Abstract
Objective
To evaluate the impact of subtotal (SG) versus total (TG) gastrectomy on the oncologic outcome of patients with cancer of the distal stomach from 28 Italian institutions.
Summary Background Data
There is controversy over whether SG and TG have a different impact on the 5-year survival probability of patients with cancer of the distal half of the stomach.
Methods
The present analysis involved 618 patients randomized during surgery to SG (315) or TG (303), provided there was at least 6 cm from the proximal edge of the tumor to the cardia, there was no intraperitoneal or distant spread, and it was possible to remove the tumor entirely. Both surgical treatments included regional lymphadenectomy.
Results
Four patients died after SG and seven after TG. Median follow-up was 72 months after SG (range 2 to 125) and 75 months after TG (range 7 to 113). Five-year survival probability as computed by the Kaplan-Meier method was 65.3% for SG and 62.4% for TG. The test of equivalence led to the conclusion that the two procedures may be considered equivalent in terms of 5-year survival probability. The analysis of survival using a multivariate Cox regression model showed a statistically significant impact on survival of tumor site, tumor spread within the gastric wall, extent of resection to the spleen plus or minus neighboring organs or structures, and relative frequency of metastasis in resected lymph nodes.
Conclusions
Both procedures have a similar survival probability. The authors believe that SG, which has been reported to be associated with a better nutritional status and quality of life, should be the procedure of choice, provided that the proximal margin of the resection falls in healthy tissue.
Cancer of the stomach is the second most common cancer in the world. It has been estimated that more than 1 million new cases were diagnosed worldwide in 1997, 1 accounting for nearly 10% of all new cancers. In the same period, deaths attributable to stomach cancer were estimated at 835,000, or 11.8% of all cancer deaths.
Even though more than a century has elapsed since Billroth and Schlatter performed the first subtotal gastrectomy (SG) and total gastrectomy (TG) for cancer, respectively, 2,3 the best surgical procedure for cancer of the distal/middle stomach is still a matter of controversy.
An extensive survey of the surgical policies of 62 centers including 16,594 patients in several European countries 4 showed that 44% of surgeons would choose TG in cancer of the antrum, histologically defined as diffuse according to the Laurén classification. 5 A similar figure was reported in a multicenter survey in Italy for both Laurén histologic subtypes. 6 In the United States, according to the Register of the American College of Surgeons, approximately 19% of patients with cancer of the distal stomach were candidates for total or near-total gastrectomy. 7 Finally, a recent analysis of the National Cancer Data Base of the United States 8 comprising 6400 patients showed that TG was used in 12.3% of patients with cancer of the antrum and pylorus. However, this percentage increased to 40% when other organs were included in the resection. The latter figure is slightly higher than that reported in a previous study. 9
An en principle TG—that is, a total gastrectomy performed even when adequate clearance of margins can be obtained by subtotal distal resection—was initially proposed by McNeer et al in the United States 10 and subsequently in France by Lortat-Jacob et al. 11 However, it never gained worldwide acceptance because the oncologic results did not appear better than those achieved with SG in several nonrandomized series. 10–15 As far as we are aware, there has been only one randomized trial comparing TG and SG in cancer confined to the antrum, carried out by the French Association on Surgical Research. 16 This trial showed no statistical difference between the two procedures in terms of 5-year survival probability. However, the power of the study was weakened because fewer patients participated in it than was planned in the statistical design. Further, the two treatments were merely compared, without allowance being made in the analysis for the possible effect of important prognostic variables (e.g., the number of metastatic lymph nodes and splenectomy).
The present study reports the results of a multicenter randomized Italian trial that investigated the effects of SG and TG in patients with cancer of the distal half of the stomach. It focused in particular on 5-year survival probability and the impact of certain prognostic factors on the oncologic outcome.
METHODS
Patients
Between April 1982 and December 1993, 1372 patients from 31 Italian institutions were screened for participation in a multicenter prospective controlled clinical trial to compare potentially curative SG and TG in patients with cancer of the distal half of the stomach. Details on eligibility criteria, surgical techniques, randomization, accrual, and follow-up modalities have been reported in a previous study. 17
There were two levels of eligibility. Before surgery, patients were considered candidates if they had a cancer of the distal half of the stomach without apparent distant metastases, were no older than 75, were in relatively good condition, and had no history of previous cancer, gastric resection, or cytotoxic chemotherapy. 18 The second level of eligibility was determined during laparotomy by assessing the following eligibility criteria: a distance of at least 6 cm from the proximal edge of the tumor to the cardia; absence of hepatic or intraperitoneal spread of the tumor or metastatic deposits in the third nodal level, according to the Japanese classification 19 ; and absence of unresectable infiltration of contiguous organs. Patients judged to be eligible at laparotomy were randomly allocated to SG or TG groups using an ordered set of sealed envelopes containing the indication of the treatment assigned according to a computer-generated random permuted blocks list. Before the patient was discharged, all information concerning eligibility criteria was sent to the coordinating center on a standard form.
Regardless of the type of operation performed (SG or TG), an effort was made to maintain a distance of at least 6 cm from the proximal edge of the tumor to the line of the anastomosis, thus minimizing the risk of leaving residual neoplastic deposits in the stomach or esophagus. 20–22 However, if patients had an involved margin of transection at the definitive histologic examination, they remained included in the evaluable set. This occurred in six patients from the SG group (four of them with a proximal clearance <6 cm) and one patient from the TG group (with a 10-cm margin of clearance). Finally, 13 patients (8 SG and 5 TG) had a distal margin infiltrated by the tumor. One patient from the SG group had both proximal and distal margins involved. Ten patients (four SG and six TG) received postoperative adjuvant chemotherapy.
