Abstract
Heterochromatin protein 1 (HP1) is a well-characterized heterochromatin component conserved from fission yeast to humans. We identified three HP1-like genes (hcpA, hcpB, and hcpC) in the Dictyostelium discoideum genome. Two of these (hcpA and hcpB) are expressed, and the proteins colocalized as green fluorescent protein (GFP) fusion proteins in one major cluster at the nuclear periphery that was also characterized by histone H3 lysine 9 dimethylation, a histone modification so far not described for Dictyostelium. The data strongly suggest that this cluster represents the centromeres. Both single-knockout strains displayed only subtle phenotypes, suggesting that both isoforms have largely overlapping functions. In contrast, disruption of both isoforms appeared to be lethal. Furthermore, overexpression of a C-terminally truncated form of HcpA resulted in phenotypically distinct growth defects that were characterized by a strong decrease in cell viability. Although genetic evidence implies functional redundancy, overexpression of GFP-HcpA, but not GFP-HcpB, caused growth defects that were accompanied by an increase in the frequency of atypic anaphase bridges. Our data indicate that Dictyostelium discoideum cells are sensitive to changes in HcpA and HcpB protein levels and that the two isoforms display different in vivo and in vitro affinities for each other. Since the RNA interference (RNAi) machinery is frequently involved in chromatin remodeling, we analyzed if knockouts of RNAi components influenced the localization of H3K9 dimethylation and HP1 isoforms in Dictyostelium. Interestingly, heterochromatin organization appeared to be independent of functional RNAi.
The correct organization of chromatin is an essential prerequisite for gene regulation and chromosome function in eukaryotes. Large blocks of heterochromatin usually specify centromeres and telomeres (20). The underlying DNA sequence at these loci mostly consists of tandem repeats and transposable elements and is packaged in higher order chromatin structures that are believed to prevent recombination events between homologous DNA sequences. Heterochromatin thus serves to maintain genomic integrity by regulating DNA accessibility. Heterochromatin was believed to be inaccessible for transcription factors and, thus, is transcriptionally silent. In the last few years, this view has significantly changed. At least in some organisms, transcriptional silencing at heterochromatic loci is reinforced by an autoregulatory feedback loop that posttranscriptionally degrades occasionally occurring transcripts via the RNA interference (RNAi) pathway (72). The resulting small interfering RNAs (siRNAs) recruit protein factors that mediate heterochromatin formation to these loci (44, 66, 70). The RNAi machinery may thus contribute to the maintenance of genomic integrity and, consequently, to mitotic and meiotic chromosome segregation (21, 71).
Since its original discovery in Drosophila melanogaster (25) as a nonhistone heterochromatin component, HP1 and its highly conserved homologues in other organisms have been characterized in detail and by now represent a paradigmatic example for proteins involved in higher order chromatin assembly (59). HP1 has been linked to gene silencing events such as position-effect variegation in Drosophila melanogaster (61), mating-type silencing in Schizosaccharomyces pombe (46), or mammalian position-effect variegation (11).
HP1-like proteins are characterized by a conserved trimodular structure that is crucial for their roles as adaptors connecting different complexes involved in formation and maintenance of heterochromatin. The N-terminal chromodomain of HP1 is necessary for binding to histone H3 tails methylated at residue lysine 9 (1, 34), a characteristic heterochromatin mark introduced by site-specific histone methyltransferases (HMTs) (57). HP1 is thus an example for proteins that read the histone code carried by specific histone modifications.
The C-terminal chromoshadow domain (CSD) is an interaction module responsible for binding a wide range of other proteins that in most cases contain a loosely conserved PxVxL motif (65, 69). The pentapeptide motif is present in the CSD itself and allows formation of homo- and heterooligomers of HP1 and its different isoforms that contribute to stability and spreading of heterochromatin domains (20). In addition, dimerization of HP1 proteins via their CSDs is required for interaction with other protein partners (3). Although oligomerization is a conserved property of HP1 proteins, the significance of isoform stoichiometry in the complexes is unclear.
The chromodomain and the CSD are separated by a less conserved linker region, the “hinge,” that contributes to heterochromatin targeting by its ability to bind DNA and RNA (45, 76).
Many organisms, for example, Drosophila melanogaster, C. elegans, and vertebrates, have two or more different HP1 isoforms. These are largely nonredundant, as shown by genetic analysis or biochemical characterization (5, 42, 64). The lethality of HP1 mutants further argues that at least some HP1 isoforms are essential (7, 12).
In several organisms, HP1 proteins are crucial for heterochromatin function at centromeres. For example, pericentromeric heterochromatin in S. pombe is required for recruitment of cohesin complexes that regulate sister chromatid cohesion and thus mitotic chromosome segregation. Consequently, a loss of the S. pombe HP1 homologue Swi6 leads to elevated rates of chromosome loss (2, 8, 52). In addition to their centromeric function, HP1 proteins are also involved in telomere capping, which serves to protect the ends of linear chromosomes that may otherwise be recognized by the DNA repair machinery (54). Loss of HP1 at telomeres causes end-to-end chromosome fusions in Drosophila (10). Association of HP1 proteins with telomeres was also observed in mammalian cells (16). Moreover, derepression of subtelomeric reporter genes correlates with delocalization of HP1 proteins (31).
Apart from their function in heterochromatin, HP1 proteins also play a role in the repression of individual euchromatic genes. In these cases, their function depends on interactions with sequence-specific repressor proteins, such as retinoblastoma protein (Rb) (51) or members of the TIF family (35, 49), and are mostly isoform specific.
Dictyostelium is a convenient model system to study various cell biological aspects, such as signal transduction, cell differentiation, cell motility, and cytokinesis. However, little is known about the subnuclear organization of its six chromosomes.
Although the Dictyostelium discoideum genome is completely sequenced (6), the information on centromeric and telomeric structures is limited. It is assumed that centromeres reside within clusters of repetitive DNA (e.g., transposons and retrotransposons) that are preferentially located at chromosome ends (6). The lack of a conserved underlying DNA sequence strongly argues that epigenetic factors probably play a role in centromere positioning, maintenance, and function in Dictyostelium.
Dictyostelium has recently been introduced as a model system to study RNAi-mediated gene silencing (38). To provide a basis for investigating RNA-mediated chromatin remodeling in Dictyostelium, we characterized well-defined heterochromatin components in this organism. We identified three genes encoding HP1-like proteins and analyzed the two isoforms that are expressed during normal growth and development.
MATERIALS AND METHODS
Plasmids.
To generate C-terminally green fluorescent protein (GFP)-tagged HcpA and HcpB, the respective cDNA was amplified using primers 5′ GAATTCAAAATGGGAAAAAGAGATAAGAG and 5′ GGATCCACTTTGTTGACCCTTATAACC for hcpA and 5′ GCTAAAAGAATTCAAAATGGGAAAAAGAG and 5′ GGATCCACTTGGCTGACCACTATAACC for hcpB and cloned into the EcoRI and BamHI sites of pDd-GFP (36). For N-terminal GFP fusions, hcpA was amplified using primers 5′ GTCGACATGGGAAAAAGAGATAAGAGAATAATAG and 5′ GGATCCTTTTAACTTTGTTGACCCTTATAACC, and hcpB was amplified with primers 5′ CAGCTAAAATTGTCGACATGGGAAAAAGAG and 5′ GGATCCTTAACTTGGCTGACCACTATAACC and cloned into the SalI and BamHI sites of pdneo2-GFP (56). For the HcpA-red fluorescent protein (RFP) fusion, the GFP coding sequence of pDd-HcpA-GFP was replaced by mutant RFP (RedStar) from p415Gal1-Redstar (30) by digestion with BglII/XhoI and ligation into BamHI/XhoI-digested pDd-HcpA-GFP.
The chromoshadow domain deletions (HcpAΔC-GFP and HcpBΔC-GFP) were designed with primers 5′ GAATTCAAAATGGGAAAAAGAGATAAGAG and 5′ ATTGGATCCACCTTCAATATAAGGTACACC for hcpA and 5′ GCTAAAAGAATTCAAAATGGGAAAAAGAG and 5′ ATTGGATCCACCTTCAATATAAGGTACACC for hcpB and cloned into the EcoRI and BamHI sites of pDd-GFP. For these constructs, identical reverse primers for both isoforms were used, since the primer binding sites were identical in both coding sequences.
Knockout constructs were generated using a blasticidin resistance cassette (68) with flanking arms of 625 bp and 501 bp for hcpA or 703 bp and 821 bp for hcpB. Primers to create recombinogenic arms were 5′ ATAGGATCCGAAGACCCTTTTAAGAAATGTTGTC and 5′ TTGAAGCTTTTTTCTTGTTCTCTTGAACTTTC as well as 5′ GCATGCCAAATCATTTAATTGATGATG and 5′ ATGGGCCCGAAGACGAATTATTTAGTAATTCTTTAAAATATG for hcpA, and 5′ ATTGGGCCCGAAGACAAAATTCACCATATAAGGGG and 5′ ATTGCATGCCCATGTATCTTAATCTGATG as well as 5′ ATTAAGCTTCATCAACAACAGCAGCAGCACC and 5′ ATTGGATCCGAAGACTTACCCCATCCAAACAATGAG for hcpB.
For both targeting constructs, the recombinogenic arms were cloned into pGEM7z-BSR, flanking the blasticidin resistance cassette. The vector pGEM7z-BSR was generated by cloning the BSR cassette (68) into the HindIII/XbaI sites of pGEM7z (Promega).
Knockout constructs were designed to disrupt the respective open reading frames (ORFs) between the N-terminal chromodomain and the nuclear localization signal (NLS) within the hinge region (data not shown). The truncated genes should thus not generate any functional, correctly localized protein.
