Abstract
Lipophilic compounds such as retinoic acid and long-chain fatty acids regulate gene transcription by activating nuclear receptors such as retinoic acid receptors (RARs) and peroxisome proliferator-activated receptors (PPARs). These compounds also bind in cells to members of the family of intracellular lipid binding proteins, which includes cellular retinoic acid-binding proteins (CRABPs) and fatty acid binding proteins (FABPs). We previously reported that CRABP-II enhances the transcriptional activity of RAR by directly targeting retinoic acid to the receptor. Here, potential functional cooperation between FABPs and PPARs in regulating the transcriptional activities of their common ligands was investigated. We show that adipocyte FABP and keratinocyte FABP (A-FABP and K-FABP, respectively) selectively enhance the activities of PPARγ and PPARβ, respectively, and that these FABPs massively relocate to the nucleus in response to selective ligands for the PPAR isotype which they activate. We show further that A-FABP and K-FABP interact directly with PPARγ and PPARβ and that they do so in a receptor- and ligand-selective manner. Finally, the data demonstrate that the presence of high levels of K-FABP in keratinocytes is essential for PPARβ-mediated induction of differentiation of these cells. Taken together, the data establish that A-FABP and K-FABP govern the transcriptional activities of their ligands by targeting them to cognate PPARs in the nucleus, thereby enabling PPARs to exert their biological functions.
Many small, biologically important lipophilic compounds regulate cellular behavior by modulating the rates of transcription of various target genes. Such compounds include, for example, retinoic acid and long-chain fatty acids (LCFA) and some of their active metabolites. These molecules activate ligand-inducible transcription factors that belong to the superfamily of nuclear hormone receptors. Retinoic acid activates the retinoic acid receptors (RARs) (9). The transcriptional activities of LCFA and their active derivatives are mediated by several classes of nuclear receptors, the best characterized of which are the peroxisome proliferator-activated receptors (PPARs) (reviewed in reference 10). Three isotypes of PPAR (α, β, and γ, also referred to as NR1C1, NR1C2, and NR1C3, respectively [Nuclear Receptor Nomenclature Committee, 1999]) that display distinct tissue distributions are known to exist. PPARα is expressed at high levels in the heart, skeletal muscle, kidney, liver, and intestines. PPARβ is widely distributed, and PPARγ is expressed at high levels in adipose tissues. The PPARs play a general role in regulating lipid metabolism and energy homeostasis. PPARα is involved in the intake of dietary LCFA in the gut, peroxisomal and mitochondrial β-oxidation in the liver, and hepatic fatty acid synthesis (10). This isotype has also been implicated in control of inflammation (11). PPARγ is a key player in adipocyte differentiation and has been implicated in modulation of the insulin sensitivity of cells (7, 17, 45) and in regulation of macrophage function (37). While the functions of PPARβ have long remained elusive, we recently found that this subtype is involved in wound healing and plays a dual role in keratinocytes. On the one hand, it mediates keratinocyte differentiation induced by inflammation-associated agents such as tumor necrosis factor alpha (TNF-α), and on the other, it functions in counteracting the apoptotic effects of TNF-α in these cells (31, 44). Hence, like the other PPAR isotypes, PPARβ appears to regulate differentiation and inflammatory responses in a cell whose functions involve active lipid metabolism.
PPARs display remarkably broad ligand selectivity (10, 13, 22-24). All three PPARs bind to and are activated by various unsaturated LCFA. In addition, PPARs are activated by some eicosanoids; 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) is a ligand for PPARγ, and hydroxyeicosatetraenoic acid and leukotriene B4 are ligands for PPARα. In recent years, a variety of synthetic compounds with selectivities toward particular PPAR isotypes have been synthesized, and some of these are used or are in clinical trials for a variety of disorders ranging from diabetes to cancer. Like other nonsteroid nuclear receptors, PPARs associate with RXR (NR2B1, NR2B2, and NR2B3) to form heterodimers that bind to particular response elements in the promoter regions of target genes and act as ligand-inducible transcription factors (30). The molecular events underlying the transcriptional activities of nuclear receptor ligands through their cognate receptors have become increasingly clear over the past years (14, 51). An important question that has remained elusive, however, relates to the mechanism by which nuclear receptor ligands, which are poorly soluble in water, reach the nucleus to bind to their cognate receptors and initiate their transcriptional activities.
In addition to nuclear receptors, many lipophilic compounds and their metabolites bind in cells to proteins that are members of the family of intracellular lipid-binding proteins (iLBPs). This family of small (14- to 15-kDa) proteins includes cellular retinol-binding proteins I and II (CRBP-I and -II), cellular retinoic acid-binding proteins (CRABP-I and -II), and nine known isotypes of fatty acid binding proteins (FABPs) (2). The iLBPs have remarkably similar three-dimensional folds. Reported X-ray crystal structures of these proteins demonstrate that the entrance to the proteins' ligand-binding pockets is flanked by two α-helices that appear to limit access to the binding site. This structure suggests that marked conformational changes may need to occur prior to exit of the ligand from the binding pocket, raising the interesting question of how such rearrangements are induced to allow ligands to leave the pocket and reach their correct sites of action. The marked evolutionary conservation of iLBPs suggests that they play important roles in the biological activities of their ligands, but the precise nature of these roles is not well understood. It has traditionally been believed that the general function of iLBPs is to solubilize and protect their ligands in aqueous spaces and to facilitate transport across the cytosol (8, 42). However, it has become increasingly clear in recent years that, in addition to these general functions, iLBPs have diverse and specific roles in regulating the metabolism and activities of their ligands (reviewed in reference 35). Our recent studies demonstrated, for example, that CRABP-II (although not CRABP-I) functions by directly delivering retinoic acid to the nuclear receptor that is activated by this hormone, i.e., RAR (4, 5, 12). These studies established that CRABP-II translocates to the nucleus upon binding of retinoic acid and that it associates directly with RAR to mediate ligand channeling to the receptor. The CRABP-II-RAR complex was found to be a short-lived intermediate which dissociates rapidly following ligand transfer. We showed further that retinoic acid channeling between CRABP-II and RAR facilitates the ligation of RAR, thereby stimulating the transcriptional activity of the receptor (4, 5, 12).
The elucidation of the function of CRABP-II as a coregulator of RAR raises the intriguing possibility that other members of the iLBP family also act in concert with nuclear receptors. In view of the observation that FABPs display ligand selectivities that are quite reminiscent of those of PPARs, (43), it is possible that some FABPs cooperate with some PPARs in mediating the transcriptional activities of their common ligands. The present study was undertaken to explore this possibility. In choosing the particular FABP and PPAR isotypes to be studied, we were guided by the tissue expression profiles of these proteins. We thus focused on possible functional interactions between adipocyte FABP (A-FABP) and PPARγ and on those between keratinocyte FABP (K-FABP) and PPARβ. (A-FABP and PPARγ and K-FABP and PPARβ are the predominant forms of the two types of proteins in adipocytes and in keratinocytes, respectively.) We show that these FABPs act in concert with these PPARs and, importantly, that they do so with strict selectivities for both receptor subtypes and ligands. The data indicate further that expression of K-FABP in keratinocytes is critical for the initiation of proper PPARβ-mediated differentiation in these cells.
MATERIALS AND METHODS
Ligands.
Wy-14643 was purchased from Cayman Chemical Co. (Ann Arbor, Mich.). Linoleic acid (C18:2) was purchased from Sigma Chemical Co. (St. Louis, Mo.). Rosiglitazone was purchased from Sigma Chemical Co. The PPARβ-selective ligand L165041 was provided by Parke-Davis. cis-Parinaric acid was purchased from Molecular Probes, Inc. (Eugene, Oreg.).
Plasmids.
rH-FABP in pMON was a gift from David Cistola; mA-FABP in pBluescript and K-FABP in the bacterial expression vector pRSET-A were provided by David Bernlohr. The PPARγ ligand-binding domain (PPARγ-LBD) and PPARβ-LBD in pRSET-A were provided by Eric Xu. rH-FABP and mA-FABP were amplified by PCR and subcloned into the mammalian expression vector pSG5. Bacterial expression vectors for glutathione S-transferase (GST)-tagged A-FABP and K-FABP were constructed by inserting the corresponding coding sequences into a pGEX-4T2 vector. mPPARα, mPPARβ, and mPPARγ were expressed by using the mammalian vector pSG5. Expression vectors for green fluorescent protein (GFP)-tagged K-FABP and A-FABP were constructed by subcloning the corresponding coding sequences into pEGFP-N2 in frame with the GFP moiety. All constructs were confirmed by sequencing. The (PPRE)3-luciferase reporter construct was provided by Ronald Evans (Salk Institute).
K-FABP containing an NES.
The nuclear export sequence (NES) MDLCQAFSDVILA was added to K-FABP by PCR using a three-step strategy with the K-FABP cDNA in pSG5 as a template. The NES-K-FABP was then cloned into pSG5 and verified by sequencing. The primary sequence of the construct contained the NES linked to the N terminus of the native K-FABP by 2 residues (EF).
Proteins.
GST-tagged and histidine-tagged proteins were expressed in Escherichia coli BL21. Protein expression was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h, except for proteins expressed by using the pRSET vector. For these, bacteria were grown at room temperature for 20 h without the addition of inducer. Bacteria were pelleted and lysed in 10 ml of HEDK100 (10 mM HEPES, 0.1 mM EDTA, 100 mM KCl, and 1 mM dithiothreitol [pH 8.0]) with 0.1% Nonidet P-40. Following treatment with DNase I and deoxycholic acid, the slurry was centrifuged and the supernatant was incubated with 1 ml of glutathione-agarose beads (for GST-tagged proteins) or Ni-charged beads (for His-tagged proteins) for 2 h at 4°C with gentle rocking. Beads were washed with HEDK100 buffer, and protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Coprecipitation assays.
