Abstract
The Escherichia coli AlkB protein protects against the cytotoxicity of methylating agents by repair of the DNA lesions 1-methyladenine and 3-methylcytosine, which are generated in single-stranded stretches of DNA. AlkB is an α-ketoglutarate- and Fe(II)-dependent dioxygenase that oxidizes the relevant methyl groups and releases them as formaldehyde. Here, we identify two human AlkB homologs, ABH2 and ABH3, by sequence and fold similarity, functional assays, and complementation of the E. coli alkB mutant phenotype. The levels of their mRNAs do not appear to correlate with cell proliferation but tissue distributions are different. Both enzymes remove 1-methyladenine and 3-methylcytosine from methylated polynucleotides in an α-ketoglutarate-dependent reaction, and act by direct damage reversal with the regeneration of the unsubstituted bases. AlkB, ABH2, and ABH3 can also repair 1-ethyladenine residues in DNA with the release of acetaldehyde.
Although single-stranded regions of DNA occur in vivo within replication forks and transcription bubbles, the susceptibility of single-stranded DNA to alkylating agents has been little investigated. The major lesions generated in single-stranded DNA are 1-alkyladenine and 3-alkylcytosine; these modification sites are protected by the complementary strand in duplex DNA (1). The 3-methylcytosine (3-meC) lesions block replication and are potentially cytotoxic (2). The Escherichia coli AlkB function counteracts toxicity by alkylating agents and its expression is induced by exposure to such agents (3, 4). Expression of E. coli AlkB in mammalian cells also confers increased resistance to alkylating agents (5). We have shown that AlkB specifically repairs alkylation damage in single-stranded DNA in vivo, and binds preferentially to single-stranded DNA in vitro (6). These results indicated that AlkB repairs 1-methyladenine (1-meA) and/or 3-meC residues in DNA, but the reaction mechanism was unknown. In an important lead, protein fold analysis combined with weak sequence homology suggested that AlkB is a member of the family of α-ketoglutarate (αKG)- and Fe(II)-dependent dioxygenases (7). These enzymes are involved in a variety of metabolic reactions; however, a fungal member of the family can perform catabolic oxidative demethylation of the free base 1-methylthymine (8). Biochemical assays with purified AlkB protein recently demonstrated that AlkB is indeed an αKG- and Fe(II)-dependent dioxygenase that oxidatively demethylates 1-meA and 3-meC residues in single-stranded as well as double-stranded DNA. The methyl group is released from the lesion as free formaldehyde, with accompanying regeneration of the unsubstituted base residue in DNA (9, 10).
Because alkylating agents are environmental carcinogens, and also are used clinically as cytotoxic anticancer drugs, it was of interest to determine whether human cells have a counterpart to the E. coli AlkB protein. Here, we identify and characterize two human AlkB homologs encoded on different chromosomes.
Materials and Methods
Single-Stranded DNA Phage Reactivation.
M13 phage was used to examine reactivation of methyl methanesulfonate (MMS)-treated single-stranded DNA in transfected E. coli alkB mutant cells expressing cloned AlkB protein or putative human homologs. In some experiments, the single-stranded φK phage was used to avoid the M13 requirement for F′ strains (11). MMS treatment of the phage and estimation of reactivation were as described (6). Cells were grown in the presence of 100 μM isopropyl β-d-thiogalactopyranoside for 3 h at 25°C to induce expression of cloned genes before transfection.
Molecular Cloning of Human ABH1, ABH2, and ABH3.
ABH1, ABH2, and ABH3 cDNAs were obtained from the IMAGE consortium and amplified by PCR using Pfu Turbo polymerase. ABH1 was subcloned into the expression vector pET15b. ABH2 and ABH3 were subcloned by using Gateway technology (Invitrogen). In this case, the amplified ORFs flanked by attB1 and attB2 sites were cloned into the entry vector p221 by recombination, and subsequently shuttled into pDEST.GST or pDEST17 for expression of GST- or hexahistidine-tagged fusion proteins, respectively.
Purification of E. coli AlkB and Human ABH1, ABH2, and ABH3 Proteins.
