Abstract
Single-dose nevirapine (sdNVP) for prevention of mother-to-child transmission of HIV-1 can select nevirapine (NVP)-resistant variants, but the frequency, duration, and clinical significance of this resistance is not well defined. We used a sensitive allele-specific PCR assay to assess the emergence and persistence of NVP-resistant variants in plasma samples from 22 women with HIV-1 subtype C infection who participated in a study of sdNVP for prevention of mother-to-child transmission of HIV-1. The women were categorized into three groups on the basis of detection of NVP resistance by standard genotype analysis. Group 1 (n = 6) had NVP resistance detected at 2 and 6 mo after sdNVP, but not at 12 mo. Group 2 (n = 9) had NVP resistance detected at 2 mo, but not 6 mo. Group 3 (n = 7) had no NVP resistance detected at any time point. Allele-specific PCR analysis for the two most common NVP resistance mutations (K103N and Y181C) detected NVP-resistant variants in most (16 of 21) samples that were negative for NVP resistance by standard genotype, at levels ranging from 0.1% to 20% 1 yr after treatment. The frequency of NVP-resistant mutations decreased over time, but persisted above predose levels for more than 1 yr in ≥23% of the women. These findings highlight the urgent need for studies assessing the impact of sdNVP on the efficacy of subsequent antiretroviral therapy containing NVP or other nonnucleoside reverse transcriptase inhibitors.
Keywords: allele-specific PCR, resistance, low-frequency mutants
The treatment of pregnant women with antiretroviral therapy, in combination with zidovudine chemoprophylaxis administered to newborns, has dramatically reduced vertical transmission of HIV in developed countries (1–5). However, perinatal transmission of HIV-1 remains a major problem in developing countries because of limited access to antiretroviral therapy (6–8). In this latter setting, the simple regimen of maternal single-dose intrapartum nevirapine (NVP) and single-dose NVP (sdNVP) for the newborn reduces the risk of mother-to-child HIV-1 transmission (MTCT) by 41% through age 18 mo (9). Moreover, sdNVP has been demonstrated to be more effective than zidovudine chemoprophylaxis alone, and to be equivalent to NVP plus zidovudine in preventing MTCT (9–12). The efficacy of sdNVP in preventing MTCT is attributed to its potent antiviral activity, rapid absorption, placental transfer, and long half-life (13, 14). As a result, its use has been recommended by the World Health Organization as one of several options to reduce MTCT in resource-limited countries [Joint UNAIDS/WHO press release (2000) (WHO, Geneva)].
NVP is a potent nonnucleoside reverse transcriptase inhibitor (NNRTI) of HIV-1 (15–17). If administered as monotherapy, NVP selects for resistant variants within 1 wk, commonly those variants with the K103N and/or Y181C mutations in reverse transcriptase, which confer cross-resistance to other approved drugs in the NNRTI class (18–20). The K103N mutation appears to have little effect on the replicative capacity of HIV-1, allowing variants with this mutation to persist long after NVP therapy has been stopped (21–23).
NVP-resistant variants have been detected by standard genotyping methods in 15–50% of mothers who have received intrapartum sdNVP (24, 25), with “fading” of the resistant population to below the detection limit of standard genotyping in most women by 6 mo. However, the persistence of low-frequency mutants in women and children who were exposed to sdNVP was recently described by two important studies. The first of these studies, by Flys et al. (26), found that NVP-resistant variants encoding K103N were detected by more-sensitive methods (LigAmp) in three of nine women and one of five infants for 12–24 mo after sdNVP. Similarly, in a cross-sectional study by Johnson et al. (27) that employed a sensitive real-time PCR method, K103N variants were detected in 16 of 40 women 4–9 mo after sdNVP.
In the present study, we used a sensitive allele-specific RT-PCR assay for K103N (AAT or AAC alleles) and Y181C (TGT allele) to analyze longitudinal samples from South African women infected with HIV-1 subtype C who had received sdNVP as part of a program to prevent MTCT (22). Our objective was to assess the extent of persistence and rates of decay of NNRTI resistance variants with these alleles.
Results
NVP Resistance After sdNVP.
All of the women studied were infected with subtype C HIV-1, as determined by standard genotype analysis. The women were categorized into three groups on the basis of detection of NVP-resistance mutations by standard genotyping (Tables 1–3, Fig. 1). Group 1 (n = 6) had NVP-resistance mutations detected at 2 mo and 6 mo, but not at 12 mo, after sdNVP. Group 2 (n = 9) had NVP resistance mutations detected at 2 mo, but not at 6 mo. Group 3 (n = 7) had no resistance mutations detected at 2 mo after sdNVP, which was the only sampling time point.
Table 1.