35S-labeled mPPARγ and mPPARβ (in pCDNA3.1) were synthesized by coupled in vitro transcription-translation using the TNT kit (Promega). For coprecipitation experiments, GST-FABP or His-FABP, immobilized on glutathione-agarose or Ni-charged Sepharose beads, respectively, were incubated with the appropriate 35S-labeled PPAR for 2 h in 1 ml of HEDK100 at 4°C with gentle rocking. Beads were washed five times in the same buffer containing 0.1% Nonidet P-40. The resulting bound proteins were analyzed by SDS-PAGE and visualized by autoradiography.
Transactivation assays.
COS-7 and COS-1 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% charcoal-treated newborn bovine calf serum (Cocalico Biologicals Inc., Reamstown, Pa.). Cells were cultured in six-well plates and were transfected with 0.3 or 1 μg of the (PPRE)3-luciferase reporter vector and 0.3 μg of pSG5 alone or harboring the cDNA for an appropriate FABP. Each well also received 0.15 or 0.3 μg of pCH110, a β-galactosidase expression plasmid, as a control for transfection efficiency. In some experiments, 50 ng of a pSG5 expression vector encoding the appropriate PPAR was cotransfected. Transfections were carried out by using Fugene (Roche Diagnostics Corporation) according to the protocol of the manufacturer. Twenty-four hours following transfection, the medium was replaced by Dulbecco's modified Eagle's medium without serum, and ligands were added from a concentrated dimethyl sulfoxide solution. Following 24 h of treatment, cells were lysed and assayed for luciferase activity by using the luciferase assay system (Promega). Luciferase activity was corrected for β-galactosidase activity, which was measured by standard procedures. All experiments were carried out in triplicate.
Labeling of PPARγ-LBD.
Purified PPARγ-LBD was covalently labeled with N-(1-pyrenebutanoyl)cysteic acid succinimidyl ester sodium salt (Molecular Probes, Inc.). Protein was incubated with a fivefold molar excess of probe at 4°C for 2 h. Labeled protein was dialyzed extensively to remove free probe. The probe/protein ratio of the labeled protein was determined from the absorbance of the probe (obtained by using the equation ɛ341 = 38,000 M−1 cm−1) and measurement of the protein concentration by a Bradford assay using bovine serum albumin as a standard. The probe/protein ratio never exceeded 0.5.
Fluorescence titrations.
Fluorescence titrations were carried out with a Fluorolog 2 DMIB spectrofluorometer (SPEX Instruments, Edison, N.J.). Protein (1 μM) was placed in a cuvette and titrated with parinaric acid from a concentrated solution in ethanol. Ligand binding was monitored by following the increase in the fluorescence of the ligand upon binding to the protein (excitation wavelength [λex], 303 nm; emission wavelength [λem], 416 nm). Ethanol concentrations never exceeded 2%. Titration curves were analyzed by fitting the data to an equation derived from simple binding theory (34) to yield the number of binding sites and the equilibrium dissociation constants (Kd). Analyses were carried out by using Origin 4.1 software (MicroCal Software Inc., Northampton, Mass.).
Fluorescence competition titrations.
Protein was complexed with parinaric acid at a molar ratio of 1. The complex was then titrated with the appropriate ligand until a plateau was reached. Binding of ligand was monitored by following the decrease in the fluorescence of parinaric acid that accompanies its dissociation from the protein. The Kd for the nonfluorescent ligand was obtained from the 50% effective concentration (EC50) of the competition curve and the previously determined Kd for parinaric acid.
Kinetics of transfer of ligands from FABP to PPARγ-LBD.
The appropriate binding protein was complexed with ligand at a molar ratio corresponding to the number of functional binding sites (determined by fluorescence titrations as described above). The holoprotein was mixed with labeled PPARγ-LBD by using a rapid mixing apparatus (HiTech, Salisbury, United Kingdom). The progress of the transfer reaction from the binding protein to the receptor was monitored by following the time-dependent decrease in the fluorescence of the probe (λex = 342 nm; λem = 377 nm) which accompanied ligand binding by the labeled PPARγ-LBD. Data were fit to a single first-order reaction equation to yield the pseudo-first-order rate constant of the transfer reaction.
Primary keratinocyte culture.
Mouse keratinocytes were isolated from epidermis as reported by Hager et al. (16) with the following modifications. The epidermis was separated from the dermis following overnight incubation at 4°C in 2.5 U of Dispase/ml. The epidermis was placed in a 50-ml centrifuge tube with 10 ml of keratinocyte serum-free culture medium (KSFM), and the tube was given 50 firm shakes. Keratinocytes were resuspended in KSFM containing 0.05 mM Ca2+ and 0.1 ng of epidermal growth factor/ml and were seeded at 5 × 104 cells per cm2. Keratinocytes were used after 2 to 3 passages.
Direct lysate RPA.
Gene-specific probes corresponding to cyclin A and involucrin were subcloned into pGEM3Zf(+) (Promega). Gene-specific antisense riboprobes were synthesized by in vitro transcription with either T7 or Sp6 RNA polymerase (Ambion). The L27 probe has been described previously (26). For all riboprobes except L27, a 1:1 ratio of [α32P]UTP to cold UTP was used; for probe L27, a 1:10 ratio was used. An RNase protection assay (RPA) was carried out as described by the manufacturer (Ambion) with the following modifications: 1 × 106 to 2 × 106 keratinocytes were lysed in 200 μl of lysis/denaturation buffer and clarified by centrifugation through a Qiashredder (Qiagen). A 45-μl volume of lysate was hybridized to 1 ng of specific cyclin A and involucrin (109 cpm/μg) and 10 ng of L27 probe (107 cpm/μg). RNase digestion (10 U of RNase A/ml and 400 U of RNase T1/ml) was carried out at 37°C for 20 to 30 min. RPA products were resolved in a 6% electrolyte-gradient denaturing polyacrylamide gel. Gels were dried and exposed to X-ray film or to a Phosphor screen. Gene-specific mRNA expressions were normalized to the specific activity of the probe and to L27 mRNA expression.
Transfection of keratinocytes with antisense K-FABP.
The expression level of K-FABP in keratinocytes was decreased by expression of an antisense construct composed of the full-length K-FABP coding sequences inserted in the reverse orientation into a pCDNA3.1 expression vector. The vector was transfected into the cells by using Superfect (Qiagen) 12 h prior to induction of differentiation.
RESULTS
FABPs enhance the transcriptional activities of PPARs.
To examine whether FABPs functionally interact with PPARs, the effects of expression of some of these binding proteins in cells on the transcriptional activities of PPARs were examined. In these experiments, the FABP and PPAR isotypes to be tested were selected based on their coexpression in different tissues. Heart FABP (H-FABP) was matched with PPARα, A-FABP was matched with PPARγ, and K-FABP was tested for possible effects on PPARβ. A luciferase reporter construct under the control of a consensus PPAR response element was cotransfected into COS-7 cells together with expression vectors for a particular PPAR isotype and for an FABP. The cells were then treated with appropriate ligands, and the expression of the reporter was examined.
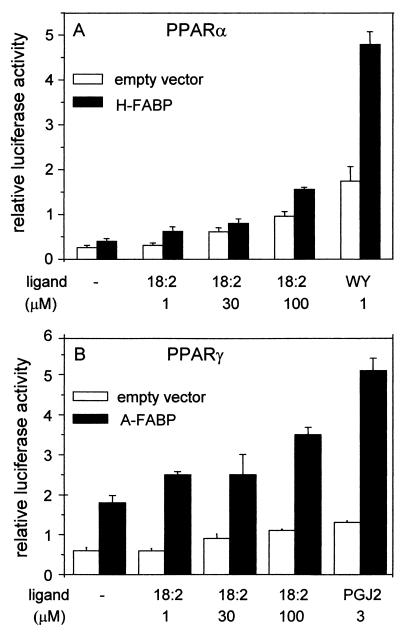
In studying the activity of PPARα, two ligands were used: linoleic acid, a naturally occurring pan-PPAR ligand, and Wy-14643, a synthetic PPARα-selective ligand (Fig. 1A). As expected, treatment with each of these ligands activated transcription of the reporter. The activation, however, was significantly enhanced upon cotransfection of H-FABP. Expression of H-FABP augmented the transcriptional activity of PPARα under all the conditions used and led to a >3-fold enhancement in the presence of 1 μM Wy-14643. The transcriptional activity of PPARγ was examined in the presence of linoleic acid as well as the natural PPARγ ligand PGJ2 (Fig. 1B). Like H-FABP for PPARα, A-FABP markedly enhanced the transcriptional activity of PPARγ both in the absence and in the presence of an exogenous ligand. As discussed in the introduction, PPARs respond to a wide variety of ligands, including naturally occurring fatty acids that are present in cells even without addition of exogenous ligands. The enhancement of transcriptional activity by the FABPs observed in the absence of added ligand (Fig. 1) most likely reflects the effect of the binding proteins on PPAR activities induced by the endogenous ligands.
FIG. 1.
H-FABP and A-FABP enhance the transcriptional activities of PPARα and PPARγ, respectively. Transactivation assays were carried out by using COS-7 cells as described in Materials and Methods. (A) PPARα was cotransfected into cells with either an empty vector or an expression vector for H-FABP. The ability of the receptor to activate expression of the luciferase reporter was examined in the absence of ligand or in the presence of either the naturally occurring pan-PPAR ligand linoleic acid (18:2) or the PPARα-selective ligand Wy-14643 (WY). (B) PPARγ was cotransfected into cells with either an empty vector or an expression vector for A-FABP. The transcriptional activity of the receptor was examined in the absence of ligand or in the presence of either linoleic acid (18:2) or the naturally occurring PPARγ ligand PGJ2. Luciferase activity was normalized to the activity of β-galactosidase.