His-tagged E. coli AlkB protein was overexpressed and purified as described (9). Expression plasmids encoding GST- or hexahistidine-tagged ABH1, ABH2, or ABH3 were transformed into E. coli Codon plus. To induce expression, 0.1 mM β-d-thiogalactopyranoside was added to cultures at A600 = 0.6, and incubation continued for 3.5 h at 25°C. Harvested cells expressing hexahistidine-tagged proteins were disrupted in 25 mM Tris-HCl, pH 8.0/300 mM NaCl/10 mM imidazole/1 mM EDTA/1 mM β-mercaptoethanol by using a French press or by treatment with lysozyme and sonication. Lysates were clarified by centrifugation at 17,000 × g for 30 min at 4°C and dialyzed against lysis buffer lacking EDTA. These lysates were loaded in batch onto Ni2+ nitrilotriacetic acid agarose resin (Qiagen, Valencia, CA). The resin was washed with lysis buffer containing 40 mM imidazole but no EDTA. Bound proteins were then eluted in 25 mM Tris⋅HCl, pH 8.0/150 mM NaCl/250 mM imidazole. When purifying GST-tagged proteins, the lysis buffer contained 50 mM Tris·HCl, pH 8.0/300 mM NaCl/1 mM EDTA/1 mM DTT. Clarified lysates were loaded batchwise onto glutathione-Sepharose resin (Amersham Pharmacia), and washed with lysis buffer lacking EDTA. Specifically bound proteins were eluted in 50 mM Tris⋅HCl, pH 8.5/150 mM NaCl/1 mM DTT/10 mM reduced glutathione. Cultures (2.4 liters) of overexpressing strains yielded 2.5 mg of GST.ABH2 or 12 mg of GST.ABH3 in soluble and 90–95% pure form. However, the enzyme activities were unstable during isolation and on storage, with ABH2 being more labile than ABH3.
Substrates and Assays of E. coli AlkB and Human ABH2 and ABH3.
Poly(dA) and poly(dC) (Amersham Pharmacia) were treated with [14C]MeI (58 mCi/mmol; 1 Ci = 37 GBq) (Amersham Pharmacia) as described (9). The specific activities of the methylated substrates were 1,860 and 700 cpm/μg, respectively. The same method was used to treat 0.6 mg poly(dA) with 250 μCi [14C] ethyl iodide (EtI; 31.5 mCi/mmol) (ICN) except that the treatment was at 37°C for 18 h. The specific activity of the ethylated poly(dA) was 120 cpm/μg.
A single stranded substrate containing [3H]cytosine residues interspersed between thymines was synthesized by PCR. The template, 5′-(A6G)9A6CAAGTCCCACGCTCACACACAATCC-3′, was replicated by using Pfu polymerase and a single primer, 5′-GGATTGTGTGTGAGCGTGGGACTTG-3′, in the presence of 150 μM dNTPs and 2.5 μM [3H]dCTP (16 Ci/mmol) (Amersham Pharmacia). To obtain a high level of methylation, the 3H-labeled PCR product was treated 8 times with 50 mM dimethyl sulfate (DMS) in 75 mM sodium cacodylate (pH 7.4) at 30°C for 2 h. Between each treatment the DMS was removed by centrifugation through a Sephadex G-25 minicolumn equilibrated in the same buffer. The level of methylation of [3H]cytosine residues was determined by formic acid hydrolysis and HPLC using a Whatman Partisil 10 cation exchange column run isocratically in 100 mM formate (pH 3.6), 2.5% methanol; 70% of the [3H]cytosines were methylated to 3-me[3H]C.
Purified AlkB protein and putative human homologs were assayed as described (9). Briefly, the enzymes were incubated with [14C]MeI- or [14C]EtI-treated substrates in 50 mM Hepes⋅KOH, pH 8/75 μM Fe(NH4)2(SO4)2/1 mM αKG/2 mM ascorbate/50 μg/ml BSA for 15 min at 37°C. The reaction was stopped by adding EDTA, and the polynucleotide substrate was ethanol precipitated. Ethanol-soluble radiolabeled material was monitored by scintillation counting. Methylated adenine and cytosine residues remaining in the 14C-methylated polynucleotides were released by acid hydrolysis and analyzed by HPLC and scintillation counting (9). 14C-ethylated adenines were analyzed by HPLC on a Phenomenex Luna 2 reverse phase column in 50 mM ammonium acetate, pH 6.5. A linear gradient of 1–46% methanol was applied to separate the ethylated adenine bases.
Detection of [14C]Aldehydes.
To determine whether AlkB released formaldehyde or acetaldehyde from 14C-ethylated poly(dA), the ethanol-soluble 14C-labeled material was derivatised with 2,4-dinitrophenylhydrazone (DNPH) (12). The DNPH derivatives were analyzed by reverse phase HPLC on a Phenomenex Hypersil column using a linear 50–90% methanol gradient in water and quantitated by scintillation counting. DNPH derivatised formaldehyde and acetaldehyde markers were monitored at A254.
Northern Hybridization.