NNRTI-resistant variants: Group 1
| Patient | Time after sdNVP, mo | Viral RNA level, copies per ml | CD4+ T cells per μl | Standard genotype results | % 103N (AAC) | % 103N (AAT) | % 103K (AAA) | % 181C (TGT) | % 181Y (TAT) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 307,000 | 182 | — | 0.006 | 0.1 | 99.9 | 0.09 | 99.9 |
| 1.9 | 209,000 | 181 | K103N, V106M | 20.3 | 3.6 | 76.1 | 1.6 | 98.4 | |
| 6.1 | 417,000 | 164 | K103N | 12.6 | 0.90 | 86.5 | 0.4 | 99.7 | |
| 14.1 | 750,000 | 161 | — | 1.4 | 0.04 | 98.5 | 0.2 | 99.8 | |
| 2 | 0 | 208,000 | 279 | — | 0.01 | 0.001 | 100 | 0.4 | 99.6 |
| 1.5 | 92,100 | 346 | K103N, G190A | 33.3 | 3.2 | 63.5 | 7.6 | 92.4 | |
| 6.0 | 79,800 | 210 | K103N | 14.3 | 11.9 | 73.8 | 0.2 | 99.8 | |
| 12.0 | 466,000 | 158 | — | 1.9 | 6.0 | 92.1 | 0.06 | 99.9 | |
| 3 | 0 | 101,000 | 309 | — | 0.03 | 0.01 | 100 | 0.2 | 99.7 |
| 2.8 | 87,600 | 389 | K103N, Y181C | 20.0 | 38.2 | 41.8 | 1.3 | 98.7 | |
| 7.3 | 113,000 | 172 | K103N | 14.2 | 3.8 | 82.0 | 0.009 | 100 | |
| 13.3 | 57,800 | 147 | — | 4.6 | 0.004 | 95.4 | 0.009 | 100 | |
| 4 | 0 | 38,500 | 641 | — | 0.07 | 0.001 | 99.9 | 0.2 | 99.8 |
| 3.1 | 23,000 | 680 | K103N, Y188C | 11.5 | 4.2 | 84.3 | 0.3 | 99.7 | |
| 6.7 | 14,700 | 673 | K103N | 1.6 | 0.78 | 97.7 | 0.05 | 100 | |
| 12.2 | 33,100 | 547 | — | 0.01 | 0.002 | 100 | 0.1 | 99.8 | |
| 5 | 0 | 112,000 | 297 | — | 0.05 | 0.01 | 99.9 | 0.1 | 99.8 |
| 1.6 | 84,000 | 211 | K103N, V106A, Y181C | 47.4 | 35.1 | 17.5 | 16.4 | 83.6 | |
| 5.6 | 209,000 | 353 | K103N | 17.9 | 10.1 | 72.0 | 0.4 | 99.6 | |
| 12.1 | 230,000 | 251 | — | 15.9 | 0.1 | 84.0 | 0.05 | 99.9 | |
| 6 | 0 | 75,800 | 256 | — | 0.08 | 0.01 | 99.9 | 0.1 | 99.9 |
| 3.1 | 603,000 | 409 | K103N | 36.1 | 25.0 | 38.9 | 1.1 | 98.9 | |
| 5.0 | 653,000 | 317 | K103N | 10.4 | 9.2 | 80.4 | 0.05 | 100 | |
| 12.1 | 441,000 | 289 | — | 8.2 | 0.1 | 91.7 | 0.1 | 99.9 | |
| Assay background | 0.01 | 0.003 | 0.3 | 0.07 | 0.1 | ||||
Resistant variants are shown in bold.
Table 2.
NNRTI-resistant variants: Group 2
| Patient | Time after sdNVP, mo | Viral RNA level, copies per ml | CD4+ T cells per μl | Standard genotype results | % 103N (AAC) | % 103 (AAT) | % 103K (AAA) | % 181C (TGT) | % 181Y (TAT) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 304,000 | 336 | — | <0.006 | <0.006 | 100 | 0.07 | 99.9 |
| 1.6 | 62,900 | 799 | K101E, Y188C | 3.2 | 0.06 | 96.7 | 1.5 | 98.5 | |
| 6 | 40,700 | 471 | — | 10.1 | <0.006 | 89.9 | 0.30 | 99.9 | |
| 12 | 106,000 | 388 | — | 0.7 | 0.02 | 99.3 | 1.3 | 98.7 | |
| 2 | 0 | 108,000 | 154 | — | <0.006 | <0.006 | 100 | 0.04 | 100 |
| 2 | 37,100 | 227 | K103N, Y188C | 26.4 | 18.4 | 55.2 | 13.4 | 86.6 | |
| 12 | 145,000 | 159 | — | 0.7 | 1.5 | 97.8 | 0.1 | 99.9 | |
| 3 | 0 | 5,320 | 736 | — | 0.2 | 0.2 | 99.6 | 0.2 | 99.8 |
| 1.6 | 8,640 | 923 | K103N, Y188C | 55.8 | 0.03 | 42.9 | 3.7 | 96.3 | |
| 6.1 | 278,000 | 911 | — | 8.1 | 0.05 | 91.6 | 0.7 | 99.3 | |