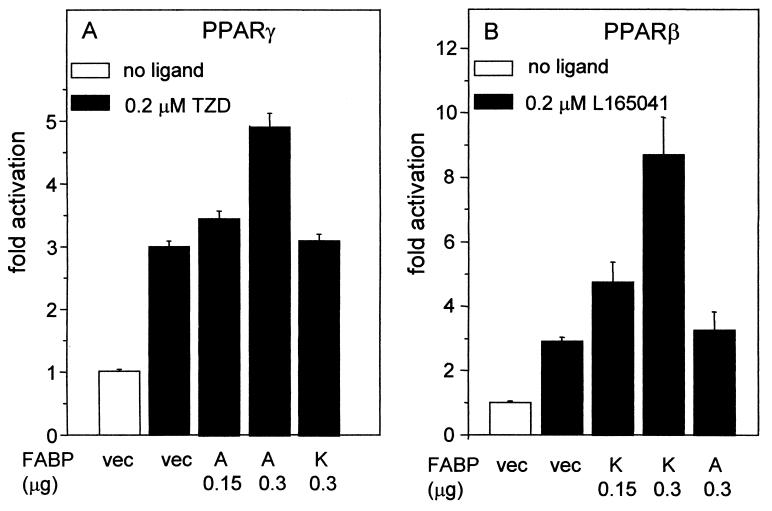
The selectivity of FABP function was further investigated by examining the effects of A-FABP and K-FABP on the transcriptional activities of PPARβ and PPARγ. In this series of experiments, synthetic ligands that are selective for PPARβ (L165041) or PPARγ (troglitazone) were used (Fig. 2). Addition of 0.2 μM troglitazone to cells overexpressing PPARγ resulted in ∼3-fold activation of the reporter. Coexpression of A-FABP in these cells markedly augmented the ability of the ligand to induce PPARγ-mediated transactivation and did so in a dose-dependent fashion (Fig. 2A). In contrast, overexpression of K-FABP had little effect on the transcriptional activity of PPARγ. On the other hand, K-FABP markedly activated PPARβ in a dose-dependent manner, while A-FABP had no effect on the activity of this receptor (Fig. 2B). Hence, A-FABP and K-FABP selectively enhance the activities of PPARγ and PPARβ, respectively.
FIG. 2.
A-FABP and K-FABP selectively enhance the transcriptional activities of PPARγ and PPARβ. Transactivation assays were carried out by using COS-7 cells as described in Materials and Methods. (A) Cells were cotransfected with PPARγ and either an empty vector (vec) or an expression vector for either K-FABP (K) or A-FABP (A). The transcriptional activity of PPARγ was examined in the absence or presence of the PPARγ-selective ligand troglitazone (TZD). (B) Cells were cotransfected with PPARβ and either an empty vector or an expression vector for either K-FABP or A-FABP. The transcriptional activity of PPARβ was examined in the absence or presence of the PPARβ-selective ligand L165041.
Ligand selectivities of FABPs and PPARs.
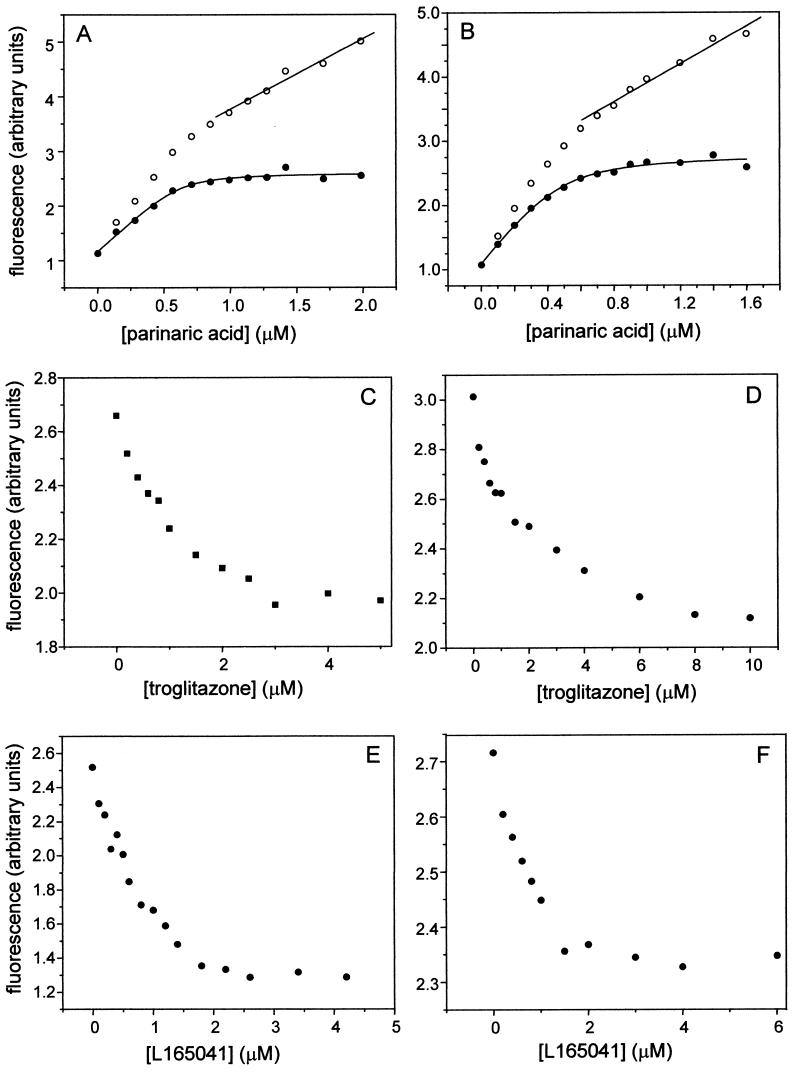
The data in Fig. 2 demonstrate that the transcriptional activities of PPARγ and PPARβ are selectively enhanced by A-FABP and K-FABP, respectively. It is possible that this behavior stems from a specific response of the FABPs to the selective PPAR isotype ligands or is mediated through selective A-FABP-PPARγ and K-FABP-PPARβ interactions. Alternatively, A-FABP may fail to augment PPARβ activity simply because it does not bind the ligand for this receptor. Similarly, K-FABP may not associate with the ligand for PPARγ. Hence, the binding affinities of K-FABP and A-FABP for the PPAR ligands used in this study were measured. Kd characterizing the interactions of the FABPs with these ligands were measured by fluorescence competition assays by using the synthetic fluorescent LCFA parinaric acid as a probe. The method requires two steps (27). In the first step, the Kd for the association of parinaric acid with a protein is measured by fluorescence titrations. Parinaric acid associates with PPARs and FABPs with high affinity, and its fluorescence is significantly enhanced upon binding to these proteins (27). The association of the probe with a protein can thus be followed by monitoring the increase in probe fluorescence upon binding. Representative titrations of A-FABP and K-FABP with parinaric acid are shown in Fig. 3A and B, respectively. Fluorescence increased in a saturable fashion upon titration with parinaric acid. Continuing addition of the probe following saturation resulted in additional increases which had a linear appearance. This linear increase stemmed from the fluorescence of unbound parinaric acid added to the mixtures (27). Titration curves were corrected for the nonspecific linear increase in fluorescence. Corrected curves (Fig. 3A and B) were analyzed by fitting to a equation derived from simple binding theory (34) to yield the number of binding sites and the Kd for the association of parinaric acid with the respective proteins. In the second step, Kd for binding of nonfluorescent ligands were measured by monitoring their abilities to compete with parinaric acid for binding. Protein was precomplexed with parinaric acid and titrated with the nonfluorescent ligand. The binding of the nonfluorescent ligand was monitored by following the decrease in the fluorescence of parinaric acid that accompanied its displacement from the ligand binding pocket (Fig. 3C to F). The Kd for the nonfluorescent ligand was extracted from the EC50 of the competition curve and the measured Kd for parinaric acid. By this method, the Kd for the association of K-FABP, A-FABP, PPARβ, and PPARγ with troglitazone and with L165041 were measured. The values (Table 1) reveal that the ligands associate with K-FABP and A-FABP with similar affinities. Hence, the selectivity of the effects of the binding proteins on the transcriptional activities of the receptors does not simply originate from differential ligand binding properties.
FIG. 3.
Determination of the ligand binding affinities of A-FABP and K-FABP. The Kd for the association of A-FABP (A) or K-FABP (B) with parinaric acid was measured by fluorescence titration. Protein (1 μM) was titrated with parinaric acid from a concentrated ethanolic solution. Fluorescence (λex = 303 nm; λem = 413 nm) was measured after each addition. Representative curves are shown. Titration curves were corrected for nonspecific increases in fluorescence due to addition of free parinaric acid. Corrected data (solid circles) were analyzed by fitting to an equation derived from binding theory to obtain the Kd and the number of ligand binding sites (solid lines through data points). The Kd for the association of troglitazone (C and D) and L165041 (E and F) with A-FABP (C and E) and K-FABP (D and F) were measured by competition fluorescence titrations. Protein was complexed with parinaric acid at a molar ratio corresponding to the measured number of binding sites. FABP-parinaric acid complexes were titrated with the appropriate ligand until a plateau was reached. Kd for the nonfluorescent ligands were calculated by using the EC50 of the competition curves and the measured Kd for parinaric acid.
