Abstract
Intraspinal microstimulation (ISMS), a novel rehabilitative therapy consisting of stimulation through fine, hair-like microwires targeted at the ventral spinal cord, has been proposed for restoring standing and walking following spinal cord injury. This study compared muscle recruitment characteristics of ISMS with those produced by peripheral nerve cuff stimulation (NCS). Thirty-three minutes of either ISMS or NCS at 1, 20 or 50 s−1 and 1.2 × threshold (T) amplitude depleted glycogen from muscle fibres of vastus lateralis and rectus femoris. ISMS and NCS were also carried out at 20 s−1 and 3.0T. Muscle serial sections were stained for glycogen and for myosin heavy chain (MHC)-based fibre types using a panel of monoclonal antibodies. The results of this study show that ISMS recruited fatigue-resistant (FR) fibres at 2.9, 1.9, 1.7 and 2.5 times their relative MHC content at 1, 20 and 50 s−1 1.2T and 20 s−1 3.0T, respectively. In contrast, NCS recruited FR fibres at 1.2, 1.0, 2.1 and 0.0 times their MHC content at 1, 20 and 50 s−1 1.2T and 20 s−1 3.0T, respectively. The proportion of FR fibres recruited by ISMS and NCS was significantly different in the 20 s−1 3.0T condition (P < 0.0001). We also report that force recruitment curves were 4.9-fold less steep (P < 0.019) for ISMS than NCS. The findings of this study provide evidence for the efficacy of ISMS and further our understanding of muscle recruitment properties of this novel rehabilitative therapy.
Spinal cord injury (SCI) represents a devastating neurological impairment with potentially life-threatening implications. The quality of life for a person with SCI often centres on the challenges to bladder and bowel function, respiration, skin and muscle health, freedom of movement, and general independence. One of the associated difficulties following SCI is the dramatic muscle atrophy and slow-to-fast transformation of skeletal muscle downstream of the lesion (Burnham et al. 1997; Castro et al. 1999). These result in a muscle that is highly fatigable and incapable of performing at workloads required for standing or stepping. Previous rehabilitative interventions for restoring stepping which have utilized functional neuromuscular stimulation (FNS) have been employed with some success (Prochazka & Mushahwar, 2001; Stein et al. 2002). Unfortunately, these systems have inherent disadvantages. Because they employ peripheral electrodes tunnelled subcutaneously for long distances, such as with epimysial and intramuscular forms of stimulation, they suffer from problems associated with electrode lead breakage (Popovic, 1992). In addition, the normal order of motor-unit recruitment is reversed so that the lowest currents recruit the fastest, most fatigable units, which leads to inappropriate, excessive force recruitment and high fatigability (Prochazka, 1993).
Recently, intraspinal microstimulation (ISMS) has been suggested for therapeutic restoration of stable stepping. This technique involves implanting microwires into the ventral horns of the lumbosacral spinal cord. Stimulation through implanted microwires has previously been effective in establishing coordinated single and multijoint movements in both intact (Mushahwar et al. 2002) and spinalized (Saigal et al. 2004) cats including consistent bilateral stepping. Furthermore, previous reports showed that ISMS produces graded force recruitment and activates muscles selectively (Mushahwar & Horch 1998, 2000b). Therefore, ISMS may avoid some of the difficulties associated with peripheral forms of FNS, including improper motor-unit recruitment pattern and electrode lead breakage because the stimulus is applied through existing central nervous system structures and the electrodes are placed in comparatively non-mobile tissue.
The purpose of this study was to compare the muscle recruitment properties of peripheral FNS in the form of a nerve cuff electrode over the femoral nerve with ISMS through electrodes targeting the quadriceps motoneurone pool. Two sets of experiments were performed to study the properties of these stimulation protocols. In the first experimental series, pulse frequency and amplitude were varied and a glycogen depletion method was used to determine which muscle fibres were activated by ISMS or by nerve cuff stimulation (NCS) protocols. In a second series of experiments, force recruitment data from quadriceps muscles were collected to analyse the functional properties of muscle under the direction of each stimulation protocol. Previous studies suggested that ISMS recruits motor units in a mixed order (Mushahwar & Horch, 2000a), but this is the first study to compare directly the recruitment properties of ISMS and NCS at the muscle level.
The results of this study show that ISMS preferentially recruited fatigue-resistant (FR) fibres out of proportion to their corresponding myosin heavy chain (MHC) content in quadriceps muscles. Contrarily, NCS chiefly recruited fast-twitch fatigable fibres. The difference between the proportion of FR fibres activated by ISMS and NCS was significantly different at the stimulation condition most likely to be used in a clinical setting. Furthermore, the rate of force recruitment with ISMS was significantly more gradual than that with NCS. The results of this investigation further our understanding of muscle recruitment by ISMS and provide support for the efficacy of ISMS as a novel rehabilitative therapy.
Methods
Animal treatment and care
All animal procedures were carried out under the guidelines of the Canadian Council for Animal Care and approved by the University of Alberta Animal Welfare Committee. Female Sprague-Dawley rats were housed in pairs in a controlled environment with 50–55% humidity, at 20°C, with alternating 12 h light–12 h dark cycles and received food and water ad libitum. Animals were randomly assigned to experimental groups for either glycogen depletion or force recruitment experiments. For all surgical experiments animals were anaesthetized using 2.5% isofluorane delivered in gaseous form. Upon completion of the experiments all animals were immediately killed by cardiac excision whilst under anaesthesia. Fifty-four animals were used for this study with a mean mass of 349.6 ± 8.7 g (± s.e.m.).
