Abstract
Vaccinia virus is a large enveloped poxvirus with more than 200 genes in its genome. Although many poxvirus genomes have been sequenced, knowledge of the host and viral protein components of the virions remains incomplete. In this study, we used gel-free liquid chromatography and tandem mass spectroscopy to identify the viral and host proteins in purified vaccinia intracellular mature virions (IMV). Analysis of the proteins in the IMV showed that it contains 75 viral proteins, including structural proteins, enzymes, transcription factors, and predicted viral proteins not known to be expressed or present in the IMV. We also determined the relative abundances of the individual protein components in the IMV. Finally, 23 IMV-associated host proteins were also identified. This study provides the first comprehensive structural analysis of the infectious vaccinia virus IMV.
Vaccinia virus is the prototype virus of the orthopoxvirus genus in the family Poxviridae, which replicates in the cytoplasm of cells (57, 104) and encodes more than 200 open reading frames (ORFs) in a 190-kb double-stranded DNA genome. Vaccinia virus infection produces multiple forms of infectious particles, namely, intracellular mature virions (IMV), intracellular enveloped virions (IEV), cell-associated enveloped virions (CEV), and extracellular enveloped virions (EEV). The IMV is the most abundant virion, with a single membrane in cells; however, the origin of the membrane is unknown (75, 77). A portion of the IMV is subsequently wrapped with two layers of Golgi membrane to form an IEV, which is transported through microtubules to the cell periphery and loses one membrane during virion egress to become a CEV (130, 140, 152, 153). The CEV remains associated with the cell surface, where actin-containing microvilli are formed, or can be released by host cell Src/Abl kinases into the medium to become an EEV (41, 42, 61, 111, 156). The IMV is robust and is known to be resistant to environmental and physical changes, whereas the CEV and EEV are very fragile, and the integrity of their outer membranes can be destroyed during purification procedures (83).
Many of the poxvirus genomes, including those of different strains of vaccinia virus, have been sequenced (http://www.poxvirus.org/viruses.asp). The genome of the vaccinia virus Western Reserve (WR) strain contains 218 potential ORFs; however, the existence of an ORF does not necessarily reveal the existence of a protein or the location of the protein. While classical biochemical and genetic approaches have generated significant knowledge of viral-gene functions, the physical composition of vaccinia virus IMV particles remains unknown.
Previous researchers have analyzed the protein composition of the vaccinia virus IMV. Initial studies of the purified IMV led to the identification on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of 17 “regions” containing 31 to 48 protein bands (103, 115, 139). Essani and Dales increased the gel resolution and detected 56 polypeptide bands on SDS-PAGE (54). Using different gel electrophoretic conditions, Oie and Ichihashi found 84 protein spots that could be assigned to 52 protein species in purified IMV (116). Unfortunately, the naming system for vaccinia virus proteins in the above studies was based on the protein migration behavior on gels, making it difficult to compare data generated in different gel electrophoresis systems. In addition, the majority of viral genes encode proteins smaller than 50 kDa that tend to cluster together and are difficult to resolve by SDS-PAGE. Moreover, N-terminal sequencing of viral protein bands on gels revealed that multiple protein bands can be derived from a single viral gene as a result of posttranslational cleavage or modification and that the 40 to 50 IMV bands seen on gels were derived from only 12 viral genes (160). Similarly, Jensen et al. found that the purified vaccinia virus core and membrane contain 30 proteins encoded by 13 viral genes and 5 host genes; additional, less abundant virion components were detected but not identified (86).
Mass spectrometry (MS), in particular, tandem MS (MS/MS), provides a powerful tool for proteome analysis because it is much more sensitive than other methods, can deal with protein mixtures, and offers a high throughput (120). Washburn et al. identified 1,484 proteins in the yeast proteome using gel-free liquid chromatography and tandem mass spectroscopy (LC/MS/MS) (175). Similar analyses have been used to identify virion proteins in human and murine cytomegalovirus, Epstein-Barr virus, and Kaposi's sarcoma-associated herpesvirus (87, 92, 172, 191). The identification of virion proteins makes it possible to start determining how the proteins interact, their stoichiometry in the viral particle, and which cellular compartments are involved in virion formation. In this study, we used LC/MS/MS analysis to determine the viral and host proteins that comprise each IMV particle. New components of vaccinia virus IMV encoded by putative ORFs were discovered, and the relative abundance of each component in the IMV was determined.
MATERIALS AND METHODS
Cells, virus, and reagents.
HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Vaccinia virus (Western Reserve strain) was propagated in HeLa cells and purified as described previously (80, 86). In brief, the infected cells were harvested when the cytotoxic pathological effect was complete. All stages of purification were performed at 4°C. The cells were centrifuged at 850 × g for 15 min to remove the medium and washed three times with phosphate-buffered saline. The pelleted cells were resuspended in TM buffer (10 mM Tris, pH 7.4, 5 mM MgCl2), and the suspension was passed 20 times in a Dounce homogenizer to break the cells. Nuclei were removed by centrifugation at 850 × g for 10 min, and the supernatant, containing virions, was laid on top of 36% sucrose solution and centrifuged at 45,000 × g for 80 min in a Beckman SW28 rotor. The virus pellet was resuspended in TM buffer, sonicated, and further purified by centrifugation through a continuous 25 to 40% sucrose gradient at 27,500 × g for 40 min in a Beckman SW28 rotor. Fractions were taken, and the virion infectivity in each fraction was analyzed by a plaque formation assay. Fractions containing virions were diluted fourfold in TM buffer and centrifuged to pellet the IMV. The purity of the IMV was confirmed by electron microscopy of negatively stained preparations. No detectable cellular debris was found in these preparations.
Tryptic and lysine C digestion of IMV particles.
Purified IMV (9 to 50 μg) were incubated at 25°C overnight with 0.5 N cyanogen bromide (Fluka) in 90% formic acid (Fluka) to cleave the proteins into smaller polypeptides, and excess reagents were removed by vacuum drying. The polypeptides were denatured and reduced at 37°C for 1 h in 6 M urea (Sigma), 2 M thiourea (Riedel-de Haën), and 10 mM dithiothreitol (Pharmacia Biotech), and then iodoacetamide (Sigma) was added to a final concentration of 20 mM and the sample was incubated at 37°C for 1 h in the dark. The peptide mixture was then diluted eightfold with 100 mM ammonium bicarbonate (Sigma), and sequencing-grade porcine trypsin (Promega) or Lys-C (Wako) was added at a substrate-to-enzyme ratio of 20:1 (wt/wt), and the mixture was incubated at 37°C overnight. The peptides formed were desalted using a C18 trap, lyophilized on a SpeedVac, and stored at −80°C.
Two-dimensional chromatography.
The peptide mixture from 50 μg of IMV was fractionated by two-dimensional chromatography (strong cation-exchange chromatography [SCX]-reverse-phase liquid chromatography). The first dimension, SCX, was eluted with a linear gradient of 0 to 300 mM KCl in 5 mM ammonium formate, pH 3.0. The peptides eluted from the SCX column were trapped in two C18 reverse-phase traps operating alternately; the bound peptides were eluted at 2-min intervals and collected on a fraction collector; a total of 22 fractions were collected. The amount of peptide in each fraction was estimated from the UV absorption at 214 nm.
Mass spectrometry.
Mass spectrometric analysis was performed on a nanoscale LC-tandem mass spectrometry (quadrupole time-of-flight mass spectrometer; QStar XL; Applied Biosystems). The instrument setup was as follows. The flow (150 μl/min) from the binary pump (Agilent 1100, with solvent A [100% deionized water] and solvent B [90% acetonitrile] [J. T. Baker], both solvents containing 0.1% formic acid) was split with two T-shaped connectors connected to a self-packed precolumn with an appropriate flow restrictor to give a column flow rate of 10 μl/min for sample loading and 200 to 300 nl/min for sample elution from the analytical column. The sample of 2 to 5 μg was injected onto the precolumn (15 mm long; 150-μm internal diameter; C18) via a 20-μl sample loop. The analytical column (C18; 15 cm long; 75-μm internal diameter) was connected to a 15-mm electrospray emitter (10-μm tip opening) by a 1-cm Teflon sleeve. The chromatographic separation was performed with a 120-min gradient profile as follows: 2% B (0 to 4.5 min), linear gradient of 2 to 10% B (4.5 to 5 min), 10 to 40% B (5 to 80 min), 40 to 50% B (80 to 100 min), 50 to 80% B (100 to 105 min), 80 to 2% B (105 to 106 min), and 2% B (106 to 120 min). The spectra of the eluted peptides were acquired in data-dependent mode by first acquiring a full MS scan from m/z 400 to 1900 for 1 second to determine the three most intense peptide peaks with charge states above 2, and then three MS/MS scans between m/z 100 and 2000 (1.5 s each) were performed for the MS-scanned parent ions with a threshold above 20 counts. Once sampled, each MS/MS precursor mass was excluded from further tandem experiments for 2 min.
The data files completed from the LC-MS runs were converted to Mascot generic-format files using the Mascot.dll script supplied with the Analyst QS software. The Mascot software package (Matrix Science) was used for database searching and protein identification using the modified vaccinia virus protein database. Peptide mass tolerance and fragment mass tolerance were set at 100 ppm and 0.25 Da, respectively, for the initial search. An alternative calibration algorithm based on Mascot protein identifications was applied to the raw data file to give mass accuracies within 20 ppm.
Proteomic analysis of IMV-derived peptides and database generation.
Individual MS/MS spectra were submitted to analysis using MASCOT version 2.0 (Matrix Science Inc.) and searched against the Human International Protein Index protein sequence database (version 3.10; 57,478 protein sequences; European Bioinformatics Institute [http://www.ebi.ac.uk/IPI/]) or against a modified vaccinia virus protein database. The modified viral-protein database combines 218 protein sequences of the WR strain of vaccinia virus obtained from http://poxvirus.org, 64 nonredundant Copenhagen strain orthologs, and 12 additional peptide sequences predicted from WR viral genome sequences using two programs, GeneMarkS (http://opal.biology.gatech.edu/GeneMark/) and fgenesV (http://softberry.com; 17). These databases allowed the identification of novel vaccinia virus proteins by an exhaustive search of all possible virus-derived peptide sequences. Proteins were scored using a probability-based MOWSE algorithm, and the scores were reported in the form −10 × log(P), where P is the probability that the observed match is a random event (123). Matches with scores higher than the 95% confidence level were regarded as significant. In all searches, phosphorylation, methionine oxidation, homoserine lactone, and carbamidomethylation of cysteine residues were considered as possible modifications. In order to increase the data confidence, the same data sets were submitted for a search against a “reverse database,” in which all peptide sequences were put in reverse order in silico; the percentage of peptides identified in the reverse database was less than 3.5%, confirming the specificity of the data search. Individual peptide interpretations were classed as significant if the MASCOT MOWSE score for the MS/MS spectra was greater than the cutoff value of 25.
Relative quantification of MS.
Quantification of protein abundance in IMV was performed based on the criteria and equations defined previously (85). In brief, the relative abundances of the proteins were estimated using an exponentially modified protein abundance index (emPAI) approach, which is based on the correlation of peptides identified in MS/MS experiments with observable peptides obtained by in silico digestion, which allows the relative abundances of proteins in a complex protein mixture to be determined. The abundance of each protein is presented as the molar and weight percentages of the total protein molecules in the IMV particles.
RESULTS
Identification of IMV proteins.