For disruption of both endogenous hcp genes, the BSR cassette of both targeting constructs was excised with XbaI and HindIII and replaced by a BSR cassette with flanking loxP sites excised from pLPBLP (9) with BcuI and HindIII.
Recombinant His-tagged HcpA and HcpB was produced by cloning the cDNAs into the NdeI and BamHI sites of pET15b (Invitrogen). Primers used for amplification of the respective cDNAs were 5′ CATATGGGAAAAAGAGATAAGAGAATAATAG and 5′ GGATCCTTTTAACTTTGTTGACCCTTATAACC for hcpA and 5′ GCTAAAATTGTAACATATGGGAAAAAGAG and 5′ GGATCCTTAACTTGGCTGACCACTATAACC for hcpB.
Induction and protein expression was performed in Escherichia coli BL21(DE3) according to the manufacturer's instructions.
For expression of 6× His-tagged fusion proteins in Dictyostelium discoideum, the coding sequences were amplified from the respective pET-15b-derived plasmids and cloned into the EcoRI and BamHI sites of pDEX-DnmA, from which the DnmA (32) coding sequence had been excised.
Strains.
Transformations to generate knockout and overexpression mutants were performed in the Ax2 parent strain. Dictyostelium transformations and cotransformations were carried out as described previously (47, 48).
Gene disruptions and subcloning were done by homologous recombination (74). Clones were analyzed by PCR as described previously (38).
For hcpA, primers 5′ CAGTTACTTGTTTCATTATGGC and 5′ CGCTACTTCTACTAATTCTAGA were used to verify site-specific integration of the BSR cassette. For hcpB, primers were 5′ AAAAGATATAGAATCTACAACTATC and 5′ CGCTACTTCTACTAATTCTAGA.
For the generation of hcpA/hcpB double knockout strains, the Ax2 strain was first transformed with the hcpA targeting construct containing a BSR cassette with flanking loxP sites. Isolation of hcpA mutant cells was carried out as described above. Transient expression of Cre-recombinase, screening for blasticidin, and G418 sensitivity were carried out as described previously (9). The generated hcpAlp strain was then transformed with the hcpB targeting construct or transformed with pDEX-RH-His-HcpA prior to hcpB targeting.
RT-PCR analysis.
Total cellular RNA was prepared as described previously (37). Total RNA was DNase-treated before cDNA synthesis to eliminate genomic DNA contaminations. Reverse transcription-PCR (RT-PCR) analysis using Revert Aid H Minus M-MulV Reverse Transcriptase (MBI) was performed according to the manufacturer's instructions. Primers used to analyze expression levels of Hcp isoforms and the unrelated thioredoxin gene in knockout and overexpression strains were hcpA forward (for), 5′CATATGGGAAAAAGAGATAAGAGAATAATAG; hcpA reverse (rev), 5′ GGATCCTTTTAACTTTGTTGACCCTTATAACC; hcpB for, 5′ GCTAAAAGAATTCAAAATGGGAAAAAGAG; hcpB rev, 5′ GGATCCACTTGGCTGACCACTATAACC; trx for, 5′ GAACGAGCTCCATGGCCAATAGAGTAATTCATG; and trx rev, 5′ CGCGGATCCTTATTTGTTTGCTTCTAGAGTACTTC.
Cell culture.
Growth curves of Ax2, hcpA mutant, and hcpB mutant were done in axenic suspension culture in HL5 medium (67) on a rotary shaker at 150 rpm. For the overexpression strains, HL5 medium supplemented with 20 μg/ml geniticin was used. The starting cell density was 1 × 105 to 3 × 105/ml for growth at 22°C and at 15°C. Cell densities were measured with a Coulter Counter every 4 to 6 h for 48 to 72 h at 22°C and every 24 h for 10 to 15 days at 15°C. All growth curves were measured in duplicate.
In order to determine cell viability, the plating efficiency of cell suspensions that had been cultured for 48 h on rotary shakers was determined. Cell densities were measured before serial dilution in phosphate buffer. Cells were plated together with Klebsiella aerogenes suspension on nonselective SM agar (67) plates and allowed to grow for 72 h. Colonies were counted, and the plating efficiency was calculated by dividing colony number by cell density times dilution factor.
Immunodetection.
Dictyostelium discoideum cells were grown on coverslips in petri dishes containing the appropriate selective medium for 20 to 24 h. Cells were then fixed in −20°C methanol for 20 min, washed three times in 1× phosphate-buffered saline (PBS), and blocked with PBG buffer (1× PBS with 3% bovine serum albumin and 0.045% cold water fish gelatin; Sigma) for 1 h at 37°C. Primary antibodies were applied in appropriate dilutions and incubated over night at 4°C.
For detection of histone H3K9 dimethylation, a polyclonal rabbit antibody (Upstate Biotechnology) diluted 1:100 was used. For staining of centrosomes, the anti-DdCP224 antibody (18) was used in a 1:100 dilution. After six washes in PBG, Cy3-conjugated secondary antibodies (Dianova, Hamburg, Germany) were applied in a 1:1,000 dilution in PBG for 1 h at 37°C. DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI) (1 mg/ml) diluted 1:15,000 in 1× PBS.
Mitotic analysis.
For mitotic assays, cells were precultured in petri dishes at 22°C and 15°C and then diluted to 3 × 105/ml at 22°C and 7 × 105/ml at 15°C, respectively. Cells were then grown on coverslips in petri dishes covered with 5 ml of HL5 medium for 20 to 24 h and fixed as described above. Mitotic cells were detected by anti-DdCP224 or by anti-α-tubulin staining. For determination of the frequency of anaphase bridges, only late mitotic cells with spindles that were ≥5 μm were counted, and the number of mitoses with DNA bridges was determined.
Statistical evaluation.
To get a significance level for the observation that overexpression of GFP-HcpA leads to a higher frequency of anaphase bridges in late mitotic cells, two procedures were applied.
Procedure 1.
As the number of anaphase bridges also depended on the number of counted cells, we adjusted the data to 90 counted cells per cell type, resulting in 11 or 12 anaphase bridges for GFP-HcpA cells and letting all other numbers be fixed. This procedure even reduced the chance to obtain a convincing level of significance. We computed the chi-squared (χ2) statistics with the following equation: 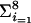 [ki − (m/8)]2/(m/8), where the ki is the respective number of anaphase bridges, with k5 = 12 or 11 and m = 29 or 28 for the total count of anaphase bridges. The calculated value was compared with the respective values obtained after randomly distributing m objects in 8 boxes, the well-known urn model in stochastics, which represents the null hypothesis we want to reject. Thus, for k5 = 12, we obtained the significance value of α = 0.0014; for k5 = 11, the significance value was α = 0.0052.
[ki − (m/8)]2/(m/8), where the ki is the respective number of anaphase bridges, with k5 = 12 or 11 and m = 29 or 28 for the total count of anaphase bridges. The calculated value was compared with the respective values obtained after randomly distributing m objects in 8 boxes, the well-known urn model in stochastics, which represents the null hypothesis we want to reject. Thus, for k5 = 12, we obtained the significance value of α = 0.0014; for k5 = 11, the significance value was α = 0.0052.
Procedure 2.
In each of the first seven cases, we have purely randomly chosen 90 trials and noted the ki numbers of the chosen trials in each of the seven cases in which there occurred anaphase bridges. Of course, k8 = 3. Like the first procedure, we determined the significance level (α) of the obtained 8-tuple (k1 to k8). This was repeated 1,000 times. For the mean of the obtained values for the significance level, we obtained an αm of 0.0032 and for the median an m of 0.0009 (see Table 3).
TABLE 3.
Frequencies of anaphase bridgesa
| Strain | % Anaphase bridges |
|---|---|
| Ax2 | 3.2 (3/94) |
| hcpA mutant | 4.1 (4/98) |
| hcpB mutant | 1.9 (2/107) |
| Vector | 2.9 (3/103) |
| GFP-HcpA | 12.9 (14/108) |
| GFP-HcpB | 0.9 (1/108) |
| HcpAΔC-GFP | 1.1 (1/95) |
| HcpBΔC-GFP | 3.3 (3/90) |
Cells were grown on coverslips for 20 to 24 h and fixed. Mitotic cells were identified by anti-DdCP224 or anti-tubulin staining. Late mitotic cells with mitotic spindles of ≥5 μm were counted (see Fig. 8C). In parentheses, absolute numbers of abnormal mitoses per number of counted mitoses are given. Results were reproduced with at least two independent clones of each strain.
Summarizing these numerical results, our experimental observations show for the significance level of at least 0.005 that overexpression of GFP-HcpA results in a higher frequency of anaphase bridges than in the other cell types.
Fluorescence microscopy.
For microscopic analysis a Leica DM IRB inverted fluorescence microscope was used, and for image acquisition a Leica DC 350F digital camera and IM50 software were used. Images were processed in Adobe Photoshop.
Electron microscopy.
Nuclei were isolated from HcpA-GFP- and HcpB-GFP-expressing cells (33) that had been treated with 100 μM thiabendazole for 4 h. The nuclei were centrifuged onto round coverslips and fixed with 2% paraformaldehyde in modified PHEM buffer [15 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 6 mM HEPES, 10 EGTA, 0.5 mM MgCl2, pH 7] (24) for 20 min. The preparations were treated with 1 mg/ml bovine serum albumin, 5% fetal calf serum, and 2% cold water fish gelatin to reduce nonspecific staining and were incubated with an anti-GFP antibody for 1 h. Five nanometer protein A gold (Department of Cell Biology, University Medical Center, Utrecht, The Netherlands) was applied for 2 h, and after extensive washes the nuclei were postfixed with 1% glutaraldehyde for 5 min. The subsequent fixation with 1% OsO4 in cacodylate buffer, dehydration, and embedment in Epon/Araldite were carried out according to standard procedures (27). Thin sections were cut using a Reichert Ultracut E, stained with uranyl acetate and lead citrate, and observed in a Jeol EM 1200 EX II.