Glycogen depletion experiments
Glycogen depletion was accomplished by stimulating one hindlimb while the contralateral hindlimb served as a sham control. For NCS experiments a bipolar nerve cuff with 1–2 mm interelectrode separation was placed around the femoral nerve, further insulated from surrounding muscles with mineral oil and sutured securely closed. For ISMS experiments a laminectomy was performed at T12–T13 to expose spinal cord segments L3–L4. Following this, 30 μm diameter, Teflon-insulated microwires (stainless steel 304, California Fine Wire Company, Grover Beach, CA, USA) were inserted 2–3 mm past the dura mater of the spinal cord. Specifically, microwire tips exposed for 30–60 μm were inserted into the quadriceps motoneurone pool in the ventral grey matter. Mono-polar stimulation with the return electrode placed in the back musculature was used for all ISMS experiments. The ISMS target area has been mapped by Mushahwar & Horch (1998, 2000b) in cats and is thought to be maintained in other species such as rabbit and humans (Sharrard, 1955; Romanes, 1964; Portal et al. 1991). Placement of microwires in the quadriceps motoneurone pool was confirmed with practice twitches and ultimately by the glycogen-depleted fibres themselves. A stimulator (STG1008, Multi-channel Systems Inc, Reutlingen, Germany) was used to deliver 400 μs, biphasic, cathodic-first, charge-balanced pulses through the electrodes at each frequency and amplitude. For both NCS and ISMS, stimulation consisted of five bouts of 5-min stimulation periods (1 s on, 1 s off) interspersed with 2-min rest periods between each bout. Since the duty cycle was 50%, total stimulation time was 12.5 min. Stimulation procedures were designed to produce depletion of glycogen in the activated quadriceps muscle fibres. Our own preliminary experiments showed that glycogen depletion of slow-twitch fibres was undetectable for up to 1 h of stimulation. Similarly, Rafuse & Gordon (1996) found that slow-twitch motor units required as long as 3 h of stimulation to produce fatigue and glycogen depletion. Previous reports demonstrated that glycogen depletion can be accelerated and enhanced with ischaemia of the selected muscles (Totosy de Zepetnek et al. 1992; Hudlicka et al. 1994). Therefore, in the present experiments, ischaemia was produced by placing sutures around the femoral artery of both stimulated and contralateral sham control hindlimbs. Muscles were made ischaemic at the beginning of the stimulation period by tightening the sutures around the femoral arteries. Sham hindlimbs were made ischaemic for the same duration. This procedure did not disrupt blood flow to the femoral nerve, which is supplied by the internal iliac artery.
Previous reports conducted ISMS at a range of amplitudes, including near-threshold levels (Mushahwar et al. 2003; Saigal et al. 2004). For near-threshold stimulation both NCS and ISMS protocols were carried out with the amplitude held at 1.2 × threshold (T). Threshold was defined as the lowest stimulation amplitude that produced a visible twitch. In light of the possibility that interneuronal pathways are differentially activated according to stimulus frequency (Minassian et al. 2004; Jilge et al. 2004) we performed stimulation at 1, 20 or 50 s−1 to determine the effect of frequency on muscle fibre type recruitment by ISMS. NCS experiments were carried out with matching frequencies as a control. Each NCS and ISMS group at the 1.2T condition contained six animals for a total of 36. High-amplitude stimulation was also performed at 3.0T to determine the recruitment properties of ISMS and NCS at stimulation amplitudes more likely to be used in clinical FNS applications. The stimulation frequency of 20 s−1 was chosen for these experiments because it represents a frequency that is similar to the maximum natural rate of motor unit firing in humans (Fuglevand & Johns, 2004 [www.colorado.edu/intphys/news/summaries.html]; Taylor & Enoka, 2004). This stimulation rate has also been successfully used in the past for ISMS experiments from our group (Saigal et al. 2004) and is likely to be the most clinically relevant frequency for ISMS therapy. Six animals were included in each of the 3.0T groups (ISMS and NCS) for a total of 12.
Following stimulation, the vastus lateralis and rectus femoris muscles were quickly excised as a group from experimental and control legs and placed into ice-cold saline for 30 s with gentle stirring. Rectus femoris and vastus lateralis muscles were chosen because they represent a large portion of the quadriceps muscle mass and they contain mixed MHC isoform based fibre types which allowed for identification of the full spectrum of fibre types. Handling and separation of these muscles was performed on a metal platform embedded in ice. Once separated, muscles were placed in a slightly stretched position on aluminium foil and frozen in melting isopentane (−156°C) cooled in liquid nitrogen. Muscles were stored at −80°C until sectioned to a thickness of 10 μm.
To determine which fibres were activated by stimulation, frozen muscle cross-sections of the rectus femoris and vastus lateralis were stained for glycogen using the periodic acid-Schiff (PAS) reaction. Activated fibres were identified as those lacking glycogen, a technique commonly known as the glycogen depletion method (Kugelberg & Edstrom, 1968; Kim et al. 1995). The absence of glycogen depletion in any of the contralateral sham control muscles confirmed that neither ischaemia due to occlusion of the femoral artery nor the muscle collection procedure induced glycogen degradation.
Muscle fibre type analysis
Immunohistochemical detection of MHC isoforms was completed on frozen muscle sections according to Putman et al. (2001, 2003). Monoclonal antibodies directed against adult MHC isoforms were harvested from hybridoma cell lines obtained from the American Type Culture Collection (Manassa, VA, USA) (Schiaffino et al. 1989) and applied at the following dilutions: anti-MHCI clone BA-D5 (MHCI, 1: 400, culture supernatant); anti-MHCIIa clone SC-71 (MHCIIa, 1: 100, culture supernatant); anti-MHCIIb clone BF-F3 (MHCIIb, 1: 400, culture supernatant). Negative controls were run in parallel by substituting a non-specific IgG (mouse IgG, 1: 2000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or by omitting the primary antibody. Each MHC stain also served as a positive control for the others. This panel of monoclonal antibodies allowed direct identification of pure type-I, -IIA, and -IIB fibres, as well as associated hybrid fibre combinations. Pure type-IID/X fibres were identified as those fibres that remained unstained by this panel of antibodies.
Stained serial sections were digitally imaged at 40× magnification (Olympus IX70, Olympus Microscopes, Melville, NY, USA) with an attached camera (Spot RT, Diagnostic Instruments, Sterling Heights, MI, USA) and analysed using the Image Pro Plus software suite (v. 4.5.0.27, Media Cybernetics, Silver Spring, MD, USA). Fibres activated by stimulation were identified by the absence of glycogen staining (Kugelberg & Edstrom, 1968; Kim et al. 1995). Image Pro Plus software was used to ensure that the identification procedure was carried out consistently. Briefly, an observer set the staining intensity threshold by which the software package identified depleted fibres. The computer then applied this threshold throughout the image, ensuring uniformity. This manner of identifying fibres cannot altogether remove subjectivity, but it can ensure that the threshold set by the observer is always applied by the computer in an unbiased manner. Serial sections stained for the various adult MHC isoforms were used to classify stimulated fibres. Glycogen depletion results for rectus femoris and vastus lateralis muscles were combined and further grouped according to the fatigue resistance of the fibre types. Type-I, -I/IIA and -IIA fibres were grouped into the fatigue-resistant category (FR) while type-IID/X and -IIB fibres were grouped into the fast fatigable category (FF) (Pette & Vrbova, 1992; Pette & Staron, 1997).