TABLE 1.
Kd characterizing the interactions of PPARβ-LBD PPARγ-LBD, A-FABP, and K-FABP with ligands
| Ligand |
Kd (nM)a
|
|||
|---|---|---|---|---|
| A-FABP | K-FABP | PPARγ-LBD | PPARβ-LBD | |
| Parinaric acid | 31.7 ± 2.3b | 42.3 ± 4.6c | 49.6 ± 1.2d | 63.8 ± 11.2c |
| Troglitazone | 47.3e | 114.0e | 50.7e | >4,000 |
| L165041 | 27.8e | 45.9e | 21.4e | 33.1 ± 1.1d |
Measured by fluorescence competition titrations as described in the legend to Fig. 3.
Mean of four measurements.
Mean of five measurements.
Mean of three measurements.
Mean of two measurements.
K-FABP and A-FABP undergo nuclear localization in response to ligands for PPARβ and PPARγ, respectively.
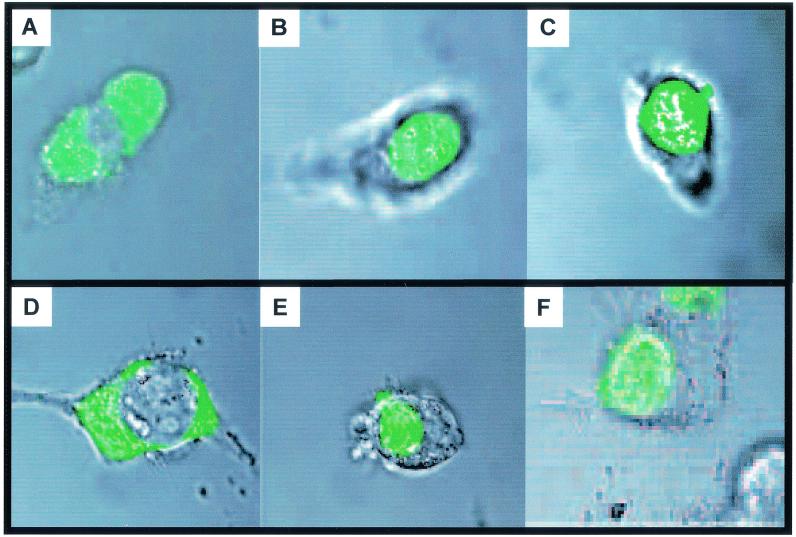
PPARs are nuclear proteins, while FABPs are usually described as cytosolic. The observations that some FABPs enhance the transcriptional activities of selected PPARs thus raise the question whether these effects are exerted directly, which would require the presence of the FABPs in the nucleus, or whether they stem from indirect activities such as classical functions of FABPs in enhancing the influx of ligands into the cells or facilitating ligand transport in the cytosol. To examine the subcellular location of FABP, constructs harboring either K-FABP or A-FABP fused with GFP were generated and transfected into COS-1 cells. The locations of the GFP-FABPs in the cells were then studied by confocal microscopy. In the absence of ligand, A-FABP was predominantly cytosolic and appeared to be excluded from the nucleus (Fig. 4A). Addition of the PPARγ ligand rosiglitazone resulted in a dramatic redistribution of A-FABP into the nucleus (Fig. 4B). Similarly, this protein localized to the nucleus in response to the natural pan-PPAR agonist linolenic acid (Fig. 4C). In contrast, A-FABP remained cytosolic following addition of the PPARβ ligand L165041 (data not shown). Like A-FABP, K-FABP was predominantly cytosolic in the absence of ligand (Fig. 4D). The cellular distribution of K-FABP did not change upon addition of the PPARγ ligand rosiglitazone (data not shown), but this binding protein located into the nucleus following addition of either the PPARβ ligand L165041 or the pan-ligand linolenic acid (Fig. 4E and F, respectively). Hence, A-FABP and K-FABP move to the nucleus in specific response to ligands for PPARγ and PPARβ, respectively.
FIG. 4.
A-FABP and K-FABP translocate into the nucleus in response to ligands for PPARγ and PPARβ, respectively. Expression vectors harboring either A-FABP (A through C) or K-FABP (D through F) tagged with GFP were transfected into COS-1 cells, and the subcellular locations of the proteins were examined by confocal microscopy. Images represent superpositions of the fluorescence and bright-field images of the same field. Ligands were added 3 to 4 h prior to imaging as follows: none (A and D), 1 μM troglitazone (PPARγ ligand) (B), 5 μM linolenic acid (C and F), and 1 μM L165041 (PPARβ ligand) (E).
Nuclear localization of K-FABP is essential for its ability to enhance the transcriptional activity of PPARβ.
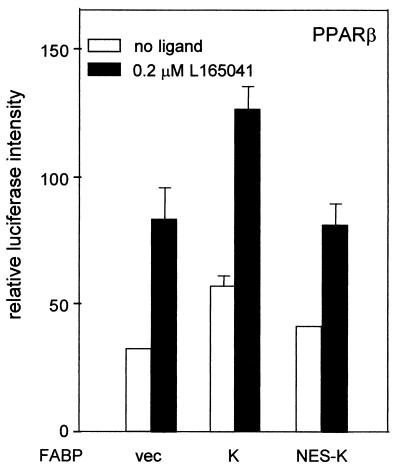
In view of the above observations, we sought to further dissect possible contributions of extranuclear versus nuclear activities of an FABP on the transcriptional activity of its cognate PPAR. To this end, a K-FABP construct that contains the NES DLCQAFSDVILA at its amino terminus was generated. NESs of this type are used by a variety of proteins to facilitate their export from the nucleus into the cytoplasm (33, 43). Hence, fusing the sequence to K-FABP will result in a shift in its subcellular distribution to the cytosol. If K-FABP modulates the activity of PPARβ through cytosolic activities such as facilitation of influx or cytosolic transport of ligands, it can be expected that similar enhancements will be observed when either native K-FABP or K-FABP containing an NES is overexpressed in cells. On the other hand, if the ability of K-FABP to augment PPARβ activity depends on nuclear function, this ability will be impaired when the protein is excluded from the nucleus. The ability of NES-K-FABP to enhance the transcriptional activity of PPARβ was examined by transactivation assays (Fig. 5). The data showed that while expression of K-FABP markedly augmented the ability of PPARβ to activate transcription of the reporter gene, overexpression of NES-K-FABP had no effect on the receptor's transcriptional activity. Thus, ligand-induced movement of K-FABP into the nucleus is essential for enabling the protein to enhance ligand-induced PPARβ-mediated transcriptional activation.
FIG. 5.
K-FABP harboring an NES (NES-K-FABP) does not enhance the transcriptional activity of PPARβ. Transactivation assays were carried out using COS-1 cells as described in Materials and Methods. Cells were cotransfected with PPARβ and either an empty vector (vec) or an expression vector for either K-FABP (K) or NES-K-FABP (NES-K). The transcriptional activity of PPARβ was examined in the absence or presence of the PPARβ ligand L165041.
A-FABP and K-FABP selectively interact with PPARγ and PPARβ, respectively, and do so in a ligand-dependent fashion.
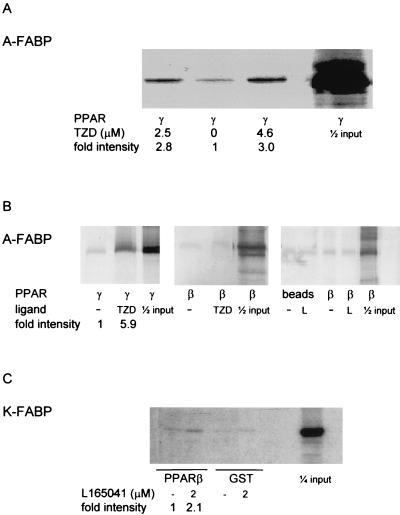
Movement of A-FABP and K-FABP into the nucleus in the presence of a cognate ligand for the “correct” PPAR colocalizes these binding proteins and receptors to the same cellular compartment under conditions where the PPARs become activated. Hence, enhancement of the transcriptional activity of PPAR by FABP may be mediated by direct interactions between the two proteins. In order to examine this possibility, coprecipitation experiments were carried out. FABPs tagged with either GST or hexahistidines were expressed in E. coli and purified by affinity chromatography. 35S-labeled PPARβ and PPARγ were synthesized by a coupled in vitro transcription-translation system. The tagged FABPs, immobilized on appropriate Sepharose beads, were incubated with 35S-labeled PPARs in the presence or absence of appropriate ligands, and the beads were centrifuged, washed, and resolved by SDS-PAGE. PPAR that coprecipitated with the FABP was then visualized by autoradiography. A weak association was observed between PPARγ and the GST-tagged or histidine-tagged A-FABP in the absence of ligand, but the interaction was significantly stabilized in the presence of the PPARγ ligand troglitazone (Fig. 6A and B). In contrast, A-FABP did not form an observable complex with PPARβ in the absence of ligand or in the presence of either troglitazone or the PPARβ-selective ligand L165041 (Fig. 6B). K-FABP associated with PPARβ in the presence of the PPARβ ligand L165041, albeit with an apparent affinity which seems to be weaker than that of the A-FABP-PPARγ association (Fig. 6C). K-FABP did not associate with PPARγ in the absence of ligand or in the presence of either troglitazone, L165041, or palmitic acid (data not shown).
FIG. 6.