Pull-down assays.
His-tagged HcpA and HcpB were purified on Ni-Sepharose beads (Amersham) according to the manufacturer's instructions.
Cell lysates from a Dictyostelium discoideum strain expressing GFP-tagged fusion proteins were prepared by sonifying 1 × 108 cells from axenic suspension culture three times for 15 s in 10 ml lysis buffer (20 mM phosphate buffer, pH 7.5, 300 mM NaCl, 10 mM imidazole, 0.5% NP-40).
For isolation of Hcp binding proteins, recombinant His-tagged HcpA or HcpB was immobilized on a Ni-Sepharose column. Cell lysates were mixed with precoated Ni-Sepharose beads and rotated for 1 to 2 h on a spinning wheel. Beads were spun down by brief centrifugation. The supernatant was stored, and the beads were washed with at least 20 column volumes of binding buffer. The respective Hcp and bound proteins were then eluted with 250 mM imidazole. Eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide (12%) gel electrophoresis. Proteins were stained with Coomassie and detected on Western blots by a monoclonal anti-GFP (Chemicon, Temecula, CA) or anti-His antibody.
The same protocol was applied for determining in vivo interactions, except that extracts from His-HcpA/GFP-HcpB- and His-HcpA/GFP-HcpA-cotransformed cell lines were directly incubated with the Ni-Sepharose beads.
Strains.
Strains used in this study were Dictyostelium discoideum Ax2, Ax2 HcpA−, Ax2 HcpB−, Ax2 GFP-HcpA, Ax2 GFP-HcpB, Ax2 His-HcpA, Ax2 His-HcpB, Ax2 HcpA-GFP, Ax2 HcpB-GFP, Ax2 HcpAΔC-GFP, and Ax2 HcpBΔ C-GFP and E. coli DH5α (Promega) and BL21(DE3) pLysS (Invitrogen).
Nucleotide sequence accession numbers.
Sequences determined in the course of this work were submitted to Dicty Base (http://dictybase.org/) under the following accession numbers: hcpA, DDB0220646; hcpB, DDB0220645; and hcpC, DDB0220647.
RESULTS
HP1-like proteins in Dictyostelium discoideum.
A database search using the human HP1α sequence and a tBLASTN algorithm revealed the presence of three putative genes encoding HP1-like proteins in the Dictyostelium discoideum genome. All three displayed the characteristic hallmarks of HP1 proteins: an N-terminal chromodomain and a C-terminal chromoshadow domain separated by a less conserved hinge region. The genes were denominated hcpA, hcpB, and hcpC (Fig. 1). HcpA displays 70% identity (77% similarity) with HcpB but only 62% identity (75% similarity) with HcpC. Sequence analysis further showed that amino acid residues within the chromodomain that are required for function, such as methyl-lysine binding, were highly conserved in the Dictyostelium proteins, whereas functionally important amino acid residues within the chromoshadow domain were less conserved (Fig. 1). The HcpA chromodomain displayed highest similarity to that of human HP1γ (79%) and slightly less to the α- and β-isoforms (73% and 77%, respectively). For comparison, the chromoshadow domain of HcpA displayed 52%, 48%, and 46% similarity to the human α-, β-, and γ-isoforms, respectively.
FIG. 1.
Alignment of HP1-like proteins. HcpA, HcpB, and HcpC were aligned with human HP1 orthologues HP1α, HP1β, and HP1γ. The highly conserved chromodomain and chromoshadow domain are underlined in black. Functionally important amino acid residues in the chromodomain are indicated as squares. Black shading indicates aromatic residues that form a pocket required for methyl-lysine binding; light gray shading indicates additional residues required for methyl-lysine recognition; dark gray shading indicates residues required for recognition of Ala 7 in the histone H3 N terminus (50). Functionally important amino acid residues in the chromoshadow domain are indicated as circles. Light gray circles indicate residues that form the CSD dimer interface (3). Residues required for recognition of the PxVxL motif in HP1-interacting proteins are shown in dark gray (central valine) and black (proline and leucine) circles (69). The nuclear localization signals within the hinge region are underlined in gray. Note that the previously described bipartite NLS within the hinge region of the human HP1 proteins (64) is missing in the Dictyostelium proteins but lies adjacent to the chromodomain. Abbreviations: Dd, Dictyostelium discoideum; Hs, Homo sapiens.
RT-PCR analysis showed that hcpA and hcpB were expressed in axenically grown cells (see below) and throughout development (data not shown). hcpC transcripts were not detected under these conditions, while the gene-specific primers readily showed a PCR product when using genomic DNA (data not shown). To see if hcpC was induced as a potential compensatory mechanism, we investigated its transcription in hcpA and hcpB knockout strains but could not detect any RT-PCR signal. Apparently, hcpC is not expressed at all or has a very restricted expression pattern. It was therefore excluded from further studies.
HcpA and HcpB display largely identical subnuclear localization.
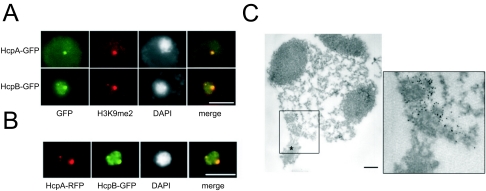
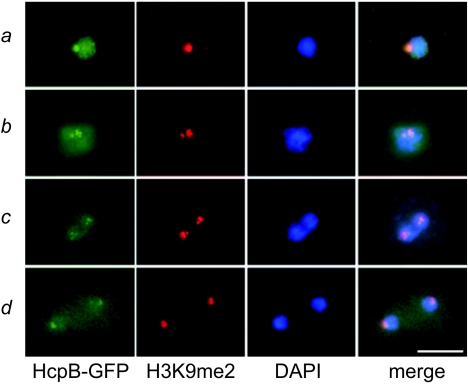
To study the subnuclear organization of Dictyostelium heterochromatin, we used C-terminally GFP-tagged HcpA and HcpB (HcpA-GFP and HcpB-GFP). Both proteins localized to one major spot at the nuclear periphery and several minor foci (Fig. 2A). Depending on expression levels, some nucleoplasmic staining, probably representing unbound HP1 molecules, was observed. Furthermore, coexpression of HcpA-RFP and HcpB-GFP showed that HcpA and HcpB colocalize (Fig. 2B), indicating that the subnuclear distribution of both isoforms is very similar. Electron microscopy revealed that the fusion proteins colocalize with electron-dense structures at the nuclear periphery close to the centrosome (shown for HcpA-GFP in Fig. 2C). These structures contain the kinetochores/centromeres of Dictyostelium chromosomes (43). Centrosomes have previously been characterized by electron microscopy and immunostaining with specific monoclonal antibodies, like DdCP224 (see below) (19).
FIG. 2.
Localization of HP1 proteins. A) HcpA-GFP and HcpB-GFP localize to one major and several minor foci at the nuclear periphery. Since the plain of the major spot is shown, only one or two minor foci are seen. Although driven by identical promoters, the fusion proteins display significant differences in expression levels. Note the increased nucleoplasmic staining by HcpB-GFP. HcpA-GFP and HcpB-GFP both colocalize with histone H3K9me2 in the majority of subnuclear foci. Bar, 5 μm. B) Cotransformed HcpA-RFP and HcpB-GFP constructs demonstrate colocalization of HP1 isoforms. In this example, both proteins are seen in one major and one minor focus at the nuclear periphery. Bar, 5 μm. C) An isolated nucleus/centrosome complex from cells expressing HcpA-GFP is shown. The centrosome is marked with an asterisk. The three dark areas represent nuclear caps (nucleoli). The image on the right shows the labeling of HcpA-GFP with 5 nm gold-coupled anti-GFP antibodies at a higher magnification. The label is predominantly found in a strictly confined area of electron-dense material close to the centrosome. The nuclear envelope has been extracted by Triton X-100 during the isolation procedure. Bar, 200 nm.
Dimethylated lysine 9 of histone 3 (H3K9me2) is a further hallmark for heterochromatin and serves as a binding site for HP1 proteins (1, 34). To test for this association in Dictyostelium, double-labeling experiments were performed and showed that both HcpA-GFP and HcpB-GFP colocalized with H3K9me2 (Fig. 2A). The same was true for the respective N-terminal GFP fusion proteins (termed GFP-HcpA and GFP-HcpB; data not shown). The specificity of the H3K9me2 antibody, originally raised against methylated human histone H3, was verified by Western blotting. It recognized a single 17-kDa protein which corresponds in size to the Dictyostelium histone H3 (data not shown).
HcpA and HcpB form homo- and heterodimers in vitro and in vivo.
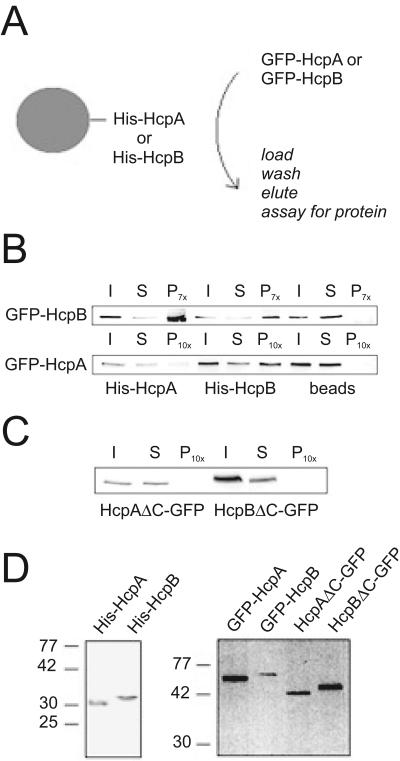
A conserved feature of HP1 proteins is their ability to form multimeric complexes by homo- and heterodimerization through their chromoshadow domains (3, 51). Since we found largely identical subnuclear distribution of both Hcp isoforms, we determined if HcpA and HcpB are capable of dimerization in vitro. Bacterially expressed His-tagged HcpA or HcpB was immobilized on Ni-Sepharose columns. Extracts from Dictyostelium discoideum cells expressing GFP-HcpA were passed over the matrix, and bound proteins were eluted with imidazole (Fig. 3A). Both His-HcpA and His-HcpB were able to retain GFP-HcpA and GFP-HcpB on the column (Fig. 3B). This showed that the Dictyostelium proteins could form both homo- and heterodimers. Relatively, His-HcpA retained much less GFP-HcpA than GFP-HcpB (Fig. 3B). This was reproducible in multiple experiments, which always showed a faint but defined band. The significance of the precipitation was confirmed by control experiments where beads without coupled Hcp protein were incubated with extracts from Dictyostelium discoideum cells expressing GFP-HcpA or GFP-HcpB. In both cases no band was detected in the precipitate (Fig. 3B).