A technique of D2 gastrectomy, as described by Nakajima and Kajitani, 23 was recommended, as follows. The entire greater omentum, superior leaf of the mesocolon, pancreatic capsule, and lesser omentum were removed en bloc with the stomach. The left gastric artery was ligated at its origin. Lymphadenectomy included dissection of node levels 1 and 2. For all tumors, lymph nodes were removed along the lesser and greater curvature; suprapyloric and infrapyloric and right paracardial lymph nodes, and those along the left gastric artery, the common hepatic artery, and the celiac axis, were also removed. For tumors involving the middle third of the stomach, the resection was planned to include the left paracardial lymph nodes and those along the splenic artery and the hilum of the spleen (standard procedure). Splenectomy was an optional procedure left to the preference of the surgeon. The tumor was finally staged according to the recent TNM classification. 24
Figure 1 shows the trial profile; 717 patients were found to be ineligible at the preoperative and intraoperative level, and 7 patients were excluded because the eligibility form had not been filled out. Twenty-six of the 648 randomized patients, accrued by three centers, were excluded by the monitoring committee because the information concerning baseline and follow-up visits was considered unreliable. Thus, the final evaluable set included 622 patients from 28 centers, 319 randomized to SG and 303 to TG.
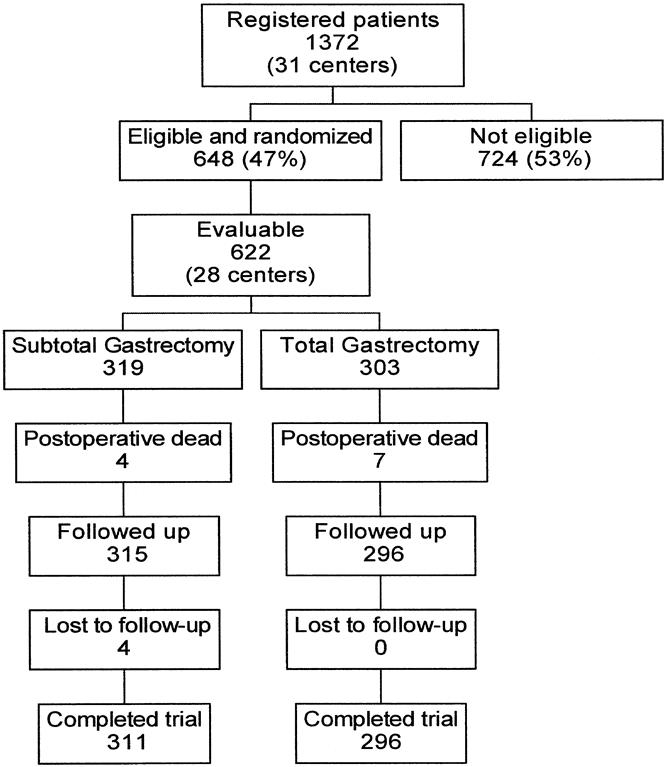
Figure 1. Profile of the subtotal/total gastrectomy trial. The evaluable set includes patients with reliable information on baseline and follow-up visits.
The study was approved by the Ethical Committee of the Istituto Nazionale Tumori, Milan, and all the eligible patients gave their signed consent to it.
The present study focuses on the primary end point of the trial—death from all causes, including postoperative deaths, which accounted for four SG and seven TG patients.
To perform this analysis, the four SG patients lost to follow-up immediately after discharge were excluded, resulting in a set of 618 subjects. The TG group also included four patients who had originally been randomized to TG but subsequently underwent SG because of intraoperative complications. These patients had healthy proximal and distal margins of transection. The two surgical groups had similar demographic characteristics (Table 1).