Multiple tissue Northern blots (Ambion 3142/3143) were probed, washed, and stripped according to the manufacturer's instructions. Each blot contained 2 μg of poly(A)+ RNA from 10 different normal tissues or 10 different cell lines derived from various carcinomas. Briefly, blots were prehybridized in hybridization buffer (Ambion) for 30 min at 42°C. Full-length ORF probes were labeled with [32P]dCTP by PCR and purified through Sephadex G-50 spin columns. The probe was added directly to the hybridization buffer to a specific activity of 106 cpm/ml. Blots were hybridized for 16 h at 42°C before being washed twice with 0.3 M NaCl/0.03 M citrate/0.1% SDS and twice with 0.15 M NaCl/0.015 M citrate/0.1% SDS at 42°C. The bound probe was visualized by fluorography. A β-actin probe was used as a control to verify integrity of the samples on the blots.
Results
Identification of Human AlkB Homologs.
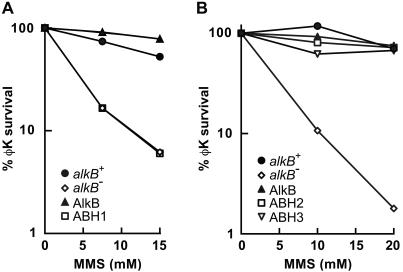
A putative human homolog, ABH, of the E. coli AlkB protein was previously reported to partially complement the MMS sensitivity of an E. coli alkB mutant (13). With the aim of further characterizing this potential homolog, we obtained two independent full-length cDNA clones of the ABH gene from the IMAGE consortium (IMAGE IDs 1337358 and 2284109). DNA sequencing showed that both cDNAs contained ORFs encoding identical proteins (data not shown). The N and C termini of these ORFs, however, differed from those reported for ABH by Wei et al. in 1996 (13), but agreed with the predicted conceptual splice of the genomic DNA sequence on chromosome 14q24 (GenBank accession no. AC008044) and also with a further cDNA (GenBank accession no. XM_007409). The ORF of the IMAGE 1337358 cDNA, which we refer to as ABH1, was subcloned into the pET15b vector and overexpressed in an alkB22 derivative of E. coli BL21.DE3. Induction by 50 μM β-d-thiogalactopyranoside at 37°C for 3 h resulted in 15% solubility of the overexpressed ABH1 protein (data not shown). E. coli alkB mutants are defective in reactivation of MMS treated single stranded DNA phage (6). Overexpression of E. coli AlkB protein complemented this phenotype, but this was not the case for human ABH1 (Fig. 1A). Moreover, the purified hexahistidine-tagged ABH1 protein did not release soluble radiolabeled material from a [14C]methyl iodide treated poly(dA) substrate in the optimised AlkB assay conditions (9) or after various modifications of this assay (data not shown). We were therefore unable to support the previous suggestion (13) that human ABH1 is a functional AlkB homolog.
Fig 1.
Complementation of E. coli alkB mutant phenotype by overexpression of human ABH2 or ABH3 proteins, but not ABH1. Reactivation of MMS-treated φK single-stranded DNA phage was monitored in various E. coli strains. (A) Host strains were alkB+ or alkB22 derivatives of E. coli BL21.DE3. Plasmid pBAR54 encodes E. coli AlkB protein and pBAR65 encodes human ABH1 (not tagged). •, alkB+/pET15b; ◊, alkB22/pET15b; ▴, alkB22/pBAR54; □, alkB22/pBAR65. (B) Host strains were alkB+ or alkB22 derivatives of E. coli AB1157 gyrA. Plasmid pBAR32 encodes E. coli AlkB protein, pGST.ABH2 encodes GST tagged ABH2 and pGST.ABH3 encodes GST-tagged ABH3. •, alkB+/pGEX6P-1; ◊, alkB22/pGEX6P-1; ▴, alkB22/pBAR32; □, alkB22/pGST.ABH2; ▿, alkB22/pGST.ABH3. The proteins overexpressed in the alkB mutant are indicated with symbols in the corner of the figures.
