Abstract
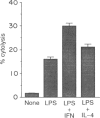
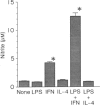
To understand the differential role of interferon-gamma (IFN-gamma) and interleukin-4 (IL-4) in the process of macrophage tumoricidal activation, we investigated the production of tumor necrosis factor-alpha (TNF-alpha) and nitric oxide in activated murine macrophages and the effects of those lymphokines on prostaglandin E2 (PGE2)-mediated down-regulation. IFN-gamma and IL-4 increased lipopolysaccharide (LPS)-induced TNF-alpha production by different mechanisms because IL-4, unlike IFN-gamma, failed to overcome the LPS-hyporesponsiveness in C3H/HeJ mice. Moreover, only IFN-gamma synergized with LPS to induce nitric oxide production and blocked eicosanoid-mediated down-regulation. These differential effects of IFN-gamma and IL-4 on the select efferent cytolytic activities may be the result of an altered or different signal transduction pathway. Because potentiation of protein kinase C (PKC) activity by IFN-gamma has been previously documented, we next studied the role of IFN-gamma and IL-4 in alteration of enzymatic activity of PKC. Two lymphokines caused translocation of PKC from cytosol to membrane with different levels, providing a biochemical basis for explaining how two lymphokines lead to different phenotypic responses. Although treatment of macrophages with IFN-gamma and IL-4 gave rise to a similar enhancing effect on macrophage TNF-alpha production, these two lymphokines appeared to differentially regulate the overall functional state of macrophages for tumour cell killing capability. Additionally, this differential regulation seems to be accomplished in part by different biochemical events.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Tkacenko V., Milsark I., Krochin N., Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986 Nov 1;164(5):1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Stenger S., Röllinghoff M., Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-gamma to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol. 1991 Feb;21(2):327–333. doi: 10.1002/eji.1830210213. [DOI] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Suzuki Y., Wheelock E. F. Interferon-gamma synergizes with tumor necrosis factor and with interleukin 1 and requires the presence of both monokines to induce antitumor cytotoxic activity in macrophages. J Immunol. 1987 Dec 15;139(12):4096–4101. [PubMed] [Google Scholar]
- Cleveland R. P., Meltzer M. S., Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain BCG. J Natl Cancer Inst. 1974 Jun;52(6):1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Gautam S., Tebo J. M., Hamilton T. A. IL-4 suppresses cytokine gene expression induced by IFN-gamma and/or IL-2 in murine peritoneal macrophages. J Immunol. 1992 Mar 15;148(6):1725–1730. [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Jansen M. M., Somers S. D., Adams D. O. Effects of bacterial lipopolysaccharide on protein synthesis in murine peritoneal macrophages: relationship to activation for macrophage tumoricidal function. J Cell Physiol. 1986 Jul;128(1):9–17. doi: 10.1002/jcp.1041280103. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Holman M. J., Klaus G. G. IL-4 promotes anti-Ig-mediated protein kinase C translocation and reverses phorbol ester-mediated protein kinase C down-regulation in murine B cells. J Immunol. 1991 Dec 1;147(11):3831–3836. [PubMed] [Google Scholar]
- Hart P. H., Cooper R. L., Finlay-Jones J. J. IL-4 suppresses IL-1 beta, TNF-alpha and PGE2 production by human peritoneal macrophages. Immunology. 1991 Mar;72(3):344–349. [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Jones C. A., Finlay-Jones J. J. Interleukin-4 suppression of monocyte tumour necrosis factor-alpha production. Dependence on protein synthesis but not on cyclic AMP production. Immunology. 1992 Aug;76(4):560–565. [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich S., Weyers M., Gong J. H., Sprenger H., Nain M., Gemsa D. Potentiation of lymphokine-induced macrophage activation by tumor necrosis factor-alpha. J Immunol. 