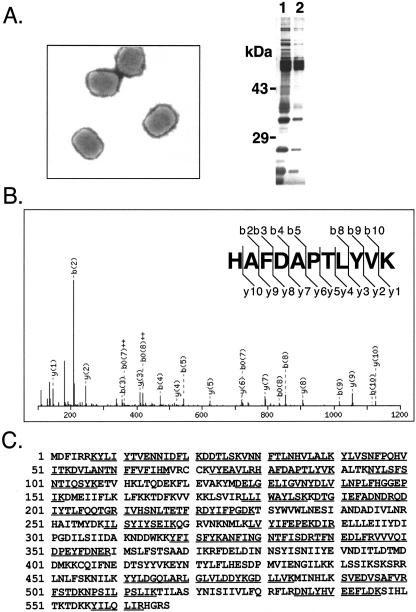
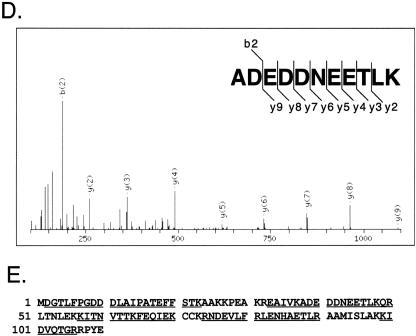
Vaccinia IMV particles were purified from the lysates of HeLa cells infected with the vaccinia virus WR strain, using two consecutive sucrose purification procedures to achieve maximal purity. The purified IMV particles were examined by electron microscopy to ensure that the virions had normal virion morphology and that cellular organelles and debris were undetectable. Proteins in purified virions were separated on 12% SDS-PAGE and silver stained (Fig. 1A). Gel electrophoresis of the purified virions followed by in-gel digestion often causes loss of proteolytic fragments generated from low-abundance proteins, and the gel matrix is inherently not optimal for resolving proteins of low molecular weight. Thus, a gel-free scheme with two-stage proteolysis was adopted to facilitate the efficient extraction of peptides from purified IMV particles. The purified IMV were completely dissolved in 90% formic acid and subjected to cyanogen bromide cleavage, followed by tryptic digestion in solution to sequentially yield a mixture of peptides. Alternatively, proteolytic digestion with Lys-C was performed in order to preserve longer peptides for better quality in data acquisition. These peptides were then analyzed by MS/MS using a modified vaccinia virus (WR) protein database constructed (see Materials and Methods) to include all predicted vaccinia virus translation products, as the commonly used vaccinia virus (WR) strain protein database does not include all predicted viral proteins or small proteins with fewer than 50 amino acids. As shown in Fig. 1B, the MS/MS spectrum of a tryptic peptide was interpreted as HAFDAPTLYVK, one of 30 unique peptides identified in a putative protein, WR062/E6R, resulting in 53% sequence coverage (Fig. 1C). Another tryptic peptide, ADEDDNEETLK, was identified in an envelope protein, A27L (Fig. 1D), that contains 71% sequence coverage, as shown in Fig. 1E. A total of three different IMV preparations were used in five independent mass spectrometry analyses. Combining the peptide data generated from trypsin and Lys-C digests, we identified 75 viral proteins in the IMV (Table 1). Because the gene names in the vaccinia virus WR strain are not as widely used as their orthologs in the Copenhagen strain, the latter gene names are used to refer to the viral translation products we identified in this work. Of these, 69 were identified by at least two trypsin- or Lys-C-generated peptides, while the other 6 (C6L, I5L, A9L, A21L, A22R, and A31R) were identified by single peptide hits in the trypsin- or Lys-C-digested samples. A further study using two-dimensional chromatographic fractionation to separate peptides prior to MS/MS was performed (SCX-LC/MS/MS), and at least one extra peptide (each) was identified for C6L, A9L, A21L, and A31R (Table 2). All the extra peptides identified in the SCX-LC/MS/MS study had MASCOT scores of at least >28. No extra peptide was identified for I5L and A22R by SCX-LC/MS/MS, although the same peptides as in one-dimensional LC/MS/MS were reproducibly detected. Both proteins are included in Table 1, because they were previously detected in the IMV (63, 84, 160).
FIG.1.
(A) Purified IMV. Electron microscopy of purified IMV particles using negative uranyl acetate staining (left) and silver staining of IMV proteins, 340 ng (lane 1) and 170 ng (lane 2), on 12% SDS-PAGE (right). (B) MS/MS spectrum of one tryptic peptide with a sequence identified as HAFDAPTLYVK and with a Mascot score of 72. (C) Amino acid sequence of the putative E6R protein. Tryptic peptides detected by MS, including the peptide in panel B, are underlined and give 53% sequence coverage. (D) MS/MS spectrum of one tryptic peptide with a sequence identified as ADEDDNEETLK and with a Mascot score of 80. (E) Amino acid sequence of A27L envelope protein. Tryptic peptides detected by MS, including the peptide in panel D, are underlined and give 71% sequence coverage.
TABLE 1.
Vaccinia virus IMV proteins identified by LC/MS/MSa
| VACV-WR ORF | Mass (Da) | VACV-CP ORF | Description | CNBr-trypsin digestion
|
CNBr-LysC digestion
|
Reference(s) | ||
|---|---|---|---|---|---|---|---|---|
| No. of unipeptides | Sequence coverage (%) | No. of unipeptides | Sequence coverage (%) | |||||
| VACV-WR_022 | 17,311 | C6L | Unknown | 1 | 7 | |||
| VACV-WR_035 | 48,842 | K4L | Phospholipase D-like | 5 | 14 | 3 | 8 | 21, 24 |
| VACV-WR_047 | 7,846 | F8L | Protein with iActA-like proline repeats not required for actin tail formation | 3 | 46 | 76 | ||
| VACV-WR_048 | 23,776 | F9L | S-S bond formation pathway thiol substrate | 5 | 27 | 2 | 12 | 146 |
| VACV-WR_049 | 52,098 | F10L | Ser/Thr kinase | 12 | 31 | 7 | 22 | 98 |
| VACV-WR_056 | 11,329 | F17R | Putative DNA-binding phosphoprotein in virus core | 8 | 56 | 5 | 54 | 86, 90, 91, 121, 182, 190 |
| VACV-WR_057 | 55,496 | E1L | Poly(A) polymerase catalytic subunit VP55 | 10 | 20 | 2 | 4 | 65 |
| VACV-WR_060 | 29,808 | E4L | DNA-dependent RNA polymerase subunit rpo30, intermediate-gene transcription factor VITF1 TFIIS-like | 8 | 28 | 3 | 15 | 1, 31 |
| VACV-WR_062 | 66,685 | E6R | Unknown | 30 | 53 | 12 | 27 | |
| VACV-WR_064 | 31,868 | E8R | Membrane protein may help wrap virosome associated with IV/IMV and core F10L kinase substrate | 11 | 52 | 3 | 13 | 51 |
| VACV-WR_066 | 10,844 | E10R | S-S bond formation pathway sulfhydryl oxidase, substrates L1R/F9L | 3 | 32 | 145 | ||
| VACV-WR_067 | 14,890 | E11L | Virion core protein | 2 | 20 | 1 | 10 | 174 |
| VACV-WR_069 | 12,347 | O2L | Nonessential glutaredoxin not part of E10R-G4L S-S bond formation pathway | 6 | 47 | 2 | 24 | 4, 88, 178 |
| VACV-WR_070 | 35,819 | I1L | DNA binding core protein | 6 | 18 | 3 | 11 | 148 |
| VACV-WR_071 | 8,493 | I2L | IMV membrane protein required for membrane fusion | 2 | 36 | 1 | 20 | 112 |
| VACV-WR_072 | 29,978 | I3L | ssDNA-binding phosphoprotein | 5 | 22 | 1 | 4 | 47, 168, 177 |
| VACV-WR_074 | 8,738 | I5L | IMV protein VP13 | 1 | 12 | 1 | 12 | 84, 160 |
| VACV-WR_076 | 48,979 | I7L | Viral core cysteine proteinase | 12 | 24 | 5 | 9 | 10, 32, 33, 89 |
| VACV-WR_077 | 77,550 | I8R | RNA-helicase, DExH-NPH-II DNA-helicase aided by L4R | 16 | 29 | 7 | 14 | 13, 56, 93, 150 |
| VACV-WR_078 | 67,997 | G1L | Insulin metalloproteinase-like has ENE and HLLEH inverse of HXXEX sites | 16 | 29 | 3 | 7 | 74 |
| VACV-WR_079 | 12,795 | G3L | Unknown | 2 | 19 | 1 | 19 | |
| VACV-WR_081 | 13,978 | G4L | S-S bond formation pathway thioredoxin-like | 3 | 29 | 2 | 12 | 72, 86, 178, 179 |
| VACV-WR_083 | 7,283 | G5.5R | DNA-dependent RNA polymerase subunit rpo7 | 3 | 47 | 8, 106 | ||
| VACV-WR_085 | 41,924 | G7L | Virion structural protein | 16 | 38 | 9 | 27 | 107, 157, 158, 160 |
| VACV-WR_087 | 38,761 | G9R | Myristylprotein | 2 | 7 | 2 | 7 | 102 |
| VACV-WR_088 | 27,262 | L1R | IMV membrane protein target of neutralizing antibody S-S bond formation pathway thiol substrate; myristylprotein | 6 | 26 | 3 | 10 | 59, 86, 102, 126, 127, 146, 184 |
| VACV-WR_090 | 40,595 | L3L | Unknown | 11 | 29 | 4 | 10 | |
| VACV-WR_091 | 28,439 | L4R | Core protein vp8 microtubule-associated, ssRNA/ssDNA-binding protein | 21 | 66 | 9 | 31 | 86, 121, 124, 160, 181, 186 |
| VACV-WR_092 | 15,034 | L5R | IMV membrane protein required for membrane fusion | 2 | 25 | 1 | 6 | 165 |
| VACV-WR_093 | 17,910 | J1R | Virion protein required for morphogenesis | 7 | 34 | 4 | 24 | 37, 38 |
| VACV-WR_095 | 38,863 | J3R | Multifunctional poly-A polymerase subunit, cap methyltransferase, and transcription elongation factor | 13 | 48 | 5 | 21 | 65, 141 |
| VACV-WR_096 | 21,328 | J4R | DNA-dependent RNA polymerase subunit rpo22 | 6 | 33 | 1 | 4 | 29, 79, 164 |
| VACV-WR_098 | 146,740 | J6R | DNA-dependent RNA polymerase subunit rpo147 | 47 | 39 | 15 | 13 | 29, 53, 79, 164 |
| VACV-WR_099 | 19,713 | H1L | Tyr/ser protein phosphatase | 8 | 49 | 4 | 26 | 69, 100, 110 |
| VACV-WR_100 | 21,529 | H2R | IMV membrane protein required for membrane fusion | 1 | 6 | 1 | 3 | 142 |
| VACV-WR_101 | 37,422 | H3L | IMV heparin binding surface protein involved in IMV maturation | 20 | 54 | 10 | 40 | 44-46, 86, 97, 160 |
| VACV-WR_102 | 93,574 | H4L | RAP94 tightly associated with DNA-dependent RNA polymerase, aids early-stage transcription, preinitiation, and termination | 27 | 38 | 9 | 13 | 2, 5, 189 |
| VACV-WR_103 | 22,287 | H5R | Morphogenesis-related, substrate of B1R kinase late gene transcription factor VLTF-4 | 4 | 28 | 2 | 15 | 14, 15, 16, 27, 94 |
| VACV-WR_104 | 36,642 | H6R | Topoisomerase type IB | 11 | 46 | 2 | 7 | 149, 151 |
| VACV-WR_106 | 96,673 | D1R | Large subunit of mRNA capping enzyme transcription termination factor VTF | 30 | 39 | 16 | 24 | 109, 173 |
