Abstract
Latent human immunodeficiency virus type 1 (HIV-1) persists even in patients treated with antiretroviral therapy. New treatment strategies are therefore needed to eradicate this latent viral reservoir without reducing immune cell function. We characterize the interleukin-7 (IL-7)-induced stimulation of primary human T cells and thymocytes and demonstrate, using the SCID-hu model, that IL-7 induces substantial expression of latent HIV while having minimal effects on the cell phenotype. Thus, IL-7 is a viable candidate to activate expression of latent HIV and may facilitate immune clearance of latently infected cells.
Latent human immunodeficiency virus (HIV) has been documented in both memory and naive T lymphocytes (9, 12, 29, 32, 35), and it is estimated to require up to 60 years to eliminate this reservoir through the use of highly active antiretroviral therapy (HAART) alone (17, 48). Thus, new treatment strategies designed to purge the pool of latently infected cells are needed.
The cell activation state plays a critical role in the ability of HIV type 1 (HIV-1) to achieve and maintain active replication (26). Cytokine treatment, which can alter the activation state of the host cell and thus alter patterns of viral infection and replication (42, 43), has been previously proposed as an adjunctive therapy both to activate the reservoir of latently infected T cells and to improve immune function in immunodeficient patients. A combination of interleukin-6 (IL-6), IL-2, and tumor necrosis factor alpha (TNF-α) induces emergence from latency in vitro (7, 10), and simultaneous treatment with IL-2 and HAART can transiently increase CD4 T cells and reduce the pool of resting cells containing replication-competent HIV in patients (11). Naive T cells treated with these cytokines become activated, however, and some cells lose naive phenotype and behavior (44, 45), which could impact their ability to mount an immune response. Previous studies showed that IL-7, in addition to stimulating T cells (13, 39, 45) and thymocytes (8, 31, 42), allows productive HIV infection of T cells while having minimal effects on naive and memory phenotypes (39, 45), prompting us to assess IL-7 as a therapeutic agent to induce activation of latent HIV-1 and to define its action on quiescent human cell populations known to harbor latent virus.
Effect of IL-7 on mature thymocytes.
The SCID-hu HIV latency model produces high levels of latently infected mature human thymocytes and peripheral naive T cells due to the decrease in cellular gene transcription during thymopoiesis (7). To precisely characterize the effects of IL-7 on cell subsets relevant to latent populations amenable to study in the SCID-hu model, 4-day whole-lobe human fetal thymic organ cultures (3, 6, 37) in serum-free medium (23, 41) were used. In our initial experiments, we monitored thymocytes for the effect of IL-7 on expression of the developmental and activation markers CD69, CD25, and CD45RA and on the IL-7 receptor α subunit, CD127. Other than the expected down regulation of CD127, we detected up regulation of CD69, a marker of positive selection, and increases in CD45RA, but only minimal change in CD25 (not shown).
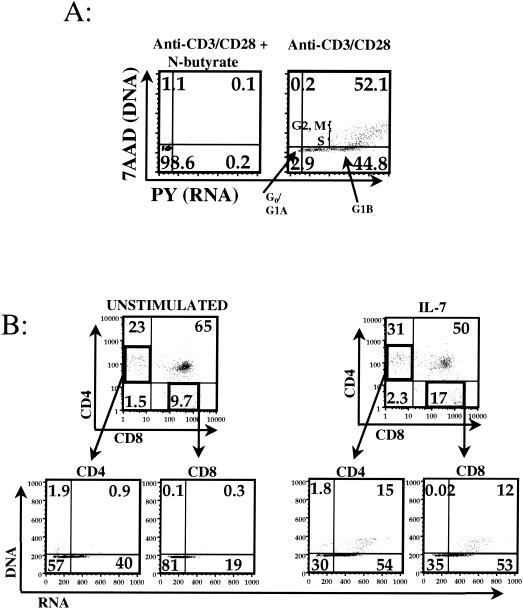
A four-color flow cytometric cell cycle assay (7, 38) was used to simultaneously examine two surface markers and cellular DNA and RNA synthesis, allowing identification of cells in different stages of the cell cycle (Fig. 1A). Both CD4+ CD8− (CD4SP) and CD8+ CD4− (CD8SP) thymocytes displayed increased percentages of cells in the G1B and S stages of the cell cycle when cultured with IL-7, increasing the percentage of activated thymocytes an average of 1.8-fold ± 0.2-fold (CD4SP) or 3.7-fold ± 1.7-fold (CD8SP) (Fig. 1B). These increases are consistent with recently reported experiments in which IL-7 increased Ki-67 staining within these subsets in human fetal thymic organ cultures (31). IL-7 induced increases in production of cellular RNA in mature thymocyte subsets (Fig. 1B), strongly suggesting to us that this cytokine should be capable of activating the expression of latent HIV-1 within the same cell subsets derived from the SCID-hu model.