Myosin heavy chain electrophoresis
To determine whether glycogen depletion of each fibre type was proportional to its whole muscle content, MHCI, MHCIIa, MHCIId/x, and MHCIIb isoform contents were quantified by SDS-PAGE in eight rectus femoris and eight vastus lateralis muscles (Bamford et al. 2003). Previous reports have shown that electrophoretic quantification of MHC isoform content provides an accurate estimation of MHC isoform based fibre type content (Hamalainen & Pette, 1996; Putman et al. 2003; Putman et al. 2004). Briefly, muscles were homogenized in ice-cold buffer containing 100 mm Na4P2O7 (pH 8.5), 5 mm EGTA, 5 mm MgCl2, 0.3 m KCl, 10 mm DTT and 5 mg ml−1 of a protease inhibitor cocktail (Complete™, Roche Diagnostics Corporation, Indianapolis, IN, USA). Samples were centrifuged at 4°C, diluted 1: 1 with glycerol and stored at −20°C until analysed. Total protein content of the extracts was analysed using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA) (Bowers-Komro et al. 1989), diluted to 0.2 μg μl−1 in Laemmli-lysis buffer (Laemmli, 1970) and boiled for 10 min. Five microlitres of each extract was electrophoresed for 24 h at 275 V and 12°C on 7% polyacrylamide gels containing glycerol, under denaturing conditions. MHC isoforms were detected by silver staining (Oakley et al. 1980) and evaluated with integrated densitometry using the Syngene GeneSnap and GeneTools software package (Chemigenius Gel Documentation System, Syngene, UK).
Force recruitment experiments
Isometric twitch force was measured during application of NCS or ISMS to generate recruitment curves for these two stimulation protocols. Nerve cuffs were placed around the femoral nerve and a laminectomy was performed to expose spinal cord segments L3–L4. The animal was fixed at the iliac crest and at the epicondyles of the femur with a stereotaxic array to prevent movement. The patellar tendon was dissected from its point of insertion and attached to a force transducer (Interface MB-5, Interface Inc., Scottsdale, AZ, USA). Stimulation (single, 400 μs, biphasic, cathodic-first, charge-balanced pulses) proceeded with the nerve cuff over the femoral nerve or with intraspinal microwires inserted within the quadriceps motoneurone pool in the ventral spinal cord (Mushahwar & Horch, 2000a). Data were amplified 100-fold and captured at a sampling rate of 1000 s−1 using a data acquisition interface (Power 1401, Cambridge Electronic Design, Cambridge, UK) with associated software (Signal v. 2.13, Cambridge Electronic Design). Stimulation amplitude was varied upwards from subthreshold levels in quasi-random order and the force of the evoked twitch was measured with rest periods of approximately 30 s between pulses to avoid potentiation between twitches. Isometric force was recorded from the same hindlimb using both NCS and ISMS methods to allow paired comparisons. In some cases multiple ISMS curves obtained by stimulating different locations in the same pool were gathered on the same hindlimb to compare against a single NCS curve. Recruitment curves were gathered from six animals providing six NCS and seven ISMS curves.
To compare twitch forces evoked by NCS and ISMS, forces were normalized to the maximum twitch force obtained by the nerve cuff for each hindlimb and plotted against pulse amplitude (μA). Linear regression was used to calculate slopes for the recruitment curves between threshold and 250 μA or between threshold and the onset of force plateau if this occurred before 250 μA.
Confirmation of microwire placement in ISMS trials
Spinal cords from ISMS glycogen depletion and force recruitment experiments were excised following stimulation, placed in a slightly stretched position on aluminium foil and frozen in melting isopentane (−156°C) cooled in liquid nitrogen. Cords were later sectioned to verify ISMS microwire placement. Typically, an electrolytic lesion is produced at the end of the experiment to aid in confirming the microwire location (Mushahwar & Horch, 1998). However, this procedure was not possible in this experiment because of the need to control muscle activation and fibre depletion in each condition. This made locating microwire tips more difficult. Thus, only 17 of 33 microwires were retrieved and their locations positively confirmed. The other 16 microwire locations were not confirmable, either because of inadvertent dislodging of the wire during extraction and sectioning, or because of an inability to find the exact tip location without the presence of an electrolytic lesion. Nevertheless, stimulation through each of these wires did produce gradual force recruitment following insertion. In our experience, this is a clear indication that the tips of these wires were located correctly within the ventral grey matter (Saigal et al. 2004).
Statistical analysis
The same statistical analysis was used for both glycogen depletion and force recruitment experiments. All analyses were performed with a computerized software statistics package (SPSS 11.0, SPSS Inc., Chicago, IL, USA). Differences between group means were determined using Student's t test with independent samples or one-way ANOVA with Scheffe's post hoc test. All planned comparisons were undertaken with one-tailed analysis, whereas unplanned comparisons were analysed using a two-tailed test. Differences were considered significant at P < 0.05. All results are presented as means ± s.e.m.
Results
Threshold levels for ISMS were significantly lower than those for NCS
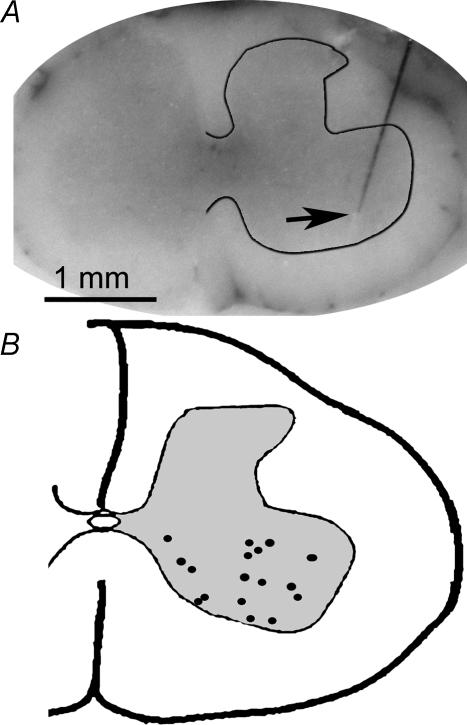
Following either the glycogen depletion or force recruitment experiments, spinal cords were extracted, frozen, and sectioned on a cryostat. The purpose of this procedure was to confirm that ISMS wires were properly located within the ventral spinal cord. Figure 1 summarizes, on a single cross-section, the tip locations for 17 of 33 intraspinal microwires used in this investigation. Mean stimulation threshold in the glycogen depletion experiments was 12.1 ± 1.0 μA and 25.0 ± 3.0 μA for ISMS and NCS, respectively, a highly significant difference (P < 0.0002). Differences in mean threshold generated during the force recruitment experiments were larger than for the glycogen depletion experiments. Mean threshold was 7.7 ± 0.1 μA and 39.1 ± 5.7 μA for ISMS and NCS, respectively (P < 0.0006).
Figure 1. ISMS electrode locations.
Frozen spinal cords were sectioned on a cryostat and representative photomicrographs (A) were taken in order to verify electrode placement within the ventral grey matter. A composite schematic for electrode tip locations (B) was generated by plotting 17 ISMS electrodes used in this study.