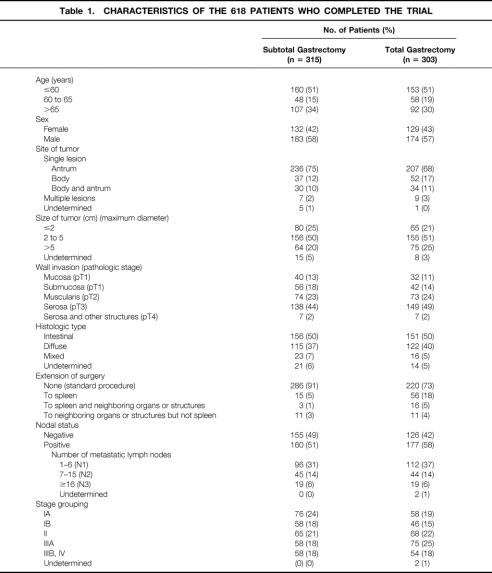
Table 1. CHARACTERISTICS OF THE 618 PATIENTS WHO COMPLETED THE TRIAL
Statistical Methods
Statistical analysis was carried out on the intention to treat the patient population, as defined by Gillings and Koch. 25
The trial protocol aimed at testing the equivalence of the two surgical procedures, defined in terms of 5-year survival probability; the rejection of the null hypothesis enables the trialist to conclude in favor of equivalence. 26 Taking TG as the reference treatment with a 5-year survival probability equal to 50%, the null hypothesis stated that the SG group was expected to have a 5-year survival probability at least 10% lower. In terms of log hazard rate ratio β, this translated into β ≥ 0.28 for the null hypothesis and β < 0.28 for the alternative hypothesis. Correspondingly, for a type I error probability of 5% (one-tailed test) and a type II error probability of 20%, a total sample size of some 600 patients was computed. 27
Time to death was calculated from the date of surgery to the date of death from any cause, censoring the follow-up time at the most recent date for living patients. Survival curves were computed using the Kaplan-Meier method. 28
A univariate Cox’s proportional hazards regression model was used to obtain the estimate β̂ of the log hazard rate ratio SG/TG; this statistic and its standard error allowed us to compute an asymptotically normal standardized deviate
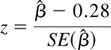 |
to test the equivalence hypothesis. To avoid any bias that might result in estimating β as a result of covariate imbalances across the two surgery groups or of balanced covariates of prognostic relevance, 27 a multivariate Cox regression model was applied. The effect of surgery was adjusted for the following covariates: age at surgery, site of tumor (antrum, body, body and antrum or multiple lesions), degree of wall invasion (pT1, pT2, pT3-pT4), extension of surgery to neighboring organs or structures (none; to the spleen or to the spleen and neighboring organs or structures; to neighboring organs or structures but not the spleen), relative frequency of metastasis in resected lymph nodes (negative; ≤25% metastatic lymph nodes; >25% metastatic lymph nodes 29 ), and involvement of transection margins (absent or present). Indicator variables were used to categorize all the covariates except for age, which was inserted into the model on a continuous scale. The relation of the log relative hazard of death versus age was investigated using the smoothed plot of martingale residuals 30 of the model excluding age; as a result, age was inserted into the model with the linear term only. The contribution of each covariate, adjusted for the effect of all the others, was tested with the likelihood ratio test.
Statistical analyses were performed using SAS 31 and S-Plus 32 software.
RESULTS
The series as a whole included mainly cancers of the antrum (72%). Half of the tumors had a maximum diameter between 2 and 5 cm, about 50% of the tumors had invaded the serosa or contiguous structures, and 55% of the patients had lymph node metastases. The surgery performed was defined as curative because no macroscopic tumor residual was left in the abdomen, but at the definitive histologic examination 15 patients in the SG group and 6 in the TG group were found to have a margin of transection infiltrated by the tumor (R1 resections).
The two surgery groups were similar for several baseline characteristics (see Table 1). However, splenectomy was performed more often in the TG group than in the SG group (chi square = 40.96, 3 df, p = 0.0010).
We report on follow-up data collected up to February 5, 1998. The distribution of follow-up time was similar in the two randomized groups; the median follow-up time was 72 months in the SG group (range 2 to 125 months) and 75 months in the TG group (range 7 to 113 months). Total deaths were 112 in the SG group and 118 in the TG group, and tumor spread accounted for 78.7% and 80.2% of the deaths, respectively. Of the few patients who received postoperative chemotherapy, two of the four in the SG group and three of the six in the TG group died within 2 years after surgery.
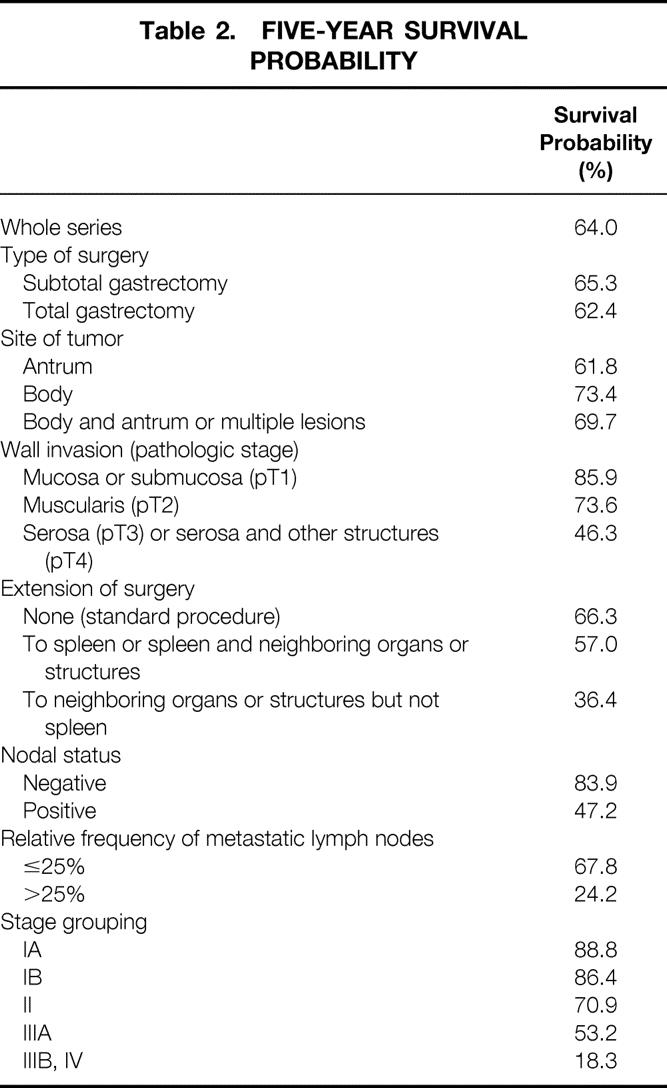
The overall survival curve is displayed in Figure 2. Figures 3 to 6 show the survival curves with regard to wall invasion categories, nodal status, TNM stage, and the two surgery groups. For clarity, Table 2 reports the 5-year survival probability with regard to type of surgical treatment/tumor characteristics.