A-FABP and K-FABP interact with PPARγ and PPARβ selectively and in a ligand-dependent fashion. Coprecipitation assays were carried out by using bacterially expressed FABPs and 35S-labeled in vitro transcribed-translated PPARs as described in Materials and Methods. (A) GST-tagged A-FABP was immobilized on glutathione-agarose and incubated with 35S-labeled PPARγ in the absence or presence of the indicated concentration of the PPARγ ligand troglitazone (TZD). (B) Histidine-tagged A-FABP was immobilized on Ni2+-Sepharose and incubated either with 35S-labeled PPARγ in the absence or presence of troglitazone or with PPARβ in the absence or presence of either troglitazone or the PPARβ ligand L165041 (L). (C) GST or GST-tagged K-FABP was immobilized on glutathione-agarose and incubated with 35S-labeled PPARβ in the absence or presence of L165041.
A-FABP channels troglitazone to PPARγ, but K-FABP does not.
The data in Fig. 6 suggest that A-FABP and K-FABP interact with PPARγ and PPARβ, respectively, and that they do so only in the presence of a ligand that is selective for the PPAR isotype with which they interact. The interactions, however, appear to be exceedingly weak (Fig. 6). We note that this is reminiscent of the characteristics of the complex that forms between CRABP-II and RAR in the presence of retinoic acid. This complex was found to be a short-lived intermediate and could not be visualized by using a variety of methodologies aimed at demonstrating protein-protein interactions, including coprecipitation assays (5, 12). We thus sought to further examine whether an FABP directly interacts with a PPAR and to explore the functional consequences of these putative interactions. We hypothesized that, like the function of CRABP-II in activating RAR, interactions between FABPs and PPARs may serve to facilitate the ligation of the PPAR by direct channeling of ligands between cognate binding proteins and receptors. We thus investigated the mechanism by which ligands move from either A-FABP or K-FABP to PPARγ.
Theoretically, transfer of a ligand from a donor protein (FABP) to an acceptor protein (PPAR) may occur by one of two possible mechanisms. In one scenario, the ligand dissociates from the donor into the aqueous phase (donor-ligand ↔ donor + ligand), followed by association with the acceptor (ligand + acceptor ↔ acceptor-ligand). In this case, the rate-limiting step for the transfer reaction will be the dissociation of the donor-ligand complex (12). Hence, the rate constant of the transfer reaction will be independent of the concentrations of the proteins.
In a second scenario, the ligand moves from the donor to the acceptor by channeling, i.e., by a process that involves direct protein-protein interactions and that bypasses the aqueous phase (donor-ligand + acceptor → acceptor-ligand + donor). In this case, the rate of the reaction will be limited by the frequency of productive collisions between the donor and the acceptor, and it will increase as the donor/acceptor ratio is increased.
The mechanism by which a ligand moves from an FABP to a PPAR may thus be studied by examining the dependence of the rate constant of the transfer reaction on the donor/acceptor ratio.
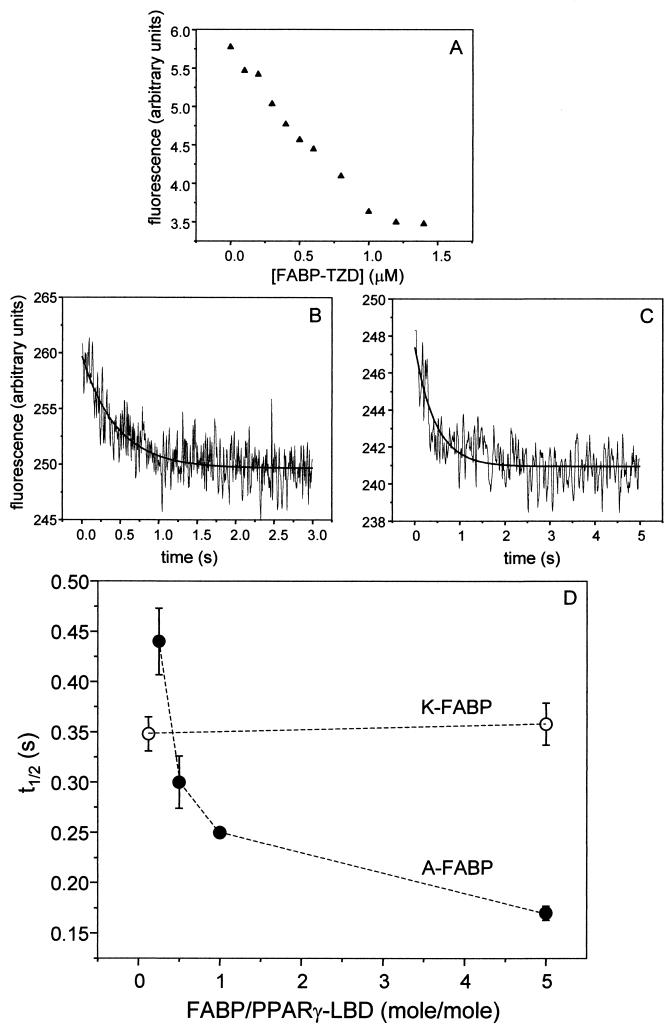
To monitor the transfer of ligand between FABP and PPAR, a fluorescence-based system was developed. The PPARγ-LBD was bacterially expressed and purified. The PPARγ-LBD was used because of technical difficulties encountered in bacterial expression of full-length PPAR. However, the question being addressed is whether the putative FABP-PPAR interactions mediate ligand channeling. If such interactions occur, they are likely to involve regions of the donor and acceptor proteins that are near the corresponding ligand binding sites; for PPAR, these would be within LBD. The report that the CRABP-II interaction domain of RAR, a protein which is homologous to PPAR, is present within the receptor's LBD (5) further supports the suitability of using the PPAR-LBD for these studies. Purified PPARγ-LBD was covalently labeled with the fluorescent probe N-(1-pyrenebutanoyl)cysteic acid succinimidyl ester (see Materials and Methods). Pyrene is an environmentally sensitive probe, i.e., its fluorescence properties respond to conformational alterations in its vicinity, such as those that accompany ligand binding by a protein. The covalent labeling did not alter the affinity of PPARγ-LBD for parinaric acid, verifying that it did not result in protein denaturation or misfolding. The ability of the protein-bound probe to serve as a readout for the movement of ligand from A-FABP to the receptor was then examined. In this control experiment, we also wanted to verify that a ligand which is initially bound to an FABP will indeed transfer to the PPAR upon mixing of the two proteins. Pyrene-labeled PPARγ-LBD was titrated with A-FABP precomplexed with troglitazone. As shown in Fig. 7A, the fluorescence of pyrene-labeled PPARγ decreased upon titration with the A-FABP-troglitazone complex to reach a plateau upon saturation. These observations indicate that the PPAR-bound pyrene moiety properly sensed movement of the ligand from the FABP to the receptor and can be used as a readout for the transfer reaction.
FIG. 7.
A-FABP directly channels troglitazone to PPARγ-LBD, while K-FABP does not. The dependence of the rate of transfer of troglitazone (TZD) from FABP to PPARγ was examined. PPARγ-LBD was covalently labeled with a pyrene moiety. (A) The labeled protein (1 μM) was titrated with A-FABP precomplexed with troglitazone. Fluorescence (λex = 342 nm; λem = 377 nm) decreased upon titration until a plateau was reached at saturation. (B and C) To determine the rate constants for ligand transfer from FABP to PPARβ-LBD, pyrene-labeled PPARβ-LBD was mixed with A-FABP (B) or K-FABP (C) precomplexed with troglitazone. Mixing was accomplished using a stopped-flow apparatus. Final protein concentrations for the representative traces shown were 1 μM PPARβ-LBD and 5 μM FABP-ligand complexes. Traces were analyzed by fitting to a single first-order reaction (solid line through data points) to obtain the pseudo-first-order rate constant of the reaction. (D) t1/2 for transfer of troglitazone from A-FABP (solid circles) or K-FABP (open circles) to PPARβ-LBD as a function of the FABP/PPAR molar ratio.
A-FABP or K-FABP was precomplexed with troglitazone and mixed with pyrene-labeled PPARγ. Mixing was accomplished by using a stopped-flow apparatus which allowed for monitoring reaction rates in the subsecond range. Transfer of troglitazone from the FABP to PPARγ was then monitored by following the time-dependent decrease in the fluorescence of pyrene-labeled PPAR which accompanied movement of the ligand from the binding protein to the receptor (Fig. 7B and C). Traces were fit to a single first-order reaction in order to obtain the apparent rate constant of the transfer reaction. To determine the mechanisms of transfer of troglitazone from the binding proteins to PPARγ, rate constants were determined at different FABP/PPARγ molar ratios (Fig. 7D). The data clearly show that the half-life (t1/2) for transfer of troglitazone from K-FABP to PPARγ was independent of the donor/acceptor ratio, indicating that it requires prior dissociation of the ligand from this binding protein, followed by association with the receptor. In contrast, the t1/2 for movement of troglitazone from A-FABP to PPARγ strongly depended on the donor/acceptor ratio. This behavior indicates that ligand transfer from A-FABP to PPARγ is mediated by direct protein-protein interactions between this binding protein and the receptor. These data establish that while K-FABP acts as a passive vehicle which binds and releases this ligand in response to concentration gradients and shifts in equilibrium conditions, A-FABP delivers troglitazone to PPARγ by channeling between the two proteins. These observations show further that the direct A-FABP-PPARγ interactions result in significant facilitation of the formation of the PPARγ-troglitazone complex and thus may be the basis for the enhancing effect of A-FABP on PPARγ-mediated transactivation (Fig. 2).
