lower panel). ip, immunoprecipitation.
ST6Gal-I deficiency attenuates autoimmune disease pathogenesis.
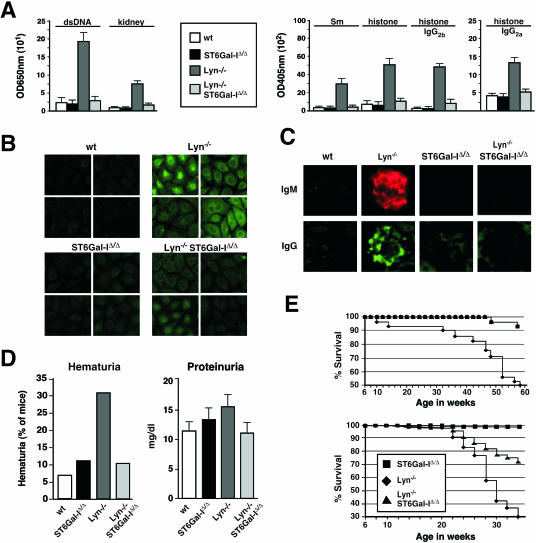
Loss of Lyn activity results in a severe autoimmune disease similar to systemic lupus erythematosus with the development of elevated autoantibody titers, nephritis, and premature death (17, 24, 49). As expected, we found increased IgM autoantibody titers with reactivity to double-stranded DNA, Sm, histone, and total kidney proteins in the sera of mice lacking Lyn. Remarkably, these autoantibody titers were absent from Lyn-deficient mice that also lacked ST6Gal-I (Fig. 7A). Using the HepG2 cell substrate in anti-nuclear antibody (ANA) analyses, sera from Lyn-null mice contained high titers of antibody to nuclear, membrane, and cytoplasmic antigens. In contrast, significant ANA titers failed to develop in the absence of ST6Gal-I (Fig. 7B). Histologic analyses revealed substantially decreased glomerular IgM and IgG deposition in Lyn-null mice also lacking ST6Gal-I (Fig. 7C). This correlated with reduced glomerular inflammation and improved kidney function in analyses of hematuria and proteinuria (Fig. 7D and data not shown). Additional factors nevertheless contribute to the spectrum of phenotypes conferred by the absence of Lyn, some of which may not be pathogenic, including splenomegaly and the reduction in peripheral B-cell numbers which both persisted in ST6Gal-I deficiency (data not shown). We explored further whether attenuated autoimmune disease signs would translate into a longer life span. Compared with Lyn-deficient mice, those that also lacked ST6Gal-I received a significant increase in average life span in two separate studies involving different environmental factors associated with separate vivaria (Fig. 7E). In one vivarium, 95% survival was noted among mice lacking ST6Gal-I or both Lyn and ST6Gal-I during the first 58 weeks of life, while less than 50% of Lyn-deficient mice survived to this age. In a second vivarium, 72% of Lyn- and ST6Gal-I-deficient mice survived for 34 weeks as compared with 30% survival among Lyn-deficient littermates.
FIG. 7.
Loss of ST6Gal-I activity attenuates autoimmune disease in Lyn deficiency. (A) Autoantibody titers (IgM) to dsDNA, kidney protein, Sm antigen, and histone, as well as IgG2 titers to histone induced by Lyn deficiency, were reduced or eliminated in the absence of ST6Gal-I (n = 12 sera from distinct mice of each genotype). (B) Similar findings were obtained in observing ANA titers against HepG2 cells in sera of 16-week-old littermates. Examples of ANA titers to both nuclear and cytoplasmic antigens (shown at a magnification of ×200) are representative of four littermate comparisons. (C) Absence of elevated glomerular deposition of IgM and IgG in Lyn-deficient mice lacking ST6Gal-I. Representative results are shown (×400). (D) Kidney dysfunction detected by elevated hematuria and proteinuria in Lyn deficiency was reduced to normal in the absence of ST6Gal-I. The results plotted represent 24 animals of each genotype. (E) ST6Gal-I deficiency increased the average life span of Lyn-null mice. Two separate studies were accomplished in different vivaria. Each study graphed represents results obtained involving distinct cohorts of mice comprising 40 to 50 littermates of each genotype.