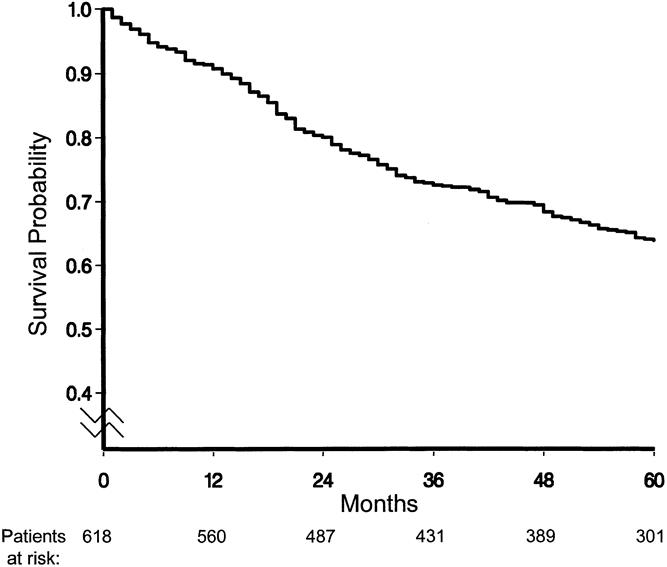
Figure 2. Survival curve for all causes of death (median follow-up: 73 months).
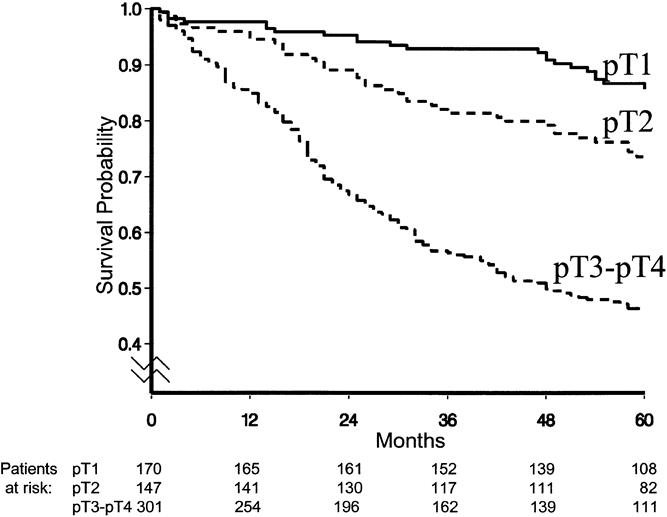
Figure 3. Survival curves with regard to wall invasion categories.
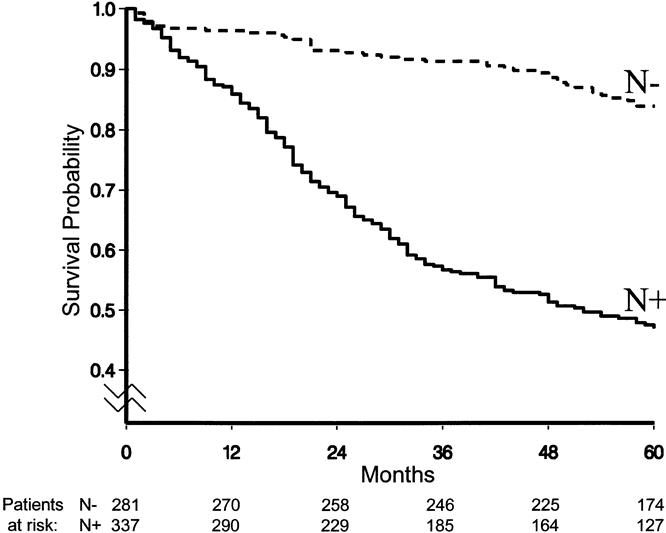
Figure 4. Survival curves with regard to nodal status.
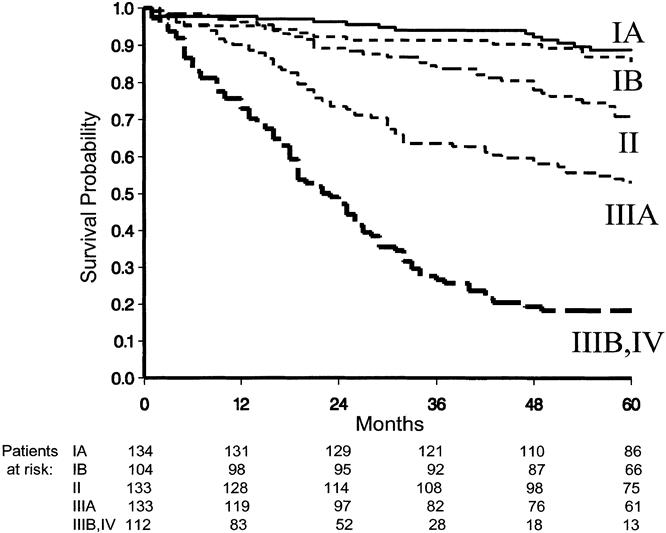
Figure 5. Survival curves with regard to TNM stage.