FIG. 3.
Homo- and heterodimer formation of HP1-like proteins in vitro. A) Scheme for pull-down analysis. Ni-Sepharose beads preloaded with either His-HcpA or His-HcpB were incubated with Dictyostelium discoideum cell lysates containing GFP-HcpA and GFP-HcpB either alone or in combination were treated as shown. B) GFP-HcpA and GFP-HcpB bind to both His-HcpA or His-HcpB but not to empty beads. I, input; S, supernatant; P, pellet (7- or 10-fold concentrated with respect to I and S). C) Neither HcpAΔC-GFP nor HcpBΔC-GFP can bind to His-HcpA. His-HcpA was immobilized on Ni-Sepharose beads and incubated with cell lysates containing either HcpAΔC-GFP or HcpBΔC-GFP. I, input; S, supernatant; P, pellet (10-fold concentrated with respect to I and S). D) Western blots showing proteins used in this study. Left, bacterially expressed His-HcpA and His-HcpB stained with an anti-His antibody. Right, cell lysates from the indicated Dictyostelium discoideum overexpression strains were probed with α-GFP antibody.
GFP fusions of HcpA or HcpB lacking the chromoshadow domain (HcpAΔC-GFP and HcpBΔC-GFP) could not form any detectable dimers with immobilized His-HcpA (Fig. 3C), indicating that the chromoshadow domain is required for dimerization. This is consistent with findings for HP1 homologues from other organisms.
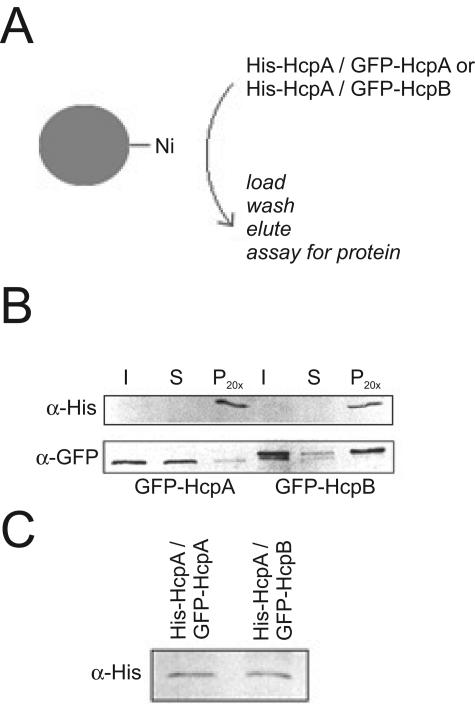
To confirm that both isoforms are also capable of homo- and heterodimerization in vivo, we generated cell lines that stably expressed both His-tagged HcpA and either GFP-HcpA or GFP-HcpB. Ni-Sepharose beads were then incubated with cell extracts from these cell lines (Fig. 4A). For both cotransformations, GFP-HcpA and GFP-HcpB could be coeluted with His-HcpA, indicating that both isoforms form oligomeric complexes in vivo (Fig. 4B).
FIG. 4.
Homo- and heterodimer formation of HP1-like proteins in vivo. A) Scheme for pull-down analysis. Ni-Sepharose beads were incubated with total cell lysates from Dictyostelium discoideum cell lines cotransformed with His-HcpA and GFP-HcpA or GFP-HcpB. B) Both GFP-HcpA and GFP-HcpB are coeluted with His-HcpA from Ni-Sepharose beads. His-HcpA is under the control of an actin 15 promoter and expressed at very low levels in the cell. It is therefore hardly detectable in the input fraction but effectively enriched in the pellet fraction. GFP-HcpB runs as a double band in this gel. C) His-HcpA is detectable in nuclear extracts of cotransformants. Nuclear extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. His-HcpA was detected with α-His antibody. Abbreviations: I, input; S, supernatant; P, pellet. Pellet fractions were 20-fold concentrated with respect to input and supernatant.
Remarkably, significantly more GFP-HcpB than GFP-HcpA was coeluted with His-HcpA, although the expression levels of His-HcpA were very similar in both cell lines (Fig. 4C) and the respective GFP-fusion proteins were present in excess over His-HcpA.
In both experiments, HcpA-homodimer formation appeared to be less favorable than HcpA-HcpB-heterodimer or HcpB-homodimer formation. This suggests that the two isoforms differently contribute to heterochromatin formation.
Although the amino acid sequences of the chromoshadow domains of Dictyostelium and mammalian HP1 proteins are significantly divergent (Fig. 1), they display the characteristic hallmarks of homo- and heterodimerization. This sequence divergence possibly confers a distinct species-specific recognition of interaction partners. For example, His-HcpA formed homo- and heterodimers with the Dictyostelium isoforms but was unable to interact with murine HP1α (data not shown).
Mitotic dynamics of Dictyostelium heterochromatin.
In interphase cells, the major heterochromatic cluster is closely associated with the centrosome, a nucleus-associated body in Dictyostelium (Fig. 5, interphase; see also Fig. 2C). Since this association was stable in randomly migrating or chemotaxing cells (data not shown), this indicated a functional relationship and was reminiscent of centromeric heterochromatin in S. pombe, which is closely connected to the nuclear membrane-embedded spindle pole body (15).
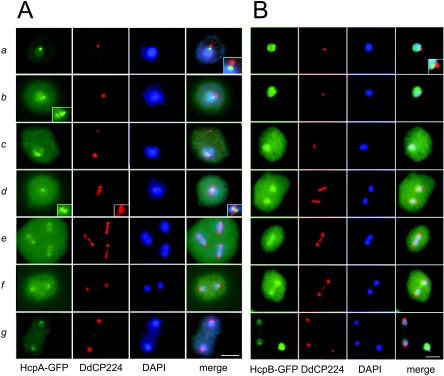
FIG. 5.
Mitotic dynamics of Dictyostelium heterochromatin. A) Cells expressing HcpA-GFP; B) cells expressing HcpB-GFP. During interphase, heterochromatin localizes next to the nucleus-associated centrosome (see inset). During prophase, the heterochromatin cluster divides in up to six spots (see inset). During prometaphase, nucleoplasmic protein enters the cytoplasm, indicated by increased cytoplasmic fluorescence. The telophase panel for HcpB-GFP shows a telophase and an interphase cell to illustrate the loss of nuclear fluorescence during mitosis. Centrosomes and mitotic spindles are stained with an anti-DdCP224 antibody. a, interphase; b, prophase; c, prometaphase; d, metaphase; e, early anaphase; f, late anaphase; g, telophase. Bar, 5 μm.
To examine if the major heterochromatic cluster represented Dictyostelium centromeres, we analyzed HcpA-GFP and HcpB-GFP distribution during mitotic stages in asynchronously growing Dictyostelium discoideum cells. Mitoses were identified by antibodies directed either against DdCP224, a Dictyostelium XMAP215 homologue that stains the centrosome and the mitotic spindle (18, 19), or against α-tubulin (28).
During interphase, both Hcp-GFP fusion proteins localized to one prominent cluster at the nuclear periphery directly opposite the centrosome and colocalized with H3K9 dimethylation. In prophase, when Dictyostelium centrosomes are duplicated, the cluster split up into several smaller foci. In some preparations, up to six spots could be distinguished that may represent the centromeres of the six Dictyostelium chromosomes (Fig. 5A, b, prophase inset). This was similar to pericentromeric heterochromatin in S. pombe that also splits up during mitotic prophase (15, 55). In prometaphase, when the duplicated spindle poles separate and penetrate the nuclear envelope, the majority of the nuclear HcpA-GFP and HcpB-GFP enters the cytoplasm, indicated by a significant increase in cytoplasmic and loss of nucleoplasmic GFP staining (Fig. 5B, c). This probably represents passive diffusion and not an active process, since a similar relocalization has been described for NLS-tagged GFP (75). Both observations argue that mitosis may not be completely closed in Dictyostelium discoideum.
In metaphase, heterochromatin localizes between a bilayered structure at the central spindle that is stained by the anti-DdCP224 antibody (Fig. 5A, d) and that probably represents the kinetochore region (58). During anaphase, the heterochromatic clusters divided and moved toward the spindle poles, leading the separated DNA masses. Similar mitotic dynamics of heterochromatin were observed for histone H3K9 dimethylation (Fig. 6). Although GFP-fused Hcp proteins stay associated with heterochromatin throughout the entire cell cycle, we reproducibly detected a significant loss of Hcp proteins at heterochromatin during late mitotic stages and an increase in cytoplasmic staining compared to interphase (Fig. 5 and 6). This is best demonstrated in Fig. 5B, g, where an interphase cell with strong HcpB-GFP centromere staining is shown next to a telophase cell with very weak GFP label. Although we cannot say if this behavior represents that of the endogenous proteins, it may indicate that heterochromatic localization of Hcp proteins is dynamically regulated during the cell cycle. Similar observations for HP1 dissociation from heterochromatin during mitosis have been made in mammalian cells (13, 23). In conclusion, the colocalization data and the mitotic behavior strongly suggested that the foci stained by the GFP-labeled HP1 isoforms represent centromeric heterochromatin.