To further examine factors that control these interactions, the mechanism of transfer of two other ligands from A-FABP to PPARγ was determined. These ligands, the fluorescent probes parinaric acid and 8-anilino-1-naphthalenesulfonate, bind to both A-FABP and PPARγ with Kd of 30 to 40 nM (Table 1; also data not shown). The t1/2 values for transfer of parinaric acid at A-FABP/PPARγ molar ratios of 1, 10, and 20 were found to be 8.7 ± 0.3, 8.9 ± 0.6, and 8.8 ± 0.7 s, respectively. The t1/2 values for movement of ANS at A-FABP/PPARγ molar ratios of 0.5, 5, and 10 were 0.04 ± 0.004, 0.04 ± 0.001, and 0.038 ± 0.002 s, respectively. Hence, movement of these ligands from the binding protein to the receptor displayed similar rate constants across a wide range of donor/acceptor ratios. The observations that, unlike troglitazone, these ligands do not induce productive interactions between A-FABP and PPARγ reveal that the selectivity of FABP-PPAR interactions is governed not only by the nature of the particular proteins but also by the presence of specific agonists.
In the absence of ectopic PPARβ, K-FABP augments the receptor's transcriptional activity only when the level of ligand is limiting.
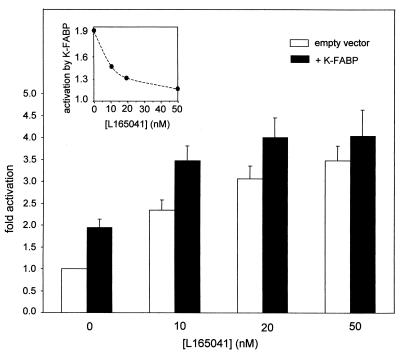
An important question that arises from the observations that FABPs potentiate PPAR-mediated transcriptional activation relates to the physiological conditions under which this activity becomes important. To address this question, the effect of K-FABP on the activity of PPARβ was investigated by transactivation assays carried out in the absence of ectopic expression of PPAR, i.e., relying on the (limiting) endogenous level of the receptor in the cells. The range of ligand doses for which the activities of PPARβ are responsive to the presence of K-FABP was studied (Fig. 8). The data revealed that expression of K-FABP markedly enhanced the transcriptional activity of PPARβ at low concentrations of the PPARβ ligand L165041. However, the ability of the binding protein to augment transcriptional rates was rapidly lost when the concentration of the ligand was raised. It could be argued that, at high ligand concentrations, K-FABP did not affect transcriptional rates because the reporter vector became saturated. However, the observations indicated that K-FABP lost its potentiating activity upon elevation of ligand concentrations even under conditions that are far from the observed maximal activity (Fig. 8, inset). Hence, K-FABP is efficacious in enhancing PPARβ activity, but only at very low ligand concentrations.
FIG. 8.
K-FABP enhances the transcriptional activity of PPARβ only under limiting ligand concentrations. Transactivation assays were carried out by using COS-7 cells as described in Materials and Methods. Cells were cotransfected either with an empty vector or with an expression vector for K-FABP. The transcriptional activity of PPARβ was examined at various concentrations of the PPARβ-selective ligand L165041. (Inset) Fold activation observed upon cotransfection of K-FABP relative to fold activation in the absence of ectopic binding protein.
K-FABP plays an important role in PPARβ-mediated keratinocyte differentiation.
Taken together, the results reported above suggest that some FABPs function to activate specific PPARs by transporting PPAR ligands to the nucleus and directly delivering them to the receptors. Such an activity will facilitate the formation of the activated form of PPARs, i.e., the liganded receptors, thereby enhancing the efficacy of PPAR action. This hypothesis implies that the presence of a particular FABP in cells may be essential for the activities of the specific PPAR with which it interacts. We thus set out to examine whether K-FABP is involved in a biological activity that is mediated by PPARβ.
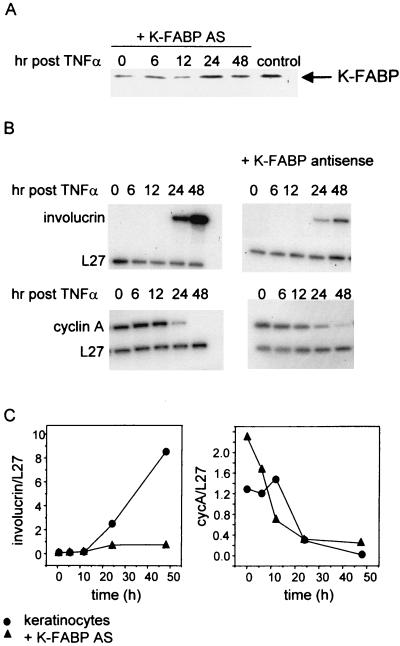
We recently demonstrated that PPARβ is critical for differentiation of keratinocytes induced by the inflammation-associated signal TNF-α (44). We showed further that, in addition to up-regulating the expression of PPARβ, treatment of keratinocytes with TNF-α also induces synthesis of a PPARβ ligand by these cells (44). In view of the observations that the activity of K-FABP is especially important at low ligand concentrations (Fig. 8), exploring the potential involvement of this protein in PPARβ activities requires a model system in which ligand levels are low (i.e., physiological). TNF-α-induced keratinocyte differentiation, a process that is mediated by PPARβ under conditions where activating ligands are generated intracellularly, is thus a particularly suitable model system for investigating possible functional interactions between K-FABP and this receptor. Primary keratinocytes were isolated and cultured. Cells were then transiently transfected with an expression vector harboring a K-FABP antisense construct 12 h prior to treatment with TNF-α. Western blot analysis demonstrated that transfection of the antisense construct significantly reduced the expression level of K-FABP, although it did not abolish it completely. The reduced expression level was maintained longer than 12 h following addition of TNF-α, the critical time frame for differentiation induction in these cells (Fig. 9A). Treatment of the keratinocytes with TNF-α induced growth arrest and differentiation, as could be seen by an increase in the expression of the mRNA of the differentiation marker involucrin and a concomitant decrease in levels of the cell cycle protein cyclin A beginning at 12 h posttreatment (Fig. 9B, left panels, and 9C). Cells expressing the K-FABP antisense construct underwent growth arrest following TNF-α treatment, as could be seen by the decrease in cyclin A expression (Fig. 9B, right, and 9C). However, these cells displayed a significant decrease in the expression of involucrin, indicating that differentiation was markedly inhibited (Fig. 9B, right, and 9C). This inhibition or delay of differentiation was observed despite the incomplete inhibition of K-FABP expression in the cells (Fig. 9A).
FIG. 9.
TNF-α-induced differentiation is inhibited or delayed in keratinocytes expressing a K-FABP antisense construct. Primary mouse prekeratinocytes were cultured, and their differentiation was induced by addition of TNF-α as described in Materials and Methods. (A) Expression of K-FABP in keratinocytes (control) or keratinocytes transfected with a vector harboring antisense (AS) K-FABP was examined by Western blotting using antibodies against K-FABP. Cells were transfected 12 h prior to treatment with TNF-α, and the expression level of K-FABP was monitored up to 48 h posttreatment. (B) Expression of the differentiation marker involucrin and the cell cycle protein cyclin A in primary keratinocytes and in keratinocytes expressing a K-FABP antisense construct was monitored by an RPA as described in Materials and Methods. (C) Relative expression levels of involucrin and cyclin A in TNF-α-treated primary keratinocytes or keratinocytes expressing a K-FABP antisense construct were measured by quantitation of the bands shown in Fig. 9B and normalization for the expression of L27.
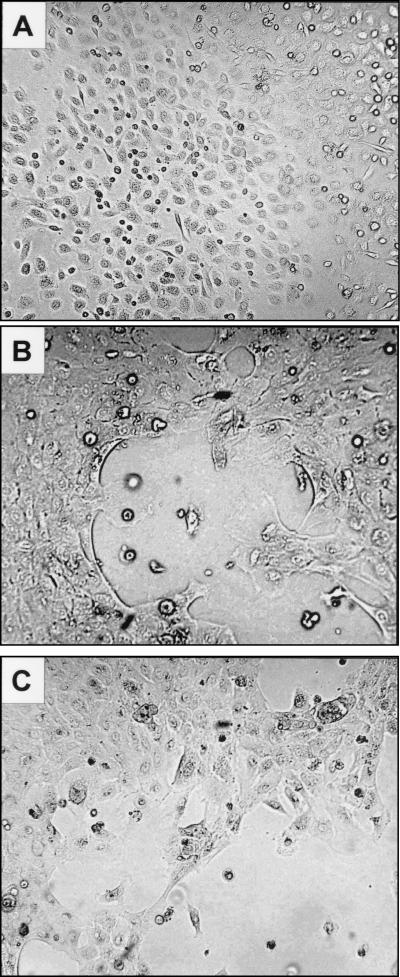
The conclusion that reduction in the expression level of K-FABP results in a marked inhibition of keratinocyte differentiation was further emphasized by examining the phenotype of the cells. Noninduced keratinocytes displayed a polygonal shape with distinct intercellular spaces, giving the cell sheet a paving stone-like appearance when cells were confluent (Fig. 10A). Upon induction, distinct spaces between cells became much less apparent. As individual cell borders became indistinct, i.e., squamous, stratification began. The differentiated cells became larger and developed phase-dense outlines (Fig. 10B). In contrast, in keratinocytes expressing a K-FABP antisense construct, intercellular spaces were retained and fewer focal areas of stratification were observed 15 h after TNF-α treatment (Fig. 10C). These observations thus demonstrate that the presence of high levels of K-FABP is essential for proper TNF-α-induced, PPARβ-mediated keratinocyte differentiation.
FIG. 10.