FIG. 1.
Effect of IL-7 on human thymocytes. (A) Anti-CD3, anti-CD28 costimulated normal peripheral blood lymphocytes, either alone (right panel) or treated with N-butyrate (left panel), are shown with the cell cycle status indicated in each quadrant, as an example of the assay used in panel B, and throughout this work. DNA is depicted on the vertical axis, and RNA is depicted on the horizontal axis. The position of the vertical axis marks the division between the G1A and G1B stages of the cell cycle and is determined by the N-butyrate-treated cells, which are blocked at the G1A-to-G1B transition. Positions indicating stages in the cell cycle are illustrated in the right panel. (B) Four-color cell cycle analysis was performed on fetal thymocytes in whole-lobe thymic organ culture either alone or in the presence of IL-7 for 4 days. Three independent experiments were performed, of which one representative is shown. Top row, CD4SP and CD8SP cell populations were backgated (arrows) and analyzed for the presence of IL-7-induced changes in cell cycle progression (bottom row).
IL-7 induces expression of latent HIV-1.
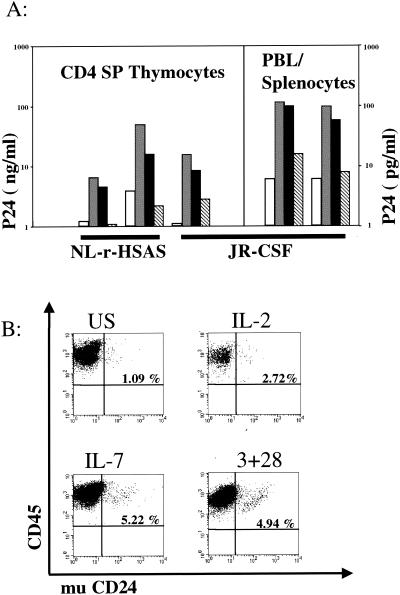
To determine whether IL-7 can force HIV-1 out of the latent state, SCID-hu mice (1, 5) were infected by intra-implant injection (7) with HIV strain JR-CSF (CCR5 tropic) or NL-r-HSAS (24) (a CXCR4-tropic strain bearing murine CD24 reporter sequences). At 4.5, 7, or 9 weeks postinfection, thymocytes or peripheral cells from infected or mock-infected mice were cultured in the presence of antiretroviral agents, as described previously (7), and then stimulated with anti-CD3 and anti-CD28 antibodies, with anti-CD3 antibodies alone, with IL-2, or with IL-7 for 3 days in vitro. IL-7 stimulated substantial p24gag production (as determined by enzyme-linked immunosorbent assay of supernatants) both from mature thymocytes and from pooled peripheral cells and, moreover, consistently induced higher p24 levels than did either anti-CD3 antibodies alone or IL-2 (Fig. 2A). Three days following stimulation, IL-7 induced more virus-encoded reporter gene (mu CD24) expression from latently infected cells from NL-r-HSAS-infected animals than did IL-2 and was nearly as effective as costimulation through CD3 and CD28 (Fig. 2B). The magnitude of reporter expression with IL-7 stimulation was also increased over that of IL-2 (Fig. 2B). IL-7 is thus the most active cytokine in terms of stimulation of latent virus that we have found to date (7).