FIG. 6.
Mitotic dynamics of HcpB-GFP and histone H3K9me2. Whereas H3K9me2 is stable during mitosis, the fluorescence intensity of HcpB-GFP at the putative centromeres and in the nucleoplasm significantly decreases. Images in vertical rows were captured with identical exposure times. a, interphase; b, prophase; c, early anaphase; d, telophase. Bar, 5 μm.
Knockout mutants of hcpA, but not of hcpB, show temperature-dependent growth defects.
To analyze the function of the two HP1 isoforms, we generated knockout strains for both hcpA and hcpB by homologous recombination (data not shown). Under standard laboratory conditions, none of the single-knockout strains displayed a significant phenotype in axenic suspension culture or during development (data not shown). However, at lower temperatures, the hcpA mutant strain but not the hcpB mutant strain displayed a slight growth defect in suspension culture compared to the parent Ax2 strain (Table 1).
TABLE 1.
Generation times of knockout and overexpression strainsa
| Temp and strain | Generation time (±SD) |
|---|---|
| 22°C | |
| Ax2 | 9.3 (±0.2) |
| hcpA mutant | 8.7 (±0.1) |
| hcpB mutant | 8.8 (±0.1) |
| Vector* | 9.3 (±1.1) |
| GFP-HcpA* | 13.5 (±0.4) |
| GFP-HcpB* | 21.8 (±0.6) |
| Vector* | 9.7 (±0.1) |
| HcpAΔC-GFP* | 28.4 (±0.4) |
| HcpBΔC-GFP* | 13.2 (±1.7) |
| 15°C | |
| Ax2 | 47.0 (±0.2) |
| hcpA mutant | 62.2 (±5.4) |
| hcpB mutant | 44.2 (±8.1) |
Mean generation times (in hours) and standard deviations from several independent clones are shown. Growth curves were measured in axenic suspension culture in HL5 medium or in HL5 medium supplemented with 20 μg/ml geniticin (indicated by asterisks).
In S. pombe, knockout of swi6 causes a similar temperature-dependent growth defect that is characterized by increased chromosome missegregation rates and can be mimicked by microtubule-destabilizing drugs such as thiabendazole (8). In contrast to that, we could not detect any defects in mitotic progression or an obvious chromosome missegregation phenotype. Furthermore, the temperature sensitivity of the hcpA mutant could not be mimicked by thiabendazole treatment, indicating that microtubule (dys)function was not involved (data not shown). Therefore, the reason for the temperature sensitivity of the hcpA mutant remains unclear.
Despite subtle differences at low temperature, the lack of a mutant phenotype under standard conditions argued for a redundancy of the two Hcp isoforms in most functions.
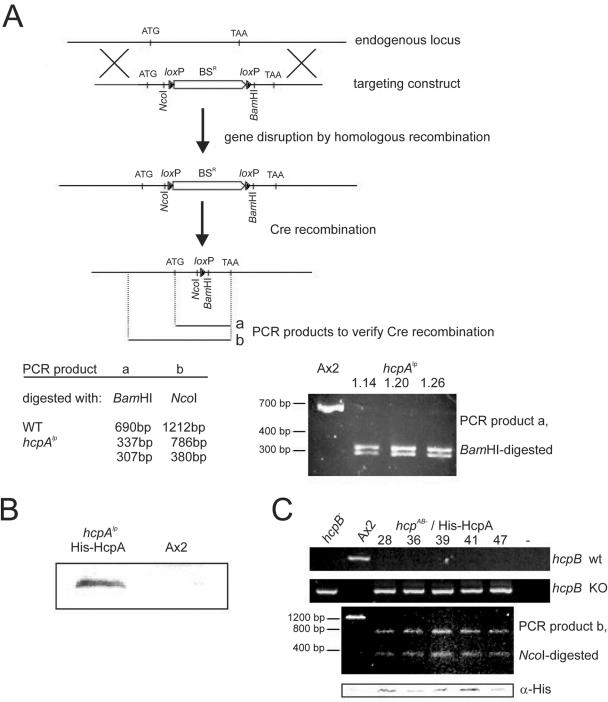
We therefore attempted to disrupt both endogenous genes to create double-knockout mutants. Since the gene disruption frequencies for the two hcp genes were reproducibly dissimilar (4% for hcpA compared to 67% for hcpB), we first created an hcpA mutant cell line that was followed by Cre-mediated excision of the blasticidin resistance cassette (Fig. 7A). Cre-mediated recombination was verified by restriction fragment length polymorphism of the PCR products that were amplified from either the wild-type hcpA gene or the disrupted hcpA gene after Cre recombination (Fig. 7A). The resulting cell line, termed hcpAlp, was then transformed with the hcpB targeting construct. Remarkably, we were not able to isolate any hcpB mutant clones in the hcpAlp background from three independent transformations, indicating that disruption of the second hcp gene greatly affected viability. Ectopic overexpression of an His-HcpA protein in the hcpAlp background (Fig. 7B) prior to hcpB targeting at least partially restored the disruption frequencies that we had observed in the Ax2 wild-type background (Table 2) and resulted in double-knockout strains. This suggested that the prior introduction of a functional gene could rescue the presumably lethal phenotype. The genetic composition of isolated clones was confirmed by PCR on the wild-type and mutated hcpB locus by PCR on the hcpAlp locus and by Western blots showing expression of His-HcpA (Fig. 7C). No obvious phenotypic differences between the hcpAB mutant-His-HcpA and the parental hcpAlp-His-HcpA cells could be observed (data not shown). These findings indicated that the two Hcp isoforms were largely redundant and that the presence of at least one isoform was essential for cell viability. The data further confirmed that ectopically expressed His-HcpA can functionally replace the endogenous gene, at least with respect to cell viability.
FIG. 7.
Disruption of endogenous genes of both Hcp isoforms. A) Scheme for hcpA gene targeting and subsequent Cre-mediated excision of the BSR cassette. Gene disruption and Cre recombination introduced restriction sites flanking the remaining loxP site at the endogenous locus. The recombinant hcpA allele (hcpAlp) is similar in size to the wild-type hcpA gene but can be distinguished from the wild-type allele by restriction digestions with the enzymes indicated. Restriction fragment analysis of PCR products derived from either wild-type (Ax2) or different recombinant clones was used to verify Cre-mediated recombination. Only the PCR products derived from the hcpAlp locus, but not from the wild-type hcpA locus, can be digested with BamHI. B) Expression of His-HcpA in hcpAlp cells. Nuclear extracts from hcpAlp-His-HcpA or Ax2 cells were probed with α-His antibody. C) Disruption of the hcpB gene. Clonal isolates of hcpAB mutant-His-HcpA cells transformed with the hcpB disruption construct were screened by PCR (see Materials and Methods) for either homologous recombination of the targeted construct (hcpB knockout [KO]) or an intact hcpB gene (hcpB wild type [WT]). Results for several independent knockout clones are shown in comparison to Ax2 wild-type cells and the single hcpB knockout cell line. The same clones were tested for presence of the Cre recombinant hcpAlp allele by PCR and restriction fragment analysis with NcoI as depicted for PCR product b in panel A. This PCR product that is longer than PCR product a (panel A) covers the 5′ noncoding region of the endogenous hcpA locus and allows us to distinguish it from the His-HcpA transgene, which only contains the coding sequence. Expression of His-HcpA was confirmed by Western blot analysis with an anti-His antibody (bottom panel).
TABLE 2.
Frequencies of hcpB gene disruptions in different background strainsa
| Strain | % Disrupted |
|---|---|
| Ax2. | 67 (28/42) |
| hcpAlp | 0 (0/200) |
| hcpAB mutant-His-HcpA | 23 (9/40) |
Number of homologous recombinations detected and total number of tested clones are given in parentheses.
Overexpression of GFP-HcpA, but not of GFP-HcpB, leads to an increased number of anaphase bridges.
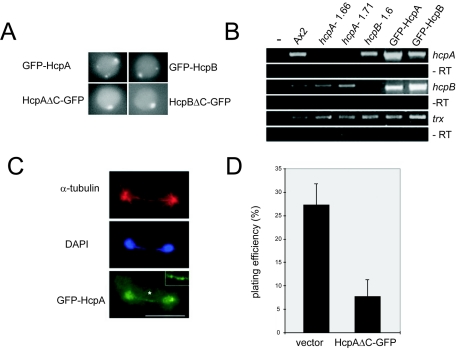
The C-terminal GFP fusion HcpA-GFP was consistently expressed at much lower levels than HcpB-GFP, with almost no nucleoplasmic fluorescence (Fig. 2A). Although both constructs were driven by an actin 15 promoter, this difference could also be detected in cotransformants expressing HcpA-RFP and HcpB-GFP fusion proteins (Fig. 2B). In contrast, N-terminal GFP fusions of HcpA and HcpB showed approximately equal expression levels with similar nucleoplasmic background (Fig. 8A). Because of the almost identical expression levels, which were confirmed by Western blots (data not shown), we used the N-terminal GFP fusions for functional analysis.
FIG. 8.