Expression of a K-FABP antisense construct inhibits or delays TNF-α-induced differentiation of keratinocytes. Primary mouse prekeratinocytes were cultured as described in Materials and Methods and were visualized by direct bright-field microscopy. (A) Cultured prekeratinocytes; (B) keratinocytes 15 h post-TNF-α-treatment; (C) keratinocytes expressing a K-FABP antisense construct 15 h post-TNF-α-treatment.
DISCUSSION
The specific roles that the multiple FABP isotypes play in the biology of their ligands is not well understood. It is usually believed that these proteins serve a general function in solubilization and trafficking of their ligands and that they affect lipid metabolism through their transport function (3, 6, 15, 36, 50). Support for the involvement of FABPs in fatty acid transport has been provided by reports that diffusion of these ligands both in vitro and in cells is facilitated in the presence of FABP (46-48). The nonspecific nature of this activity has been emphasized by the observation that bovine serum albumin could replace FABP in establishing intracellular diffusive flux of fatty acids (29). Other roles for FABPs have been implied by some studies. It has been suggested that I-FABP, H-FABP, and A-FABP may facilitate the dissociation of fatty acid from membranes (19, 21, 28, 40). It has also been reported that H-FABP associates with the scavenger receptor CD36 (41), although the functional significance of this observation remains unclear. Recently, the possibility that a connection exists between the activities of FABPs and PPARs has been explored. It was reported that L-FABP associates with specific nuclear membrane proteins in the presence of long-chain fatty acids or synthetic PPAR ligands (25). Overexpression of A-FABP and K-FABP was reported to inhibit the transcriptional activities of all three PPAR subtypes both in the absence and in the presence of exogenous ligand (18). It was also recently shown that L-FABP interacts with PPARα and PPARγ both in the absence and in the presence of ligands and that decreased expression of L-FABP in cells results in down-regulation of the transcriptional activities of ligands for all three PPAR isotypes (49). The mechanism by which L-FABP may exert these seemingly nonselective effects has not been clarified. We note as well that the basis of the inconsistency between the report that A-FABP and K-FABP inhibit PPAR activity nonselectively (18) and the data in the present report is not clear to us.
Here, we provide evidence that some FABPs act in concert with PPARs and that this activity is highly selective for particular FABP-PPAR pairs: A-FABP specifically enhances the activity of PPARγ, while K-FABP activates PPARβ. The data demonstrate further that A-FABP and K-FABP relocate from the cytosol to the nucleus in response to particular PPAR ligands and that the nuclear localization of K-FABP is critical for allowing this protein to augment the activity of its cognate receptor. Interestingly, we found that both the ligand-induced nuclear localization of the FABPs and their ability to enhance transcriptional activity are highly selective for the ligand of a particular PPAR isotype. This is so despite the apparent lack of selectivity in ligand binding by the FABPs (Table 1). These observations suggest that FABPs employ different modes of binding toward different ligands. In other words, they suggest that some ligands induce correct alterations in the FABP structure, leading to activation of the proteins, while others do not.
In addition to their functional interactions, A-FABP and K-FABP were found to physically associate with PPARγ and PPARβ, respectively. These interactions were specific for particular protein pairs and depended on the presence of particular ligands. The observed FABP-PPAR interactions were found to be weak, suggesting that the resulting complex is transient in nature, i.e., it comprises a short-lived intermediate which dissociates rapidly following transfer of the ligand. Nevertheless, examination of the mechanism by which a ligand moves from FABPs to PPARγ established that direct and selective FABP-PPAR interactions do occur and are functionally important. These studies revealed that A-FABP delivers troglitazone to PPARγ through direct association between the binding protein and the receptor. The selectivity of these interactions was confirmed by the observations that, unlike A-FABP, K-FABP does not interact with PPARγ, i.e., movement of troglitazone from K-FABP to the receptor proceeds through simple diffusion. A-FABP-mediated channeling of ligands to PPARγ significantly facilitated the ligation of the receptor, providing a rationale for understanding the ability of this binding protein (which is not shared by K-FABP) to enhance PPARγ transcriptional activity. A-FABP and K-FABP thus appear to function in a manner similar to that of CRABP-II in regulating the activity of nuclear hormone receptors, i.e., their association with particular ligands in the cytosol leads to translocalization into the nucleus, where they form a short-lived complex with a cognate PPAR. This complex mediates ligand channeling to the receptor, thereby facilitating its activation and enhancing its transcriptional activity. The importance of FABPs for biological activities mediated by PPARs is demonstrated by the observation that the presence of K-FABP is essential for the ability of PPARβ to properly induce differentiation of keratinocytes. Taken together, the data reveal that the A-FABP-PPARγ and K-FABP-PPARβ pairs cooperate tightly in regulating transcription in adipocytes and keratinocytes, respectively. Hence, the tissue-specific functions of PPARs are found to be closely supported by the tissue-specific expression of particular FABPs.
Functional interactions between FABPs and PPARs are also suggested by examination of mice and cells that are deficient in FABP expression. It has recently been reported that PPARβ plays a central role in neuronal differentiation (39) and that reduction in K-FABP expression in the neuronal cell line PC12 results in inhibition of differentiation of these cells (1). Taken together with our data demonstrating the importance of K-FABP and its cognate receptor, PPARβ, in inducing differentiation in keratinocytes, these observations raise the intriguing possibility that the same FABP-PPAR pair plays a similar role in mediating neuronal differentiation. Regarding A-FABP, it has been reported that mice in whom expression of this protein has been inhibited, like their wild-type counterparts, develop obesity when fed with a high-fat diet. However, unlike control mice, A-FABP−/− animals do not become insulin resistant or diabetic. This striking phenotype was observed despite the fact that disruption of A-FABP in the null mice is compensated for by up-regulation of K-FABP in their adipose tissues (20). Interestingly, mice that are heterozygous for PPARγ, like A-FABP-null mice, display increased insulin sensitivity compared to wild-type animals (32). Although the exact role of PPARγ in regulating insulin sensitivity is not clear at present, the similarity of the phenotypes of A-FABP−/− mice and mice deficient in PPARγ expression suggests that the two proteins act in concert. In view of the present study, the observation that up-regulation of K-FABP expression does not functionally compensate for the loss of A-FABP in A-FABP−/− mice appears to reflect the PPAR selectivity of these FABPs. It should also be noted that PPARγ has been reported to be essential for adipocyte differentiation both in vitro and in vivo (38), while A-FABP-null mice do not display an adipose tissue deficit (20). This apparent conflict is resolved by considering that the hypothesis put forward here does not propose that FABPs are absolutely essential for PPAR activity under all conditions. Clearly, PPARs can be activated by ligands in the absence of a binding protein, most likely due to the ability of these small ligands to enter the nucleus by free diffusion. Our data indicate, however, that FABPs function by facilitating ligand delivery, i.e., they act to enhance transcriptional activation by providing increased fluxes. Indeed, we show that the potentiating activity of FABP is especially significant when cellular levels of ligands are limiting, i.e., under conditions that necessitate enhanced efficiency of ligand delivery. These observations suggest that while FABPs are dispensable at high ligand concentrations, they become essential at low concentrations. A prediction that can be derived from these observations is that mice lacking A-FABP will display a more severe phenotype under conditions of ligand deficiency.
While the present study establishes that some FABPs physically and functionally cooperate with specific PPARs, several important questions remain to be explored. For example, the mechanisms through which FABPs locate to the nucleus in response to correct ligands is not understood at present. Similarly, considering the close similarities of the overall folding of FABPs, the specific structural features that allow them to discriminate between particular PPAR isotypes remain to be elucidated. Finally, it is worth noting that the existence of at least nine isotypes of FABP suggest that some of these proteins may have roles that are different from the functions of A-FABP and K-FABP described here. The complete scope of the biological functions of FABPs has thus only begun to be elucidated.
Acknowledgments
We thank David Cistola and Eric Xu for FABP and PPAR constructs, and we thank David Bernlohr for constructs and antibodies.
N.-S. Tan, N. S. Shaw, N. Vinckenbosch, and P. Liu contributed equally to this work.
This work was supported by grants from the NIH (grant CA68150 to N.N.), and from the Swiss National Science Foundation and Etat de Vaud (to W.W. and B.D.). The assistance of the Novartis Foundation in supporting N.N.'s sabbatical leave in Lausanne is gratefully acknowledged.