DISCUSSION
The ST6Gal-I sialyltransferase modulates B-cell immune function by mechanisms that include CD22-dependent regulation of IgM BCR trafficking, activation, and endocytosis. Loss of ST6Gal-I induces Shp-1 recruitment to CD22 while elevating IgM colocalization with CD22 and clathrin, coincident with attenuated immune signaling and an enhanced rate of methyl-β-cyclodextrin-sensitive CD22-dependent IgM endocytosis. These events are constitutive in ST6Gal-I deficiency, occurring prior to BCR activation, and correlate with significantly reduced tyrosine phosphorylation of Igα/β, Syk, and PLC-γ2 upon IgM stimulation. Similar effects of ST6Gal-I deficiency were observed among MD4 transgenic B-cell populations expressing antigen receptors to hen egg lysozyme but which had never been exposed to antigen. These findings imply that diminished B-cell activation responses conferred by ST6Gal-I deficiency are not developmental phenotypes acquired by altered antigen receptor signaling during B-cell ontogeny, but rather reflect a role of ST6Gal-I protein glycosylation in promoting mature B-cell immune function.
ST6Gal-I- and CD22-dependent regulation of IgM signaling and endocytosis.
Attenuation and extinction of B-cell immune signaling have been associated with IgM internalization in fused lipid raft-clathrin domains, interactions of IgM BCRs with CD22 and Shp-1, and disruption of IgM BCR interaction with Igα/β heterodimers (7, 9, 11, 30, 41, 43, 46). The B-cell phenotype induced by ST6Gal-I deficiency is reminiscent of these findings. Increased IgM BCR endocytosis was associated with elevated IgM-clathrin colocalization among GM1-positive membrane domains. Absence of ST6Gal-I increased the level of IgM-CD22 colocalization, which was further observed among clathrin domains. In addition, no change in Igα/β expression at the B-cell surface was detected on ST6Gal-I-deficient B cells, implying the disruption of IgM-Igα/β complexes prior to IgM endocytosis (data not shown). While CD22 may be excluded from lipid rafts, we concur with a recent report (6) that the majority of CD22 normally resides among clathrin-rich domains. Nevertheless, almost half of colocalized IgM-CD22 is outside of clathrin-rich domains in ST6Gal-I deficiency, implying that CD22 associates with the BCR and dampens immune responsiveness prior to arrival in clathrin-rich domains and subsequent endocytosis. Interestingly, a 40% reduction of cell surface IgM levels was invariably observed on B cells lacking ST6Gal-I, CD22, and Lyn, either singly or in combination (16; data not shown). Therefore, the level of cell surface IgM BCR expression does not correlate with the magnitude of immune responses to anti-IgM stimulation and implicates instead altered BCR colocalization and trafficking as responsible for dampened immune signaling in ST6Gal-I deficiency. The mechanism of CD22-dependent trafficking of cell surface IgM is not resolved; however, CD22 Siglec ligand binding may be involved in this process.
ST6Gal-I sialylation in CD22 Siglec-dependent and -independent binding.
The physiologic role of CD22 Siglec binding has been difficult to perceive. As CD22 is the only molecule detected on the B-cell surface with Siglec binding activity for counter-receptors produced by ST6Gal-I (5, 19), it would be expected that CD22 mutations specifically disabling Siglec binding would recapitulate phenotypes observed in ST6Gal-I deficiency. CD22 Siglec mutations targeted to the mouse germ line and studied among primary B cells have indeed produced phenotypes similar to those observed in ST6Gal-I deficiency, including attenuated BCR-induced proliferation (32). In contrast, an exogenous approach to disrupt CD22 Siglec ligands using glycan ligand mimetics increased antigen receptor-stimulated Ca2+ mobilization when added to established B-cell lines, suggesting a reduction of CD22 Siglec-dependent association with IgM complexes (19). Moreover, study of a B-cell line derived from CD22-deficient mice revealed augmented Ca2+ mobilization upon expression of mutant CD22 molecules disabled in Siglec binding (18). Such findings appear difficult to reconcile. From the earliest discordances observed among CD22 mutant mice, it seems likely that mouse strain differences may contribute to alterations in the outcome involving CD22 function in BCR-induced proliferation (25, 27, 28, 32, 38). In addition, the use of primary B cells may be relevant, as a study using the Daudi B-cell line reported no increase in CD22 endocytosis in the absence of CD22 Siglec ligands (50).