ISMS caused greater depletion of fatigue-resistant fibres at high amplitudes of stimulation
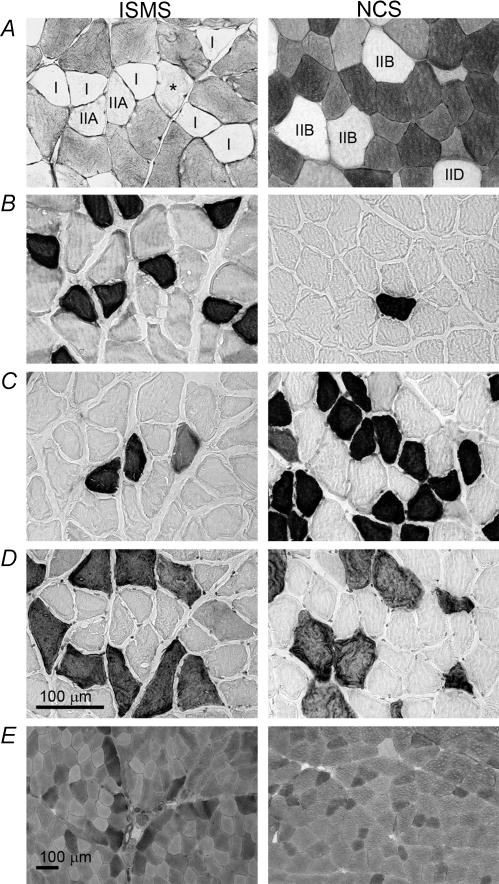
Glycogen depletion experiments were carried out in order to determine which fibres were activated by either NCS or ISMS under different stimulation conditions. Figure 2 shows an example of the method used to identify glycogen-depleted fibres in one ISMS animal and one NCS animal, both stimulated at 1 s−1 1.2T. Depleted fibres were identified by the PAS stain (Fig. 2A) and then characterized according to their MHC content in serial sections (type-I, -IIA, -IIB in Fig. 1B–D, respectively). In the example shown, ISMS primarily depleted type-I and -IIA fibres while NCS depleted type-IIB and -IID fibres. PAS staining of control muscles demonstrated that neither the ischaemia, nor the extraction procedure produced detectable glycogen depletion (Fig. 2E). In the 1.2T condition, the mean number of depleted fibres per animal was 176.6 ± 27.5 and 181.2 ± 44.6 for ISMS and NCS, respectively. In the 3.0T condition, the mean number of fibres depleted per animal was 150.5 ± 49.5 and 1215.7 ± 363.6 for ISMS and NCS, respectively. The sum of all fibres depleted and analysed in this study was 14 637.
Figure 2. Glycogen depletion protocol.
Representative photomicrographs of histological and immunohistochemical stains were used to identify glycogen content (periodic acid Schiff's) (A), type-I fibres (anti-MHCI) (B), type-IIA fibres (anti-MHCIIa) (C) and type-IIB fibres (anti-MHCIIb) (D). The scale bar in D applies to panels A–D. Control stains for glycogen (E) were performed on sham control hindlimb muscle in order to verify that neither the ischaemia nor the extraction procedure produced glycogen depletion. Fibres were identified by their reaction with the corresponding MHC antibody, while type-IID/X fibres were identified by the absence of immunohistochemical staining. Although the IIA fibre marked with an asterisk in A appears depleted to the naked eye, it fell just below the threshold for detection according to computer analysis.
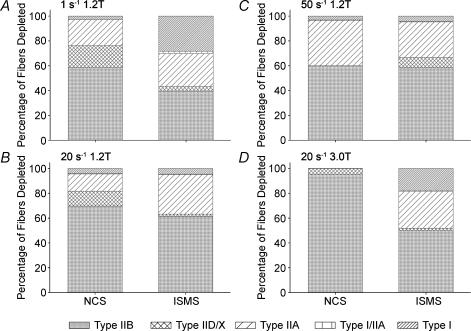
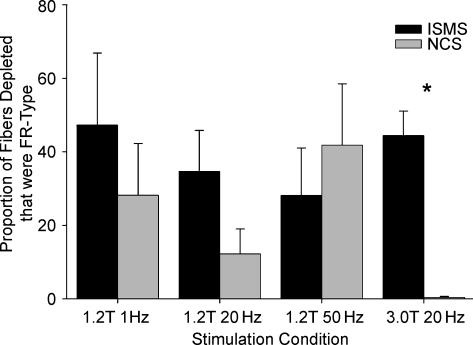
Figure 3 shows the cumulative proportion of each fibre type depleted in each stimulation condition from all animals. The total number of fibres depleted in the 1 s−1 1.2T, 20 s−1 1.2T, 50 s−1 1.2T and 20 s−1 3.0T were 726, 1481, 1055 and 7294, respectively, for NCS, and 1249, 1178, 751 and 1188, respectively, for ISMS. NCS stimulation at 3.0T depleted significantly more fibres than any other condition (P < 0.004). All other conditions were not significantly different in terms of total fibres depleted. The only condition that resulted in greater depletion of type I, I/IIA and IIA fibres by ISMS was 20 s−1 3.0T. Figure 4 shows the proportion of glycogen-depleted fibres that were FR type (type I + type I/IIA + type IIA) in each experimental group (n = 6 animals per group). At 1.2T, the proportion of FR fibres recruited by ISMS was 1.7- (P < 0.23), 2.8- (P < 0.059) and 0.67-fold (P < 0.24) that of NCS at 1, 20 and 50 s−1 frequencies, respectively. Although both 1 and 20 s−1 ISMS recruited proportionally more FR fibres than NCS, this trend did not reach statistical significance in either case. Specifically, in the 1.2T conditions, some ISMS samples did not display depleted FR fibres, producing large variances in the data (e.g. min 0.0%, max 97.8% in the 1 s−1 condition). In contrast, the 3.0T condition produced consistent and highly significant increases (P < 0.0001) in the proportion of FR fibres recruited by ISMS over NCS. Only 0.4% of fibres recruited by 20 s−1 3.0T NCS were FR fibres, whereas 44.4% of the total fibres depleted by ISMS at 20 s−1 3.0T were FR.
Figure 3. Glycogen depletion results.
Graphs show MHC type-I, -I/IIA, -IIA, -IID/X and -IIB fibres depleted by either NCS or ISMS as a proportion of the total fibres depleted in 1 s−1 1.2T (A), 20 s−1 1.2T (B), 50 s−1 1.2T (C) and 20 s−1 3.0T (D) conditions. Six animals were used for each stimulation condition.
Figure 4. Fatigue-resistant fibres depleted in each condition.
Proportion of glycogen-depleted fibres that were FR type (MHC type-I, -I/IIA and -IIA) in each experimental group (n = 6) by either NCS or ISMS. Results are shown as means ± s.e.m. for both 1.2T and 3.0T. Statistical significance was set at P < 0.05.