Distinct phenotypes of cells expressing Hcp fusion proteins. A) Live-cell images of GFP-HcpA-, GFP-HcpB-, HcpAΔC-GFP-, and HcpBΔC-GFP-expressing cells. All images were taken with identical exposure times. Both truncated fusion proteins lacking the CSD still localize to the major heterochromatic cluster but do not form additional smaller foci. B) Expression levels of hcpA, hcpB, and thioredoxin in different background strains determined by semiquantitative RT-PCR. hcpA and hcpB transcript levels are significantly enriched in strains overexpressing GFP-HcpA or GFP-HcpB, respectively, but are not detectable in the knockout strains. Due to partial cross-sensitivity, primers used for hcpB can also bind to hcpA. Note the increase in signal intensity but slightly lower size of the PCR product in the GFP-HcpA overexpression strain. Numbers of PCR cycles for amplification were 32 (hcpA, hcpB) and 27 (thioredoxin). −RT, minus reverse transcription. C) Anaphase-bridge phenotype in the GFP-HcpA strain. A late mitotic cell, as identified by a long mitotic spindle, is shown. DAPI stain shows a DNA bridge between the main DNA masses. GFP-HcpA is found in small foci on the DNA bridge. The asterisk indicates the area of magnification in the upper right corner. Bar, 5 μm. D) Cell viability, as determined by plating efficiency, is significantly impaired by overexpression of HcpAΔC-GFP compared to the control transformant.
Interestingly, when studying mitotic heterochromatin dynamics, we observed striking phenotypes that were particularly enriched in cell lines expressing GFP-HcpA.
Compared to the control transformation, we found a more than fourfold increase in DNA bridges between the separated DNA masses in late mitotic cells (Table 3).
Statistical evaluation of the data (see Materials and Methods) showed on the significance level of a maximum of 0.005 that overexpression of GFP-HcpA resulted in a higher frequency of anaphase bridges than in the other cell types.
Anaphase bridges were reminiscent of the abnormal separation of dicentric chromosomes in, for example, Drosophila melanogaster. GFP-HcpA fluorescence frequently localized to these DNA bridges (Fig. 8C), indicating that heterochromatin was involved in these structures. Furthermore, overexpression of GFP-HcpA caused pronounced growth defects in axenic suspension culture compared to control transformants (Table 1).
Overexpression of GFP-HcpB did not increase the number of anaphase bridges but still caused growth defects that were even more severe than those observed for GFP-HcpA-expressing strains (Tables 1 and 2). The results were reproduced with several independent clones, but the reason for slow growth could not be elucidated. In order to show that the recombinant proteins were indeed overexpressed compared to the wild type, we performed semiquantitative RT-PCR analysis of both strains. This showed that the mRNA levels of the respective Hcp isoforms were significantly increased in comparison to the endogenous transcripts (Fig. 8B). Although we could not test for expression levels of the endogenous protein, it is very likely that the recombinant Hcp isoforms were also more abundant than the respective wild-type proteins.
In order to gain further insight into the function of HP1 proteins in heterochromatin, we overexpressed truncated HcpA or HcpB protein lacking the C-terminal chromoshadow domain (HcpAΔC-GFP and HcpBΔC-GFP). We assumed that these proteins still localize to heterochromatin (73) but prevent the formation of higher order chromatin structures, since HcpAΔC-GFP was not able to dimerize in vitro (Fig. 3C). Hence, the mutant proteins should compete with both Hcp wild-type forms for binding sites and result in a dominant-negative loss-of-function phenotype when expressed in sufficient amounts.
The truncated proteins were, at least in part, localized to the same major heterochromatic focus as the full-length proteins. This was confirmed by colocalization with H3K9me2 staining and close proximity to the centrosome as identified by tubulin staining (data not shown). Staining of the minor subnuclear foci was, however, strongly reduced (Fig. 8A), suggesting distinct CSD-dependent and CSD-independent targeting of HP1 proteins in Dictyostelium. Overexpression of the truncated HcpA protein led to severe growth defects in axenic suspension culture (Table 1). Microscopic analysis of cells in suspension culture revealed that approximately half of the cells, compared to less than 5% of the control transformant, were abnormally rounded and had aberrantly condensed DNA. However, these did not represent prometaphase cells, since no significant mitotic defects could be detected (data not shown). Moreover, the plating efficiency on bacterial lawns of the HcpAΔC-GFP strain was more than threefold reduced compared to the control strain (Fig. 8D). Interestingly, overexpression of HcpAΔC-GFP, although expressed at levels similar to those of full-length GFP-HcpA, did not elicit increased numbers of anaphase bridges as seen for full-length HcpA (Table 2). It appears that overexpression of functional HcpA, which is able to interact with other proteins via its CSD, is required for this defect.
The decrease in cell viability of the HcpAΔC-GFP overexpression strain supported our assumption that the truncated protein had a dominant-negative function. This was, however, not as severe as a complete loss-of-function mutation in both Hcp isoforms.
RNAi-mediated heterochromatin formation in Dictyostelium?
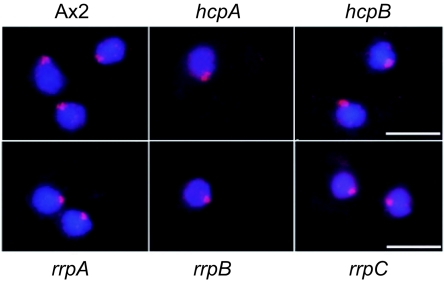
In Drosophila melanogaster (62) and S. pombe (22), HP1 loss-of-function mutants influence H3K9 methylation levels and their subnuclear distribution. In Dictyostelium discoideum, the knockout mutants of individual HP1 genes did not cause significant changes in histone H3K9me2 subnuclear distribution (Fig. 9). This indicated that either the two isoforms are redundant for recruiting histone methyltransferases (HMTs) or that HMTs act upstream of HP1.
FIG. 9.
H3K9me2 localization in hcp and rrp knockout mutants. Knockout of either hcpA or hcpB has no effect on H3K9me2 levels or its subnuclear distribution. Similarly, knockouts of the RNA-dependent RNA polymerase genes rrpA, rrpB, or rrpC that are involved in RNA-mediated posttranscriptional gene silencing do not affect H3K9me2 levels and distribution. DAPI is in blue and H3K9me2 is in red. Bar, 5 μm.
The RNAi machinery and their products, short interfering RNAs (siRNAs), are implicated in DNA methylation and in chromatin organization (22, 41). In Dictyostelium there is at least a correlation between the RNAi pathway and the silencing of a major heterochromatin component, the retrotransposon DIRS-1 (32). We therefore examined if RNA-directed RNA polymerases (RdRP), which are involved in RNA-mediated gene silencing, were required for heterochromatin formation in Dictyostelium. No obvious defects could be detected in H3K9 methylation (Fig. 9) or localization of the HP1 proteins (data not shown) in either of the three knockout strains of the RdRP genes rrpA, rrpB, and rrpC (38). Although we cannot rule out that in Dictyostelium RdRPs play a minor or a redundant role in heterochromatin formation, it is possible that, like in other organisms, there are parallel pathways for heterochromatin formation, including RNAi-independent ones (26).
DISCUSSION
Even though centromeric sequences have not yet been clearly defined in the Dictyostelium discoideum genome, we provide cytological indications that centromeres are clustered during G2 phase in a single domain at the nuclear periphery, where H3K9 dimethylation and the majority of HP1 proteins colocalize. The mitotic behavior of this cluster and its localization next to the centrosome strongly support this conclusion. Interestingly, DIRS retrotransposons that are believed to constitute the Dictyostelium centromeres also cluster during interphase and mitosis (6). Telomeres may also cluster near the spindle pole body in meiotic prophase (60), but this is not necessarily the case in mitotic cells. In mitotically cycling cells of S. pombe (15), S. cerevisiae (17), and Plasmodium falciparum (14), telomeres form clusters at the nuclear periphery. Since centromeres are probably subtelocentric in Dictyostelium, the main heterochromatic cluster may also contain the proximal telomeres. The distal telomeres may be located elsewhere at the nuclear periphery and could be represented by the additional minor heterochromatic foci. Alternatively, these may contain other heterochromatic domains of unknown function.
Both HP1 isoforms, when expressed as GFP fusion proteins, are very similar with regard to subnuclear localization and association with heterochromatin during the cell cycle. Despite their likely centromeric localization, we did not detect any mitotic defects that could be assigned to centromere or kinetochore function in either knockout strain. This suggests that the HP1 isoforms are either redundant or, what we believe to be less likely, not at all required for centromere function. Null mutants of a single HP1 isoform are fully viable under standard growth conditions and only display subtle growth phenotypes at lower temperatures. This was rather surprising, since single HP1 isoforms in other organisms such as Drosophila melanogaster are essential for viability or have at least distinct important functions that cannot be fully compensated for by the other isoforms.
We were not able to generate viable double-knockout strains in the wild-type background, but we readily obtained them when a rescue construct expressing HcpA had previously been introduced into the cells. This strongly suggested that HcpA and HcpB were redundant in terms of viability. Further support came from the observation that overexpression of a C-terminally truncated HcpA, assumed to display a dominant-negative effect, led to severe growth defects and significantly reduced cell viability.
Although the two HP1 isoforms are very similar in sequence and single knockouts did not display obvious phenotypes, overexpression of full-length HcpA and HcpB had distinguishable effects on growth of mitotically cycling cells. The aberrant chromosome configurations found in the HcpA overexpression strain resembled those in HP1 null strains of Drosophila melanogaster (10), where they are caused by telomere fusions. In mammals, overexpression of HP1 isoforms as N-terminal GFP fusions has been reported to cause telomere fusions and changes in telomere length by disturbed interaction of telomerase with telomeric sequences (63).