| VACV-WR_107 | 16,935 | D2L | Virion core protein | 5 | 36 | 3 | 21 | 52 |
| VACV-WR_108 | 27,973 | D3R | Virion core protein | 7 | 41 | 1 | 3 | 52 |
| VACV-WR_111 | 73,784 | D6R | 70kDa small subunit of early gene transcription factor VETF | 14 | 27 | 4 | 9 | 28, 66 |
| VACV-WR_112 | 17,900 | D7R | DNA-dependent RNA polymerase subunit rpo18 | 1 | 6 | 1 | 8 | 3, 125 |
| VACV-WR_113 | 35,424 | D8L | IMV membrane protein binds cell surface chondroitin may effect viral entry | 11 | 51 | 5 | 20 | 80, 86, 114 |
| VACV-WR_116 | 72,264 | D11L | ATPase, nucleoside triphosphate phosphohydrolase-I, NPH-I transcription elongation, termination, release factor | 14 | 25 | 6 | 14 | 30, 93, 131 |
| VACV-WR_117 | 33,330 | D12L | Small subunit of mRNA capping enzyme transcription termination factor VTF | 11 | 41 | 6 | 33 | 113, 173 |
| VACV-WR_118 | 61,852 | D13L | Rifampicin target associates with inner surface immature virus membrane | 1 | 2 | 2 | 3 | 11, 108, 161, 162 |
| VACV-WR_121 | 8,922 | A2.5L | S-S bond formation pathway CxxxC links SH-oxidase E10R and thioredoxin G4L | 4 | 36 | 3 | 42 | 147 |
| VACV-WR_122 | 72,578 | A3L | p4b precursor of core protein 4b | 46 | 65 | 15 | 34 | 121, 135, 160 |
| VACV-WR_123 | 30,908 | A4L | 39-kDa core protein complexes with core protein p4a/4a | 14 | 55 | 5 | 13 | 49, 86, 121 |
| VACV-WR_124 | 18,984 | A5R | DNA-dependent RNA Polymerase subunit rpo19 | 5 | 45 | 2 | 12 | 6 |
| VACV-WR_125 | 43,147 | A6L | unknown | 5 | 14 | 6 | 23 | |
| VACV-WR_126 | 82,220 | A7L | 82-kDa large subunit of early gene transcription factor VETF | 23 | 33 | 14 | 22 | 66 |
| VACV-WR_128 | 12,102 | A9L | IMV membrane protein required for morphogenesis | 1 | 11 | 187 | ||
| VACV-WR_129 | 102,209 | A10L | Precursor p4a of core protein 4a, complexes with A4L | 69 | 62 | 20 | 23 | 86, 121, 160, 171 |
| VACV-WR_132 | 7,691 | A13L | IMV membrane protein | 5 | 55 | 2 | 42 | 86, 160, 169 |
| VACV-WR_133 | 9,987 | A14L | Phosphorylated IMV membrane protein required for morphogenesis | 3 | 25 | 1 | 8 | 18, 86, 133, 134, 160, 167 |
| VACV-WR_135 | 10,944 | A15L | Unknown | 2 | 32 | 1 | 14 | |
| VACV-WR_136 | 43,396 | A16L | Soluble myristylprotein | 7 | 25 | 1 | 5 | 102 |
| VACV-WR_137 | 22,984 | A17L | IMV membrane protein undergoes phosphorylation and proteolytic processing; required for morphogenesis | 5 | 21 | 3 | 17 | 50, 86, 95, 133, 160, 183 |
| VACV-WR_138 | 56,679 | A18R | DNA helicase effects elongation and termination of postreplicative viral transcription | 8 | 17 | 1 | 4 | 93, 188 |
| VACV-WR_140 | 13,636 | A21L | IMV membrane protein required for membrane fusion | 1 | 10 | 166 | ||
| VACV-WR_142 | 21,865 | A22R | Palmitylprotein; Holliday junction endonuclease; resolves viral DNA concatemers into unit length genomes | 1 | 5 | 63, 67 | ||
| VACV-WR_144 | 133,279 | A24R | DNA-dependent RNA polymerase subunit rpo132 | 35 | 34 | 14 | 13 | 9, 78 |
| VACV-WR_148 | 84,299 | A25L | Gene fragment, cowpox A-type inclusion protein | 34 | 44 | 13 | 19 | 62 |
| VACV-WR_149 | 57,957 | A26L | p4c protein; IMV membrane protein required for directing IMV into A-type inclusion body | 23 | 49 | 10 | 23 | 105 |
| VACV-WR_150 | 12,564 | A27L | IMV surface protein roles in IMV-cell attachment, fusion, and microtubule transport | 10 | 71 | 3 | 24 | 39, 86, 101, 132, 138, 160 |
| VACV-WR_151 | 16,319 | A28L | IMV membrane protein required for membrane fusion | 3 | 23 | 1 | 8 | 143, 144 |
| VACV-WR_152 | 35,370 | A29L | DNA-dependent RNA polymerase rpo35 | 6 | 21 | 2 | 9 | 9 |
| VACV-WR_153 | 8,740 | A30L | IMV protein for association of dense viroplasm with viral membranes during morphogenesis | 2 | 54 | 107, 157, 158, 159 | ||
| VACV-WR_154 | 14,200 | A31R | Unknown | - | 1 | 13 | ||
| VACV-WR_167 | 15,043 | A42R | Profilin-like | 8 | 57 | 2 | 12 | 20 |
| VACV-WR_171 | 13,654 | A45R | Inactive Cu-Zn superoxide dismutase-like in virion | 4 | 25 | 1 | 7 | 7 |
| VACV-WR_172 | 27,618 | A46R | Toll/IL1-receptor [TIR]-like suppresses TIR-dependent signal transduction host defense modulator | 2 | 7 | 25, 155 | ||
The data presented in Table 1 are compiled from three different IMV preparations. Tryptic digestions of different IMV preparations revealed 92% reproducibility, and Lys-C digestions revealed 87% reproducibility. Peptides generated by both trypsin and Lys-C are required to obtain a total of 75 viral proteins: 65 proteins (86.7%) were detected in peptides generated by either trypsin or Lys-C digestions, 9 proteins (12%) were detected only in trypsin-digested peptides, and 1 protein (1.3%) was detected only in Lys-C-digested peptides. VACV, vaccinia virus; CP, Copenhagen strain; ssDNA, single-stranded DNA; ssRNA, single-stranded RNA.
TABLE 2.
Viral proteins identified by LC/MS/MS by a single tryptic peptide and confirmed by SCX-LC/MS/MS
| VACVa-WR ORF | Reverse-phase LC/MS/MS
|
SCX followed by reverse-phase LC/MS/MS
|
Reference for IMV association | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of total hits | No. of unique peptides | Mascot score | Peptide sequence (CNBr-trypsin)b | No. of total hits | No. of unique peptides | Mascot score | Peptide sequence (CNBr-LysC) | ||
| VACV-WR_022(C6L) | 1 | 1 | 30 | YYDGNIYELAK | 3 | 2 | 66 | ADSFSLESDSIK | |
| 32 | IDAVRYYDGNIYELAK | ||||||||
| VACV-WR_074(I5L) | 2 | 1 | 39 | VISGAALIVK | 3 | 1 | 72 | VISGAALIVK | 160 |
| VACV-WR_128(A9L) | 2 | 1 | 35 | LRPNSFWFVVVR | 4 | 2 | 46 | SCYTAILK | 187 |
| 28 | RNESSINSNSSPK | ||||||||
| VACV-WR_140(A21L) | 5 | 1 | 42 | LFSYNFTTSGIK | 6 | 4 | 40 | NVPIPCSK | 166 |
| 49 | INEVNNNK | ||||||||
| 40 | DVDTLYCDK | ||||||||
| 31 | NFICVDDRLFSYNFTTSGIK | ||||||||
| VACV-WR_142(A22R) | 1 | 1 | 32 | DNSVRVLDI(p)SK | 1 | 1 | 34 | DNSVRVLDI(p)SK | 63 |
| VACV-WR_154(A31R) | 1 | 1 | 33 | YLSGGGIYHDDLVVLGK | 4 | 2 | 31 | VTINNLK | |
| 33 | YLSGGGIYHDDLVVLGK | ||||||||
VACV, vaccinia virus.
(p), predicted phosphorylated site.
Of the 75 viral proteins identified, 65 were consistent with previous reports of their presence in IMV particles (references in Table 1); these were 22 membrane proteins (H3L, D8L, H2L, L1R, L5R, A2.5, A9L, A13L, A14L, A17L, A21L, A25L, A26L, A27L, A28L, E8R, E10R, F9L, I2L, I5L, J1R, and D13L), 12 core proteins (A3L, A4L, A10L, A30L, F17R, E11L, I1L, I3L, G7L, L4R, D2L, and D3R), 24 enzymes [kinase (F10L), phosphatase (H1L), glutaredoxins (G4L and O2L), topoisomerase (H6R), proteinases (G1L and I7L), ATPase (D11L), poly(A) polymerase subunits (E1L and J3R), capping enzymes (D1R and D12L), DNA-dependent RNA polymerase complexes (A5R, A24R, A29L, J4R, J6R, H4L, E4L, G5.5R, and D7R), helicases (I8R and A18R), and endonuclease (A22R)], 3 viral transcription factors (A7L, H5R, and D6R), and 4 proteins (A42R, A45R, A46R, and F8L) interacting with host proteins. This shows that our proteomic data are reliable and consistent with previous results. More importantly, the proteomic data revealed 10 new viral proteins present in IMV, consisting of the products of seven former putative ORFs (E6R, G3L, L3L, A6L, A15L, A31R, and C6L) and three proteins (K4L, G9R, and A16L) previously with unknown locations and functions. K4L shows homology with phospholipase D but is nonessential for viral growth in cell cultures (21, 24, 122), while both G9R and A16L have been identified as myristylated proteins in virus-infected cells (102).
Six viral proteins, I6L, G5R, A12L, A14.5, A32L, and B1R, have been cited in the literature as being IMV associated but were not identified in our analyses (Table 3) (19, 34, 43, 48, 68, 99, 160). All six proteins were not detected in LC/MS/MS using different IMV preparations, although three proteins (A12L, A32L, and B1R) were detected in SCX-LC/MS/MS, suggesting that the abundances of these three proteins were below the threshold of LC/MS/MS and that SCX chromatography was necessary to reduce the sample complexity in order to enhance the detection sensitivity (Table 3). Consistent with our interpretation, A32L was previously shown to be difficult to detect in purified IMV (34). The other three proteins (I6L, G5R, and A14.5L) were not identified in either LC/MS/MS or SCX-LC/MS/MS analyses. Several possible reasons may explain why they were not detected in both MS/MS analyses. First, MS is biased against small and hydrophobic proteins. A14.5L contains 53 amino acids, producing at most two tryptic peptides (19). It is also very hydrophobic, with a grand average of hydropathicity index of 1.438 (96). These features of A14.5L may have jeopardized its detection in MS analyses. Second, MS, despite great sensitivity, can only detect peptides at femtomole range. Third, peptides that are too close to be resolved in MS will also be ignored by data acquisition software. We speculate that these technical reasons might explain the lack of detection of G5R and I6L.
TABLE 3.