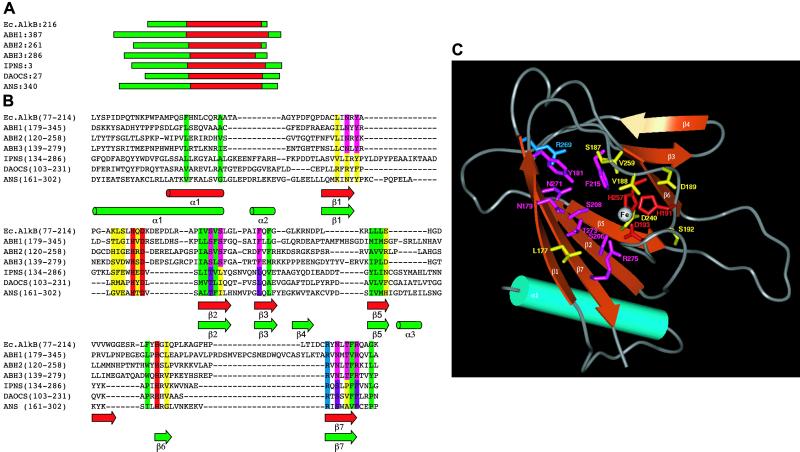
blast searches of the NCBI human DNA databases revealed a different potential AlkB homolog (Fig. 2). This ORF, described as “similar to prostate cancer antigen-1,” was predicted from the conceptual splice of DNA sequences on chromosome 12q23.3 (accession no. XP_058581). Searching the databases with XP_058581 revealed yet another possible AlkB homolog. This second protein known as prostate cancer antigen-1 is encoded by the DEPC-1 gene located on chromosome 11q11 (accession no. NP_631917). Both these proteins are of unknown function. We refer to the two proteins as ABH2 (XP_058581) and ABH3 (NP_631917), having 30.8 and 23.1% core region identity to E. coli AlkB, respectively (Fig. 2A). Analysis of ABH2 and ABH3 for known protein domains by using the program domainfishing (14) indicated that ABH2 and ABH3 were similar to αKG- and Fe(II)-dependent dioxygenases in their C-terminal regions. By using the known crystal structures of three αKG- and Fe(II)-dependent dioxygenases (15–17) and the predicted secondary structures of AlkB (18), ABH2, and ABH3, a well defined alignment within the core region of these enzymes was obtained (Fig. 2 A and B). Both ABH2 and ABH3 contain the Fe(II)-binding motif, HXDXNH, which is characteristic of αKG-dependent dioxygenases, and a conserved arginine that may be involved in αKG interactions (4). Considerable structural and sequence variation occurred outside the core regions. Based on the superimposed core regions of the three crystal structures, a 3D model was constructed for the core of ABH3 (19) (Fig. 2C), which exhibits a jellyroll topology containing one 3- and one 4- β-strand sheets.
Fig 2.
(A) Approximate location of the core of the αKG–Fe(II) dioxygenase fold within AlkB, its putative human homologs, and three crystal structures from the αKG–Fe(II) dioxygenase superfamily: IPNS (isopenicillin N synthase from Aspergillus nidulans) (15), DAOCS (deacetoxycephalosporin C synthase from Streptomyces clavuligerus) (16), and ANS (anthocyanidin synthase from Arabidopsis thaliana) (17). (B) Multiple alignment within the core region of the AlkB homologs and the three crystal structures described above. Predicted secondary structures of ABH2 and ABH3 were calculated with the program PSI-PRED (19). The location of average secondary structure elements are shown as cylinders for α-helices and solid arrows for β-strands. These are colored green for known secondary structure locations (crystal structures) and red for predicted secondary structure locations (AlkB homologs). Residue background colors indicate the following properties: green, conserved hydrophobics; red, coordinate the Fe(II) ion; blue, conserved within the substrate binding pocket for all sequences; violet, conserved within the substrate binding pocket of the crystal structures only; magenta, conserved within the binding pocket for all AlkB homologs only; yellow, within the substrate binding pocket for all sequences but not conserved. (C) Three-dimensional model for the core region of ABH3. By using the known crystal structures of the three αKG-dependent DNA dioxygenases as templates and the alignment (B), a three-dimensional model was constructed for the core of ABH3 by using the program 3D-JIGSAW (24). The overall quality of side-chain packing and stereochemistry of the final model were checked by using program QUANTA 2000 (Accelrys, Orsay, France). The conserved α-helix is colored cyan. The β-strands, forming the jellyroll structure, are colored orange. The approximate location of the Fe(II) ion is represented as a white sphere. Side chains are colored according to the scheme described in B.
Full length ABH2 and ABH3 cDNAs were obtained from the IMAGE consortium (IDs 5179189 and 5484474, respectively). The ORFs were subcloned to obtain plasmid constructs (pGST.ABH2 and pGST.ABH3) encoding GST-tagged fusion proteins. These plasmids were transformed into an E. coli alkB22 mutant, and their ability to complement the defective reactivation of a MMS treated single-stranded DNA phage was examined. At the MMS doses used, both plasmids fully complemented the alkB phenotype so that phage reactivation was similar to that in an alkB+ strain or in the alkB22 mutant overexpressing E. coli AlkB protein (Fig. 1B). This complementation provided evidence that both ABH2 and ABH3 are functional human homologs of the E. coli AlkB protein.
Enzymatic Activities of ABH2 and ABH3.