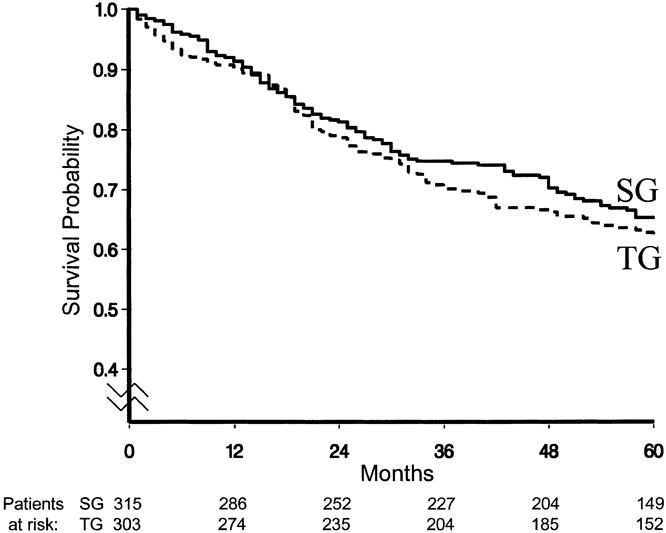
Figure 6. Survival curves with regard to surgical treatment.
Table 2. FIVE-YEAR SURVIVAL PROBABILITY
The test of equivalence (z = −2.89) led to the rejection of the null hypothesis and to the conclusion that the two surgical procedures could be considered equivalent in terms of 5-year survival probability. The hazard rate ratio SG/TG was 0.89; however, the two-sided 95% confidence interval was 0.68 to 1.17, indicating that the prognostic advantage of SG was not statistically significant.
The initial multivariate Cox model, in addition to the covariates mentioned in the statistics section, also included the first-order interaction terms surgery × site, with the goal of investigating whether the hazard rate ratio SG/TG changed in the three sites (antrum; body; and body and antrum or multiple lesions). Because this interaction was not found to be statistically significant, it was deleted from the model, leading to the results reported in Table 3. The adjusted surgery hazard rate ratio was 1.01 (two-sided 95% confidence interval 0.76 to 1.33), supporting the conclusion that the surgical procedures had similar effects on survival.
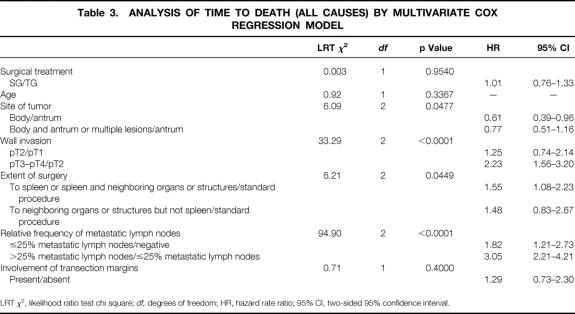
Table 3. ANALYSIS OF TIME TO DEATH (ALL CAUSES) BY MULTIVARIATE COX REGRESSION MODEL
LRT χ2, likelihood ratio test chi square; df, degrees of freedom; HR, hazard rate ratio; 95% CI, two-sided 95% confidence interval.
As expected, site of tumor, wall invasion, extension of surgery, and the relative frequency of metastatic lymph nodes were found to have a significant impact. In particular, the tumors localized in the antrum appeared to have an unfavorable prognosis compared with the others. Tumors staged pT3 or pT4 had a hazard rate significantly greater than those staged pT2. An extension of surgery to the spleen or to the spleen and neighboring organs or structures was associated with a statistically significantly worse prognosis with respect to a standard procedure. Patients with a relative frequency of positive lymph nodes ≤25% had a statistically significantly worse prognosis than patients with negative nodes. The prognosis became even worse when the relative frequency of involved nodes exceeded 25%. The aforementioned Cox model was extended to investigate the possible effect of the calendar period of surgery on overall survival, but no statistically significant contribution was found.
DISCUSSION
This study focuses on the impact that two different surgical procedures for gastric cancer have on 5-year survival probability. Both procedures included a D2 lymphadenectomy (Japanese classification 19 ) for two reasons. First, the D2 dissection has had acceptable morbidity and perioperative mortality rates in the experience of Italian surgeons. 33,34 Second, the review of both large (a few thousand patients) retrospective series of Western and Japanese patients 7,33,35 and randomized clinical trials 36–39 have shown so far no benefit from extended lymphadenectomy.
A 64% 5-year survival probability after gastrectomy for cancer was found in the present study. This figure compares well with the 75% reported by the National Cancer Center in Tokyo 40 in a study comprising 2500 patients with a nodal status similar to our series; however, early gastric cancer accounted for 49% of the entire series in that study. Our results, which are better than those found in other Western surgical series, probably reflect the relatively high prevalence of pT1 cancer in this patient population, as well as the exclusion of patients with tumors of the upper third of the stomach, who are well known to have a worse prognosis. 41
There was no difference in survival probability in patients assigned to SG or TG. This was true not only for patients with cancer of the antrum, a finding consistent with the conclusions of a previous randomized clinical trial on a limited number of patients, 16 but also for those with cancer of the middle third of the stomach. In conclusion, it would appear that there is no advantage in extending the resection to the stomach in toto, provided that the proximal margin of transection is in healthy tissue.