| 12.1 | 30,100 | 686 | — | 0.05 | 0.05 | 99.9 | 0.9 | 99.1 | |
| 4 | 0 | 42,600 | 544 | — | 0.07 | 0.01 | 99.9 | 0.2 | 99.8 |
| 1.6 | 18,400 | 1164 | K103N | 18.8 | 0.03 | 80.8 | 1.1 | 98.9 | |
| 6.1 | 85,500 | 675 | — | 0.02 | 0.01 | 100 | 0.1 | 99.9 | |
| 12.1 | 42,300 | 532 | — | 0.03 | <0.03 | 100 | 0.20 | 99.8 | |
| 5 | 0 | 34,600 | 249 | — | 0.08 | <0.03 | 100 | 0.6 | 99.4 |
| 1.8 | 13,800 | 310 | K103N | 39.8 | <0.1 | 59.8 | 5.3 | 94.7 | |
| 6.5 | 123,000 | 202 | — | 10.1 | 0.1 | 90.0 | 0.7 | 99.3 | |
| 12.1 | 33,700 | 156 | — | 0.003 | 0.02 | 100 | 0.2 | 99.8 | |
| 6 | 0 | 90,800 | 422 | — | 0.2 | 0.01 | 99.8 | 0.1 | 99.9 |
| 3.5 | 150,000 | 530 | K103N | 8.6 | 0.01 | 91.4 | 0.07 | 100 | |
| 9.3 | 58,200 | 431 | — | 2 | 0.006 | 98.0 | 0.08 | 100 | |
| 13.2 | 138,000 | 515 | — | 0.03 | 0.02 | 100 | 0.2 | 99.8 | |
| 7 | 0 | 62,200 | 119 | — | 0.008 | <0.004 | 100 | 0.2 | 99.8 |
| 2 | 297,000 | 194 | K103N, Y181C, Y188C | 25.6 | 13.4 | 61.1 | 19.6 | 80.3 | |
| 6 | 669,000 | 37 | — | 5.4 | 0.7 | 93.9 | 0.1 | 99.9 | |
| 12 | 750,000 | 6 | — | 0.009 | 0.01 | 100 | 0.1 | 99.9 | |
| 8 | 0 | 224,000 | 135 | — | 0.01 | <0.01 | 100 | 0.1 | 99.9 |
| 1.6 | 120,000 | 127 | K103N, Y181C | 9.7 | 3.0 | 87.3 | 17.9 | 82.1 | |
| 6.1 | 145,000 | 120 | — | 0.05 | 0.01 | 100 | 0.07 | 99.9 | |
| 12.8 | 242,000 | 109 | — | 0.01 | 0.01 | 100 | 0.04 | 100 | |
| 9 | 0 | 63,600 | 334 | — | 0.03 | <0.03 | 100 | 0.3 | 99.7 |
| 1.6 | 100,000 | 346 | K103N, V106M | 7.0 | 1.7 | 91.3 | 12.9 | 87.1 | |
| 5 | 172,000 | 425 | — | 6.5 | 0.07 | 93.4 | 0.04 | 99.9 | |
| 11.7 | 148,000 | 344 | — | 21.6 | 0.05 | 78.3 | 0.08 | 99.8 | |
| Assay background | 0.01 | 0.003 | 0.3 | 0.07 | 0.1 | ||||
Resistant variants are shown in bold.
Table 3.
NNRTI-resistant variants: Group 3
| Patient | Time after sdNVP, mo | Viral RNA level, copies per ml | CD4+ T cells per μl | % 103N (AAC) | % 103K (AAA) | % 181C (TGT) | % 181 (TAT) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 348,000 | 538 | 0.01 | 100 | 0.03 | 100 | |
| 1.8 | 46,000 | 561 | 0.6 | 99.4 | 0.6 | 99.4 | ||
| 2 | 0 | 70,200 | 507 | 0.01 | 100 | 0.001 | 100 | |
| 1.7 | 11,300 | 661 | 0.01 | 100 | 2.9 | 97.1 | ||
| 3 | 0 | 173,000 | 480 | 0.02 | 100 | 0.06 | 100 | |
| 1.7 | 38,300 | 321 | 0.2 | 99.8 | 0.3 | 99.7 | ||
| 4 | 0 | 13,600 | 651 | 0.06 | 100 | 0.03 | 100 | |
| 1.5 | 80,000 | 1,029 | 15.3 | 84.7 | 1.7 | 98.3 | ||
| 5 | 0 | 15,800 | 221 | 0.01 | 100 | 0.05 | 100 | |
| 1.5 | 15,400 | 309 | ND | ND | 2.7 | 97.3 | ||
| 6 | 0 | 16,400 | 305 | 0.01 | 100 | ND | ND | |
| 3.2 | 26,300 | 244 | 0.01 | 100 | ND | ND | ||
| 7 | 0 | 117,000 | 384 | 0.1 | 100 | 0.06 | 99.4 | |
| 1.4 | 12,600 | 475 | 0.03 | 100 | 0.08 | 99.2 | ||
| Assay background | 0.01 | 0.3 | 0.07 | 0.1 | ||||
All samples were negative for NNRTI resistance mutations by standard genotype analysis. Resistant variants are shown in bold. ND, not determined.