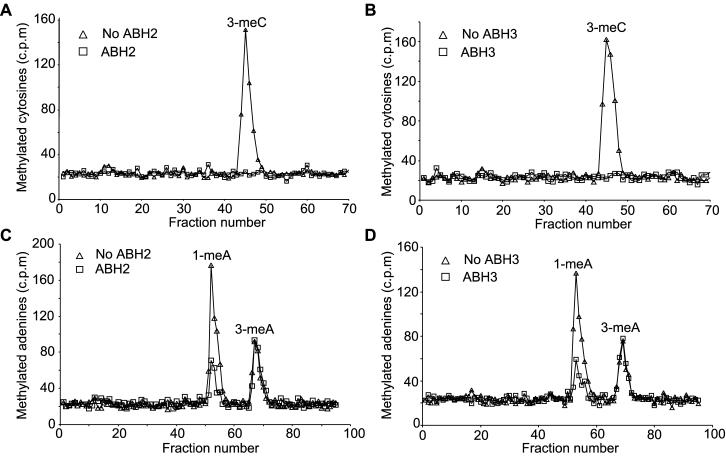
The GST- and hexahistidine- tagged ABH2 and ABH3 proteins were overexpressed in E. coli and purified by affinity chromatography. E. coli AlkB protein removes 1-meA and 3-meC from single stranded DNA (9). The ability of the two human proteins to repair these DNA modifications was therefore examined. The purified proteins were incubated with [14C]MeI-treated poly(dA) or poly(dC) substrates, and the removal of 1-meA and 3-meC monitored by acid hydrolysis of the substrate and HPLC chromatography. Initially, assay conditions of pH 8 and 75 μM Fe(II) that are optimal for AlkB were used, but these were subsequently modified in the case of ABH2, which had greater activity at pH 7.5 and 25 μM Fe(II). Both GST- and hexahistidine-tagged ABH2 and ABH3 removed both 1-meA and 3-meC from the methylated polynucleotides (Fig. 3). These activities were totally dependent on αKG, stimulated by ascorbate and fully inhibited by EDTA (data not shown). These properties are characteristic of αKG- and Fe(II)-dependent dioxygenases. ABH2 and ABH3, like AlkB, are therefore members of this family of enzymes. AlkB oxidizes the methyl groups of 1-meA and 3-meC in DNA to release formaldehyde (9, 10). By assaying the release of ethanol-soluble [14C]formaldehyde from 14C-methylated poly(dA) or poly(dC), ABH2 was ≈4-fold more active on 1-meA than on 3-meC, whereas ABH3 was slightly more active (≈2-fold) on 3-meC than 1-meA (data not shown). Both enzymes were also similarly active on a [14C]MeI-treated oligonucleotide (50% GC) annealed to a complementary strand, which was verified to be >90% double stranded by E. coli exonuclease III digestion (data not shown).
Fig 3.
Repair of 1-methyladenine and 3-meC residues by ABH2 and ABH3. ABH2 or ABH3 (1 nmol) were incubated with [14C]MeI-treated poly(dC) (A and B) or poly(dA) (C and D) for 30 min under standard assay conditions. The 14C-labeled methylated bases remaining in the substrates were analyzed by HPLC and scintillation counting. ▵, no enzyme; □, ABH2 or ABH3.
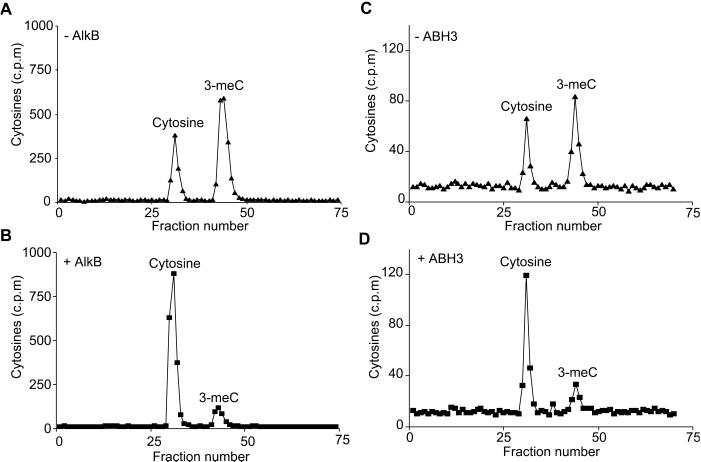
E. coli AlkB directly reverts 1-meA in DNA to adenine by oxidative demethylation (9, 10). Here, we demonstrate that AlkB also directly reverts 3-meC to cytosine, and show that ABH2 and ABH3 have the same activity. A single stranded oligonucleotide substrate containing [3H]cytosine was synthesized by PCR using a single primer and then repeatedly methylated with DMS until 70% of the cytosines were converted to 3-meC. AlkB, ABH2, or ABH3 were incubated with this substrate and reversion of 3-me[3H]C to [3H]cytosine examined by acid hydrolysis and HPLC chromatography. Both AlkB and ABH3 directly reverted 3-meC to cytosine (Fig. 4), confirming that ABH3 acts by the same mechanism as AlkB; only partial reversion was achieved with ABH2 (data not shown) because of its lower activity on 3-meC and the lability of this enzyme.