FIG. 2.
Effect of IL-7 on HIV-1 latency. (A) CD4SP thymocytes or pooled peripheral blood lymphocytes (PBL) and splenocytes were isolated from infected (HIVNL-r-HSAs or HIVJR-CSF) SCID-hu Thy/Liv implants and were cultured for three days in the presence of Protease Inhibitor, and under the following stimulation conditions: unstimulated (white), costimulated (grey), IL-7 (black), and IL-2 (striped). HIV-1 p24gag protein concentrations in the supernatants were measured to assess virus production. Each grouping of four bars represents one experiment. Unstimulated peripheral cells fell below the limit of detection (6 pg/ml). (B) Expression of virus-encoded reporter (mu CD24) indicating activation of latent virus. Mature CD4 SP thymocytes from Nl-r-HSAs infected implants were treated for 3 days without stimulation (US) or with IL-2, IL-7, or anti-CD3/CD28 costimulation as indicated. Quadrant gates were set on isotype controls.
Effect of IL-7 on quiescent human peripheral blood T lymphocytes.
Our finding that IL-7 induced expression of latent virus from peripheral blood and splenocytes in the SCID-hu model suggested that this cytokine would be effective in purging latent virus from peripheral T lymphocytes in patients. This may only be clinically relevant, however, if quiescent cells targeted by the cytokine are able to maintain functional phenotype. This was assessed by monitoring various activation and maturation markers. Quiescent human lymphocytes were purified from normal human peripheral blood and depleted of cells expressing HLA-DR, CD25, CD69, CD14, and/or CD19 as described previously (26). In some experiments, either CD4 or CD8, in combination with either CD45RA or CD45RO, was also negatively selected to generated four quiescent populations: CD45RA−/CD4−, CD45RA−/CD8−, CD45R0−/CD4−, and CD45R0−/CD8−. Cells were cultured for 3 to 6 days in medium alone, stimulated with anti-CD3, costimulated with anti-CD3 and anti-CD28, or treated with 10 ng of IL-7/ml and assessed for changes in expression of activation markers and for effects on RNA and DNA synthesis.
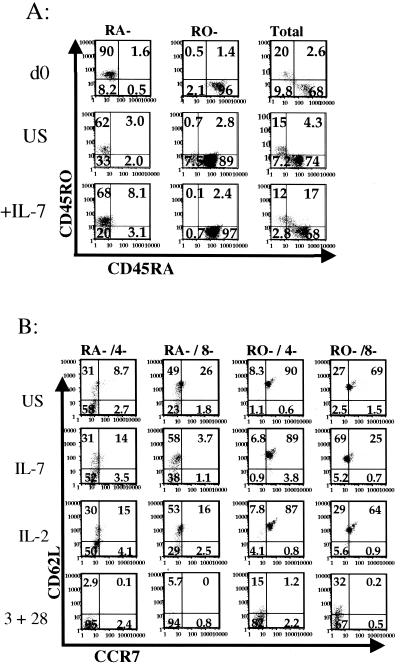
Both CD45RO− and CD45RA− cells displayed increased levels of CD25 in the presence of IL-7, although increased expression of CD69, an early marker of activation, was not seen. CD27 was up regulated by both IL-2 and IL-7 but not by costimulation (not shown). Although we observed a slight increase in the percentage of CD45RA− and CD45RO− quiescent cells that expressed CD45RA and in the mean fluorescence intensity for this marker, we observed minimal changes in the expression of CD45RO (Fig. 3A), CD4, and CD8 (not shown). The IL-7 receptor was down regulated in all subsets treated with IL-7, suggesting that binding and internalization of the cytokine was occurring (not shown).
FIG. 3.