Fibre type recruitment by ISMS and NCS in mixed fast-twitch muscle
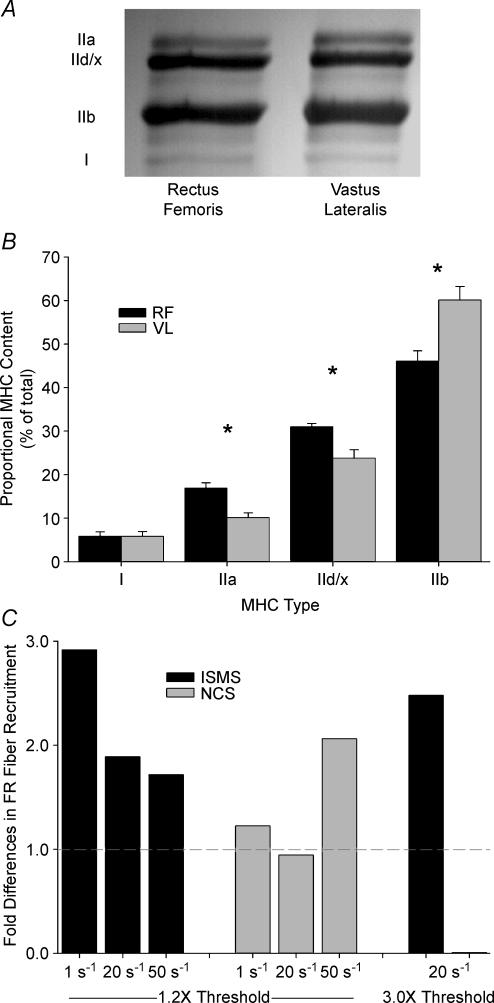
Electrophoresis was performed to measure the MHC-isoform contents of activated muscles. This allowed us to determine whether the number of FR fibres activated by ISMS or NCS was proportional to their distribution within the vastus lateralis and rectus femoris, or if these fibres were selectively recruited. The MHC-isoform contents of the vastus lateralis and rectus femoris are shown in Fig. 5. There was no difference in MHCI content between rectus femoris and vastus lateralis (P = 0.99), but the rectus femoris displayed significantly more MHCIIa (P < 0.001), MHCIId/x (P < 0.003), and less MHCIIb (P < 0.003). For the purposes of this study the MHC-isoform contents of the rectus femoris and vastus lateralis were grouped into FR and FF categories. FR MHC content was 22.8 ± 2.0% and 16.0 ± 1.4% in the rectus femoris and vastus lateralis, respectively (P < 0.02). These data were further summarized to compare with the grouped rectus femoris and vastus lateralis glycogen depletion results. Using these broad classifications, the proportion of FR and FF fibres were 19.4 ± 1.5% and 80.6 ± 1.5%, respectively. Comparison of the cumulative proportion of FR fibres depleted in each stimulation condition revealed that ISMS recruited FR fibres at 2.9-, 1.9-, 1.7- and 2.5-fold the FR MHC content at 1, 20 and 50 s−1 1.2T and 20 s−1 3.0T, respectively. In contrast, NCS recruited FR fibres at 1.2, 1.0, 2.1 and 0.0 times the FR MHC content at 1, 20 and 50 s−1 1.2T and 20 s−1 3.0T, respectively (Fig. 5C).
Figure 5. Depletion of fatigue-resistant fibres as a function of myosin heavy chain content.
Relative MHC isoform content in 8 rectus femoris and 8 vastus lateralis muscles from 8 different animals as determined by one-dimensional electrophoresis. A, representative gel blot of rectus femoris and vastus lateralis muscles with type-IIa, -IId/x, -IIb and -I MHC bands delineated. B, bar-plot showing results of SDS-PAGE for MHC isoform content in rectus femoris (RF) and vastus lateralis (VL) muscles. C, after grouping MHC isoform content results into FR and FF categories the total number of FR fibres depleted by each condition was divided by the FR MHC content yielding fold differences in the recruitment of FR fibre types. Note that statistical significance in B was set at P < 0.05.
ISMS recruited force more gradually than NCS
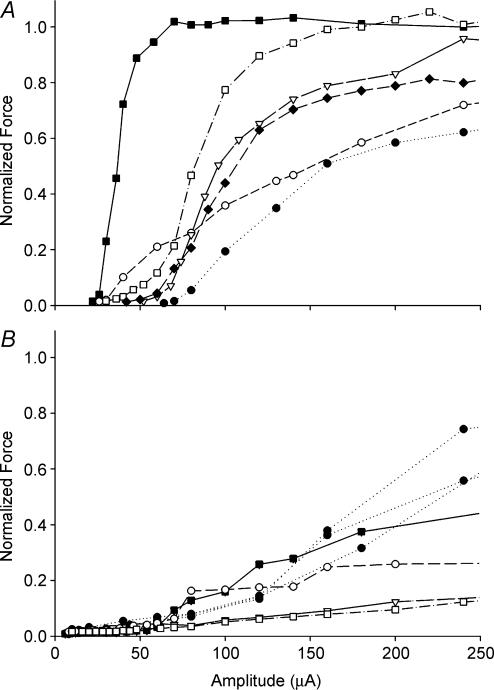
Force recruitment experiments were performed to compare the functional characteristics of ISMS and NCS with the results from the glycogen depletion experiments. Twitch recruitment curves obtained via intraspinal and nerve cuff stimulation from 0 to 250 μA are shown in Fig. 6. The mean slope of the recruitment curves for NCS was 0.020 ± 0.006 and was thus 4.9-fold steeper (P < 0.02) than the mean slope of recruitment curves for ISMS (0.0041 ± 0.0008).
Figure 6. Peak twitch force results.
Force recruitment curves produced by either NCS of the femoral nerve (A) or ISMS in the quadriceps motoneurone pool in the ventral grey matter (B). Force is normalized to the peak twitch force produced by the nerve cuff in each hindlimb and is expressed as a function of pulse amplitude in microamps. The same symbols in A and B indicate that the NCS and ISMS curves were obtained from the same animals.
A charge level of 90 nC has previously been identified as the maximum level for safe charge injection in central nervous system tissue (Agnew & McCreery, 1990). Since the pulse width for all stimulation experiments was 400 μs, the maximum safe charge of 90 nC corresponds to 225 μA. Mean peak twitch force at 225 μA was 1.56 ± 0.49 N and 0.34 ± 0.06 N for NCS and ISMS, respectively (P < 0.03). At 225 μA, ISMS through a single microwire recruited a mean of 36.8 ± 11.1% of twitch force recruited by NCS at the same charge level.