Fig 4.
Direct reversion of 3-meC to cytosine by E. coli AlkB and human ABH3. A thymine-rich oligodeoxynucleotide containing several [3H]cytosine residues was heavily methylated with DMS and then incubated with or without 0.23 nmol AlkB (A and B) or 0.84 nmol ABH3 (C and D) at 37°C for 30 min. Purified ABH3 was less active than AlkB; therefore, a reduced amount of substrate was incubated with ABH3 so that reversal of 3-meC to cytosine was readily seen. The [3H]cytosine residues remaining in the oligonucleotide were analyzed by HPLC and scintillation counting.
Repair of Ethylated DNA Bases.
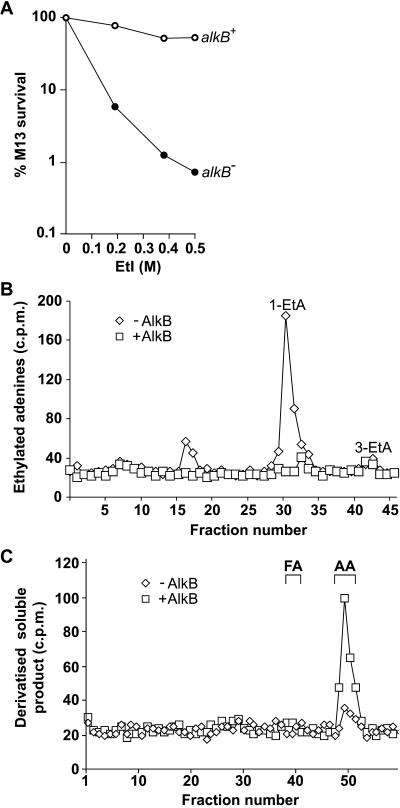
Bacterial and human 3-methyladenine-DNA glycosylases and O6-methylguanine-DNA methyltransferases also repair bulkier ethylated adducts, but usually with lesser efficiency (20). AlkB uses a completely different mechanism to these enzymes to remove aberrant methyl groups from DNA. It was therefore of interest to determine whether this activity can also repair ethyl adducts. To investigate whether E. coli AlkB can repair ethyl lesions generated in single stranded DNA, the ability of an alkB mutant to reactivate an EtI-treated single-stranded DNA phage was examined. Survival of the ethylated M13 phage was greatly decreased in the alkB mutant compared with the wild type, indicating that AlkB protein repairs DNA ethylation damage (Fig. 5A). A single-stranded substrate containing 1-ethyladenine (1-etA) was prepared by treating poly(dA) with [14C]EtI. In standard assay conditions, E. coli AlkB protein released [14C]ethanol-soluble material from this substrate. Analysis of the ethylated bases remaining in the polynucleotide showed that AlkB had completely removed the 1-etA lesions. A small amount of 3-etA was also present in the substrate, but was not removed by AlkB activity (Fig. 5B). AlkB therefore repaired 1-etA but not 3-etA, which parallels its previously observed activity on 1-meA but not 3-meA (9). By quantifying the release of [14C]ethanol soluble material, AlkB was observed to repair 1-etA with a similar but slightly lower efficiency than 1-meA (data not shown). ABH2 and ABH3 proteins were also shown to remove 1-etA from 14C-ethylated poly(dA). Both human proteins repaired 1-etA inefficiently, however, at ≈0.5% of the rate of 1-meA as followed by measuring the release of radioactive material from 14C-ethylated poly(dA) and 14C-methylated poly(dA) in parallel, and also by measuring the reduction of 1-etA remaining in the polynucleotide substrate (data not shown).
Fig 5.
Repair of 1-ethyladenine by E. coli AlkB. (A) Defective reactivation of EtI treated M13 single-stranded DNA phage in an E. coli alkB mutant. Host strains were alkB+ and alkB117:Tn3 derivatives of F′/ABII57. ○, alkB+; •, alkB117:Tn3. (B) Removal of 1-etA from [14C]EtI-treated poly(dA) by 10 pmols AlkB protein. The 14C-ethylated adenines remaining in the substrate were examined by HPLC. ◊, No AlkB; □, +AlkB. (C) AlkB oxidizes 1-etA to release acetaldehyde. AlkB (10 pmols) was incubated with [14C]EtI-treated poly(dA), and the [14C]ethanol soluble material derivatized with DNPH. ◊, No AlkB; □, +AlkB. DNPH derivatives of formaldehyde and acetaldehyde were run as markers and monitored at A254.