The identities of CD22 binding partners depend upon the methodologies employed. IgM-CD22 interaction has been reported in ranges from 0.5% to 18% by coprecipitation and protein cross-linking (21, 30, 50). Those methods can detect closely adjacent and even direct interactions but may also disrupt selective in situ binding and can rely upon strict intermolecular distances. Quantitative fluorescent deconvolution microscopy cannot achieve this degree of spatial resolution; however, this technique can analyze native and intact B-cell surfaces. Our findings by this method are consistent with recent studies (6) that report a substantial although minor fraction of total cell surface IgM (∼30%) is typically colocalized with CD22 in wild-type unstimulated peripheral B cells. A significant basal level of IgM-CD22 colocalization is consistent with the normal role of CD22 expression in modulating peripheral naïve B-cell activation thresholds. It is not clear, however, whether IgM-CD22 colocalization on B cells expressing ST6Gal-I involves a subset of total cell surface glycoproteins lacking CD22 Siglec ligands due to the phenomenon of microheterogeneity of glycan linkage formation in the Golgi apparatus. It remains possible that increased colocalization of IgM with CD22 in ST6Gal-I deficiency results from the absence of CD22 Siglec binding.
Many proteins are glycosylated by ST6Gal-I in B cells, and all would appear to be CD22 Siglec ligands by lectin coprecipitation from homogenized cell extracts (16; data not shown). However, CD22 interactions are likely more selective in situ. On the resting intact B-cell surface, CD22 Siglec binding was recently shown to predominantly involve cis ligands resulting in homotypic multimeric CD22 complexes (15). Our results by quantitative fluorescent deconvolution microscopy of entire intact as well as naïve B-cell surfaces appear consistent with those findings. We observed more dispersed CD22 expression at the cell surface in ST6Gal-I deficiency as well as a reduced fraction of total cell surface CD22 colocalized with IgM. These findings suggest that reduced homotypic CD22 binding occurs in the absence of Siglec ligands concurrent with increased heterotypic CD22 interactions among other cell surface molecules, including the IgM BCR, possibly by enabling CD22 Siglec-independent binding contributed by the conserved extracellular non-Siglec Ig-like domains. This may explain the significant amount of IgM cross-linking to CD22 observed on intact cell surfaces that lack Siglec ligands (50).
Control of Shp-1 recruitment to CD22 by ST6Gal-I protein glycosylation.
The attenuation of BCR signaling by CD22 is attributed to Shp-1 recruitment following activation (7, 9, 11, 43). In wild-type B cells, a low level of Shp-1 coprecipitation with CD22 is observed prior to IgM cross-linking, with maximal Shp-1 recruitment occurring after several minutes of BCR stimulation. In contrast, ST6Gal-I deficiency induced maximal Shp-1 recruitment to CD22 prior to IgM stimulation and the resultant increase in phosphotyrosine on CD22. Nevertheless, constitutive Shp-1 recruitment to CD22 in ST6Gal-I deficiency may not simply reflect the absence of CD22 Siglec ligands. No similar increase in Shp-1 recruitment to CD22 was reported among B cells bearing mutant CD22 molecules with either deleted or likely inactive Siglec binding domains (32). It is possible that the absence of CD22 sialylation by ST6Gal-I alters CD22 conformation in promoting Shp-1 recruitment when CD22 is colocalized with IgM BCRs. Or perhaps ST6Gal-I deficiency increases access of Shp-1 to CD22 by altering glycoprotein trafficking and residence among membrane microdomains. It is widely accepted that Shp-1 recruitment to CD22 reflects binding of Shp-1 src homology 2 (SH2) domain sequences to phosphotyrosine within CD22 ITIM domains. Remarkably, the level of phosphotyrosine detected on CD22 in ST6Gal-I deficiency was reduced to negligible levels in the further absence of Lyn, and yet the constitutively high level of Shp-1 recruitment to CD22 remained in the absence of immune stimulation. Shp-1 recruitment was not sustained following BCR stimulation of ST6Gal-I-deficient B cells, revealing that Lyn function increases the half-life of CD22-Shp-1 complexes following BCR activation. While the mechanism of CD22-Shp-1 complex formation in ST6Gal-I deficiency remains to be established, it is possible that detection of phosphotyrosine by antibody binding is thwarted by ST6Gal-I deficiency or a mechanism exists by which CD22 and Shp-1 complexes can form independent of CD22 tyrosine phosphorylation.