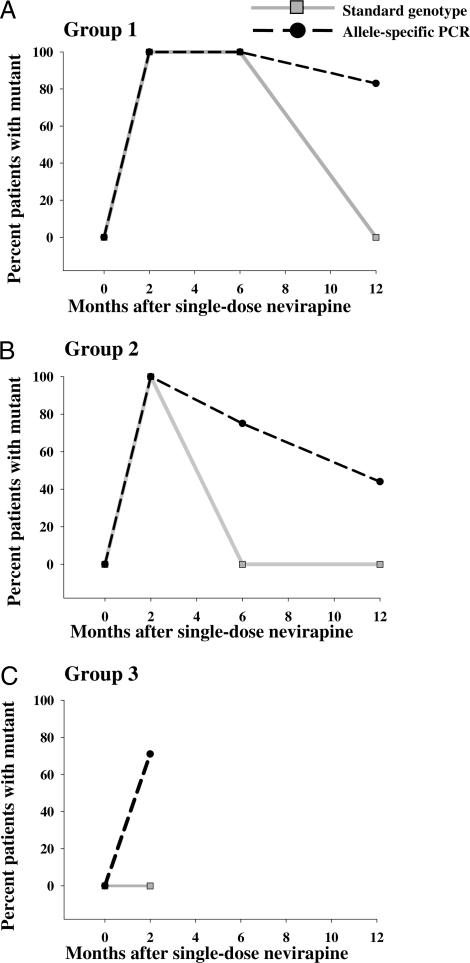
Fig. 1.
Percentage of patients with NNRTI-resistant variants 103N and/or 181C. The percentage of patients with 103N and/or 181C mutations detected by standard genotyping (gray lines and squares) was compared with the percentage of patients with mutations detected by allele-specific PCR (dashed lines and circles). Samples were divided into three groups on the basis of standard genotype results: group 1, positive for a NNRTI-resistant mutation at 2 and 6 mo (A); group 2, positive at 2 mo only (B); and group 3, never positive (C).
The percentage of NVP-resistant variants K103N (AAA to AAT or AAC) and Y181C (TAT to TGT) was determined by allele-specific RT-PCR. Predose baseline samples had negligible levels of mutations (≤0.1%) at both codons, similar to the assay background level. Results from analysis of all patient samples are shown in Fig. 1 and Tables 1–3.
103N Mutants.
Consistent with standard genotype results, all 2-mo samples from groups 1 and 2 had 103N mutations detectable by allele-specific RT-PCR, with the AAC codon ranging from ≈3.2% to 55.8% of the virus population, and the less-frequent AAT codon ranging from background to 38.2% (Tables 1 and 2). In group 1, samples from 6 mo after sdNVP showed a decrease in the mutant populations at codon 103 (AAC) to ≈12% of the virus population. At 12 mo after sdNVP, five of six women still had detectable levels of AAC mutation (frequencies of 1.4–15.9%), but the AAT mutation had declined to undetectable levels in three of these women. In group 2, most women (six of eight) had 103N AAC variants detected 6 mo after sdNVP (2.0–10.1% of the virus population), despite negative standard genotypes. AAT mutations were detected in only one of nine women, at a frequency of 0.7%. At 12 mo after sdNVP, only three of nine women still had detectable levels of AAC mutation, with frequencies ranging from 0.7% to 21.6%. In group 3, three of seven women had the AAC mutation detected 2 mo after sdNVP (0.2–15.3% of the virus population), despite negative standard genotypes.
181C Mutants.
Analysis of codon 181 revealed that, 2 mo after sdNVP, five of six patients in group 1 and eight of nine in group 2 had minor populations of Y181C ranging from 1.1% to 19.6% of the virus populations. This mutant population was detected in four samples from group 1 and six samples from group 2 by standard genotype. In group 3, most women (five of six) had detectable levels of 181C at 2 mo, ranging from 0.3% to 2.9%, despite negative standard genotypes. At 6 mo, 181C was still detectable in two of six group 1 women (with a frequency of 0.4%) and two of nine group 2 women with frequencies of 0.3% and 0.7%, respectively. At 12 mo, the Y181C mutant population had declined to below the limit of detection in all patients from group 1 and in all but two patients from group 2. Overall, more than half of the patients in groups 1 and 2 (9 of 15) had 103N and/or 181C mutations 1 yr after sdNVP.
Average Frequency of 103N and 181C Mutations Over Time.
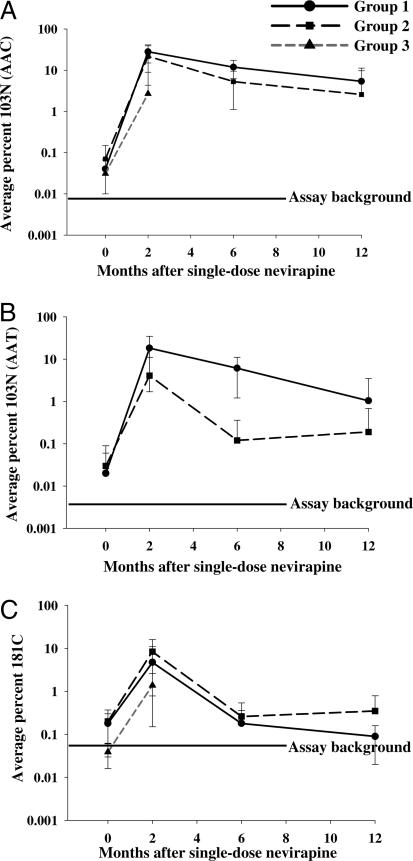
To better understand the variability in selection and decay of different mutants among the women studied, we plotted the average frequency of 103N and 181C variants over time (Fig. 2). The average frequency of the 103N AAC mutation in group 1 patient samples increased to 28% 2 mo after therapy and then decreased to 5% by 12 mo (Fig. 2A). Patient samples in group 2 showed a similar pattern of decline at somewhat lower average levels, with the average AAC mutant population increasing to ≈22% 2 mo after treatment and then declining over the next 10 mo. Importantly, 1 yr after treatment, the 103N AAC mutation persisted in these two patient groups at an average of 3–5% of the viral population. Two months after treatment, patient samples in group 3 had an average AAC mutation frequency of 3% (later samples were not available for analysis owing to the design of the parent study).