Dictyostelium telomeres appear not to be composed of specific hexanucleotide repeats, and a homolog for a telomerase gene was not found in the genome. Intriguingly, rRNA gene sequences from the extrachromosomal rRNA gene copies can be found at the ends of Dictyostelium chromosomes (6). It is therefore likely that telomeres in Dictyostelium are maintained by a DNA recombination mechanism that fuses rRNA gene sequences to the chromosome ends. We suggest that overexpression of HcpA leads to alterations in chromatin structure; at telomeres, these may impair proper telomere maintenance by interfering with DNA recombination or with the resolution of aberrant recombination intermediates. Since we did not observe these phenotypes in cells expressing C-terminally truncated forms of HcpA, only overexpression of functional HcpA can elicit the defect in chromosome distribution. Furthermore, overexpression of full-length GFP-HcpB has no effects on chromosome segregation. This argued for a specific (mal)function of HcpA that was not elicited by HcpB. The two isoforms differ especially in their dimerization preferences. With overexpression of HcpA, the stoichiometry of the proteins may be disturbed and possibly lead to abnormally high concentrations of HcpA in the oligomers. Also, the unbalanced oligomer composition probably changes the interaction with other protein partners and may alter the formation and the dynamics of chromatin structure.
The functionality of the GFP-tagged HcpA and HcpB proteins was supported by the observations that the fusion proteins maintained their ability to specifically form homo- and heterodimers in vitro and in vivo and, as expected, colocalized with H3K9me2. Furthermore, it has been shown that N-terminal GFP fusions of HP1 maintain their functions in various other organisms (4, 29, 73).
The observed phenotype of HP1 overexpression strains argues that additional binding occurred at sites that are not covered in the wild type. This could be due to spreading out of heterochromatic loci as observed in position-effect variegation. Alternatively, other parts of chromatin may be aberrantly targeted by excess HP1, since overexpression resulted in increased nucleoplasmic staining.
H3K9 dimethylation serves as a binding site for HP1 proteins (1, 34), and binding of HP1 feeds back on histone methylation. However, no change in the H3K9 methylation pattern was observed in any of the mutant strains. This was in contrast to Drosophila melanogaster, where a knockout of HP1 causes ectopic H3K9 methylation in euchromatin (62). Our analysis suggests that both isoforms can equally contribute to histone methyltransferase (HMT) activity or that the functional interaction between HMT and HP1 proteins is not required in Dictyostelium. Alternatively, HMT is acting upstream of HcpA and HcpB.
Heterochromatic regions are, at least in part, controlled by the RNA interference machinery (39, 40, 53, 72). However, in Dictyostelium, single knockouts of RrpC, RrpB, and the strictly required RNAi component RrpA had no influence on the localization of the heterochromatic markers (HcpA, HcpB, and H3K9) dimethylation. Similar to other organisms, several pathways may exist to establish and maintain heterochromatin. Alternatively, fluorescence microscopy was not sensitive enough to detect subtle changes in chromatin organization.
In Dictyostelium discoideum, we have recently shown that epigenetic gene silencing of transposable elements requires components of the RNAi machinery and/or DNA methylation (32). Our analysis thus introduces a new model system to elucidate the involvement of RNAi in epigenetic gene silencing by histone modification and/or DNA methylation.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Ne 285/8, SPP1129 “Epigenetics”).
Ralf Gräf (Munich University) is acknowledged for antibodies, Ingo Schubert and Zuzana Jasencakova (IPK Gatersleben) are acknowledged for help with the first immunostaining experiments, and Markus Maniak (Kassel University) is acknowledged for support with microscopy and for generously providing diverse reagents. H. Ziezold (FB 17, University of Kassel) is acknowledged for expert support with the statistical evaluation. We thank P. A. Silver (Harvard Medical School, Boston, Mass.) for providing the anti-GFP antibody and Ingo Schubert (IPK Gatersleben) for discussions and comments on the manuscript.
REFERENCES
- 1.Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120-124. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, P., J. F. Maure, J. F. Partridge, S. Genier, J. P. Javerzat, and R. C. Allshire. 2001. Requirement of heterochromatin for cohesion at centromeres. Science 294:2539-2542. [DOI] [PubMed] [Google Scholar]
- 3.Brasher, S. V., B. O. Smith, R. H. Fogh, D. Nietlispach, A. Thiru, P. R. Nielsen, R. W. Broadhurst, L. J. Ball, N. V. Murzina, and E. D. Laue. 2000. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 19:1587-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheutin, T., A. J. McNairn, T. Jenuwein, D. M. Gilbert, P. B. Singh, and T. Misteli. 2003. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299:721-725. [DOI] [PubMed] [Google Scholar]
- 5.Couteau, F., F. Guerry, F. Muller, and F. Palladino. 2002. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. EMBO Rep. 3:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichinger, L., J. A. Pachebat, G. Glockner, M. A. Rajandream, R. Sucgang, M. Berriman, J. Song, R. Olsen, K. Szafranski, Q. Xu, B. Tunggal, S. Kummerfeld, M. Madera, B. A. Konfortov, F. Rivero, A. T. Bankier, R. Lehmann, N. Hamlin, R. Davies, P. Gaudet, P. Fey, K. Pilcher, G. Chen, D. Saunders, E. Sodergren, P. Davis, A. Kerhornou, X. Nie, N. Hall, C. Anjard, L. Hemphill, N. Bason, P. Farbrother, B. Desany, E. Just, T. Morio, R. Rost, C. Churcher, J. Cooper, S. Haydock, N. van Driessche, A. Cronin, I. Goodhead, D. Muzny, T. Mourier, A. Pain, M. Lu, D. Harper, R. Lindsay, H. Hauser, K. James, M. Quiles, M. Madan Babu, T. Saito, C. Buchrieser, A. Wardroper, M. Felder, M. Thangavelu, D. Johnson, A. Knights, H. Loulseged, K. Mungall, K. Oliver, C. Price, M. A. Quail, H. Urushihara, J. Hernandez, E. Rabbinowitsch, D. Steffen, M. Sanders, J. Ma, Y. Kohara, S. Sharp, M. Simmonds, S. Spiegler, A. Tivey, S. Sugano, B. White, D. Walker, J. Woodward, T. Winckler, Y. Tanaka, G. Shaulsky, M. Schleicher, G. Weinstock, A. Rosenthal, E. C. Cox, R. L. Chisholm, R. Gibbs, W. F. Loomis, M. Platzer, R. R. Kay, J. Williams, P. H. Dear, A. A. Noegel, B. Barrell, and A. Kuspa. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature 435:43-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eissenberg, J. C., G. D. Morris, G. Reuter, and T. Hartnett. 1992. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekwall, K., J. P. Javerzat, A. Lorentz, H. Schmidt, G. Cranston, and R. Allshire. 1995. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269:1429-1431. [DOI] [PubMed] [Google Scholar]
- 9.Faix, J., L. Kreppel, G. Shaulsky, M. Schleicher, and A. R. Kimmel. 2004. A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanti, L., G. Giovinazzo, M. Berloco, and S. Pimpinelli. 1998. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2:527-538. [DOI] [PubMed] [Google Scholar]
- 11.Festenstein, R., S. Sharghi-Namini, M. Fox, K. Roderick, M. Tolaini, T. Norton, A. Saveliev, D. Kioussis, and P. Singh. 1999. Heterochromatin protein 1 modifies mammalian PEV in a dose- and chromosomal-context-dependent manner. Nat. Genet. 23:457-461. [DOI] [PubMed] [Google Scholar]
- 12.Filesi, I., A. Cardinale, S. van der Sar, I. G. Cowell, P. B. Singh, and S. Biocca. 2002. Loss of heterochromatin protein 1 (HP1) chromodomain function in mammalian cells by intracellular antibodies causes cell death. J. Cell Sci. 115:1803-1813. [DOI] [PubMed] [Google Scholar]
- 13.Fischle, W., B. S. Tseng, H. L. Dormann, B. M. Ueberheide, B. A. Garcia, J. Shabanowitz, D. F. Hunt, H. Funabiki, and C. D. Allis. 2005. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438:1116-1122. [DOI] [PubMed] [Google Scholar]
- 14.Freitas-Junior, L. H., E. Bottius, L. A. Pirrit, K. W. Deitsch, C. Scheidig, F. Guinet, U. Nehrbass, T. E. Wellems, and A. Scherf. 2000. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407:1018-1022. [DOI] [PubMed] [Google Scholar]
- 15.Funabiki, H., I. Hagan, S. Uzawa, and M. Yanagida. 1993. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 121:961-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Cao, M., R. O'Sullivan, A. H. Peters, T. Jenuwein, and M. A. Blasco. 2004. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 36:94-99. [DOI] [PubMed] [Google Scholar]
- 17.Gotta, M., T. Laroche, A. Formenton, L. Maillet, H. Scherthan, and S. M. Gasser. 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134:1349-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf, R., C. Daunderer, and M. Schliwa. 2000. Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J. Cell Sci. 113(Part 10):1747-1758. [DOI] [PubMed] [Google Scholar]
- 19.Graf, R., U. Euteneuer, T. H. Ho, and M. Rehberg. 2003. Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 14:4067-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal, S. I., and D. Moazed. 2003. Heterochromatin and epigenetic control of gene expression. Science 301:798-802. [DOI] [PubMed] [Google Scholar]
- 21.Hall, I. M., K. Noma, and S. I. Grewal. 2003. RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc. Natl. Acad. Sci. USA 100:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall, I. M., G. D. Shankaranarayana, K. Noma, N. Ayoub, A. Cohen, and S. I. Grewal. 2002. Establishment and maintenance of a heterochromatin domain. Science 297:2232-2237. [DOI] [PubMed] [Google Scholar]
- 23.Hirota, T., J. J. Lipp, B. H. Toh, and J. M. Peters. 2005. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438:1176-1180. [DOI] [PubMed] [Google Scholar]