Endogenous control of ST6Gal-I function.
The immune modulatory capability of ST6Gal-I may be normally regulated among B cells, perhaps as indicated by unmasking of CD22 Siglec binding following immune stimulation. Although no change in ST6Gal-I RNA levels occurs subsequent to BCR activation of wild-type B cells (data not shown), multiple changes in B-cell surface glycan linkages occur which mimic those observed on ST6Gal-I-deficient B cells bearing unmasked CD22 (10, 36) (see Fig. S3 in the supplemental material). Decreased ST6Gal-I function may result from the induction of glycosyltransferases that compete for glycoprotein substrates, by the expression or activation of endogenous sialidases, or perhaps from proteolysis in the stem region abolishing glycosyltransferase substrate access in the Golgi apparatus by releasing the catalytic domain as a secreted fragment. Except in the instance of a rapid desialylation at the cell surface, it appears unlikely that these mechanisms are involved during the early postactivation responses that result in rapid Shp-1 recruitment to CD22 and IgM endocytosis. Interestingly, the normal induction of BCR endocytosis in response to IgM stimulation among wild-type B cells, which also results in a cell surface IgM half-life of less than 2 h, was not affected by the loss of CD22 or Lyn (data not shown). The similar decrease of IgM BCR cell surface half-life in ST6Gal-I deficiency, which in contrast is CD22 dependent and occurs in the absence of IgM stimulation, likely represents a distinct pathway to endocytosis that typically employs ST6Gal-I and CD22 function to restrain IgM BCR internalization in promoting naive mature B-cell immune function.
Posttranslational modifications contributing to intracellular immune signal transduction pathways include kinase and phosphatase enzymes that regulate protein phosphorylation. Less evident has been whether protein glycosylation, confined to extracellular compartments and topologically separated from protein phosphorylation, may participate in the formation of these intracellular signals. We have observed humoral immune deficits in the absence of protein glycosylation by ST6Gal-I that result in diminished tyrosine phosphorylation of proteins associated with the BCR complex, in findings that reflect both cell-type-specific and glycoprotein-selective roles for ST6Gal-I. Such focused purpose is increasingly evident among the discerned functions of mammalian glycosyltransferases, as further exemplified by the specificity of ST8Sia-II and -IV in neural NCAM function, FT-8 in lung epithelial transforming growth factor β receptor signaling, and GnT-4a in pancreatic β-cell glucose transporter expression (1, 26, 47, 48). Diminished B-cell immune responses observed in ST6Gal-I deficiency reflect constitutive disruption of a CD22-dependent mechanism that controls glycoprotein-selective cell surface modulation of IgM trafficking and colocalization with CD22-Shp-1 complexes among unstimulated mature B cells coincident with enhancing the rate of IgM endocytosis. Therefore, ST6Gal-I operates with CD22 in an immune regulatory pathway that converges with clathrin-dependent IgM endocytosis in establishing thresholds for naïve mature peripheral BCR activation. Diminishing ST6Gal-I function spares B-cell viability yet has a therapeutic effect in the context of the severe systemic lupus erythematosus phenotype due to Lyn deficiency and possibly other autoimmune disease syndromes arising from abnormal and hyperimmune B-cell activity.