Fig. 2.
Average percent of NNRTI-resistant variants 103N (AAC and AAT) (A and B, respectively) and 181C (C) for each patient group. Longitudinal plasma samples were obtained from patients before and after sdNVP therapy, and the percentage of each mutant population was measured by allele-specific PCR. The average percentage for each group was then calculated. Solid lines with circles represent group 1, dashed lines with squares represent group 2, and dark-gray short dashes with triangles indicate group 3. The background level for each assay is shown.
The average frequency of the AAT 103N mutation followed a similar pattern (Fig. 2B). Two months after sdNVP therapy, the average frequency of this mutation increased to 18% in group 1 patient samples and to 4% in group 2, declining to 1% and 0.2% respectively, at 1 yr. Even though the frequency of this mutation was low relative to AAC, the AAT mutation in both groups persisted above the baseline frequency of 0.003% for more than a year after treatment. The AAT mutation was not detected in group 3 patient samples at 2 mo.
The average frequency of the 181C TGT mutation in group 1 patient samples increased to 4% 2 mo after sdNVP and then decreased over the next 10 mo to 0.1% (Fig. 2C). Group 2 showed a similar pattern; however, the average frequency of the 181C mutation was 2-fold greater (8%) at 2 mo after treatment, and then declined to 0.4%. In samples from women in group 3 at 2 mo after sdNVP treatment, the average frequency of the 181C mutation was 1%.
Estimated Percentage of Women with NVP-Resistant Variants.
From our data, we estimated the proportion of women in the South African MTCT study who would be expected to have NVP-resistant variants 2, 6, and 12 mo after sdNVP. For this purpose, we multiplied the proportion of women represented in each group by the percentage of patients with mutations in each group at different time points and extrapolated the results to all 335 women enrolled in the study (Table 4). Using this calculation, we estimate that 83%, ≥34%, and ≥23% of the women in the trials would have NVP-resistant variants at 2, 6, and 12 mo, respectively.
Table 4.
Expected percentage of women with NVP-resistant mutations
| Group | Proportion of women receiving sdNVP | % of women with NVP-resistant mutations after sdNVP |
|||||
|---|---|---|---|---|---|---|---|
| 2 mo |
6 mo |
12 mo |
|||||
| % of group* | % of total† | % of group* | % of total† | % of group* | % of total† | ||
| 1 | 0.14 | 100 | 14 | 100 | 14 | 83 | 12 |
| 2 | 0.26 | 100 | 26 | 75 | 20 | 44 | 11 |
| 3 | 0.60 | 71 | 43 | NA | NA | NA | NA |
| Total | 83 | ≥34 | ≥23 | ||||
NA, samples were not available.
*(Number of women with any detectable NVP resistance mutation/total number of women in the group) × 100.
†Percentage of women with any NVP resistance mutation × proportion of treated women in the group.
Discussion
NVP-resistant variants are detected by standard genotype in 15–40% of HIV-1 infected women who receive sdNVP (24, 25). These variants have been reported to fade below the limit of detection for this analytical method within 6–12 mo of sdNVP (25). Standard genotypes, however, can consistently detect only mutations that comprise 20% of the virus population (28–30). As a consequence, long-term persistence of NVP-resistant variants could be underestimated. To address this possibility, we used an allele-specific RT-PCR assay that quantifies 103N and 181C variants down to 0.1% to test longitudinal samples from 22 HIV-1 subtype C-infected women who received sdNVP as participants in a Soweto, South Africa, MTCT study (22).
Analysis of the 22 baseline samples before exposure to sdNVP revealed negligible levels of mutations (0.1% or less) at both codons, similar to the assay background level, indicating that any subsequent increase in these mutant populations was related to sdNVP. We found that 91% of the 22 women studied had 103N and/or 181C mutations 2 mo after sdNVP, with mutant frequencies ranging from 0.2% to 55.8% of the virus population. After adjusting for the proportion of patients represented in each group, this result suggests that 83% of the women receiving sdNVP in the Soweto MTCT study would be expected to have NVP-resistant variants 2 mo after sdNVP, assuming that these women are typical of those in the parent study. This value is approximately twice the proportion reported in studies that used standard genotype for detection (25, 31, 32). This finding is consistent with our prior report that mutants comprising 20% of the viral population are not reliably detected by standard genotyping (22, 29). Our current findings are also supported by a recent study showing that the majority of women have NVP resistance a median of 8 wk after sdNVP and that 33% of these mutants are missed by standard genotype analysis (27). Even this high rate of NVP resistance may underestimate the true value, because adherence to sdNVP in this study relied on verbal report. Also, the detailed kinetics of appearance and decay of NVP resistance after a single dose have not been determined, and we may have missed low peak values in some patients because of infrequent sampling.