Virion proteins cited in literature but not identified by LC/MS/MS
| VACVa-WR ORF | LC/MS/MS | SCX-LC/MS/MS
|
Reference showing IMV association | |||
|---|---|---|---|---|---|---|
| No. of unique peptides | No. of total hits | No. of unique peptides | Mascot score | Peptide sequence (CNBr-LysC) | ||
| VACV-WR_075(I6L) | 0 | 0 | 0 | 48,68 | ||
| VACV-WR_082(G5R) | 0 | 0 | 0 | 43 | ||
| VACV-WR_131(A12L) | 0 | 8 | 5 | 57 | SSSSSTSASK | 160,180 |
| 41 | NLLAQIGGDAAVK | |||||
| 56 | DGQIVQAVTNAGK | |||||
| 40 | VGEINHDLLGIDSVNAGK | |||||
| 25 | NLAVRSSYDDYIETVNK | |||||
| VACV-WR_134(A14.5L) | 0 | 0 | 0 | 19 | ||
| VACV-WR_155(A32L) | 0 | 1 | 1 | 29 | NCFQEK | 34 |
| VACV-WR_183(B1R) | 0 | 4 | 3 | 41 | NQWVVGPLIGK | 99,128 |
| 29 | ASNIVLDQIDK | |||||
| 53 | LYLVDYGLVSK | |||||
VACV, vaccinia virus.
Relative abundances of viral proteins in the IMV.
The quantity of each viral component within the IMV particle is an important issue, but it has been difficult to address in the past. Crude estimation of the quantity of each protein by gel staining intensity is not suitable for complex structure analysis. While recent advances in differential protein analyses have provided a means for comparing relative protein expression between two populations, there is a need for a method giving the relative quantification of a single protein in a complex population. Although quantification of particular proteins of interest using isotope-labeled synthetic peptides, termed Protein-AQUA, is in principle applicable to comprehensive analyses (12, 64), it is hampered by the high cost of isotope-labeled peptides and the difficulty of quantitative digestion of proteins in gel (73).
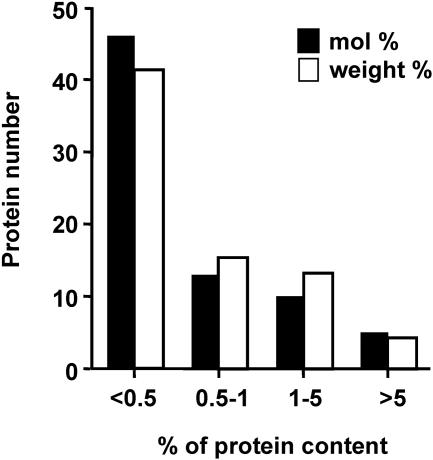
Recently, Ishihama et al. demonstrated that it is feasible to quantify label-free protein components using an emPAI approach (85), which fully utilizes the advantages of the wide dynamic detection range provided by LC/MS analysis and reliable protein search algorithms. This approach to determining the relative abundances of proteins in a complex protein mixture is based on the correlation of peptides identified by tandem MS with observable peptides obtained by in silico digestion. The abundance of each protein determined in this study is presented as the molar and weight percentages of the total protein molecules in IMV particles (Table 4). The most abundant viral-protein group (weight percentage, >5) consisted of four core proteins (A4L, A10L, F17R, and A3L). The relatively abundant protein group (5 > weight percentage > 1) consisted of seven envelope proteins (A27L, A25L, A14L, H3L, A26L, D8L, and A13L,), two core proteins (L4R and G7L), four proteins involved in transcription (D11L, J6R, A24R, and D1R), and one novel protein (E6R). Eleven of the above 18 abundant proteins have been previously identified as abundant proteins in IMV particles (86, 160). The intermediate-abundance protein group (1% > weight percentage > 0.5%) contained 15 viral proteins, and the less abundant group (weight percentage, <0.5%) contained 41 viral proteins. The distribution of viral proteins based on different molar or weight abundances in IMV revealed a similar distribution (Fig. 2); the low abundances of many proteins explain why they have escaped previous proteomic detection.
TABLE 4.
Relative viral protein abundances in vaccinia virus IMVa
| VACV-WR ORF | VACV-CP ORF | Description | Try-emPAI
|
Reference for previous proteomic identification | |||
|---|---|---|---|---|---|---|---|
| Rank by wt % | Average emPAI | Mol% | Weight % | ||||
| VACV-WR_123 | A4L | 39-kDa core protein complexes with core protein p4a/4a | 1 | 81.73 | 20.10 | 24.04 | 86 |
| VACV-WR_129 | A10L | Precursor p4a of core protein 4a, complexes with A4L | 2 | 7.75 | 1.91 | 7.54 | 86,160 |
| VACV-WR_056 | F17R | Putative DNA binding phosphoprotein in virus-core | 3 | 69.00 | 16.97 | 7.44 | 86 |
| VACV-WR_122 | A3L | p4b precursor of core protein 4b | 4 | 8.04 | 1.98 | 5.55 | 160 |
| VACV-WR_150 | A27L | IMV surface protein roles in IMV-cell attachment, fusion, and microtubule transport | 5 | 34.21 | 8.41 | 4.09 | 86,160 |
| VACV-WR_091 | L4R | Core protein vp8 microtubule-associated, ssRNA/ssDNA binding protein | 6 | 13.00 | 3.20 | 3.52 | 86,160 |
| VACV-WR_148 | A25L | Gene fragment, cowpox A-type inclusion protein | 7 | 4.23 | 1.04 | 3.40 | |
| VACV-WR_133 | A14L | Phosphorylated IMV membrane protein required for morphogenesis | 8 | 33.77 | 8.31 | 3.21 | 86,160 |
| VACV-WR_101 | H3L | IMV heparin binding surface protein involved in IMV maturation | 9 | 8.65 | 2.13 | 3.08 | 86,160 |
| VACV-WR_149 | A26L | Gene fragment, cowpox A-type inclusion protein | 10 | 5.11 | 1.26 | 2.82 | |
| VACV-WR_116 | D11L | ATPase, nucleoside triphosphate phosphohydrolase-I, NPH-I transcription elongation, termination, release factor | 11 | 3.76 | 0.93 | 2.59 | |
| VACV-WR_113 | D8L | IMV membrane protein binds cell surface chondroitin, may effect viral entry | 12 | 7.09 | 1.74 | 2.39 | 86,160 |
| VACV-WR_062 | E6R | Unknown | 13 | 2.78 | 0.68 | 1.77 | |
| VACV-WR_132 | A13L | IMV membrane protein | 14 | 23.42 | 5.76 | 1.71 | 86,160 |
| VACV-WR_098 | J6R | DNA-dependent RNA polymerase subunit rpo147 | 15 | 1.04 | 0.25 | 1.45 | |
| VACV-WR_144 | A24R | DNA-dependent RNA polymerase subunit rpo132 | 16 | 1.00 | 0.25 | 1.27 | |
| VACV-WR_106 | D1R | Large subunit of mRNA capping enzyme transcription termination factor VTF | 17 | 1.14 | 0.28 | 1.05 | |
| VACV-WR_085 | G7L | Virion structural protein | 18 | 2.52 | 0.62 | 1.01 | 160 |
| VACV-WR_167 | A42R | Profilin like | 19 | 5.88 | 1.45 | 0.84 | |
| VACV-WR_078 | G1L | Insulin metalloproteinase-like; has ENE and HLLEH inverse of HXXEX sites | 20 | 1.16 | 0.28 | 0.75 | |
| VACV-WR_064 | E8R | Membrane protein may help wrap virosome; associates with IV/IMV and core F10L kinase substrate | 21 | 2.40 | 0.59 | 0.73 | |
| VACV-WR_071 | I2L | Unknown | 22 | 9.00 | 2.21 | 0.73 | |
| VACV-WR_077 | I8R | RNA helicase, DExH-NPH-II DNA helicase aided by L4R | 23 | 0.90 | 0.22 | 0.66 | |
| VACV-WR_126 | A7L | 82-kDa large subunit of early gene transcription factor VETF | 24 | 0.83 | 0.20 | 0.65 | |
| VACV-WR_117 | D12L | Small subunit of mRNA capping enzyme transcription termination factor VTF | 25 | 2.02 | 0.50 | 0.64 | |
| VACV-WR_124 | A5R | DNA-dependent RNA polymerase subunit rpo19 | 26 | 3.45 | 0.85 | 0.62 | |
| VACV-WR_108 | D3R | Virion core protein | 27 | 2.30 | 0.57 | 0.61 | |
| VACV-WR_099 | H1L | Tyr/Ser protein phosphatase | 28 | 3.22 | 0.79 | 0.60 | |
| VACV-WR_095 | J3R | Multifunctional poly(A) polymerase subunit, cap methyltransferase, and transcription elongation factor | 29 | 1.59 | 0.39 | 0.59 | |
| VACV-WR_093 | J1R | Virion protein required for morphogenesis | 30 | 3.34 | 0.82 | 0.57 | |
| VACV-WR_102 | H4L | RAP94 tightly associated with DNA-dependent RNA polymerase, aids early-stage transcription preinitiation and termination | 31 | 0.63 | 0.16 | 0.56 | |
| VACV-WR_103 | H5R | Morphogenesis-related, substrate of B1R kinase late-gene transcription factor VLTF-4 | 32 | 2.38 | 0.59 | 0.50 | |
| VACV-WR_076 | I7L | Viral core cysteine proteinase | 33 | 1.07 | 0.26 | 0.50 | |
| VACV-WR_048 | F9L | S-S bond formation pathway thiol substrate | 34 | 1.95 | 0.48 | 0.44 | |
| VACV-WR_069 | O2L | Nonessential glutaredoxin not part of E10R-G4L S-S bond formation pathway | 35 | 3.22 | 0.79 | 0.38 | |
| VACV-WR_104 | H6R | Topoisomerase type IB | 36 | 1.05 | 0.26 | 0.37 | |
| VACV-WR_107 | D2L | Virion core protein | 37 | 2.18 | 0.54 | 0.35 | |
| VACV-WR_111 | D6R | 70-kDa small subunit of early-gene transcription factor VETF | 38 | 0.49 | 0.12 | 0.34 | |
| VACV-WR_151 | A28L | Unknown, putative signal peptide | 39 | 2.06 | 0.51 | 0.32 | |
| VACV-WR_083 | G5.5R | DNA-dependent RNA polymerase subunit rpo7 | 40 | 4.60 | 1.13 | 0.32 | |
| VACV-WR_153 | A30L | IMV protein for association of dense viroplasm with viral membranes during morphogenesis | 41 | 3.77 | 0.93 | 0.31 | |
| VACV-WR_049 | F10L | Ser/Thr kinase | 42 | 0.62 | 0.15 | 0.31 | |
| VACV-WR_090 | L3L | Unknown | 43 | 0.67 | 0.16 | 0.26 | |
| VACV-WR_137 | A17L | IMV membrane protein undergoes phosphorylation and proteolytic processing; required for morphogenesis | 44 | 1.18 | 0.29 | 0.26 | 86,160 |
| VACV-WR_060 | E4L | DNA-dependent RNA polymerase subunit rpo30, intermediate-gene transcription factor; VITF1 TFIIS like | 45 | 0.86 | 0.21 | 0.24 | |
| VACV-WR_057 | E1L | Poly(A) polymerase catalytic subunit VP55 | 46 | 0.41 | 0.10 | 0.22 | |
| VACV-WR_035 | K4L | Phospholipase D like | 47 | 0.46 | 0.11 | 0.21 | |
| VACV-WR_152 | A29L | DNA-dependent RNA polymerase rpo35 | 48 | 0.63 | 0.15 | 0.21 | |
| VACV-WR_079 | G3L | Unknown | 49 | 1.70 | 0.42 | 0.21 | |
| VACV-WR_135 | A15L | Unknown | 50 | 1.98 | 0.49 | 0.21 | |
| VACV-WR_096 | J4R | DNA-dependent RNA polymerase subunit rpo22 | 51 | 1.00 | 0.25 | 0.20 | |
| VACV-WR_072 | I3L | ssDNA binding phosphoprotein | 52 | 0.64 | 0.16 | 0.18 | |
| VACV-WR_070 | I1L | DNA binding core protein | 53 | 0.49 | 0.12 | 0.17 | |
| VACV-WR_171 | A45R | Inactive Cu-Zn superoxide dismutase like in virion | 54 | 1.24 | 0.30 | 0.16 | |
| VACV-WR_125 | A6L | Unknown | 55 | 0.37 | 0.09 | 0.15 | |
| VACV-WR_088 | L1R | IMV membrane protein target of neutralizing antibody S-S bond formation pathway thiol substrate; myristyl protein | 56 | 0.57 | 0.14 | 0.15 | 86 |
| VACV-WR_136 | A16L | Soluble myristyl protein | 57 | 0.32 | 0.08 | 0.13 | |
| VACV-WR_081 | G4L | S-S bond formation pathway; thioredoxin like | 58 | 0.93 | 0.23 | 0.12 | 86 |
| VACV-WR_066 | E10R | S-S bond formation pathway sulfhydryl oxidase, substrates L1R/F9L | 59 | 1.03 | 0.25 | 0.11 | |
| VACV-WR_121 | A2.5L | S-S bond formation pathway CXXXC links SH oxidase E10R and thioredoxin G4L | 60 | 1.20 | 0.30 | 0.10 | |
| VACV-WR_112 | D7R | DNA-dependent RNA polymerase subunit rpo18 | 61 | 0.58 | 0.14 | 0.10 | |
| VACV-WR_138 | A18L | DNA helicase effects elongation and termination of postreplicative viral transcription | 62 | 0.18 | 0.05 | 0.10 | |
| VACV-WR_074 | I5L | IMV protein VP13 | 63 | 1.15 | 0.28 | 0.10 | 160 |
| VACV-WR_047 | F8L | Protein with iActA-like proline repeats not required for actin tail formation | 64 | 1.24 | 0.30 | 0.09 | |
| VACV-WR_087 | G9R | Myristyl protein | 65 | 0.23 | 0.06 | 0.08 | |
| VACV-WR_100 | H2R | Unknown | 66 | 0.38 | 0.09 | 0.08 | |
| VACV-WR_092 | L5R | Putative membrane protein | 67 | 0.39 | 0.10 | 0.06 | |
| VACV-WR_142 | A22R | Palmityl protein; Holliday junction endonuclease; resolves viral DNA concatemers into unit-length genomes | 68 | 0.26 | 0.06 | 0.05 | |
| VACV-WR_128 | A9L | IMV membrane protein required for morphogenesis | 69 | 0.46 | 0.11 | 0.05 | |
| VACV-WR_067 | E11L | Virion core protein | 70 | 0.33 | 0.08 | 0.05 | |
| VACV-WR_118 | D13L | Rifampin target associates with inner surface immature virus membrane | 71 | 0.08 | 0.02 | 0.05 | |
| VACV-WR_140 | A21L | Unknown | 72 | 0.33 | 0.08 | 0.04 | |
| VACV-WR_022 | C6L | Unknown | 73 | 0.26 | 0.04 | ||
| VACV-WR_172 | A46R | Toll/IL1-receptor (TIR) likesuppresses TIR-dependent signal transduction host defense modulator | 74 | 0.15 | 0.04 | 0.04 | |
| VACV-WR_154 | A31R | Unknown | 75 | ||||
Data presented in Table 4 are compiled from three different IMV preparations. VACV, vaccinia virus; CP, Copenhagen strain; ssRNA, single-stranded RNA; ssDNA, single-stranded DNA.