Supplementary Material
Acknowledgments
We thank Linda Walker and Maria Conejo for excellent technical support. Fluorescent microscopy was performed with the UCSD Cancer Center Digital Imaging Shared Resource Facility directed by James Feramisco. CD22-deficient and ST6Gal-I/CD22 doubly deficient mice were provided by James C. Paulson of The Scripps Research Institute (La Jolla, Calif.).
J.D.M. is a founder of Abaron Biosciences, Inc., a company that is developing drugs related to the research described in the manuscript. The University of California—San Diego is also an equity holder. The terms of this arrangement have been reviewed and approved by the University of California—San Diego, in accordance with its conflict of interest policies.
This research was funded by NIH grants HL57345 (J.D.M.), AI050143 (J.C.P.), and GM25042 (B.E.C.). J.D.M. is supported as an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Angata, K., J. M. Long, O. Bukalo, W. Lee, A. Ditayev, A. Wynshaw-Boris, M. Schachner, M. Fukuda, and J. D. Marth. 2004. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 279:32603-32613. [DOI] [PubMed] [Google Scholar]
- 2.Baum, L. 2002. Developing a taste for sweets. Immunity 16:5-8. [DOI] [PubMed] [Google Scholar]
- 3.Chan, V. W. F., F. Meng, P. Soriano, A. L. DeFranco, and C. A. Lowell. 1997. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal transduction and down-regulation. Immunity 7:69-81. [DOI] [PubMed] [Google Scholar]
- 4.Chui, D., G. Sellakumar, R. Green, M. Sutton-Smith, T. McQuistan, K. Marek, H. Morris, A. Dell, and J. D. Marth. 2001. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl. Acad. Sci. USA 98:1142-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, B. E., O. Blixt, N. V. Bovin, C. P. Danzer, D. Chui, J. D. Marth, L. Nitschke, and J. C. Paulson. 2002. Constitutively unmasked CD22 on B cells of ST6Gal I knockout mice: novel sialoside probe for murine CD22. Glycobiology 12:563-571. [DOI] [PubMed] [Google Scholar]
- 6.Collins, B. E., B. A. Smith, P. Bengston, and J. C. Paulson. 2006. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat. Immunol. 7:199-206. [DOI] [PubMed] [Google Scholar]
- 7.Cornall, R. J., J. G. Cyster, M. L. Hibbs, A. R. Dunn, K. L. Otipoby, E. A. Clark, and C. C. Goodnow. 1998. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating B cell signaling and selection. Immunity 8:497-508. [DOI] [PubMed] [Google Scholar]
- 8.Crocker, P. R., and A. Varki. 2001. Siglecs in the immune system. Immunology 103:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyster, J., and C. C. Goodnow. 1997. Tuning antigen receptor signaling by CD22: integrating cues from antigens and the microenvironment. Immunity 6:509-517. [DOI] [PubMed] [Google Scholar]
- 10.Danzer, C. P., B. E. Collins, O. Blixt, J. C. Paulson, and L. Nitschke. 2003. Transitional and marginal zone B cells have a high proportion of unmasked CD22: implications for BCR signaling. Int. Immunol. 15:1137-1147. [DOI] [PubMed] [Google Scholar]
- 11.Doody, G. M., L. B. Justement, C. C. Delibrias, R. J. Matthews, J. Lin, M. L. Thomas, and D. T. Fearon. 1995. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 269:242-244. [DOI] [PubMed] [Google Scholar]
- 12.Ellies, L. G., D. Ditto, G. G. Levy, M. Wahrenbrock, D. Ginsburg, A. Varki, D. T. Le, and J. D. Marth. 2002. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc. Natl. Acad. Sci. USA 99:10042-10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floyd, H., L. Nitschke, and P. R. Crocker. 2000. A novel subset of murine B cells that expresses unmasked forms of CD22 is enriched in bone marrow: implications for B-cell homing to the bone marrow. Immunology 101:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodnow, C. C., J. Crosbie, S. Adelstein, T. B. Lavoie, S. J. Smith-Gill, R. A. Brink, H. Pritchard-Briscoe, J. S. Wotherspoon, R. H. Loblay, and K. Raphael. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334:676-682. [DOI] [PubMed] [Google Scholar]