Discussion
Overview
The purpose of this study was to compare the muscle recruitment characteristics of NCS, a peripheral method of stimulation, with the novel rehabilitative therapy, ISMS. In the first set of experiments, muscle fibres activated at three different frequencies and two different amplitudes by each stimulation protocol were identified by the glycogen depletion method and classified according to their corresponding MHC-based fibre types. In the second set of experiments, data were gathered from ISMS and NCS twitch force recruitment curves to assess force recruitment characteristics. During high-amplitude stimulation ISMS activated a significantly larger proportion of fatigue-resistant fibres than NCS. In accordance with this result we found that NCS twitch force recruitment was steeper than ISMS. ISMS through a single electrode produced a considerable amount of force with more gradual recruitment. Whole nerve stimulation through the nerve cuff required significantly more charge at threshold but produced higher forces at the same charge level. Other work from our laboratory has shown that ISMS probably recruits motoneurones through interneurones or afferent pathways (Mushahwar et al. 2003). This would explain the more gradual recruitment of force and increased activation of fatigue-resistant muscle fibres by ISMS compared with NCS. This study corroborates and enhances previous evidence for the efficacy of ISMS and is the first work to compare directly the muscle recruitment properties of ISMS and NCS.
ISMS causes greater depletion of fatigue-resistant fibres at high amplitudes of stimulation
In this study, glycogen depletion was enhanced using ischaemia. Our preliminary experiments, as well as the work of others (Rafuse & Gordon, 1996), showed that stimulation alone required up to 3 h to produce detectable glycogen depletion in slow-twitch muscle fibres. Therefore, ischaemia was induced in the quadriceps muscles, but not in the femoral nerve, by occlusion of arterial flow through the femoral artery. PAS staining in control muscles showed that ischaemia alone did not induce detectable glycogen depletion, thus proving the validity of this technique. Glycogen depletion via ISMS or NCS resulted in differences in the proportions of FR fibres depleted. In the 1.2T condition these differences approached significance at 20 s−1 (P < 0.059) but not in the 1 or 50 s−1 conditions.
In all of the 1.2T conditions, there were at least a few ISMS samples that did not deplete FR fibres. Although fibre recruitment resulting from low-amplitude stimulation may be especially sensitive to electrode placement, at 3.0T small differences in electrode placement are less likely to affect the outcome due to increased current spread. Indeed, the differences between ISMS and NCS at 3.0T were particularly consistent, where the former recruited a significantly higher proportion of FR fibres (Fig. 3). It is interesting that the 3.0T ISMS condition did not deplete a significantly larger number of fibres than other ISMS conditions. This suggests a limited spread of current as has been shown in the past (Mushahwar & Horch, 1997; Pikov & McCreery, 2004; Lemay & Grill, 2004; Snow et al. 2005).
When comparing the proportion of FR fibres depleted by each condition it is illuminating to consider the contents of MHC isoforms in rectus femoris and vastus lateralis muscles. Electrophoretic analyses revealed that 19.4% of the total MHC content can be placed in the FR category. Thus, the cumulative proportion of FR fibres recruited by ISMS represents a 2.9-, 1.9-, 1.7- and 2.5-fold increase over the FR MHC content at 1, 20 and 50 s−1 1.2T and at 20 s−1 3.0T, respectively (Fig. 4). In contrast, NCS recruited FR fibres at 1.2-, 1.0-, 2.1- and 0.0-fold the FR MHC content at 1, 20 and 50 s−1 1.2T and at 20 s−1 3.0T, respectively. Although ISMS preferentially recruited FR fibres in the rectus femoris and vastus lateralis, only the 50 s−1 1.2T NCS condition did the same.
Surprisingly, the 50 s−1 condition not only recruited FR fibres out of proportion to their MHC isoform content, but 50 s−1 NCS depleted a larger proportion of FR fibres than the 50 s−1 ISMS condition (Figs 3 and 4). The proportion of FR fibres depleted by ISMS did not decrease significantly compared with other frequencies. Instead, there was an increase in the number of FR fibres depleted by NCS at 50 s−1. Other authors have used peripheral, low amplitude stimulation at 100 s−1 to produce contractions which may contain both peripheral and central components (Collins et al. 2001; Collins et al. 2002). These authors suggested that their results were due to motoneurone activation through Ia afferents and the generation of plateau potentials. Although the frequencies used in this study were not as high as the 100 s−1 used by Collins et al. (2001, 2002), it is possible that the higher proportion of FR fibres at the 50 s−1 1.2T NCS condition is due to activation of Ia afferents. These afferents make physiologically normal connections to motoneurones and could recruit fibres in a near-normal order. Although our data suggest that it is possible for NCS-type systems at 50 s−1 to recruit FR fibres, they would probably still suffer from problems encountered by peripheral stimulation methods (Popovic, 1992). Furthermore, this result would only be present at lower stimulation amplitudes where low levels of force are produced. Indeed, our results suggest that at 3.0T stimulation this effect is abolished due to the overriding influence of whole nerve stimulation through motor axons at higher amplitudes.
There is one report of surface stimulation recruiting fibres in a mixed manner (Thomas et al. 2002), but the results of this experiment are difficult to interpret (Enoka, 2002). It is likely that the authors showed neither normal recruitment nor truly random recruitment. Rather, it is more likely that the authors showed pseudorandom recruitment order due to the spread of current from the point-source surface electrode used in these experiments. Although the largest axons are characterized by their low recruitment thresholds (Henneman et al. 1965), the spatial orientation of axons in the nerve trunk could result in a situation where the smallest axons receive current above threshold level while larger axons, located further away do not. This ‘depth effect’ phenomenon could also explain the pseudorandom recruitment order reported by Singh et al. (2000) when using intramuscular BION™ electrodes.
ISMS recruits force more gradually than NCS
Similar to other work in our lab (Snow et al. 2005) we found that the mean slope for ISMS force recruitment curves was 4.9-fold lower than for NCS. A range of force recruitment curves generated by ISMS has previously been reported for electrodes targeting feline quadriceps motoneurone pools (Mushahwar & Horch, 2000a) and intermediate grey matter (Aoyagi et al. 2004). It has been proposed that differences in electrode placement within a motoneurone pool may activate different subsets of motoneurones targeting different compartments in skeletal muscle (English et al. 1993; Lieber & Friden, 2000). The fibre type distribution of these compartments may vary, and thus the force recruitment characteristics can change depending on microwire placement. This may point to another advantage of activating motoneurones in the ventral horn. Not only is it possible to activate muscles selectively with ISMS (Mushahwar & Horch, 1998, 2000a), this selectivity may also extend to subsets of motoneurone pools composed of different types of motor units. Thus, ISMS seems capable of activating the muscles in a more gradual manner, as required for postural support for extended periods, and in a steep manner as needed for standing from a chair. A multielectrode array has been proposed for ISMS (Mushahwar & Horch, 1997; Snow et al. 2005) and our results suggest that such a system could be used not just to activate individual muscles selectively but populations of fibre types within the muscle. This would represent a significant improvement over current forms of peripheral nerve stimulation.