Following the data from the literature 20 and from this institution, 21 it was recommended in this protocol that at least 6 cm be maintained proximally from the tumor (or at least 3 cm if the cancer was confined to the muscular layer) to achieve an adequate proximal clearance of the tumor.
The multivariate analysis confirms the negative prognostic impact on survival of the extension of surgery to the spleen or to the spleen and neighboring organs and structures, of deep tumoral penetration through the gastric wall, and of metastatic involvement of the lymph nodes.
It is not surprising that the extension of surgery to the surrounding organs is associated with a worse prognosis: this procedure is usually performed when cancer is locally advanced (pT4) and has invaded the neighboring structures. More intriguing is the interpretation of the effect of splenectomy on the final outcome, because this procedure is usually performed as part of TG or when the spleen is inadvertently injured during removal of the stomach. However, the literature has already reported a lower 5-year survival probability for patients in whom gastrectomy is associated with splenectomy. 42–44 Nevertheless, this report is the first to show an adverse prognostic impact of splenectomy on multivariate analysis on a large number of patients. This seems to suggest that splenectomy should not be performed as a routine adjunctive procedure to gastrectomy unless there are metastatic nodes at the splenic hilum that cannot be removed without splenectomy.
In terms of the prognostic role of lymph node metastases, the classification recently proposed by Yu et al 29 was adopted in our study. Because it is based on the ratio of invaded-to-removed nodes, it appears to be more simple, convenient, and reproducible than the UICC TNM staging system 45 or the Japanese staging system, 46,47 and it can pinpoint patients with different 5-year survival probability equally well. 29
An unexpected finding was the better outcome of patients with cancer of the gastric body compared with those with cancer of the antrum. A possible explanation is the smaller distal clearance that can be achieved in antral tumors as a result of the anatomic boundaries with the pancreas. This finding is in keeping with a recent report of only 58% 5-year survival probability in nonobstructing cancer of the antrum. 48
In conclusion, there are several advantages in performing a more conservative operation (SG) in patients with cancer of the lower or middle stomach. In fact, TG is technically a more demanding procedure than SG and is more often associated with splenectomy, which has an adverse effect on postoperative complications and on susceptibility to infections. 18,36,43,49,50 Further, TG involves a longer postoperative hospital stay (and consequently a higher cost), as previously shown. 18 Finally, patients who undergo TG have a lower calorie intake and require more meals per day to maintain an acceptable nutritional status, which nonetheless is always more depleted than that of patients who undergo SG. 51–53 This results in a poorer quality of life for these patients. 53–56
We believe that a modern surgical strategy for cancer of the distal half of the stomach should involve conservative procedures that can achieve the same outcome as more radical surgery while producing a better quality of life for patients.
Acknowledgments
The following institutions and investigators participated in the Italian Gastrointestinal Trial Study Group. They are listed in order of the number of cases recruited.
Istituto Nazionale Tumori, Milano: Gennari, L., Bozzetti, F., Bonfanti, G.; Divisione Chirurgia Generale, Policlinico Careggi, Firenze: Boffi, L., Siliani, L.; Ospedale Fatebenefratelli, Roma: Cucchiara, G., Daffinà, A.; Istituto Clinica Chirurgica Università Cattolica Sacro Cuore, Roma: Crucitti, F., Pacelli, F.; Divisione Chirurgia Generale, Ospedale Morgagni, Forlì: Vio, A., Folli, S.; Istituto Nazionale Tumori Pascale, Napoli: Parisi, V., Ruffolo, F.; Chirurgia I, Ospedale di Legnano: Repossini, L., Wizeman, G.; Unità Operativa Chirurgia Generale Silvestrini, Perugia: Mercati, U., Mariotti, A.; Divisione Chirurgia Generale, Pizzamiglio, Ospedale Niguarda, Milano: Russo, A., Spada, G., Sartori, P.;Divisione Chirurgia Generale, Ospedale S. Anna, Como: Peruzzo, L., Brenna, A.; Istituto Clinica Chirurgica Policlinico, Bari: Rubino, M., Palasciano, N.; Chirurgia II, Ospedale di Cremona, Cremona: Alquati, P., Scurelli, A.; Ospedale San Raffaele, Milano: Di Carlo, V., Cordio, S.; Clinica Chirurgica, Ospedale L. Sacco, Milano: Rovati, V., Casati, M.; Clinica Chirurgica VI, Policlinico Umberto I, Università degli Studi La Sapienza, Roma: Ribotta, G., D’Amato, A.; Clinica Chirurgica, Ospedale Maggiore Policlinico, Milano: Montorsi, M., Fumagalli, U.; Ospedale Regina Elena, Roma: Santoro, E., Garofalo, A., Carlini, M.; Divisione Chirurgica, Ospedale Maggiore, Parma: Battistini, C., Bocchi, P.; Divisione Chirurgia Generale, Ospedale Busto Arsizio, Busto Arsizio: Branchini, L.; Chirurgia I, Ospedale Uboldo, Cernusco sul Naviglio: Mor, C., Penati, M.; Ospedale S. Filippo Neri, Roma: Terranova, V., Garavello, A.; Clinica Chirurgica, Ospedale Cattinara, Trieste: Leggeri, A., Bortul, M.; Chirurgia Generale I, Ospedale S. Paolo, Milano: Dagrada, T., Moro, G.; Clinica Chirurgica Generale, Ospedale San Gerardo, Monza: Lavorato, F., Galastri, L.; Divisione Chirurgica Generale, Ospedale Civico, Carrara: Sicari, A., Fialdini, G.; Policlinico I Padiglione Monteggia, Ospedale Policlinico, Milano: Germiniani, R., Spinola, A.; II Chirurgia, Ospedale Civile, Ravenna: Carosi, V., Orselli, G.; Ospedale Consorziale Policlinico, Bari: Chiarito, G.