FIG. 2.
Distribution of viral proteins with different abundances in IMV. All the viral proteins identified in this study were quantified as described in Materials and Methods and divided into four categories based on the protein contents in IMV, as shown below the graph. The black columns show the molar percentages of the total proteins in IMVs, while the white columns show the weight percentages.
Identification of host proteins associated with the IMV.
The host protein content of vaccinia virus IMV was determined by comparing the detected peptides with a Human International Protein Index protein sequence database, and 23 host proteins associated with vaccinia virus IMV particles were identified (Table 5). All were present at low abundance (intermediate and less abundant categories) in virions and could be divided into several categories, namely, calcium binding proteins (annexin A1 and A2), chaperon proteins (cyclophilin A, Hsc71, and Hsp90), cytoskeleton proteins (actin, tubulin, and myosin), chromosome architecture proteins (histone and HMG1), translation components (eIF4A-1, eIF1α-1-1, and 60S ribosomal protein), protein transport/vesicular-trafficking proteins (ADP ribosylation factors1/3 and 4, Rab7, Rab10, and ubiquitin), and proteins involved in redox regulation (thioredoxin and peroxiredoxin 1). Of these host proteins, cyclophilin A, β-actin, and ubiquitin have been previously reported as components of vaccinia virus IMV particles (35, 86, 176), and another 13 have been reported in other viruses (Table 5) (22, 87, 92, 172, 191). Thus, seven host proteins (60S acidic ribosomal proteins, 66-kDa protein, ADP ribosylation factor 4, HMG1, peroxiredoxin 1, Rab10, and thioredoxin) were found to be associated with vaccinia virus IMV and have not yet been detected in other viruses.
TABLE 5.
Host proteins identified in vaccinia virus IMVa
| Accession no. | Mass (Da) | Description | CNBr-trypsin digestion
|
CNBr-LysC digestion
|
Association with known virion particlesb | Reference(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of unipeptides | Sequence coverage (%) | emPAI | Protein content (mol%) | No. of unipeptides | Sequence coverage (%) | emPAI | Protein content (mol%) | |||||
| IPI00008530 | 34,252 | 60S acidic ribosomal protein P0 | 2 | 6 | 0.52 | 0.13 | ||||||
| IPI00333428 | 66,265 | 66-kDa protein | 1 | 1 | 0.06 | 0.02 | 1 | 1 | 0.06 | 0.07 | ||
| IPI00215914 | 20,684 | ADP ribosylation factor 1 and/or 3 | 1 | 5 | 0.29 | 0.07 | 1 | 9 | 0.58 | 0.68 | HCMV | 172 |
| IPI00215918 | 20,367 | ADP ribosylation factor 4 | 2 | 9 | 0.67 | 0.16 | ||||||
| IPI00218918 | 38,559 | Annexin A1 | 2 | 8 | 0.33 | 0.08 | HCMV, MCMV | 92, 172 | ||||
| IPI00455315 | 38,449 | Annexin A2 | 7 | 26 | 0.51 | 0.13 | HCMV, KSHV | 172, 185, 191 | ||||
| IPI00021439 | 41,710 | Actin, cytoplasmic 1 | 8 | 30 | 0.57 | 0.14 | 2 | 5 | 0.12 | 0.14 | ASFV, HCMV, HIV, KSHV, MCMV, VV | 55, 86, 92, 119, 172, 191 |
| IPI00639885 | 50,109 | Elongation factor 1-alpha 1 | 9 | 21 | 0.63 | 0.16 | 2 | 5 | 0.12 | 0.14 | HCMV, HIV, MCMV | 40, 92, 172 |
| IPI00025491 | 46,125 | Eukaryotic initiation factor 4A-I | 2 | 4 | 0.23 | 0.06 | 3 | 8 | 0.27 | 0.31 | HCMV | 172 |
| IPI00003865 | 70,854 | Heat shock cognate 71-kDa protein | 16 | 28 | 0.90 | 0.22 | 5 | 11 | 0.45 | 0.52 | HCMV, HIV | 71, 172 |
| IPI00382470 | 98,052 | Heat shock protein HSP 90-alpha 2 | 3 | 10 | 0.22 | 0.05 | 2 | 9 | 0.25 | 0.30 | DHBV, HCMV, KSHV, | 81, 172, 191 |
| IPI00334775 | 84,790 | Heat shock protein HSP 90-beta | 11 | 16 | 0.86 | 0.21 | 6 | 9 | 0.32 | 0.37 | DHBV, HCMV, KSHV, | 81, 172, 191 |
| IPI00419258 | 24,747 | High-mobility group protein 1 | 3 | 20 | 1.51 | 0.37 | ||||||
| IPI00003935 | 13,781 | Histone H2B.q | 1 | 7 | 0.47 | 0.12 | 1 | 6 | 0.58 | 0.68 | MCMV, SV40 | 36, 92 |
| IPI00019502 | 226,392 | Myosin-9 (myosin heavy chain, nonmuscle IIa) | 7 | 5 | 0.22 | 0.05 | 1 | 1 | 0.02 | 0.03 | HCMV, KSHV | 172, 191 |
| IPI00419585 | 17,870 | Peptidyl-prolyl cis-trans isomerase A (cyclophilin A) | 3 | 21 | 0.78 | 0.19 | 2 | 13 | 0.67 | 0.78 | HCMV, HIV, KSHV, SIV, VSV, VV | 23, 26, 35, 60, 163, 172, 191 |
| IPI0000874 | 22,096 | Peroxiredoxin 1 | 5 | 23 | 0.76 | 0.19 | ||||||
| IPI00016513 | 22,527 | Ras-related protein Rab-10 | 2 | 11 | 0.52 | 0.13 | 1 | 6 | 0.23 | 0.27 | ||
| IPI00016342 | 23,475 | Ras-related protein Rab-7 | 3 | 16 | 0.70 | 0.17 | 1 | 8 | 0.33 | 0.39 | KSHV | 191 |
| IPI00216298 | 11,599 | Thioredoxin | 1 | 7 | 0.47 | 0.12 | 1 | 7 | 0.47 | 0.55 | ||
| IPI00387144 | 50,120 | Tubulin alpha-ubiquitous chain | 9 | 26 | 0.63 | 0.16 | 5 | 13 | 0.36 | 0.42 | ASFV, HCMV | 55, 172 |
| IPI00011654 | 49,727 | Tubulin-beta-2 chain | 7 | 19 | 0.48 | 0.12 | 5 | 13 | 0.61 | 0.71 | ASFV | 55 |
| P62988 (Swiss-Prot ID) | 8,565 | Ubiquitin | 5 | 30 | 4.44 | 1.09 | 3 | 29 | 2.70 | 3.15 | ASFV, baculovirus, HIV, HSV, MuLV, SIV, VV | 55, 70, 86, 117, 118, 176 |
Twenty-three host proteins are compiled from both trypsin-digested and Lys-C-digested virion preparations. The data reproducibility among different virus preparations reached 67% for trypsin digestions and 87% for Lys-C digestions.
ASFV, African swine fever virus; DHBV, duck hepatitis virus; HCMV, human cytomegalovirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; KSHV, Kaposi's sarcoma-associated herpesvirus; MCMV, murine cytomegalovirus; MuLV, murine leukemia virus; SIV, simian immunodeficiency virus; SV40, simian virus 40; VSV, vesicular stomatitis virus; VV, vaccinia virus.
DISCUSSION
Proteomic techniques are widely used to identify protein components of large complexes that participate in different biological processes. MS remains the most suitable tool, as it is fast, sensitive, and widely applicable. Current high-sensitivity methods using MS for protein identification have greatly lowered detection limits, making it easy to analyze proteins with low cell copy numbers. In addition, the quality of bioinformatics continues to improve, making the prediction of gene products deduced from genome information increasingly accurate when MS data are matched to known protein sequences. The use of nano-LC for the high-resolution separation of digested peptides and nano-electrospray ionization/MS analysis allows protein detection in the low femtomole range in routine analysis. This approach can also be used to complement traditional biochemical methods of elucidating viral structures and functions and determining the number and nature of proteins within a virus. In this study, we identified 75 viral and 23 host proteins associated with purified vaccinia virus IMV particles, a significant increase from previous studies (86, 160). Ten novel viral proteins were identified in IMV (Table 1). EEV-specific viral proteins, such as F13L, A33R, A34R, A36R, and B5R, and viral nonstructural proteins, such as A11R, were not detected in our analyses, supporting the idea that the 10 novel viral proteins are not just copurified lysate contaminants (129, 137). If we include the additional six virion proteins cited from the literature (Table 3), there are a total of 81 viral proteins in IMV.