Effect of IL-7 on surface phenotype of quiescent human lymphocytes. Total, CD45RA−, or CD45RO− quiescent lymphocytes were cultured in the presence or absence of IL-7. Developmental and stimulation markers were examined using flow cytometric methods. (A) Total, CD45RA−, or CD45RO− quiescent lymphocytes were cultured for 4 days as described previously and then analyzed for expression of CD45RA (x axis) and CD45RO (y axis) in the presence (bottom row) and absence (middle row) of IL-7, as well as on day 0 (top row). (B) CD45RA−/4−, CD45RA−/8−, CD45R0−/4−, and CD45R0−/8− quiescent lymphocytes were isolated and cultured under the following stimulation conditions: unstimulated, IL-7, IL-2, or anti-CD3 and anti-CD28 costimulation for 3 days. Lymphocyte subsets were then analyzed for changes in expression of CCR7 (x axis) and CD62-L (y axis).
Significantly, when stimulated with IL-7 or IL-2, CCR7 and CD62L remained substantially unchanged in memory (CD45RA−) subsets, although CCR7 was slightly up regulated in the CD45RO−/CD4− subset and slightly down regulated in the CD45RO−/CD8− subset (Fig. 3B). In contrast, both CD62L and CCR7 were dramatically down regulated by costimulation (Fig. 3B). Thus, in general, the overall expression profile in these highly purified quiescent lymphocyte subsets remained stable when they were stimulated with IL-7.
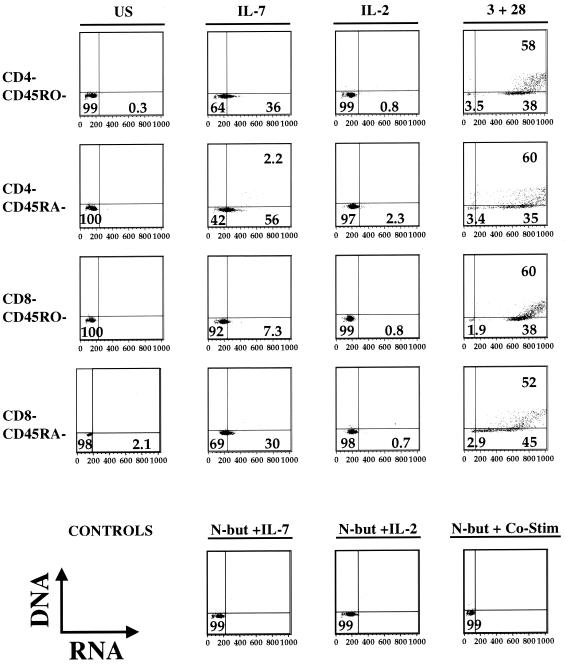
Although treatment with IL-7 does not appear to negatively perturb the phenotype of the quiescent populations described above, elimination of the viral reservoir in the periphery may be achievable only if quiescent peripheral lymphocytes are capable of responding sufficiently to the stimulatory effects of IL-7. We therefore tested the ability of this cytokine to induce cell cycle progression in these same four subpopulations. IL-7, in contrast to IL-2, increased cellular RNA expression and entry into G1B in all subpopulations analyzed (Fig. 4). The observed increase in RNA is maintained for at least 15 days in culture (not shown).
FIG. 4.
Effect of IL-7 on cell cycle status of quiescent human lymphocytes. Quiescent CD45RA−/CD4−, CD45RA−/CD8−, CD45R0−/CD4−, and CD45R0−/CD8− lymphocytes were isolated, and cultured under the following stimulation conditions: unstimulated, IL-7, IL-2, or anti-CD3 and anti-CD28 costimulation. After 3 days, cells were stained to detect DNA (y axis) and RNA (x axis) and analyzed to determine cell cycle status. Control stimulations performed in the presence of cell cycle inhibitor (N-acetyl butyric acid) and were used to set quadrant positions (bottom row). Seven similar experiments were performed.