Footnotes
Correspondence: Federico Bozzetti, MD, Istituto Nazionale Tumori, Via G. Venezian 1, 20133 Milan, Italy.
Accepted for publication April 6, 1999.
References
- 1.World Health Organization. The world health report. Geneva: WHO; 1997.
- 2.Billroth T. Offenes Schreiben an Herrn Dr. L. Wittelshofer. Wien Med Wochenschr 1881; 31:161–165.
- 3.Schlatter K. A unique case of complete removal of the stomach-successful esophago-enterostomy recovery. Med Rec 1897; 52: 909–914. [Google Scholar]
- 4.Heberer G, Teichmann RK, Kramling HJ, Gunther MD. Results of gastric resection for carcinoma of the stomach: The European experience. World J Surg 1988; 12: 374–381. [DOI] [PubMed] [Google Scholar]
- 5.Laurén P. The two histologic main types of gastric carcinoma: Diffuse and so-called intestinal type carcinoma. Acta Path Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 6.Santoro E, Garofalo A. Il cancro dello stomaco negli ospedali italiani. Rome: Edizioni Scientifiche Romane; 1988.
- 7.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993; 218: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundhal SA, Menck HR, Mansour EG, Winchester DP. The national cancer data base report on gastric carcinoma. Cancer 1997; 80: 2333–2341. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence W, Menck HR, Steele GD, Winchester DP. The national cancer data base report on gastric cancer. Cancer 1995; 75: 1734–1744. [DOI] [PubMed] [Google Scholar]
- 10.McNeer G, Bowden L, Booher RJ, McPeak JC. Elective total gastrectomy for cancer of the stomach: End results. Ann Surg 1974; 180: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lortat-Jacob JL, Giuli R, Estenne B, Clot PH. Intrepret de la gastrectomie totale pour le traitement des cancer de l’estomac. Etude des 482 interventions radicales. Chirurgie 1975; 101: 59–67. [PubMed] [Google Scholar]
- 12.Launois B, Cardin JL, Bardaxoglou E, et al. Management of cancer of the stomach: Total gastrectomy versus sub-total gastrectomy. Hepato-Gastroenterol 1991; 38: 45–52. [PubMed] [Google Scholar]
- 13.Le Treut YP, Echimane A, Hans D, et al. Cancers de l’antre gastrique. Que peut-on attendre de l’élargissement de principe de l’exérèses. Etude retrospective de 73 cas. Presse Med 1985; 14: 1319–1327. [PubMed] [Google Scholar]
- 14.Gennari L, Bozzetti F, Bonfanti G, et al. Subtotal versus total gastrectomy for cancer of the lower two-thirds of the stomach: A new approach to an old problem. Br J Surg 1986; 73: 534–538. [DOI] [PubMed] [Google Scholar]
- 15.Gazzaniga GM. Controlli a distanza di 120 gastrectomie totali. Min Chir 1982; 37: 268–278. [PubMed] [Google Scholar]
- 16.Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 1989; 209: 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozzetti F, Bonfanti G, Boffi L, et al. Studio policentrico randomizzato sul confronto tra gastrectomia totale e subtotale nei tumori della metà distale dello stomaco. Chirurgia 1993; 6: 428–434. [Google Scholar]
- 18.Bozzetti F, Marubini E, Bonfanti G, et al. Total versus subtotal gastrectomy: Surgical morbidity and mortality rates in a multicenter Italian randomized trial. Ann Surg 1997; 226: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japanese Research Society for Gastric Cancer. The general rules for the gastric cancer study in surgery and pathology. Jpn J Surg 1981; 11: 127–145. [DOI] [PubMed] [Google Scholar]
- 20.Eker R. Carcinomas of the stomach: Investigation of the lymphatic spread of gastric carcinomas after total and partial gastrectomy. Acta Chir Scand 1951; 101: 112–126. [PubMed] [Google Scholar]
- 21.Bozzetti F, Bonfanti G, Bufalino R. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg 1989; 196: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozzetti F, Bonfanti G, Regalia E. How long is a 6-cm margin of resection in the stomach? Eur J Surg Oncol 1992; 18: 481–483. [PubMed] [Google Scholar]
- 23.Nakajima T, Kajitani T. Surgical treatment of gastric cancer with special reference to lymph node dissection. Excerpta Med 1981; 207–225. [Google Scholar]
- 24.Sobin LH, Wittekind CH. TNM classification. In: UICC. TNM classification of malignant tumours, 5th ed. New York: Wiley-Liss; 1997: 59–62.
- 25.Gillings D, Koch G. The application of the principle of intention to treat to the analysis of clinical trials. Drug Info J 1991; 25: 411–424. [Google Scholar]
- 26.Blackwelder WC. Proving the null hypothesis in clinical trials. Controlled Clinical Trials 1982; 3: 345–353. [DOI] [PubMed] [Google Scholar]
- 27.Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester: John Wiley and Sons; 1995.