Furthermore, the relative abundance of each viral and host protein in IMV was determined by calculating the emPAI value, providing important quantification information for proteomic experiments. The most abundant viral proteins were in general agreement with previous reports (86), validating our quantification methods. In addition, our analysis also revealed that a significant proportion of virion proteins were present at low abundance. It is worth noting that the quantification method based on the emPAI value is fairly new, and the relative abundance would be better utilized to compare proteins of different abundance groups (Fig. 2) rather than to compare proteins within the same low-abundance group.
In addition to the viral proteins, 23 IMV-associated host proteins were identified. Compared with the data reproducibility of 75 viral proteins obtained from different IMV preparations, the host proteins indeed revealed somewhat more variation from one preparation to another. Several, such as ARF1/3, ARF 4, Rab-7, and Rab-10, are involved in transport and vesicle trafficking, and their association with IMV particles may be due to the intracellular route taken by the IMV during virion egress. Some host proteins, such as tubulin, actin, annexin A2, cyclophilin A, Hsc71, and Hsp90, have been identified in other virions, such as human cytomegalovirus, murine cytomegalovirus, and adenovirus (Table 3). It is worth noting that our previous study (82) showed a transient association of Hsp90 with viral factories in cells, while no Hsp90 was detected by immunoblot analysis in purified IMV. Since MS/MS is much more sensitive than immunoblot detection, this discrepancy could be due to the low abundance of Hsp90 associated with viral particles. Although we cannot rule out the possibility that some of the cytoskeleton and chaperon proteins could be fortuitously associated with viral particles during IMV preparation, as previously shown by Franke and Hruby (58), other abundant host proteins, such as myosin and vimentin, were not detected in purified IMV. Also, we did not detect host proteins that are known to be on vaccinia virus EEV, i.e., CD46, CD59, CD29, CD71, CD81, and MHC-1 (170). We also did not detect CD55, moesin, and cofilin, which were frequently identified in other viruses, such as human T-cell leukemia/lymphoma virus type 1, human immunodeficiency virus, and human cytomegalovirus, implying that host protein association with vaccinia virus IMV has some selectivity (92, 119, 136, 154, 172). Interestingly, six host proteins were uniquely identified in vaccinia virus, and their roles in vaccinia virus biology need to be studied in the future.
Acknowledgments
This work was supported by grants from the Academia Sinica and the National Science Council (NSC94-2627-M-001-005 and NSC91-3112-P-001-057-Y), Taiwan, Republic of China.
REFERENCES
- 1.Ahn, B. Y., P. D. Gershon, E. V. Jones, and B. Moss. 1990. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol. Cell. Biol. 10:5433-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, B. Y., P. D. Gershon, and B. Moss. 1994. RNA polymerase-associated protein Rap94 confers promoter specificity for initiating transcription of vaccinia virus early stage genes. J. Biol. Chem. 269:7552-7557. [PubMed] [Google Scholar]
- 3.Ahn, B. Y., E. V. Jones, and B. Moss. 1990. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5′ poly(A) leader on its early transcript. J. Virol. 64:3019-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, B. Y., and B. Moss. 1992. Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascorbate reductase activities. Proc. Natl. Acad. Sci. USA 89:7060-7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn, B. Y., and B. Moss. 1992. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc. Natl. Acad. Sci. USA 89:3536-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn, B. Y., J. Rosel, N. B. Cole, and B. Moss. 1992. Identification and expression of rpo19, a vaccinia virus gene encoding a 19-kilodalton DNA-dependent RNA polymerase subunit. J. Virol. 66:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almazan, F., D. C. Tscharke, and G. L. Smith. 2001. The vaccinia virus superoxide dismutase-like protein (A45R) is a virion component that is nonessential for virus replication. J. Virol. 75:7018-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amegadzie, B. Y., B. Y. Ahn, and B. Moss. 1992. Characterization of a 7-kilodalton subunit of vaccinia virus DNA-dependent RNA polymerase with structural similarities to the smallest subunit of eukaryotic RNA polymerase II. J. Virol. 66:3003-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amegadzie, B. Y., M. H. Holmes, N. B. Cole, E. V. Jones, P. L. Earl, and B. Moss. 1991. Identification, sequence, and expression of the gene encoding the second-largest subunit of the vaccinia virus DNA-dependent RNA polymerase. Virology 180:88-98. [DOI] [PubMed] [Google Scholar]
- 10.Ansarah-Sobrinho, C., and B. Moss. 2004. Role of the I7 protein in proteolytic processing of vaccinia virus membrane and core components. J. Virol. 78:6335-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldick, C. J., Jr., and B. Moss. 1987. Resistance of vaccinia virus to rifampicin conferred by a single nucleotide substitution near the predicted NH2 terminus of a gene encoding an Mr 62,000 polypeptide. Virology 156:138-145. [DOI] [PubMed] [Google Scholar]
- 12.Barr, J. R., V. L. Maggio, D. G. Patterson, Jr., G. R. Cooper, L. O. Henderson, W. E. Turner, S. J. Smith, W. H. Hannon, L. L. Needham, and E. J. Sampson. 1996. Isotope dilution—mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin. Chem. 42:1676-1682. [PubMed] [Google Scholar]
- 13.Bayliss, C. D., and G. L. Smith. 1996. Vaccinia virion protein I8R has both DNA and RNA helicase activities: implications for vaccinia virus transcription. J. Virol. 70:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaud, G., and R. Beaud. 1997. Preferential virosomal location of underphosphorylated H5R protein synthesized in vaccinia virus-infected cells. J. Gen. Virol. 78:3297-3302. [DOI] [PubMed] [Google Scholar]
- 15.Beaud, G., and R. Beaud. 2000. Temperature-dependent phosphorylation state of the H5R protein synthesised at the early stage of infection in cells infected with vaccinia virus ts mutants of the B1R and F10L protein kinases. Intervirology 43:67-70. [DOI] [PubMed] [Google Scholar]
- 16.Beaud, G., R. Beaud, and D. P. Leader. 1995. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase. J. Virol. 69:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besemer, J., A. Lomsadze, and M. Borodovsky. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L kinase. J. Virol. 73:3534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betakova, T., E. J. Wolffe, and B. Moss. 2000. The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses. J. Virol. 74:4085-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasco, R., N. B. Cole, and B. Moss. 1991. Sequence analysis, expression, and deletion of a vaccinia virus gene encoding a homolog of profilin, a eukaryotic actin-binding protein. J. Virol. 65:4598-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose, S., M. Mathur, P. Bates, N. Joshi, and A. K. Banerjee. 2003. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol. 84:1687-1699. [DOI] [PubMed] [Google Scholar]
- 24.Boursnell, M. E., I. J. Foulds, J. I. Campbell, and M. M. Binns. 1988. Non-essential genes in the vaccinia virus HindIII K fragment: a gene related to serine protease inhibitors and a gene related to the 37K vaccinia virus major envelope antigen. J. Gen. Virol. 69:2995-3003. [DOI] [PubMed] [Google Scholar]
- 25.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV(CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 70:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown, N. G., D. Nick Morrice, G. Beaud, G. Hardie, and D. P. Leader. 2000. Identification of sites phosphorylated by the vaccinia virus B1R kinase in viral protein H5R. BMC Biochem. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broyles, S. S., and B. S. Fesler. 1990. Vaccinia virus gene encoding a component of the viral early transcription factor. J. Virol. 64:1523-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broyles, S. S., and B. Moss. 1986. Homology between RNA polymerases of poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and transcriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc. Natl. Acad. Sci. USA 83:3141-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broyles, S. S., and B. Moss. 1987. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. J. Virol. 61:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broyles, S. S., and M. J. Pennington. 1990. Vaccinia virus gene encoding a 30-kilodalton subunit of the viral DNA-dependent RNA polymerase. J. Virol. 64:5376-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrd, C. M., T. C. Bolken, and D. E. Hruby. 2002. The vaccinia virus I7L gene product is the core protein proteinase. J. Virol. 76:8973-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd, C. M., and D. E. Hruby. 2005. A conditional-lethal vaccinia virus mutant demonstrates that the I7L gene product is required for virion morphogenesis. Virol. J. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassetti, M. C., M. Merchlinsky, E. J. Wolffe, A. S. Weisberg, and B. Moss. 1998. DNA packaging mutant: repression of the vaccinia virus A32 gene results in noninfectious, DNA-deficient, spherical, enveloped particles. J. Virol. 72:5769-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro, A. P., T. M. Carvalho, N. Moussatche, and C. R. Damaso. 2003. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J. Virol. 77:9052-9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, Y. H., J. P. MacGregor, D. A. Goldstein, and M. R. Hall. 1979. Histone modifications in simian virus 40 and in nucleoprotein complexes containing supercoiled viral DNA. J. Virol. 30:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu, W. L., and W. Chang. 2002. Vaccinia virus J1R protein: a viral membrane protein that is essential for virion morphogenesis. J. Virol. 76:9575-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu, W. L., P. Szajner, B. Moss, and W. Chang. 2005. Effects of a temperature sensitivity mutation in the J1R protein component of a complex required for vaccinia virus assembly. J. Virol. 79:8046-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung, C. S., J. C. Hsiao, Y. S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cimarelli, A., and J. Luban. 1999. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 73:5388-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cudmore, S., P. Cossart, G. Griffiths, and M. Way. 1995. Actin-based motility of vaccinia virus. Nature 378:636-638. [DOI] [PubMed] [Google Scholar]
- 42.Cudmore, S., I. Reckmann, G. Griffiths, and M. Way. 1996. Vaccinia virus: a model sustem for actin-membrane interactions. J. Cell Sci. 109:1739-1747. [DOI] [PubMed] [Google Scholar]
- 43.da Fonseca, F. G., A. S. Weisberg, M. F. Caeiro, and B. Moss. 2004. Vaccinia virus mutants with alanine substitutions in the conserved G5R gene fail to initiate morphogenesis at the nonpermissive temperature. J. Virol. 78:10238-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 74:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J. Virol. 74:7518-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis, R. E., and C. K. Mathews. 1993. Acidic C terminus of vaccinia virus DNA-binding protein interacts with ribonucleotide reductase. Proc. Natl. Acad. Sci. USA 90:745-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeMasi, J., S. Du, D. Lennon, and P. Traktman. 2001. Vaccinia virus telomeres: interaction with the viral I1, I6, and K4 proteins. J. Virol. 75:10090-10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demkowicz, W. E., J. S. Maa, and M. Esteban. 1992. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J. Virol. 66:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derrien, M., A. Punjabi, M. Khanna, O. Grubisha, and P. Traktman. 1999. Tyrosine phosphorylation of A17 during vaccinia virus infection: involvement of the H1 phosphatase and the F10 kinase. J. Virol. 73:7287-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doglio, L., A. De Marco, S. Schleich, N. Roos, and J. Krijnse Locker. 2002. The vaccinia virus E8R gene product: a viral membrane protein that is made early in infection and packaged into the virions' core. J. Virol. 76:9773-9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dyster, L. M., and E. G. Niles. 1991. Genetic and biochemical characterization of vaccinia virus genes D2L and D3R which encode virion structural proteins. Virology 182:455-467. [DOI] [PubMed] [Google Scholar]
- 53.Ensinger, M. J. 1987. Phenotypic characterization of temperature-sensitive mutants of vaccinia virus with mutations in a 135,000-Mr subunit of the virion-associated DNA-dependent RNA polymerase. J. Virol. 61:1842-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Essani, K., and S. Dales. 1979. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology 95:385-394. [DOI] [PubMed] [Google Scholar]
- 55.Esteves, A., M. I. Marques, and J. V. Costa. 1986. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology 152:192-206. [DOI] [PubMed] [Google Scholar]
- 56.Fathi, Z., and R. C. Condit. 1991. Genetic and molecular biological characterization of a vaccinia virus temperature-sensitive complementation group affecting a virion component. Virology 181:258-272. [DOI] [PubMed] [Google Scholar]
- 57.Fenner, F. 1990. Poxviruses, p. 2113-2133. In B. Fields and D. M. Knipe (ed.), Virology. Raven Press, New York, N.Y.