- 24.Hitt, A. L., J. H. Hartwig, and E. J. Luna. 1994. Ponticulin is the major high affinity link between the plasma membrane and the cortical actin network in Dictyostelium. J. Cell Biol. 126:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James, T. C., and S. C. Elgin. 1986. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6:3862-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia, S. T., K. Noma, and S. I. S. Grewal. 2004. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304:1971-1976. [DOI] [PubMed] [Google Scholar]
- 27.Kalt, A., and M. Schliwa. 1996. A novel structural component of the Dictyostelium centrosome. J. Cell Sci. 109(Part 13):3103-3112. [DOI] [PubMed] [Google Scholar]
- 28.Kilmartin, J. V., B. Wright, and C. Milstein. 1982. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J. Cell Biol. 93:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirschmann, D. A., R. A. Lininger, L. M. Gardner, E. A. Seftor, V. A. Odero, A. M. Ainsztein, W. C. Earnshaw, L. L. Wallrath, and M. J. Hendrix. 2000. Down-regulation of HP1Hsalpha expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 60:3359-3363. [PubMed] [Google Scholar]
- 30.Knop, M., F. Barr, C. G. Riedel, T. Heckel, and C. Reichel. 2002. Improved version of the red fluorescent protein (drFP583/DsRed/RFP). BioTechniques 33:592-598. [DOI] [PubMed] [Google Scholar]
- 31.Koering, C. E., A. Pollice, M. P. Zibella, S. Bauwens, A. Puisieux, M. Brunori, C. Brun, L. Martins, L. Sabatier, J. F. Pulitzer, and E. Gilson. 2002. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhlmann, M., B. E. Borisova, M. Kaller, P. Larsson, D. Stach, J. Na, L. Eichinger, F. Lyko, V. Ambros, F. Soderbom, C. Hammann, and W. Nellen. 2005. Silencing of retrotransposons in Dictyostelium by DNA methylation and RNAi. Nucleic Acids Res. 33:6405-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuriyama, R., C. Sato, Y. Fukui, and S. Nishibayashi. 1982. In vitro nucleation of microtubules from microtubule-organizing center prepared from cellular slime mold. Cell Motil. 2:257-272. [DOI] [PubMed] [Google Scholar]
- 34.Lachner, M., D. O'Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116-120. [DOI] [PubMed] [Google Scholar]
- 35.Lechner, M. S., G. E. Begg, D. W. Speicher, and F. J. Rauscher. 2000. Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell. Biol. 20:6449-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 37.Maniak, M., U. Saur, and W. Nellen. 1989. A colony-blot technique for the detection of specific transcripts in eukaryotes. Anal. Biochem. 176:78-81. [DOI] [PubMed] [Google Scholar]
- 38.Martens, H., J. Novotny, J. Oberstrass, T. L. Steck, P. Postlethwait, and W. Nellen. 2002. RNAi in Dictyostelium: the role of RNA-directed RNA polymerases and double-stranded RNase. Mol. Biol. Cell. 13:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathieu, O., and J. Bender. 2004. RNA-directed DNA methylation. J. Cell Sci. 117:4881-4888. [DOI] [PubMed] [Google Scholar]
- 40.Matzke, M., W. Aufsatz, T. Kanno, L. Daxinger, I. Papp, M. F. Mette, and A. J. Matzke. 2004. Genetic analysis of RNA-mediated transcriptional gene silencing. Biochim. Biophys. Acta 1677:129-141. [DOI] [PubMed] [Google Scholar]
- 41.Matzke, M. A., and J. A. Birchler. 2005. RNAi-mediated pathways in the nucleus. Nat. Rev. Genet. 6:24-35. [DOI] [PubMed] [Google Scholar]
- 42.Meehan, R. R., C. F. Kao, and S. Pennings. 2003. HP1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. EMBO J. 22:3164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moens, P. B. 1976. Spindle and kinetochore morphology of Dictyostelium discoideum. J. Cell Biol. 68:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motamedi, M. R., A. Verdel, S. U. Colmenares, S. A. Gerber, S. P. Gygi, and D. Moazed. 2004. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119:789-802. [DOI] [PubMed] [Google Scholar]
- 45.Muchardt, C., M. Guilleme, J. S. Seeler, D. Trouche, A. Dejean, and M. Yaniv. 2002. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1alpha. EMBO Rep. 3:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama, J., A. J. Klar, and S. I. Grewal. 2000. A chromodomain protein, Swi6, performs imprinting functions in fission yeast during mitosis and meiosis. Cell 101:307-317. [DOI] [PubMed] [Google Scholar]
- 47.Nellen, W., S. Datta, C. Reymond, A. Sivertsen, S. Mann, T. Crowley, and R. A. Firtel. 1987. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 28:67-100. [DOI] [PubMed] [Google Scholar]
- 48.Nellen, W., and R. A. Firtel. 1985. High-copy-number transformants and cotransformation in Dictyostelium. Gene 39:155-163. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen, A. L., J. A. Ortiz, J. You, M. Oulad-Abdelghani, R. Khechumian, A. Gansmuller, P. Chambon, and R. Losson. 1999. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18:6385-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen, P. R., D. Nietlispach, H. R. Mott, J. Callaghan, A. Bannister, T. Kouzarides, A. G. Murzin, N. V. Murzina, and E. D. Laue. 2002. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416:103-107. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 52.Nonaka, N., T. Kitajima, S. Yokobayashi, G. Xiao, M. Yamamoto, S. I. Grewal, and Y. Watanabe. 2002. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4:89-93. [DOI] [PubMed] [Google Scholar]
- 53.Pal-Bhadra, M., B. A. Leibovitch, S. G. Gandhi, M. Rao, U. Bhadra, J. A. Birchler, and S. C. Elgin. 2004. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303:669-672. [DOI] [PubMed] [Google Scholar]
- 54.Perrini, B., L. Piacentini, L. Fanti, F. Altieri, S. Chichiarelli, M. Berloco, C. Turano, A. Ferraro, and S. Pimpinelli. 2004. HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell. 15:467-476. [DOI] [PubMed] [Google Scholar]
- 55.Pidoux, A. L., S. Uzawa, P. E. Perry, W. Z. Cande, and R. C. Allshire. 2000. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J. Cell Sci. 113(Part 23):4177-4191. [DOI] [PubMed] [Google Scholar]
- 56.Rauchenberger, R., U. Hacker, J. Murphy, J. Niewohner, and M. Maniak. 1997. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr. Biol. 7:215-218. [DOI] [PubMed] [Google Scholar]
- 57.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 58.Rehberg, M., and R. Graf. 2002. Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell. 13:2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richards, E. J., and S. C. Elgin. 2002. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108:489-500. [DOI] [PubMed] [Google Scholar]
- 60.Scherthan, H. 2001. A bouquet makes ends meet. Nat. Rev. Mol. Cell. Biol. 2:621-627. [DOI] [PubMed] [Google Scholar]
- 61.Schotta, G., A. Ebert, R. Dorn, and G. Reuter. 2003. Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin. Cell Dev. Biol. 14:67-75. [DOI] [PubMed] [Google Scholar]
- 62.Schotta, G., A. Ebert, V. Krauss, A. Fischer, J. Hoffmann, S. Rea, T. Jenuwein, R. Dorn, and G. Reuter. 2002. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 21:1121-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma, G. G., K. K. Hwang, R. K. Pandita, A. Gupta, S. Dhar, J. Parenteau, M. Agarwal, H. J. Worman, R. J. Wellinger, and T. K. Pandita. 2003. Human heterochromatin protein 1 isoforms HP1(Hsalpha) and HP1(Hsbeta) interfere with hTERT-telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol. Cell. Biol. 23:8363-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smothers, J. F., and S. Henikoff. 2001. The hinge and chromo shadow domain impart distinct targeting of HP1-like proteins. Mol. Cell. Biol. 21:2555-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smothers, J. F., and S. Henikoff. 2000. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 10:27-30. [DOI] [PubMed] [Google Scholar]
- 66.Sugiyama, T., H. Cam, A. Verdel, D. Moazed, and S. I. Grewal. 2005. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl. Acad. Sci. USA 102:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sussman, M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions, p. 9-29. In J. A. Spudich (ed.), Methods in cell biology, vol. 28. Academic Press, Orlando, Fla. [DOI] [PubMed] [Google Scholar]
- 68.Sutoh, K. 1993. A transformation vector for Dictyostelium discoideum with a new selectable marker bsr. Plasmid 30:150-154. [DOI] [PubMed] [Google Scholar]
- 69.Thiru, A., D. Nietlispach, H. R. Mott, M. Okuwaki, D. Lyon, P. R. Nielsen, M. Hirshberg, A. Verreault, N. V. Murzina, and E. D. Laue. 2004. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 23:489-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verdel, A., S. Jia, S. Gerber, T. Sugiyama, S. Gygi, and S. I. Grewal. 2004. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 28:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volpe, T., V. Schramke, G. L. Hamilton, S. A. White, G. Teng, R. A. Martienssen, and R. C. Allshire. 2003. RNA interference is required for normal centromere function in fission yeast. Chromosome Res. 11:137-146. [DOI] [PubMed] [Google Scholar]
- 72.Volpe, T. A., C. Kidner, I. M. Hall, G. Teng, S. I. Grewal, and R. A. Martienssen. 2002. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297:1833-1837. [DOI] [PubMed] [Google Scholar]
- 73.Wang, G., A. Ma, C. M. Chow, D. Horsley, N. R. Brown, I. G. Cowell, and P. B. Singh. 2000. Conservation of heterochromatin protein 1 function. Mol. Cell. Biol. 20:6970-6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witke, W., W. Nellen, and A. Noegel. 1987. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 6:4143-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zang, J. H., G. Cavet, J. H. Sabry, P. Wagner, S. L. Moores, and J. A. Spudich. 1997. On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell. 8:2617-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao, T., T. Heyduk, C. D. Allis, and J. C. Eissenberg. 2000. Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J. Biol. Chem. 275:28332-28338. [DOI] [PubMed] [Google Scholar]