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 29.Yu W, Choi GS, Whang I, Suh IS. Comparison of five systems for staging lymph node metastasis in gastric cancer. Br J Surg 1997; 84: 1305–1309. [PubMed] [Google Scholar]
- 30.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika 1990; 77: 216–218. [Google Scholar]
- 31.SAS Institute Inc. SAS/STAT user’s guide, version 6, 4th ed, volume 2. Cary, NC: SAS Institute Inc.; 1989.
- 32.Statistical Sciences, S-PLUS guide to statistical and mathematical analysis, version 3.3. Seattle: StatSci, a division of MathSoft, Inc.; 1995.
- 33.Bozzetti F. Rationale for extended lymphadenectomy in gastrectomy for carcinoma. J Am Coll Surg 1995; 180: 505–508. [PubMed] [Google Scholar]
- 34.Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality after D2 gastrectomy for gastric cancer: Results of the Italian gastric cancer study group prospective multicenter surgical study. J Clin Oncol 1998; 16: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 35.Otsuji E, Yamaguchi T, Sawai K, et al. Recent advances in surgical treatment have improved the survival of patients with gastric carcinoma. Cancer 1998; 82: 1233–1238. [PubMed] [Google Scholar]
- 36.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Cooperative Group. Br J Cancer 1999; 79: 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson CS, Chung SCS, Woods SDS, et al. A prospective randomized trial compared R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg 1994; 220: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988; 75: 110–112. [DOI] [PubMed] [Google Scholar]
- 39.Bonenkamp JJ, Hermans J, Sasako M, van de Velde C. Extended lymph-node dissection for gastric cancer. Dutch Gastric Cancer Group. N Engl J Med 1999; 340: 908–914. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987; 11: 418–425. [DOI] [PubMed] [Google Scholar]
- 41.Marubini E, Bonfanti G, Bozzetti F, et al. A prognostic score for patients resected for gastric cancer. Eur J Cancer 1993; 29a: 845–850. [DOI] [PubMed] [Google Scholar]
- 42.Koga S, Kishimoto H, Tanaka K, Kawaguchi H. Clinical and pathologic evaluation of patients with recurrence of gastric cancer more than five years postoperatively. Am J Surg 1978; 136: 317–321. [DOI] [PubMed] [Google Scholar]
- 43.Brady MS, Rogarko A, Deut LL, Shiu MH. Effects of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg 1991; 126: 359–364. [DOI] [PubMed] [Google Scholar]
- 44.Maehara Y, Moriguchi S, Yoshida M, et al. Splenectomy does not correlate with length of survival in patients undergoing curative total gastrectomy for gastric carcinoma. Cancer 1991; 67: 3006–3009. [DOI] [PubMed] [Google Scholar]
- 45.Hermanek P, Sobin LH. TNM classification of malignant tumours. 4th ed, 2d revision Berlin: Springer; 1992.
- 46.Kajitani T. Japanese Research Society for Gastric Cancer. The general rules for gastric cancer study in surgery and pathology. Part 1. Clinical classification. Jpn J Surg 1981; 11: 127–139. [DOI] [PubMed] [Google Scholar]
- 47.Adachi Y, Oshiro T, Okuyama T, et al. A simple classification of lymph node level in gastric carcinoma. Am J Surg 1995; 169: 382–385. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Sakuma A. A clinicopathological study on upper gastric cancer with special reference to the choice of operative procedure. J Jpn Soc Surg 1977; 78: 395–407. [Google Scholar]
- 49.Otsuji E, Yamaguchi T, Sawai K, et al. End results of simultaneous splenectomy in patients undergoing total gastrectomy for gastric carcinoma. Surgery 1996; 120: 40–44. [DOI] [PubMed] [Google Scholar]
- 50.Shaw JHF, Print CG. Postsplenectomy sepsis. Br J Surg 1989; 76: 1074–1081. [DOI] [PubMed] [Google Scholar]
- 51.Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg 1998; 22: 254–261. [DOI] [PubMed] [Google Scholar]
- 52.Bozzetti F, Ravera E, Cozzaglio L, et al. Comparison of nutritional status after total or subtotal gastrectomy. Nutrition 1990; 6: 371–375. [PubMed] [Google Scholar]
- 53.Braga M, Molinari M, Zuliani W, et al. Surgical treatment of gastric adenocarcinoma: Impact on survival and quality of life. A prospective ten-year study. Hepato-Gastroenterol 1996; 43: 187–193. [PubMed] [Google Scholar]
- 54.Jentschura D, Winkler M, Strohmeier N, et al. Quality-of-life after curative surgery for gastric cancer: A comparison between total gastrectomy and subtotal gastric resection. Hepato-Gastroenterol 1997; 44: 1137–1142. [PubMed] [Google Scholar]
- 55.Korenaga D, Orita H, Okuyama T, et al. Quality of life after gastrectomy in patients with carcinoma of the stomach. Br J Surg 1992; 79: 248–250. [DOI] [PubMed] [Google Scholar]
- 56.Larsson J, Åkerlind I, Permerth J, Hörnqvist JO. The relation between nutritional state and quality of life in surgical patients. Eur J Surg 1994; 160: 329–334. [PubMed] [Google Scholar]