Twelve of 14 patients (86%) who had samples available 6 mo after sdNVP (groups 1 and 2 combined) had the 103N and/or 181C mutation detected with frequencies ranging from 0.3% to 17.9%. Furthermore, 9 of 15 patients (60%) who had samples 12 mo after sdNVP had 103N and/or 181C detected, with mutation frequencies ranging from 0.1% to 21%. We extrapolated from the data in groups 1–3 that no fewer than 34% and 23% of the 335 women enrolled in the Soweto MTCT study would be expected to have 103N and/or 181C mutations at 6 and 12 mo after sdNVP, respectively. Thus, by our calculations, more than one in five women who received sdNVP would have had minor populations of NVP-resistant variants 1 yr after dosing that were not detected by standard genotyping.
The average frequencies of the 103N (AAC and AAT) and 181C mutations were similar for patient samples from groups 1 and 2, whereas the average frequencies of 103N (AAC) and 181C mutations were lower in samples from group 3. Allele-specific PCR did not detect any 103N AAT mutations in group 3 samples. In all groups, the average frequency of the 103N (AAC) mutation was greater than that of the 181C mutation, indicating an overall lower frequency for 181C. The reason for these differences is not clear. The frequency of both the 103N and 181C mutations declined with time but persisted above pretreatment levels for ≥12 mo in group 1 and 2 patient samples. We found that the length of time that each mutation persists varied among women; however, the average frequency for group 1 and group 2 patients 12 mo after sdNVP was ≈2%. These values are similar to frequencies of 103N found in a smaller study 12–24 mo after the administration of sdNVP (0.8–3.5%) (26), although the latter study reported only AAC mutation frequencies. This study also used a more sensitive LigAmp assay for detecting low-frequency mutations.
The long-term persistence of NVP-resistant variants is potentially important for women who will receive sdNVP to block transmission in subsequent pregnancies or who will require antiretroviral therapy for progressive disease (7–10, 14, 26, 27, 33, 34). A recent report suggests that sdNVP may decrease the efficacy of a subsequent NVP-containing treatment regimen, even if NVP-resistant variants are not detected by standard genotyping methods (32). This observation raises the possibility that NVP-resistant variants, even at the low frequencies found in the present study, could compromise future treatments containing NNRTIs and limit the subsequent efficacy of sdNVP for prevention of MTCT.
In conclusion, NVP-resistant variants can be detected by allele-specific RT-PCR in the majority of women given sdNVP to prevent MTCT. This more-sensitive assay detects resistance for ≥6 mo after the standard genotype becomes negative for these mutations. The frequency of the 103N and/or 181C mutations declined with time but persisted above pretherapy levels for ≥12 mo in 60% of the women who had NVP resistance detected by standard genotype 2 mo after sdNVP. Additional studies are needed to assess the clinical significance of NVP-resistant variants, particularly in relation to subsequent treatment with antiretroviral therapy and subsequent pregnancies.
Materials and Methods
Clinical Specimens.
Plasma samples were collected from 22 women participating in a prospective MTCT cohort at Chris Hani Baragwanath Hospital, Soweto, South Africa. Sample collection was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, and all participants provided written informed consent for viral resistance testing. Baseline and follow-up plasma samples were selected randomly and analyzed from 22 of 335 women who had received sdNVP in the MTCT study. In the present study, all women had a blood draw scheduled at 6 wk postpartum. Thereafter, blood draws were performed only if the first sample was found to have resistant mutations by standard genotyping. Plasma samples were collected from the 22 women at baseline before exposure to sdNVP, and at a median of 2 mo (from 1.5 to 3.5 mo; n = 22), 6 mo (from 5 to 9.3 mo; n = 14), and 12 mo (from 11.7 to 14.1 mo; n = 15) thereafter.
Standard Genotype.
Standard genotype analysis was performed on all specimens at an accredited laboratory in South Africa, employing the HIV-1 ViroSeq genotyping system (Abbott/Celera, Abbott Park, IL), and data were analyzed by using both ViroSeq software and the Stanford database. One standard genotype was obtained for each time point, and sequence discrepancies were settled by reviewing the specific sequence chromatogram.
Viral RNA Extraction and Amplification.
RNA was extracted from virus pelleted from 250 to 1,000 μl of plasma (containing a minimum of 10,000 copies of HIV-1 RNA) as described (22, 29). First-round, real-time RT-PCR amplification of a 637-bp fragment of pol including reverse transcriptase codons 22–234 was performed by using HIV-1 subtype C-specific primers 1946F 5′-AAACAATGGCCATTGACAGAAGA-3′ and 2583R 5′-GTTCATACCCCATCCAAAGAAATG-3′, with PCR product monitored by SYBR green fluorescence as described (22). All primers used in the assay were subtype C-specific and specially designed to amplify subtype C virus found in South Africa; other primer sets may be required for non-C subtypes. The patient samples were run in triplicate. No-template controls and HIV RNA standards (3 × 106 to 300 copies) were included on the same 96-well plate. To confirm amplification specificity, PCR products were subjected to thermal denaturation analysis of 70–80°C, with readings taken every 0.1°C for 3 sec. Samples that did not yield the correct melting temperature (Tm) for the amplified product were removed from the analysis.