- 15.Han, S., B. E. Collins, P. Bengston, and J. C. Paulson. 2005. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan crosslinking. Nat. Chem. Biol. 1:93-97. [DOI] [PubMed] [Google Scholar]
- 16.Hennet, T., D. Chui, J. C. Paulson, and J. D. Marth. 1998. Immune regulation by the ST6Gal sialyltransferase. Proc. Natl. Acad. Sci. USA 95:4504-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbs, M. L., D. M. Darlinton, J. Armes, D. Grail, G. Hodgson, R. Maglitto, S. A. Stacker, and A. R. Dunn. 1995. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell 83:301-311. [DOI] [PubMed] [Google Scholar]
- 18.Jin, L., P. A. McLean, B. G. Neel, and H. H. Wortis. 2002. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med. 195:1199-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelm, S., J. Gerlach, R. Brossmer, C. P. Danzer, and L. Nitschke. 2002. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 195:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitagawa, H., and J. C. Paulson. 1994. Differential expression of five sialyltransferase genes in human tissues. J. Biol. Chem. 269:17872-17878. [PubMed] [Google Scholar]
- 21.Leprince, C., K. E. Draves, R. L. Geahlen, J. A. Ledbetter, and E. A. Clark. 1993. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc. Natl. Acad. Sci. USA 90:3236-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe, J. B. 2001. Glycosylation, immunity, and autoimmunity. Cell 104:809-812. [DOI] [PubMed] [Google Scholar]
- 23.Lowe, J. B., and J. D. Marth. 2003. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72:643-691. [DOI] [PubMed] [Google Scholar]
- 24.Nishizumi, H., I. Taniuchi, Y. Yamanashi, D. Kitamura, D. Ilic, S. Mori, T. Watanabe, and T. Yamamoto. 1995. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity 3:549-560. [DOI] [PubMed] [Google Scholar]
- 25.Nitschke, L., R. Carsetti, B. Ocker, G. Kohler, and M. C. Lamers. 1997. CD22 is a negative regulator of B-cell receptor signaling. Curr. Biol. 7:133-143. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsubo, K., S. Takamatsu, M. T. Minowa, A. Yoshida, M. Takeuchi, and J. D. Marth. 2005. Dietary and genetic control of glucose transporter-2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123:1307-1321. [DOI] [PubMed] [Google Scholar]
- 27.O'Keefe, T. L., G. T. Williams, S. L. Davies, and M. S. Neuberger. 1996. Hyperresponsive B cells in CD22-deficient mice. Science 274:798-801. [DOI] [PubMed] [Google Scholar]
- 28.Otipoby, K. L., K. B. Andersson, K. E. Draves, S. J. Klaus, A. G. Farr, J. D. Kerner, R. M. Perlmutter, C. L. Law, and E. A. Clark. 1996. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature 384:634-637. [DOI] [PubMed] [Google Scholar]
- 29.Otipoby, K. L., K. E. Draves, and E. A. Clark. 2001. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J. Biol. Chem. 276:44315-44322. [DOI] [PubMed] [Google Scholar]
- 30.Peaker, C., J. G., and M. S. Neuberger. 1993. Association of CD22 with the B cell antigen receptor. Eur. J. Immunol. 23:1358-1363. [DOI] [PubMed] [Google Scholar]
- 31.Phillips, M. L., E. Nudelman, F. C. Gaeta, M. Perez, A. K. Singhal, S. Hakomori, and J. C. Paulson. 1990. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-LeX. Science 250:1130-1132. [DOI] [PubMed] [Google Scholar]
- 32.Poe, J. C., Y. Fujimoto, M. Hasegawa, K. M. Haas, A. S. Miller, I. G. Sanford, C. B. Bock, M. Fujimoto, and T. F. Tedder. 2004. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat. Immunol. 5:1078-1087. [DOI] [PubMed] [Google Scholar]