AlkB oxidizes the methyl group of 1-meA in DNA to release formaldehyde (9, 10). Depending on whether AlkB oxidizes 1-etA in DNA initially at carbon-1 or carbon-2 of the ethyl group, the product released could be either formaldehyde or acetaldehyde. To determine the nature of the aldehyde released, AlkB was incubated with [14C]EtI treated poly(dA) and the ethanol soluble 14C-product derivatized with DNPH and examined by HPLC. The radiolabeled product co-chromatographed with the DNPH derivative of acetaldehyde and not formaldehyde (Fig. 5C). AlkB therefore oxidizes at carbon-1 of the ethyl adduct generating acetaldehyde.
Tissue Distribution of ABH2 and ABH3.
The mRNAs of ABH2 and ABH3 were clearly detected in all of 10 different human tissues and several different cell lines derived from carcinomas by Northern hybridization, but were present at variable levels. ABH2 mRNA was present at relatively high levels in liver and bladder, whereas ABH3 was highest in spleen, prostate, bladder, and colon tissues. In the cell lines derived from carcinomas, ABH2 mRNA was relatively high in HeLa, whereas ABH3 mRNA was barely detectable in HeLa cells or in two of three cell lines derived from Burkitt's lymphomas (data not shown). There was no obvious correlation between expression of the ABH2 and ABH3 genes and the proliferative status of the tissue or cell type. Yellow fluorescent protein fusion constructs with the two human proteins showed that ABH2 localized to cell nuclei, where it was present diffusely throughout the nucleoplasm and accumulated in nucleoli, whereas ABH3 was present diffusely in nuclei and to a lesser extent in cytoplasm. Neither ABH2 nor ABH3 showed the punctate cytoplasmic staining typical of mitochondrial proteins (data not shown).
Discussion
We have identified two human homologs, ABH2 and ABH3, of the E. coli AlkB protein by complementation of the alkB mutant phenotype. Like AlkB, both proteins have conserved domains and biochemical requirements characteristic of αKG-dependent dioxygenases, and are therefore new members of this family of enzymes. Both proteins had DNA repair activities similar to AlkB, and removed both 1-meA and 3-meC from single- and double-stranded DNA substrates. The relative efficiency of repair of the two aberrant bases differed between ABH2 and ABH3, but this effect was small and may not have biological significance. One clear difference between AlkB and the human enzymes was the rapid repair of both 1-meA and 1-etA by AlkB, whereas both human proteins were only weakly active on 1-etA. Our findings suggest that ABH2 and ABH3 have similar activities in DNA repair. Redundancy of human DNA repair enzymes is not unprecedented. Other examples include the uracil–DNA glycosylases, UNG and SMUG1, and the thymine–DNA glycosylases, TDG and MBD4 (21). The occurrence of alternative or backup DNA repair activities emphasizes the complex task of counteracting DNA damage in the large genomes of higher eukaryotes. Even though we were unable to detect an AlkB-like function for the previously reported ABH1 protein, highly conserved residues in AlkB, ABH2, and ABH3 are also present in ABH1 (Fig. 2B). It is therefore possible that ABH1 has a related activity, but with a different substrate specificity to AlkB, ABH2, and ABH3.
The molecular model of the ABH3 core (Fig. 2C) indicates residues involved in catalysis and substrate specificity of the AlkB family of enzymes. The conserved residues of AlkB, ABH2, and ABH3 (shown by the magenta and blue colored side chains in Fig. 2C) may be essential for the very similar or identical function of these enzymes. Subtle differences in their relative activities and specificities may be caused by variation in side chains in and around the substrate-binding pocket (shown by yellow colored side chains in Fig. 2C). The 3D model will help to identify relevant target residues for site-directed mutagenesis. However, high-resolution crystal structures of human AlkB homologues would be required before detailed atomic level screening and analysis can be used to design small molecule antagonists of these enzymes. Such inhibitors could be useful adjuncts to cancer therapy that uses simple alkylating agents.
AlkB and its human homologs enzymatically reverse 1-meA and 3-meC in DNA to adenine and cytosine by oxidative demethylation, indicating an accurate mode of DNA repair. Other activities that directly reverse DNA damage are the suicidal O6-methylguanine–DNA methyltransferases that transfer the DNA methyl group to a cysteine residue in the protein (22–24), light requiring photolyases that monomerize cyclobutyl pyrimidine dimers and the Bacillus subtilis spore SP lyase, which cleaves the unique spore photoproduct (5-thyminyl-5,6-dihydrothymine dimer) to two thymine residues by a free-radical mechanism (25). Photolyase and SP lyase have not been found in human cells. Thus, the only two known mechanisms for direct reversal of DNA base damage in mammalian cells are caused by the O6-methylguanine-DNA methyltransferase and the activities characterized here of the ABH2 and ABH3 DNA dioxygenases.