1988 Mar 1;140(5):1511–1518. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Hori K., Ehrke M. J., Mace K., Mihich E. Effect of recombinant tumor necrosis factor on tumoricidal activation of murine macrophages: synergism between tumor necrosis factor and gamma-interferon. Cancer Res. 1987 Nov 15;47(22):5868–5874. [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Littman B. H., Dastvan F. F., Carlson P. L., Sanders K. M. Regulation of monocyte/macrophage C2 production and HLA-DR expression by IL-4 (BSF-1) and IFN-gamma. J Immunol. 1989 Jan 15;142(2):520–525. [PubMed] [Google Scholar]
- Luettig B., Decker T., Lohmann-Matthes M. L. Evidence for the existence of two forms of membrane tumor necrosis factor: an integral protein and a molecule attached to its receptor. J Immunol. 1989 Dec 15;143(12):4034–4038. [PubMed] [Google Scholar]
- McBride W. H., Economou J. S., Nayersina R., Comora S., Essner R. Influences of interleukins 2 and 4 on tumor necrosis factor production by murine mononuclear phagocytes. Cancer Res. 1990 May 15;50(10):2949–2952. [PubMed] [Google Scholar]
- Meltzer M. S., Benjamin W. R., Farrar J. J. Macrophage activation for tumor cytotoxicity: induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J Immunol. 1982 Dec;129(6):2802–2807. [PubMed] [Google Scholar]
- Meltzer M. S. Macrophage activation for tumor cytotoxicity: characterization of priming and trigger signals during lymphokine activation. J Immunol. 1981 Jul;127(1):179–183. [PubMed] [Google Scholar]
- Meltzer M. S., Tucker R. W., Sanford K. K., Leonard E. J. Interaction of BCG-activated macrophages with neoplastic and nonneoplastic cell lines in vitro : quantitation of the cytotoxic reaction by release of tritiated thymidine from prelabeled target cells. J Natl Cancer Inst. 1975 May;54(5):1177–1184. doi: 10.1093/jnci/54.5.1177. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Kleinerman E. S., Saito T., Oppenheim J. J. Role of interleukin 1 in promoting human monocyte-mediated tumor cytotoxicity. J Immunol. 1985 Jul;135(1):314–320. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Rennick D. Role of interleukin 2, interleukin 4, and alpha, beta, and gamma interferon in stimulating macrophage antibody-dependent tumoricidal activity. J Exp Med. 1988 Feb 1;167(2):712–717. doi: 10.1084/jem.167.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum M. G., Donato N. J. Tumor necrosis factor alpha: a multifaceted peptide hormone. Crit Rev Immunol. 1989;9(1):21–44. [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Somers S. D., Erickson K. L. Regulation of murine macrophage function by IL-4. I. Activation of macrophages by a T-T cell hybridoma is due to IL-4. Cell Immunol. 1989 Aug;122(1):178–187. doi: 10.1016/0008-8749(89)90158-5. [DOI] [PubMed] [Google Scholar]
- Somers S. D., Weiel J. E., Hamilton T. A., Adams D. O. Phorbol esters and calcium ionophore can prime murine peritoneal macrophages for tumor cell destruction. J Immunol. 1986 Jun 1;136(11):4199–4205. [PubMed] [Google Scholar]
- Stenger S., Solbach W., Röllinghoff M., Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-alpha production by macrophages is induced by the synergistic action of interferon (IFN)-gamma and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-gamma and IL 4. Eur J Immunol. 1991 Jul;21(7):1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Zlotnik A., Woodward J. G. Induction of class I and class II MHC antigen expression on murine bone marrow-derived macrophages by IL-4 (B cell stimulatory factor 1). J Immunol. 1988 Mar 1;140(5):1542–1547. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Chen G. J., Huang D. S., Scuderi P., Watson R. R. Production of tumor necrosis factor alpha by resident and activated murine macrophages. J Leukoc Biol. 1992 Mar;51(3):251–255. doi: 10.1002/jlb.51.3.251. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Russell S. W. Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol. 1981 Feb;126(2):424–427. [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]