- 58.Franke, C. A., and D. E. Hruby. 1987. Association of non-viral proteins with recombinant vaccinia virus virions. Arch. Virol. 94:347-351. [DOI] [PubMed] [Google Scholar]
- 59.Franke, C. A., E. M. Wilson, and D. E. Hruby. 1990. Use of a cell-free system to identify the vaccinia virus L1R gene product as the major late myristylated virion protein M25. J. Virol. 64:5988-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 61.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 62.Funahashi, S., T. Sato, and H. Shida. 1988. Cloning and characterization of the gene encoding the major protein of the A-type inclusion body of cowpox virus. J. Gen. Virol. 69:35-47. [DOI] [PubMed] [Google Scholar]
- 63.Garcia, A. D., and B. Moss. 2001. Repression of vaccinia virus Holliday junction resolvase inhibits processing of viral DNA into unit-length genomes. J. Virol. 75:6460-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerber, S. A., J. Rush, O. Stemman, M. W. Kirschner, and S. P. Gygi. 2003. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. USA 100:6940-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gershon, P. D., B. Y. Ahn, M. Garfield, and B. Moss. 1991. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell 66:1269-1278. [DOI] [PubMed] [Google Scholar]
- 66.Gershon, P. D., and B. Moss. 1990. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc. Natl. Acad. Sci. USA 87:4401-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grosenbach, D. W., S. G. Hansen, and D. E. Hruby. 2000. Identification and analysis of vaccinia virus palmitylproteins. Virology 275:193-206. [DOI] [PubMed] [Google Scholar]
- 68.Grubisha, O., and P. Traktman. 2003. Genetic analysis of the vaccinia virus I6 telomere-binding protein uncovers a key role in genome encapsidation. J. Virol. 77:10929-10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan, K. L., S. S. Broyles, and J. E. Dixon. 1991. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature 350:359-362. [DOI] [PubMed] [Google Scholar]
- 70.Guarino, L. A., G. Smith, and W. Dong. 1995. Ubiquitin is attached to membranes of baculovirus particles by a novel type of phospholipid anchor. Cell 80:301-309. [DOI] [PubMed] [Google Scholar]
- 71.Gurer, C., A. Cimarelli, and J. Luban. 2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol. 76:4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gvakharia, B. O., E. K. Koonin, and C. K. Mathews. 1996. Vaccinia virus G4L gene encodes a second glutaredoxin. Virology 226:408-411. [DOI] [PubMed] [Google Scholar]
- 73.Havlis, J., and A. Shevchenko. 2004. Absolute quantification of proteins in solutions and in polyacrylamide gels by mass spectrometry. Anal. Chem. 76:3029-3036. [DOI] [PubMed] [Google Scholar]
- 74.Hedengren-Olcott, M., C. M. Byrd, J. Watson, and D. E. Hruby. 2004. The vaccinia virus G1L putative metalloproteinase is essential for viral replication in vivo. J. Virol. 78:9947-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heuser, J. 2005. Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic “honeycomb” surface coat. J. Cell Biol. 169:269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higley, S., and M. Way. 1997. Characterization of the vaccinia virus F8L protein. J. Gen. Virol. 78:2633-2637. [DOI] [PubMed] [Google Scholar]
- 77.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux. 1999. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 73:1503-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hooda-Dhingra, U., D. D. Patel, D. J. Pickup, and R. C. Condit. 1990. Fine structure mapping and phenotypic analysis of five temperature-sensitive mutations in the second largest subunit of vaccinia virus DNA-dependent RNA polymerase. Virology 174:60-69. [DOI] [PubMed] [Google Scholar]
- 79.Hooda-Dhingra, U., C. L. Thompson, and R. C. Condit. 1989. Detailed phenotypic characterization of five temperature-sensitive mutants in the 22- and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA polymerase. J. Virol. 63:714-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsiao, J. C., C. S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu, J., D. O. Toft, and C. Seeger. 1997. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 16:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung, J. J., C. S. Chung, and W. Chang. 2002. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J. Virol. 76:1379-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichihashi, Y., and M. Oie. 1996. Neutralizing epitope on penetration protein of vaccinia virus. Virology 220:491-494. [DOI] [PubMed] [Google Scholar]
- 84.Ichihashi, Y., M. Oie, and T. Tsuruhara. 1984. Location of DNA-binding proteins and disulfide-linked proteins in vaccinia virus structural elements. J. Virol. 50:929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishihama, Y., Y. Oda, T. Tabata, T. Sato, T. Nagasu, J. Rappsilber, and M. Mann. 2005. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteomics. 4:1265-1272. [DOI] [PubMed] [Google Scholar]
- 86.Jensen, O. N., T. Houthaeve, A. Shevchenko, S. Cudmore, T. Ashford, M. Mann, G. Griffiths, and J. Krijnse Locker. 1996. Identification of the major membrane and core proteins of vaccinia virus by two-dimensional electrophoresis. J. Virol. 70:7485-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson, G. P., S. J. Goebel, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1991. Vaccinia virus encodes a protein with similarity to glutaredoxins. Virology 181:378-381. [DOI] [PubMed] [Google Scholar]
- 89.Kane, E. M., and S. Shuman. 1993. Vaccinia virus morphogenesis is blocked by a temperature-sensitive mutation in the I7 gene that encodes a virion component. J. Virol. 67:2689-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kao, S. Y., and W. R. Bauer. 1987. Biosynthesis and phosphorylation of vaccinia virus structural protein VP11. Virology 159:399-407. [DOI] [PubMed] [Google Scholar]
- 91.Kao, S. Y., E. Ressner, J. Kates, and W. R. Bauer. 1981. Purification and characterization of a superhelix binding protein from vaccinia virus. Virology 111:500-508. [DOI] [PubMed] [Google Scholar]
- 92.Kattenhorn, L. M., R. Mills, M. Wagner, A. Lomsadze, V. Makeev, M. Borodovsky, H. L. Ploegh, and B. M. Kessler. 2004. Identification of proteins associated with murine cytomegalovirus virions. J. Virol. 78:11187-11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koonin, E. V., and T. G. Senkevich. 1992. Vaccinia virus encodes four putative DNA and/or RNA helicases distantly related to each other. J. Gen. Virol. 73:989-993. [DOI] [PubMed] [Google Scholar]
- 94.Kovacs, G. R., and B. Moss. 1996. The vaccinia virus H5R gene encodes late gene transcription factor 4: purification, cloning, and overexpression. J. Virol. 70:6796-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krijnse-Locker, J., S. Schleich, D. Rodriguez, B. Goud, E. J. Snijder, and G. Griffiths. 1996. The role of a 21-kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment. J. Biol. Chem. 271:14950-14958. [DOI] [PubMed] [Google Scholar]
- 96.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 97.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin, S., and S. S. Broyles. 1994. Vaccinia protein kinase 2: a second essential serine/threonine protein kinase encoded by vaccinia virus. Proc. Natl. Acad. Sci. USA 91:7653-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin, S., W. Chen, and S. S. Broyles. 1992. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J. Virol. 66:2717-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu, K., B. Lemon, and P. Traktman. 1995. The dual-specificity phosphatase encoded by vaccinia virus, VH1, is essential for viral transcription in vivo and in vitro. J. Virol. 69:7823-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maa, J. S., J. F. Rodriguez, and M. Esteban. 1990. Structural and functional characterization of a cell surface binding protein of vaccinia virus. J. Biol. Chem. 265:1569-1577. [PubMed] [Google Scholar]
- 102.Martin, K. H., D. W. Grosenbach, C. A. Franke, and D. E. Hruby. 1997. Identification and analysis of three myristylated vaccinia virus late proteins. J. Virol. 71:5218-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCrae, M. A., and J. F. Szilagyi. 1975. Preparation and characterisation of a subviral particle of vaccinia virus containing the DNA-dependent RNA polymerase activity. Virology 68:234-244. [DOI] [PubMed] [Google Scholar]
- 104.McFadden, G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKelvey, T. A., S. C. Andrews, S. E. Miller, C. A. Ray, and D. J. Pickup. 2002. Identification of the orthopoxvirus p4c gene, which encodes a structural protein that directs intracellular mature virus particles into A-type inclusions. J. Virol. 76:11216-11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meis, R. J., and R. C. Condit. 1991. Genetic and molecular biological characterization of a vaccinia virus gene which renders the virus dependent on isatin-beta-thiosemicarbazone (IBT). Virology 182:442-454. [DOI] [PubMed] [Google Scholar]
- 107.Mercer, J., and P. Traktman. 2005. Genetic and cell biological characterization of the vaccinia virus A30 and G7 phosphoproteins. J. Virol. 79:7146-7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohandas, A. R., and S. Dales. 1995. Involvement of spicules in the formation of vaccinia virus envelopes elucidated by a conditional lethal mutant. Virology 214:494-502. [DOI] [PubMed] [Google Scholar]
- 109.Morgan, J. R., L. K. Cohen, and B. E. Roberts. 1984. Identification of the DNA sequences encoding the large subunit of the mRNA-capping enzyme of vaccinia virus. J. Virol. 52:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Najarro, P., P. Traktman, and J. A. Lewis. 2001. Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J. Virol. 75:3185-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Newsome, T. P., N. Scaplehorn, and M. Way. 2004. SRC mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science 306:124-129. [DOI] [PubMed] [Google Scholar]
- 112.Nichols, R. J., E. Stanista, and P. Traktman. 2005. Presented at the joint meeting of the three divisions of the International Union of Microbiological Societies, 2005: XIII International Congress of Virology, San Francisco, California, 23 to 28 July 2005.