- 33.Powell, L. D., R. K. Jain, K. L. Matta, S. Sabesan, and A. Varki. 1995. Characterization of sialoligosaccharide binding by recombinant soluble and native cell-associated CD22. J. Biol. Chem. 270:7523-7532. [DOI] [PubMed] [Google Scholar]
- 34.Priatel, J. J., D. Chui, N. Hiraoka, C. J. T. Simmons, K. B. Richardson, D. M. Page, M. Fukuda, N. M. Varki, and J. D. Marth. 2000. The ST3Gal-I sialyltransferase controls CD8+ T cell homeostasis by modulating O-glycan biosynthesis. Immunity 12:273-283. [DOI] [PubMed] [Google Scholar]
- 35.Putnam, M. A., A. E. Moquin, M. Merrihew, C. Outcalt, E. Sorge, A. Caballero, T. A. Gondre-Lewis, and J. R. Drake. 2003. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J. Immunol. 170:905-912. [DOI] [PubMed] [Google Scholar]
- 36.Razi, N., and A. Varki. 1998. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl. Acad. Sci. USA 95:7469-7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen, S. D. 2004. Ligands for L-selectin: homing, inflammation and beyond. Annu. Rev. Immunol. 22:129-156. [DOI] [PubMed] [Google Scholar]
- 38.Sato, S., A. S. Miller, M. Inaoki, C. B. Bock, P. J. Jansen, M. L. Tang, and T. F. Tedder. 1996. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity 5:551-562. [DOI] [PubMed] [Google Scholar]
- 39.Sgroi, D., A. Varki, S. Braesch-Andersen, and I. Stamenkovic. 1993. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J. Biol. Chem. 268:7011-7018. [PubMed] [Google Scholar]
- 40.Smith, K. G. C., K. M. Tarlinton, G. M. Doody, M. L. Hibbs, and D. T. Fearon. 1998. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 187:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoddart, A., A. P. Jackson, and F. M. Brodsky. 2005. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol. Biol. Cell 16:2339-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashima, S., Y. Tachida, T. Nakagawa, T. Hamamoto, and S. Tsuji. 1999. Quantitative analysis of expression of mouse sialyltransferase genes by competitive PCR. Biochem. Biophys. Res. Commun. 260:23-27. [DOI] [PubMed] [Google Scholar]
- 43.Tedder, T. F., J. Tuscano, S. Sato, and J. H. Kehrl. 1997. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu. Rev. Immunol. 15:481-504. [DOI] [PubMed] [Google Scholar]
- 44.Torres, R. M., H. Flaswinkel, M. Reth, and K. Rajewsky. 1996. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science 272:1804-1808. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji, S., A. K. Datta, and J. C. Paulson. 1996. Systematic nomenclature for sialyltransferases. Glycobiology 6:v-vii. [DOI] [PubMed] [Google Scholar]
- 46.Vilen, B. J., T. Nakamura, and J. C. Cambier. 1999. Antigen-stimulated dissociation of BCR mIg from Ig-alpha/Ig-beta: implications for receptor desensitization. Immunity 10:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, X., S. Inoue, J. Gu, E. Miyoshi, K. Noda, W. Li, Y. Mizuno-Horikawa, M. Nakano, M. Asahi, M. Takahashi, N. Uozumi, S. Ihara, S. H. Lee, Y. Ikeda, Y. Yamaguchi, Y. Aze, Y. Tomiyama, J. Fujji, K. Suzuki, A. Kondo, S. D. Shapiro, C. Lopez-Otin, T. Kuwaki, M. Okabe, K. Honke, and N. Taniguchi. 2005. Dysrregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA 102:15791-15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinhold, B., R. Seidenfaden, I. Rockle, M. Muhlenhoff, F. Schertzinger, S. Conzelmann, J. D. Marth, R. Gerardy-Schahn, and H. Hildebrandt. 2005. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by NCAM deletion. J. Biol. Chem. 280:42971-42977. [DOI] [PubMed] [Google Scholar]
- 49.Xu, Y., K. W. Harder, N. D. Huntington, M. L. Hibbs, and D. M. Tarlinton. 2005. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 22:9-18. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, M., and A. Varki. 2004. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology 14:939-949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.