Although NCS recruited higher forces, ISMS through a single wire recruited mean force approaching 37% of that normalized to the nerve cuff at 225 μA. This result is surprising given that it was produced with only one electrode and a single pulse. Other authors have compared single pulses with 50 s−1 stimulation trains and concluded that tetanic force levels produced by single ISMS wires can match those of whole nerve stimulation through peripheral nerve cuffs (Snow et al. 2005). The use of higher frequencies, multiple electrodes and/or interleaved stimulation protocols might allow ISMS to activate the muscle fully without producing damaging effects. This approach has already proven successful in creating consistent, fatigue-resistant weight-bearing stepping in spinalized animals (Saigal et al. 2004).
Potential mechanisms for ISMS stimulation results
At the 1.2T condition the differences between NCS and ISMS did not reach significance. In fact the NCS and ISMS results from near-threshold stimulation were so similar that it is tempting to speculate that both stimulation procedures might have been acting through similar mechanisms. It has already been shown that ISMS activates afferent fibres before motoneurones (Mushahwar et al. 2003) and may also activate interneurones contained within the ventral spinal cord. In the NCS condition, Ia afferents were probably responsible for the twitch response at near-threshold conditions, otherwise known as the Hoffmann (H) reflex (Zehr, 2002). Ia afferents directly synapse on motoneurones, and thus the resulting synaptic activation of motoneurones produces a normal or near-normal physiological recruitment order. Reversed recruitment order is commonly seen at higher amplitudes with peripheral methods such as NCS or surface stimulation (Levy et al. 1990). As charge levels increase, ISMS is expected to activate more interneurones and afferents thereby recruiting force more gradually than NCS. In contrast, direct stimulation of motoneurone axons during NCS leads to rapid recruitment of fast-twitch motor units and abolition of the slow-twitch response at higher amplitudes. Neurophysiologically, this is confirmed by the increasing dominance of the muscle-wave response and disappearance of the H-reflex as stimulus amplitude increases beyond the maximal H-reflex response (Zehr, 2002). If stimulus amplitude is increased further, F-waves will be produced by NCS because of antidromic activation of motor axons in possible combination with the Ia afferent reflex arc (Mesrati & Vecchierini, 2004). In the present study, as stimulation amplitude increased, NCS reached peak twitch force quickly due to the preferential activation of FF fibres. In contrast, ISMS twitch force continued to rise gradually due to the delayed recruitment of large, fast-twitch fatigable motor units. Our results with 3.0T stimulation demonstrated that ISMS preferentially recruited FR fibres in rectus femoris and vastus lateralis muscle while NCS almost exclusively recruited FF fibres (Fig. 3). Ultimately, the pattern of ISMS activation is similar to the physiologically normal activation of motor units in which the smallest, most fatigue-resistant motor units are activated first, holding the larger units in reserve for activities requiring large force or power (Henneman 1957, 1965).
Previous work has provided insight into how muscle fibre type is specified over the long term. Periods of tonic motoneurone activity promote a slow-fibre program while periods of inactivity interspersed with bursts of high frequency activity promote the fast-fibre program (Pette & Vrbova, 1992). Evidence for this position has been enhanced by the discovery of the calcineurin signalling pathway, which transduces this activity pattern signal into the nucleus and alters gene expression (Schiaffino & Serrano, 2002). In accordance with this theory there is a dramatic slow-to-fast transformation of muscle fibre type following SCI (Burnham et al. 1997; Castro et al. 1999). Due to the plastic nature of skeletal muscle (Pette & Staron, 1997), previous authors have performed chronic low-frequency stimulation through a peripheral FNS system to rehabilitate skeletal muscle following SCI. The ultimate goal of these interventions is the up-regulation of FR fibre types and restoration of fatigue resistance (Martin et al. 1992; Andersen et al. 1996).
Although fatigue-resistant movements are an important goal for FNS it is also important to ensure that the potential for high force production in stimulated muscle is preserved over the long-term. The mixed fibre type content of intact quadriceps muscle (Fig. 5) represents the capacity of this muscle to undertake both fatigue-resistant and high-force movements. Thus, maintaining mixed muscle fibre types should remain the goal for FNS therapies following SCI. Unfortunately, long-term stimulation with peripheral forms of FNS entails the possibility of complete transformation of the muscle such that no fast fibres remain to produce high-force movements. Andersen et al. (1996) performed 30 min of stimulation through surface electrodes three times a week at 60 s−1 and approximately 3.25–7.25T for up to 12 months in five male spinal cord injured subjects. Following stimulation, fibres expressing pure IID/X MHC (the fastest isoform expressed in humans) dropped from 37.2 ± 15.5% to 2.3 ± 1.4% of the total fibre content with concomitant increases in pure IIA fibre content from 21.2 ± 13.9% to 91.2 ± 2.8% (Andersen et al. 1996). This dramatic transformation is to be expected from peripheral forms of stimulation, which entail a reversed recruitment order of motor unit firing. The fastest fibres, which undergo a phasic duty cycle in intact muscle, are also the first activated by peripheral systems, thus being highly susceptible to transformation. In contrast, ISMS through a single electrode allowed for fibre type selectivity and recruited a mixed fibre type at both 1.2T and 3.0T conditions. The capacity of ISMS to recruit slow fibres while leaving fast fibres in reserve should aid in maintaining a mixed fibre type and should prove helpful in rescuing the normal mixed-muscle phenotype in chronic SCI cases. Further studies with ISMS should be designed to address the capacity of this novel rehabilitative therapy to rescue the normal mixed-muscle phenotype in a chronic SCI model.
Acknowledgments
The authors thank Mrs Jean Pearcey, Ms Catherine O'Brien and Ms Sabrina Rashid for excellent technical assistance. This study was funded by research grants from the National Institutes of Health (NIH), the Canadian Institute of Health Research (CIHR), the Alberta Heritage foundation for Medical Research (AHFMR) and the National Sciences and Engineering Research Council of Canada (NSERC). J.A.B. was supported by an NSERC Postgraduate Scholarship. C.T.P. and V.K.M. are both AHFMR Medical Scholars.