- 113.Niles, E. G., G. J. Lee-Chen, S. Shuman, B. Moss, and S. S. Broyles. 1989. Vaccinia virus gene D12L encodes the small subunit of the viral mRNA capping enzyme. Virology 172:513-522. [DOI] [PubMed] [Google Scholar]
- 114.Niles, E. G., and J. Seto. 1988. Vaccinia virus gene D8 encodes a virion transmembrane protein. J. Virol. 62:3772-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Obijeski, J. F., E. L. Palmer, L. G. Gafford, and C. C. Randall. 1973. Polyacrylamide gel electrophoresis of fowlpox and vaccinia virus proteins. Virology 51:512-516. [DOI] [PubMed] [Google Scholar]
- 116.Oie, M., and Y. Ichihashi. 1981. Characterization of vaccinia polypeptides. Virology 113:263-276. [DOI] [PubMed] [Google Scholar]
- 117.Ott, D. E., L. V. Coren, E. N. Chertova, T. D. Gagliardi, and U. Schubert. 2000. Ubiquitination of HIV-1 and MuLV Gag. Virology 278:111-121. [DOI] [PubMed] [Google Scholar]
- 118.Ott, D. E., L. V. Coren, T. D. Copeland, B. P. Kane, D. G. Johnson, R. C. Sowder II, Y. Yoshinaka, S. Oroszlan, L. O. Arthur, and L. E. Henderson. 1998. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J. Virol. 72:2962-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ott, D. E., L. V. Coren, B. P. Kane, L. K. Busch, D. G. Johnson, R. C. Sowder II, E. N. Chertova, L. O. Arthur, and L. E. Henderson. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pandey, A., and M. Mann. 2000. Proteomics to study genes and genomes. Nature 405:837-846. [DOI] [PubMed] [Google Scholar]
- 121.Pedersen, K., E. J. Snijder, S. Schleich, N. Roos, G. Griffiths, and J. K. Locker. 2000. Characterization of vaccinia virus intracellular cores: implications for viral uncoating and core structure. J. Virol. 74:3525-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pedersen, K. M., B. Finsen, J. E. Celis, and N. A. Jensen. 1998. Expression of a novel murine phospholipase D homolog coincides with late neuronal development in the forebrain. J. Biol. Chem. 273:31494-31504. [DOI] [PubMed] [Google Scholar]
- 123.Perkins, D. N., D. J. Pappin, D. M. Creasy, and J. S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551-3567. [DOI] [PubMed] [Google Scholar]
- 124.Ploubidou, A., V. Moreau, K. Ashman, I. Reckmann, C. Gonzalez, and M. Way. 2000. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 19:3932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quick, S. D., and S. S. Broyles. 1990. Vaccinia virus gene D7R encodes a 20,000-dalton subunit of the viral DNA-dependent RNA polymerase. Virology 178:603-605. [DOI] [PubMed] [Google Scholar]
- 126.Ravanello, M. P., and D. E. Hruby. 1994. Characterization of the vaccinia virus L1R myristylprotein as a component of the intracellular virion envelope. J. Gen. Virol. 75:1479-1483. [DOI] [PubMed] [Google Scholar]
- 127.Ravanello, M. P., and D. E. Hruby. 1994. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J. Virol. 68:6401-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rempel, R. E., and P. Traktman. 1992. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J. Virol. 66:4413-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Resch, W., A. S. Weisberg, and B. Moss. 2005. Vaccinia virus nonstructural protein encoded by the A11R gene is required for formation of the virion membrane. J. Virol. 79:6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rietdorf, J., A. Ploubidou, I. Reckmann, A. Holmstrom, F. Frischknecht, M. Zettl, T. Zimmermann, and M. Way. 2001. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat. Cell Biol. 3:992-1000. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez, J. F., J. S. Kahn, and M. Esteban. 1986. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proc. Natl. Acad. Sci. USA 83:9566-9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodriguez, J. F., and G. L. Smith. 1990. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 18:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodriguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 1997. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J. Virol. 71:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodriguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 1998. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J. Virol. 72:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosel, J., and B. Moss. 1985. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J. Virol. 56:830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saifuddin, M., C. J. Parker, M. E. Peeples, M. K. Gorny, S. Zolla-Pazner, M. Ghassemi, I. A. Rooney, J. P. Atkinson, and G. T. Spear. 1995. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 182:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanderson, C. M., F. Frischknecht, M. Way, M. Hollinshead, and G. L. Smith. 1998. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J. Gen. Virol. 79:1415-1425. [DOI] [PubMed] [Google Scholar]
- 138.Sanderson, C. M., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J. Gen. Virol. 81:47-58. [DOI] [PubMed] [Google Scholar]
- 139.Sarov, I., and W. K. Joklik. 1972. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology 50:579-592. [DOI] [PubMed] [Google Scholar]
- 140.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schnierle, B. S., P. D. Gershon, and B. Moss. 1992. Cap-specific mRNA (nucleoside-O2′-)-methyltransferase and poly(A) polymerase stimulatory activities of vaccinia virus are mediated by a single protein. Proc. Natl. Acad. Sci. USA 89:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway. J. Virol. 78:2348-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Senkevich, T. G., A. S. Weisberg, and B. Moss. 2000. Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis. Virology 278:244-252. [DOI] [PubMed] [Google Scholar]
- 146.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA 97:12068-12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Senkevich, T. G., C. L. White, A. Weisberg, J. A. Granek, E. J. Wolffe, E. V. Koonin, and B. Moss. 2002. Expression of the vaccinia virus A2.5L redox protein is required for virion morphogenesis. Virology 300:296-303. [DOI] [PubMed] [Google Scholar]
- 148.Shchelkunov, S. N., O. I. Riazankina, P. B. Gashnikov, A. B. Totmenin, V. E. Chizhikov, and E. G. Malygin. 1991. Molecular biological study of the vaccinia virus genome. IV. The late nonstructural 36K protein of vaccinia virus is vitally important. Mol. Biol. 25:396-404. [PubMed] [Google Scholar]
- 149.Shuman, S. 1998. Vaccinia virus DNA topoisomerase: a model eukaryotic type IB enzyme. Biochim. Biophys. Acta 1400:321-337. [DOI] [PubMed] [Google Scholar]
- 150.Shuman, S. 1992. Vaccinia virus RNA helicase: an essential enzyme related to the DE-H family of RNA-dependent NTPases. Proc. Natl. Acad. Sci. USA 89:10935-10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shuman, S., and B. Moss. 1987. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc. Natl. Acad. Sci. USA 84:7478-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith, G. L., and M. Law. 2004. The exit of vaccinia virus from infected cells. Virus Res. 106:189-197. [DOI] [PubMed] [Google Scholar]
- 153.Smith, G. L., B. J. Murphy, and M. Law. 2003. Vaccinia virus motility. Annu. Rev. Microbiol. 57:323-342. [DOI] [PubMed] [Google Scholar]
- 154.Spear, G. T., N. S. Lurain, C. J. Parker, M. Ghassemi, G. H. Payne, and M. Saifuddin. 1995. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses, Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV). J. Immunol. 155:4376-4381. [PubMed] [Google Scholar]
- 155.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stokes, G. V. 1976. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J. Virol. 18:636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szajner, P., H. Jaffe, A. S. Weisberg, and B. Moss. 2003. Vaccinia virus G7L protein interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions. J. Virol. 77:3418-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Szajner, P., A. S. Weisberg, and B. Moss. 2004. Physical and functional interactions between vaccinia virus F10 protein kinase and virion assembly proteins A30 and G7. J. Virol. 78:266-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Szajner, P., A. S. Weisberg, E. J. Wolffe, and B. Moss. 2001. Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions. J. Virol. 75:5752-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahashi, T., M. Oie, and Y. Ichihashi. 1994. N-terminal amino acid sequences of vaccinia virus structural proteins. Virology 202:844-852. [DOI] [PubMed] [Google Scholar]
- 161.Tartaglia, J., and E. Paoletti. 1985. Physical mapping and DNA sequence analysis of the rifampicin resistance locus in vaccinia virus. Virology 147:394-404. [DOI] [PubMed] [Google Scholar]
- 162.Tartaglia, J., A. Piccini, and E. Paoletti. 1986. Vaccinia virus rifampicin-resistance locus specifies a late 63,000 Da gene product. Virology 150:45-54. [DOI] [PubMed] [Google Scholar]
- 163.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 164.Thompson, C. L., U. Hooda-Dhingra, and R. C. Condit. 1989. Fine structure mapping of five temperature-sensitive mutants in the 22- and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA polymerase. J. Virol. 63:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Townsley, A. C., T. G. Senkevich, and B. Moss. 2005. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 79:10988-10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Townsley, A. C., T. G. Senkevich, and B. Moss. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79:9458-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Traktman, P., K. Liu, J. DeMasi, R. Rollins, S. Jesty, and B. Unger. 2000. Elucidating the essential role of the A14 phosphoprotein in vaccinia virus morphogenesis: construction and characterization of a tetracycline-inducible recombinant. J. Virol. 74:3682-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tseng, M., N. Palaniyar, W. Zhang, and D. H. Evans. 1999. DNA binding and aggregation properties of the vaccinia virus I3L gene product. J. Biol. Chem. 274:21637-21644. [DOI] [PubMed] [Google Scholar]
- 169.Unger, B., and P. Traktman. 2004. Vaccinia virus morphogenesis: a13 phosphoprotein is required for assembly of mature virions. J. Virol. 78:8885-8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vanderplasschen, A., E. Mathew, M. Hollinshead, R. B. Sim, and G. L. Smith. 1998. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc. Natl. Acad. Sci. USA 95:7544-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van Meir, E., and R. Wittek. 1988. Fine structure of the vaccinia virus gene encoding the precursor of the major core protein 4a. Arch. Virol. 102:19-27. [DOI] [PubMed] [Google Scholar]
- 172.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vos, J. C., M. Sasker, and H. G. Stunnenberg. 1991. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 10:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang, S. P., and S. Shuman. 1996. A temperature-sensitive mutation of the vaccinia virus E11 gene encoding a 15-kDa virion component. Virology 216:252-257. [DOI] [PubMed] [Google Scholar]
- 175.Washburn, M. P., D. Wolters, and J. R. Yates III. 2001. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19:242-247. [DOI] [PubMed] [Google Scholar]
- 176.Webb, J. H., R. J. Mayer, and L. K. Dixon. 1999. A lipid modified ubiquitin is packaged into particles of several enveloped viruses. FEBS Lett. 444:136-139. [DOI] [PubMed] [Google Scholar]
- 177.Welsch, S., L. Doglio, S. Schleich, and J. Krijnse Locker. 2003. The vaccinia virus I3L gene product is localized to a complex endoplasmic reticulum-associated structure that contains the viral parental DNA. J. Virol. 77:6014-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.White, C. L., T. G. Senkevich, and B. Moss. 2002. Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly. J. Virol. 76:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.White, C. L., A. S. Weisberg, and B. Moss. 2000. A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis. J. Virol. 74:9175-9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Whitehead, S. S., and D. E. Hruby. 1994. Differential utilization of a conserved motif for the proteolytic maturation of vaccinia virus proteins. Virology 200:154-161. [DOI] [PubMed] [Google Scholar]
- 181.Wilcock, D., and G. L. Smith. 1994. Vaccinia virus core protein VP8 is required for virus infectivity, but not for core protein processing or for INV and EEV formation. Virology 202:294-304. [DOI] [PubMed] [Google Scholar]
- 182.Wittek, R., M. Hanggi, and G. Hiller. 1984. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J. Virol. 49:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wolffe, E. J., D. M. Moore, P. J. Peters, and B. Moss. 1996. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J. Virol. 70:2797-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53-63. [DOI] [PubMed] [Google Scholar]
- 185.Wright, J. F., A. Kurosky, E. L. Pryzdial, and S. Wasi. 1995. Host cellular annexin II is associated with cytomegalovirus particles isolated from cultured human fibroblasts. J. Virol. 69:4784-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang, W. P., and W. R. Bauer. 1988. Purification and characterization of vaccinia virus structural protein VP8. Virology 167:578-584. [PubMed] [Google Scholar]
- 187.Yeh, W. W., B. Moss, and E. J. Wolffe. 2000. The vaccinia virus A9L gene encodes a membrane protein required for an early step in virion morphogenesis. J. Virol. 74:9701-9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang, W., and D. H. Evans. 1993. DNA strand exchange catalyzed by proteins from vaccinia virus-infected cells. J. Virol. 67:204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang, Y., B. Y. Ahn, and B. Moss. 1994. Targeting of a multicomponent transcription apparatus into assembling vaccinia virus particles requires RAP94, an RNA polymerase-associated protein. J. Virol. 68:1360-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang, Y. F., and B. Moss. 1991. Vaccinia virus morphogenesis is interrupted when expression of the gene encoding an 11-kilodalton phosphorylated protein is prevented by the Escherichia coli lac repressor. J. Virol. 65:6101-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]