Acknowledgments
We thank Peter Robins for help in protein purification.
Abbreviations
3-meC, 3-methylcytosine
1-meA, 1-methyladenine
αKG, α-ketoglutarate
MMS, methyl methanesulfonate
DMS, dimethyl sulfate
DNPH, 2,4-dinitrophenylhydrazone
1-etA, 1-ethyladenine
EtI, ethyl iodide
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bodell W. J. & Singer, B. (1979) Biochemistry 18, 2860-2863. [DOI] [PubMed] [Google Scholar]
- 2.Boiteux S. & Laval, J. (1982) Biochimie 64, 637-641. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H., Yamamoto, Y. & Sekiguchi, M. (1983) J. Bacteriol. 153, 1301-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedgwick, B. & Lindahl, T. (2002) Oncogene Rev., in press. [DOI] [PubMed]
- 5.Chen B. J., Carroll, P. & Samson, L. (1994) J. Bacteriol. 176, 6255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinglay S., Trewick, S. C., Lindahl, T. & Sedgwick, B. (2000) Genes Dev. 14, 2097-2105. [PMC free article] [PubMed] [Google Scholar]
- 7.Aravind L. & Koonin, E. V. (2001) Genome Biol. 2, research0007.1-0007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornburg L. D., Lai, M.-T., Wishnok, J. S. & Stubbe, J. (1993) Biochemistry 32, 14023-14033. [DOI] [PubMed] [Google Scholar]
- 9.Trewick S. C., Henshaw, T. F., Hausinger, R. P., Lindahl, T. & Sedgwick, B. (2002) Nature 419, 174-178. [DOI] [PubMed] [Google Scholar]
- 10.Falnes P. O., Johansen, R. F. & Seeberg, E. (2002) Nature 419, 178-182. [DOI] [PubMed] [Google Scholar]
- 11.Kodaira K.-I., Oki, M., Kakikawa, M., Kimoto, H. & Taketo, A. (1996) J. Biochem. 119, 1062-1069. [DOI] [PubMed] [Google Scholar]
- 12.Houlgate P. R., Dhingra, K. S., Nash, S. J. & Evans, W. H. (1989) Analyst 114, 355-360. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y., Carter, K. C., Wang, R. & Shell, B. K. (1996) Nucleic Acids Res. 24, 931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras-Moreira B. & Bates, P. A. (2002) Bioinformatics 18, 1141-1142. [DOI] [PubMed] [Google Scholar]
- 15.Roach P. L., Clifton, I. J., Fulop, V., Harlos, K., Barton, G. J., Hajdu, J., Andersson, I., Schofield, C. J. & Baldwin, J. E. (1995) Nature 375, 700-704. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.-J., Lloyd, M. D., Harlos, K., Clifton, I. J., Baldwin, J. E. & Schofield, C. J. (2001) J. Mol. Biol. 308, 937-948. [DOI] [PubMed] [Google Scholar]
- 17.Wilmouth R. C., Turnbull, J. J., Welford, R. W. D., Clifton, I. J. & Prescott, A. G. (2002) Structure (London) 10, 93-103. [DOI] [PubMed] [Google Scholar]
- 18.Aravind L., Walker, D. R. & Koonin, E. V. (1999) Nucleic Acids Res. 27, 1223-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones D. T. (1999) J. Mol. Biol. 292, 195-202. [DOI] [PubMed] [Google Scholar]
- 20.Tudek B., Van Zeeland, A. A., Kusmierek, J. T. & Laval, J. (1998) Mutat. Res. 407, 169-176. [DOI] [PubMed] [Google Scholar]
- 21.Wood R. D., Mitchell, M., Sgouros, J. & Lindahl, T. (2001) Science 291, 1284-1289. [DOI] [PubMed] [Google Scholar]
- 22.Olsson M. & Lindahl, T. (1980) J. Biol. Chem. 255, 10569-10571. [PubMed] [Google Scholar]
- 23.Foote R. S., Mitra, S. & Pal, B. C. (1980) Biochem. Biophys. Res. Commun. 97, 654-659. [DOI] [PubMed] [Google Scholar]
- 24.Bogden J. M., Eastman, A. & Bresnick, E. (1981) Nucleic Acids Res. 9, 3089-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebeil R. & Nicholson, W. L. (2001) Proc. Natl. Acad. Sci. USA 98, 9038-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates P. A. & Sternberg, M. J. E. (1999) Proteins Suppl. 3, 47-54. [DOI] [PubMed] [Google Scholar]