Allele-Specific RT-PCR.
The products of the first amplification were diluted to ≈107 total copies per reaction mixture, and a second round of PCR was performed with nondiscriminating primers (2090F 5′-AAG TGG AGA AAA TTA GTA GAT TTC AGG GA-3′ and 2222R 5′-ATC CCC CAC ATC TAG TAC TGT CAC TG-3′ for codon 103 or 2298F 5′-CAC CAG GGA TTA GAT ATC AAT ATA ATG TG-3′ and 2450R 5′-CTA CAT ACA AGT CAT CCA TAT ATT GA-3′ for codon 181), as well as with primers that amplified specific alleles (AAC 103N: 2218R 5′-CCC ACA TCT AGT ACT GTC ACT GAT TGG-3′ and AAT 103N: 5′-CCC ACA TCT AGT ACT GTC ACT GAT TCA-3′ or TGT 181C: 2450R 5′-CTA CAT ACA AGT CAT CCA TAT ATT GCC-3′) or wild-type sequences (AAA 103K: 2218R 5′-CCC ACA TCT AGT ACT GTC ACT GAT TCT-3′ or TAT 181Y: 2450R 5′-CTA CAT ACA AGT CAT CCA TAT ATT GCT-3′), by using SYBR green fluorescence to detect the amplified product, as described in ref. 22. To minimize the contribution of nonspecific amplification to the fluorescent signal, fluorescence was measured at a temperature just below the Tm of the correct product but above the Tm of smaller, nonspecific products. To confirm amplification specificity, the PCR products were subjected to a melting curve analysis as described above. All analyses were done in triplicate, and results are reported as percent mutant or wild type, which was determined by dividing the mutant or wild-type population detected by the total population detected. The values for mutant and wild-type populations for each sample were normalized to 100%. Results with a coefficient of variation 100% were repeated.
Acknowledgments
We thank Ann Wiegand and Mary Kearney for valuable help and discussion; Sarah Cohen for sample management; Dr. Adrian Puren and staff for diagnostic work; and Dr. Claudia Chezzi, Candice Pillay, and Matshediso Ntsala for genotyping data. This work was supported, in part, by grants to J. Mellors from the National Cancer Institute [Science Applications International Corporation (SAIC) contract 20XS190A] and the National Institute of Allergy and Infectious Diseases (Virology Support Subcontract of the Adult AIDS Clinical Trials Central Group U01AI38858). The parent study was funded by the National Department of Health of South Africa.
Glossary
ABBREVIATIONS
- NVP
nevirapine
- sdNVP
single-dose NVP
- MTCT
mother-to-child transmission of HIV-1
- NNRTI
nonnucleoside reverse transcriptase inhibitor.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Anonymous AIDS. 2001;15:761–770. [Google Scholar]
- 2.Connor E. M., Sperling R. S., Gelber R., Kiselev P., Scott G., O’Sullivan M. J., VanDyke R., Bey M., Shearer W., Jacobson R. L., et al. N. Engl. J. Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 3.Fiscus S. A., Adimora A. A., Funk M. L., Schoenbach V. J., Tristram D., Lim W., McKinney R., Rupar D., Woods C., Wilfert C. Pediatr. Infect. Dis. J. 2002;21:664–668. doi: 10.1097/00006454-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Coll O., Fiore S., Floridia M., Giaquinto C., Grosch-Worner I., Guiliano M., Lindgren S., Lyall H., Mandelbrot L., Newell M. L., et al. AIDS. 2002;16(Suppl 2):S1–S18. [PubMed] [Google Scholar]
- 5.Ioannidis J. P., Abrams E. J., Ammann A., Bulterys M., Goedert J. J., Gray L., Korber B. T., Mayaux M. J., Mofenson L. M., Newell M. L., et al. J. Infect. Dis. 2001;183:539–545. doi: 10.1086/318530. [DOI] [PubMed] [Google Scholar]
- 6.Dabis F., Ekpini E. R. Lancet. 2002;359:2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 7.Stringer E. M., Sinkala M., Stringer J. S., Mzyece E., Makuka I., Goldenberg R. L., Kwape P., Chilufya M., Vermund S. H. AIDS. 2003;17:1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringer J. S., Rouse D. J., Sinkala M., Marseille E. A., Vermund S. H., Stringer E. M., Goldenberg R. L. Lancet. 2003;362:1850–1853. doi: 10.1016/S0140-6736(03)14907-0. [DOI] [PubMed] [Google Scholar]
- 9.Jackson J. B., Musoke P., Fleming T., Guay L. A., Bagenda D., Allen M., Nakabiito C., Sherman J., Bakaki P., Owor M., et al. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 10.Guay L. A., Musoke P., Fleming T., Bagenda D., Allen M., Nakabiito C., Sherman J., Bakaki P., Ducar C., Deseyve M., et al. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 11.Loutfy M. R., Walmsley S. L. Drugs. 2004;64:471–488. doi: 10.2165/00003495-200464050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Taha T. E., Kumwenda N. I., Hoover D. R., Fiscus S. A., Kafulafula G., Nkhoma C., Nour S., Chen S., Liomba G., Miotti P. G., Broadhead R. L. J. Am. Med. Assoc. 2004;292:202–209. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]