REFERENCES
- 1.Allen, G. W., J. W. Liu, and M. De Leon. 2000. Depletion of a fatty acid-binding protein impairs neurite outgrowth in PC12 cells. Brain Res. Mol. Brain Res. 76:315-324. [DOI] [PubMed] [Google Scholar]
- 2.Banaszak, L., N. Winter, Z. Xu, D. A. Bernlohr, S. Cowan, and T. A. Jones. 1994. Lipid-binding proteins: a family of fatty acid and retinoid transport proteins. Adv. Protein Chem. 45:89-151. [DOI] [PubMed] [Google Scholar]
- 3.Bass, N. M. 1990. Fatty acid-binding protein expression in the liver: its regulation and relationship to the zonation of fatty acid metabolism. Mol. Cell. Biochem. 98:167-176. [DOI] [PubMed] [Google Scholar]
- 4.Budhu, A., R. Gillilan, and N. Noy. 2001. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J. Mol. Biol. 305:939-949. [DOI] [PubMed] [Google Scholar]
- 5.Budhu, A. S., and N. Noy. 2002. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol. Cell. Biol. 22:2632-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burczynski, F. J., M. N. Zhang, P. Pavletic, and G. Q. Wang. 1997. Role of fatty acid binding protein on hepatic palmitate uptake. Can. J. Physiol. Pharmacol. 75:1350-1355. [PubMed] [Google Scholar]
- 7.Chawla, A., E. J. Schwarz, D. D. Dimaculangan, and M. A. Lazar. 1994. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135:798-800. [DOI] [PubMed] [Google Scholar]
- 8.Coe, N. R., and D. A. Bernlohr. 1998. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1391:287-306. [DOI] [PubMed] [Google Scholar]
- 9.Dalman, F. C., L. J. Sturzenbecker, A. A. Levin, D. A. Lucas, G. H. Perdew, M. Petkovitch, P. Chambon, J. F. Grippo, and W. B. Pratt. 1991. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form “docking” complexes with hsp90. Biochemistry 30:5605-5608. [DOI] [PubMed] [Google Scholar]
- 10.Desvergne, B., and W. Wahli. 1999. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20:649-688. [DOI] [PubMed] [Google Scholar]
- 11.Devchand, P. R., H. Keller, J. M. Peters, M. Vazquez, F. J. Gonzalez, and W. Wahli. 1996. The PPARα-leukotriene B4 pathway to inflammation control. Nature 384:39-43. [DOI] [PubMed] [Google Scholar]
- 12.Dong, D., S. E. Ruuska, D. J. Levinthal, and N. Noy. 1999. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J. Biol. Chem. 274:23695-23698. [DOI] [PubMed] [Google Scholar]
- 13.Forman, B. M., J. Chen, and R. M. Evans. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 94:4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass, C. K., D. W. Rose, and M. G. Rosenfeld. 1997. Nuclear receptor coactivators. Curr. Opin. Cell Biol. 9:222-232. [DOI] [PubMed] [Google Scholar]
- 15.Glatz, J. F., E. Van Breda, and G. J. Van der Vusse. 1998. Intracellular transport of fatty acids in muscle. Role of cytoplasmic fatty acid-binding protein. Adv. Exp. Med. Biol. 441:207-218. [DOI] [PubMed] [Google Scholar]
- 16.Hager, B., J. R. Bickenbach, and P. Fleckman. 1999. Long-term culture of murine epidermal keratinocytes. J. Investig. Dermatol. 112:971-976. [DOI] [PubMed] [Google Scholar]
- 17.Hamm, J. K., A. K. el Jack, P. F. Pilch, and S. R. Farmer. 1999. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Ann. N. Y. Acad. Sci. 892:134-145. [DOI] [PubMed] [Google Scholar]
- 18.Helledie, T., M. Antonius, R. V. Sorensen, A. V. Hertzel, D. A. Bernlohr, S. Kolvraa, K. Kristiansen, and S. Mandrup. 2000. Lipid-binding proteins modulate ligand-dependent trans-activation by peroxisome proliferator-activated receptors and localize to the nucleus as well as the cytoplasm. J. Lipid Res. 41:1740-1751. [PubMed] [Google Scholar]
- 19.Herr, F. M., J. Aronson, and J. Storch. 1996. Role of portal region lysine residues in electrostatic interactions between heart fatty acid binding protein and phospholipid membranes. Biochemistry 35:1296-1303. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil, G. S., R. S. Johnson, R. J. Distel, R. Ellis, V. E. Papaioannou, and B. M. Spiegelman. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 274:1377-1379. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, K. T., and J. Storch. 1996. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J. Biol. Chem. 271:13317-13323. [DOI] [PubMed] [Google Scholar]
- 22.Kersten, S., B. Desvergne, and W. Wahli. 2000. Roles of PPARs in health and disease. Nature 405:421-424. [DOI] [PubMed] [Google Scholar]
- 23.Kliewer, S. A., S. S. Sundseth, S. A. Jones, P. J. Brown, G. B. Wisely, C. S. Koble, P. Devchand, W. Wahli, T. M. Willson, J. M. Lenhard, and J. M. Lehmann. 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 94:4318-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krey, G., O. Braissant, F. L'Horset, E. Kalkhoven, M. Perroud, M. G. Parker, and W. Wahli. 1997. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 11:779-791. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, J. W., D. J. Kroll, and P. I. Eacho. 2000. Ligand-dependent interaction of hepatic fatty acid-binding protein with the nucleus. J. Lipid Res. 41:1390-1401. [PubMed] [Google Scholar]
- 26.Lemberger, T., B. Staels, R. Saladin, B. Desvergne, J. Auwerx, and W. Wahli. 1994. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 269:24527-24530. [PubMed] [Google Scholar]
- 27.Lin, Q., S. E. Ruuska, N. S. Shaw, D. Dong, and N. Noy. 1999. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry 38:185-190. [DOI] [PubMed] [Google Scholar]
- 28.Liou, H. L., and J. Storch. 2001. Role of surface lysine residues of adipocyte fatty acid-binding protein in fatty acid transfer to phospholipid vesicles. Biochemistry 40:6475-6485. [DOI] [PubMed] [Google Scholar]
- 29.Luxon, B. A., and M. T. Milliano. 1997. Cytoplasmic codiffusion of fatty acids is not specific for fatty acid binding protein. Am. J. Physiol. 273:C859-C867. [DOI] [PubMed] [Google Scholar]
- 30.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalik, L., B. Desvergne, N. S. Tan, S. Basu-Modak, P. Escher, J. Rieusset, J. M. Peters, G. Kaya, F. J. Gonzalez, J. Zakany, D. Metzger, P. Chambon, D. Duboule, and W. Wahli. 2001. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR) α and PPARβ mutant mice. J. Cell Biol. 154:799-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles, P. D., Y. Barak, W. He, R. M. Evans, and J. M. Olefsky. 2000. Improved insulin sensitivity in mice heterozygous for PPAR-γ deficiency. J. Clin. Investig. 105:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroianu, J. 1999. Nuclear import and export: transport factors, mechanisms and regulation. Crit. Rev. Eukaryot. Gene Expr. 9:89-106. [DOI] [PubMed] [Google Scholar]
- 34.Norris, A. W., L. Cheng, V. Giguere, M. Rosenberger, and E. Li. 1994. Measurement of subnanomolar retinoic acid binding affinities for cellular retinoic acid binding proteins by fluorometric titration. Biochim. Biophys. Acta 1209:10-18. [DOI] [PubMed] [Google Scholar]
- 35.Noy, N. 2000. Retinoid-binding proteins: mediators of retinoid action. Biochem. J. 348:481-495. [PMC free article] [PubMed] [Google Scholar]
- 36.Ockner, R. K., and J. A. Manning. 1976. Fatty acid binding protein. Role in esterification of absorbed long chain fatty acid in rat intestine. J. Clin. Investig. 58:632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricote, M., J. T. Huang, J. S. Welch, and C. K. Glass. 1999. The peroxisome proliferator-activated receptor (PPARγ) as a regulator of monocyte/macrophage function. J. Leukoc. Biol. 66:733-739. [DOI] [PubMed] [Google Scholar]
- 38.Rosen, E. D., P. Sarraf, A. E. Troy, G. Bradwin, K. Moore, D. S. Milstone, B. M. Spiegelman, and R. M. Mortensen. 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4:611-617. [DOI] [PubMed] [Google Scholar]
- 39.Saluja, I., J. G. Granneman, and R. P. Skoff. 2001. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia 33:191-204. [PubMed] [Google Scholar]
- 40.Smith, E. R., and J. Storch. 1999. The adipocyte fatty acid-binding protein binds to membranes by electrostatic interactions. J. Biol. Chem. 274:35325-35330. [DOI] [PubMed] [Google Scholar]
- 41.Spitsberg, V. L., E. Matitashvili, and R. C. Gorewit. 1995. Association and coexpression of fatty-acid-binding protein and glycoprotein CD36 in the bovine mammary gland. Eur. J. Biochem. 230:872-878. [DOI] [PubMed] [Google Scholar]
- 42.Storch, J., and A. E. Thumser. 2000. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 1486:28-44. [DOI] [PubMed] [Google Scholar]
- 43.Sweitzer, T. D., D. C. Love, and J. A. Hanover. 2000. Regulation of nuclear import and export. Curr. Top. Cell Regul. 36:77-94. [DOI] [PubMed] [Google Scholar]
- 44.Tan, N. S., L. Michalik, N. Noy, R. Yasmin, C. Pacot, M. Heim, B. Fluhmann, B. Desvergne, and W. Wahli. 2001. Critical roles of PPAR β/δ in keratinocyte response to inflammation. Genes Dev. 15:3263-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tontonoz, P., E. Hu, and B. M. Spiegelman. 1995. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator-activated receptor gamma. Curr. Opin. Genet. Dev. 5:571-576. [DOI] [PubMed] [Google Scholar]
- 46.Vork, M. M., J. F. Glatz, and G. J. van der Vusse. 1991. Does fatty acid-binding protein facilitate the diffusion of oleic acid? Biochem. J. 280:835-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vork, M. M., J. F. Glatz, and G. J. van der Vusse. 1993. On the mechanism of long chain fatty acid transport in cardiomyocytes as facilitated by cytoplasmic fatty acid-binding protein. J. Theor. Biol. 160:207-222. [DOI] [PubMed] [Google Scholar]
- 48.Weisiger, R. A. 1999. Saturable stimulation of fatty acid transport through model cytoplasm by soluble binding protein. Am. J. Physiol. 277:G109-G119. [DOI] [PubMed] [Google Scholar]
- 49.Wolfrum, C., C. M. Borrmann, T. Borchers, and F. Spener. 2001. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 98:2323-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodford, J. K., J. R. Jefferson, W. G. Wood, T. Hubbell, and F. Schroeder. 1993. Expression of liver fatty acid binding protein alters plasma membrane lipid composition and structure in transfected L-cell fibroblasts. Biochim. Biophys. Acta 1145:257-265. [DOI] [PubMed] [Google Scholar]
- 51.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]