References
- Agnew WF, McCreery DB. Neural Prostheses: Fundamental Studies. Englewood Cliffs, NJ, USA: Prentice Hall; 1990. [Google Scholar]
- Andersen JL, Mohr T, Biering-Sorensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES) Pflugers Arch. 1996;431:513–518. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Mushahwar VK, Stein RB, Prochazka A. Movements elicited by electrical stimulation of muscles, nerves, intermediate spinal cord, and spinal roots in anesthetized and decerebrate cats. IEEE Trans Neural Syst Rehabil Eng. 2004;12:1–11. doi: 10.1109/TNSRE.2003.823268. [DOI] [PubMed] [Google Scholar]
- Bamford JA, Lopaschuk GD, MacLean IM, Reinhart ML, Dixon WT, Putman CT. Effects of chronic AICAR administration on the metabolic and contractile phenotypes of rat slow- and fast-twitch skeletal muscles. Can J Physiol Pharmacol. 2003;81:1072–1082. doi: 10.1139/y03-110. [DOI] [PubMed] [Google Scholar]
- Bowers-Komro DM, Yamada Y, McCormick DB. Substrate specificity and variables affecting efficiency of mammalian flavin adenine dinucleotide synthetase. Biochemistry. 1989;28:8439–8446. doi: 10.1021/bi00447a025. [DOI] [PubMed] [Google Scholar]
- Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997;35:86–91. doi: 10.1038/sj.sc.3100364. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993;73:857–867. doi: 10.1093/ptj/73.12.857. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve. 2002;25:763–764. doi: 10.1002/mus.10117. [DOI] [PubMed] [Google Scholar]
- Hamalainen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown MD, Egginton S, Dawson JM. Effect of long-term electrical stimulation on vascular supply and fatigue in chronically ischemic muscles. J Appl Physiol. 1994;77:1317–1324. doi: 10.1152/jappl.1994.77.3.1317. [DOI] [PubMed] [Google Scholar]
- Jilge B, Minassian K, Rattay F, Dimitrijevic MR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol Cybern. 2004;91:359–376. doi: 10.1007/s00422-004-0511-5. [DOI] [PubMed] [Google Scholar]
- Kim CK, Bangsbo J, Strange S, Karpakka J, Saltin B. Metabolic response and muscle glycogen depletion pattern during prolonged electrically induced dynamic exercise in man. Scand J Rehabil Med. 1995;27:51–58. [PubMed] [Google Scholar]
- Kugelberg E, Edstrom L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968;31:415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemay MA, Grill WM. Modularity of motor output evoked by intraspinal microstimulation in cats. J Neurophysiol. 2004;91:502–514. doi: 10.1152/jn.00235.2003. [DOI] [PubMed] [Google Scholar]
- Levy M, Mizrahi J, Susak Z. Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng. 1990;12:150–156. doi: 10.1016/0141-5425(90)90136-b. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72:1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- Mesrati F, Vecchierini MF. F-waves: neurophysiology and clinical value. Neurophysiol Clin. 2004;34:217–243. doi: 10.1016/j.neucli.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–416. doi: 10.1038/sj.sc.3101615. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10:68–81. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Proposed specifications for a lumbar spinal cord electrode array for control of lower extremities in paraplegia. IEEE Trans Rehabil Eng. 1997;5:237–243. doi: 10.1109/86.623015. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Ann N Y Acad Sci. 1998;860:531–535. doi: 10.1111/j.1749-6632.1998.tb09096.x. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Muscle recruitment through electrical stimulation of the lumbo-sacral spinal cord. IEEE Trans Rehabil Eng. 2000a;8:22–29. doi: 10.1109/86.830945. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Trans Rehabil Eng. 2000b;8:11–21. doi: 10.1109/86.830944. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Prochazka A, Ellaway PH, Guevremont LG, Gaunt RA. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience 2003; 2003. Microstimulation in CNS excites axons before neuronal cell bodies. Program No. 276.6.2003, online. [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Pikov V, McCreery DB. Mapping of spinal cord circuits controlling the bladder and external urethral sphincter functions in the rabbit. Neurourol Urodyn. 2004;23:172–179. doi: 10.1002/nau.20008. [DOI] [PubMed] [Google Scholar]
- Popovic DB. Functional electrical stimulation for lower extremities. In: Stein RB, Peckham PH, Popovic DB, editors. Neural Prostheses: Replacing Motor Function After Disease or Disuse. New York: Oxford University Press; 1992. pp. 233–251. [Google Scholar]
- Portal JJ, Corio M, Viala D. Localization of the lumbar pools of motoneurones which provide hindlimb muscles in the rabbit. Neurosci Lett. 1991;124:105–107. doi: 10.1016/0304-3940(91)90832-e. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Comparison of natural and artificial control of movement. IEEE Trans Rehabil Eng. 1993;1:7–17. [Google Scholar]
- Prochazka A, Mushahwar VK. Spinal cord function and rehabilitation – an overview. J Physiol. 2001;533:3–4. doi: 10.1111/j.1469-7793.2001.0003b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Dixon WT, Pearcey JA, MacLean IM, Jendral MJ, Kiricsi M, Murdoch GK, Pette D. Chronic low-frequency stimulation upregulates uncoupling protein-3 in transforming rat fast-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1419–R1426. doi: 10.1152/ajpregu.00421.2004. [DOI] [PubMed] [Google Scholar]
- Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Sultan KR, Wassmer T, Bamford JA, Skorjanc D, Pette D. Fiber-type transitions and satellite cell activation in low-frequency-stimulated muscles of young and aging rats. J Gerontol a Biol Sci Med Sci. 2001;56:B510–B519. doi: 10.1093/gerona/56.12.b510. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. I. comparisons of the capacity for regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries. J Neurophysiol. 1996;75:268–281. doi: 10.1152/jn.1996.75.1.268. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The motor pools of the spinal cord. Prog Brain Res. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:430–440. doi: 10.1109/TNSRE.2004.837754. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–575. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Sharrard WJ. The distribution of the permanent paralysis in the lower limb in poliomyelitis; a clinical and pathological study. J Bone Joint Surg Br. 1955;37-B:540–558. doi: 10.1302/0301-620X.37B4.540. [DOI] [PubMed] [Google Scholar]
- Singh K, Richmond FJ, Loeb GE. Recruitment properties of intramuscular and nerve-trunk stimulating electrodes. IEEE Trans Rehabil Eng. 2000;8:276–285. [PubMed] [Google Scholar]
- Snow S, Horch KW, Mushahwar VK. Intraspinal microstimulation using cylindrical multielectrodes. IEEE Trans Biomed Eng. 2006 doi: 10.1109/TBME.2005.857638. (in press) [DOI] [PubMed] [Google Scholar]
- Stein RB, Chong SL, James KB, Kido A, Bell GJ, Tubman LA, Belanger M. Electrical stimulation for therapy and mobility after spinal cord injury. Prog Brain Res. 2002;137:27–34. doi: 10.1016/s0079-6123(02)37005-5. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Enoka RM. Quantification of the factors that influence discharge correlation in model motor neurons. J Neurophysiol. 2004;91:796–814. doi: 10.1152/jn.00802.2003. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Nelson G, Than L, Zijdewind I. Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles. Muscle Nerve. 2002;25:797–804. doi: 10.1002/mus.10111. [DOI] [PubMed] [Google Scholar]
- Totosy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol. 1992;67:1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]