- 13.Mirochnick M., Fenton T., Gagnier P., Pav J., Gwynne M., Siminski S., Sperling R. S., Beckerman K., Jimenez E., Yogev R., et al. J. Infect. Dis. 1998;178:368–374. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 14.Musoke P., Guay L. A., Bagenda D., Mirochnick M., Nakabiito C., Fleming T., Elliott T., Horton S., Dransfield K., Pav J. W., et al. AIDS. 1999;13:479–486. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 15.D’Aquila R. T., Hughes M. D., Johnson V. A., Fischl M. A., Sommadossi J. P., Liou S. H., Timpone J., Myers M., Basgoz N., Niu M., Hirsch M. S. Ann. Intern. Med. 1996;124:1019–1030. doi: 10.7326/0003-4819-124-12-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kilby J. M., Saag M. S. Adv. Exp. Med. Biol. 1996;394:291–298. doi: 10.1007/978-1-4757-9209-6_26. [DOI] [PubMed] [Google Scholar]
- 17.Tucker T. J., Lumma W. C., Culberson J. C. Methods Enzymol. 1996;275:440–472. doi: 10.1016/s0076-6879(96)75026-7. [DOI] [PubMed] [Google Scholar]
- 18.Deeks S. G. J. Acquired Immune Defic. Syndr. 2001;26(Suppl 1):S25–S33. doi: 10.1097/00042560-200103011-00004. [DOI] [PubMed] [Google Scholar]
- 19.Havlir D. V., Eastman S., Gamst A., Richman D. D. J. Virol. 1996;70:7894–7899. doi: 10.1128/jvi.70.11.7894-7899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richman D. D., Havlir D., Corbeil J., Looney D., Ignacio C., Spector S. A., Sullivan J., Cheeseman S., Barringer K., Pauletti D., et al. J. Virol. 1994;68:1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dykes C., Fox K., Lloyd A., Chiulli M., Morse E., Demeter L. M. Virology. 2001;285:193–203. doi: 10.1006/viro.2001.0920. [DOI] [PubMed] [Google Scholar]
- 22.Palmer S., Boltz V., Maldarelli F., Kearney M., Halvas E. K., Rock D., Falloon J., Davey R. T., Jr., Dewar R. L., Metcalf J. A., et al. AIDS. 2006;20:701–710. doi: 10.1097/01.aids.0000216370.69066.7f. [DOI] [PubMed] [Google Scholar]
- 23.Lecossier D., Shulman N. S., Morand-Joubert L., Shafer R. W., Joly V., Zolopa A. R., Clavel F., Hance A. J. J. Acquired Immune Defic. Syndr. 2005;38:37–42. doi: 10.1097/00126334-200501010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Eshleman S. H., Jackson J. B. AIDS Rev. 2002;4:59–63. [PubMed] [Google Scholar]
- 25.Eshleman S. H., Mracna M., Guay L. A., Deseyve M., Cunningham S., Mirochnick M., Musoke P., Fleming T., Glenn Fowler M., Mofenson L. M., et al. AIDS. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 26.Flys T., Nissley D. V., Claasen C. W., Jones D., Shi C., Guay L. A., Musoke P., Mmiro F., Strathern J. N., Jackson J. B., et al. J. Infect. Dis. 2005;192:24–29. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 27.Johnson J. A., Li J. F., Morris L., Martinson N., Gray G., McIntyre J., Heneine W. J. Infect. Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 28.Gunthard H. F., Wong J. K., Ignacio C. C., Havlir D. V., Richman D. D. AIDS Res. Hum. Retroviruses. 1998;14:869–876. doi: 10.1089/aid.1998.14.869. [DOI] [PubMed] [Google Scholar]
- 29.Palmer S., Kearney M., Maldarelli F., Halvas E. K., Bixby C. J., Bazmi H., Rock D., Falloon J., Davey R. T., Jr., Dewar R. L., et al. J. Clin. Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuurman R., Brambilla D., de Groot T., Huang D., Land S., Bremer J., Benders I., Boucher C. A. AIDS Res. Hum. Retroviruses. 2002;18:243–248. doi: 10.1089/088922202753472801. [DOI] [PubMed] [Google Scholar]
- 31.Abrams E. J. AIDS Rev. 2004;6:131–143. [PubMed] [Google Scholar]
- 32.Jourdain G., Ngo-Giang-Huong N., Le Coeur S., Bowonwatanuwong C., Kantipong P., Leechanachai P., Ariyadej S., Leenasirimakul P., Hammer S., Lallemant M. N. Engl. J. Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence J., Mayers D. L., Hullsiek K. H., Collins G., Abrams D. I., Reisler R. B., Crane L. R., Schmetter B. S., Dionne T. J., Saldanha J. M., et al. N. Engl. J. Med. 2003;349:837–846. doi: 10.1056/NEJMoa035103. [DOI] [PubMed] [Google Scholar]
- 34.Lallemant M., Jourdain G., Le Coeur S., Mary J. Y., Ngo-Giang-Huong N., Koetsawang S., Kanshana S., McIntosh K., Thaineua V. N. Engl. J. Med. 2004;351:217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]