IL-7 is required for thymocyte development (34), enhances T-cell function and survival, (2, 4, 25), and has been reported to induce proliferation of naive CD45RA+ cell populations in peripheral blood while maintaining their naive phenotype (21, 39, 45). Our finding that IL-7 increased both RNA expression and DNA synthesis in mature thymocytes and induced RNA expression in stringently purified, quiescent CD4 and CD8 as well as CD45RA+ and CD45RO+ T cells is consistent with previously reported results for murine (46) and human (13, 15, 42) cells and with IL-7's ability to induce latent virus expression in the SCID-hu system.
Consistent with previous data (21, 45, 47), IL-7 altered cell surface expression of a limited set of activation antigens on quiescent T cells, most dramatically the IL-2 receptor. Further, IL-7 induced only minimal perturbations in the expression of the naive homing and adhesion molecules CCR7 and CD62L, suggesting that IL-7 treatment would not impair cell migration patterns or behavior. Together, our data point to an IL-7-induced activation state distinct from that produced by costimulation, characterized by retention of phenotypic and functional markers and progression to the early G1B stage of the cell cycle.
While our results in the SCID-hu model directly pertain to activation of latent virus in the naive quiescent cell subset, our cell cycle analysis of memory cells suggests that latent virus in this subset would behave similarly. It remains unclear whether IL-7 activation of latent virus occurs through a direct effect on the long terminal repeat. Signaling through the IL-7 receptor occurs through activation of Janus family kinases (JAK1/3), which then recruit signal transducers and activators of transcription (STAT) family members (STAT1, STAT3, and STAT5). In addition, IL-7 can activate the PI3 kinase cascade via an interaction with STAT3 (33). Very recent studies suggest that only the STAT5-mediated IL-7 signaling pathway is involved in IL-7-induced effects on HIV expression during de novo infection (15). Activation of the latent reservoir may behave similarly.
Any potential immunotherapy must be considered for its effects on immune function, on viral pathogenesis, and on latently infected cells. Although it is thought that any increase in viral production associated with cytokine stimulation would be mitigated by HAART, several reports show that cytokines can increase HIV replication (16, 42). Recent work has demonstrated a correlation between CD4-T-cell depletion and increased circulating levels of IL-7 (20, 30). This correlation is important in light of IL-7's emerging role as a regulator of T cell homeostasis, both through peripheral expansion (19, 40) and through thymus-dependent mechanisms (28). IL-7 has also been linked to emergence of syncytium-inducing isolates during end-stage HIV disease (27). Further, increased levels of IL-7 are seen in some autoimmune diseases (14, 22), and chronic elevation of IL-7 in animal models has led to lymphoproliferative disorders (18, 36). Thus, a more detailed understanding of the in vivo effects of cytokine stimulation under conditions of HAART and HIV infection is warranted.
Our results indicate that IL-7 has minimal effects on T-cell phenotype and suggest that this cytokine is an excellent candidate for further study as an adjunctive therapy designed to purge the pool of latently infected T cells.
Acknowledgments
We thank G. Bristol, R. Cortado, and A. Kacena for valuable technical assistance and B. Jamieson for helpful discussions.
This work was supported by NIH grants AI36059 and AI36554, the UCLA CFAR, and the James B. Pendleton Charitable Trust. D.D.S.-A. was supported by a UCLA AIDS Institute fellowship. Y.D.K. was supported by NIDCR grant T32DF0796.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Amos, C. L., A. Woetmann, M. Nielsen, C. Geisler, N. Odum, B. L. Brown, and P. R. Dobson. 1998. The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): a survival factor in activated human T cells. Cytokine 10:662-668. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, G., and E. J. Jenkinson. 1995. The role of the thymus during T-lymphocyte development in vitro. Semin. Immunol. 7:177-183. [DOI] [PubMed] [Google Scholar]
- 4.Boise, L. H., A. J. Minn, C. H. June, T. Lindsten, and C. B. Thompson. 1995. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc. Natl. Acad. Sci. USA 92:5491-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 6.Bonyhadi, M. L., L. Su, J. Auten, J. M. McCune, and H. Kaneshima. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1073-1080. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. [DOI] [PubMed] [Google Scholar]
- 8.Chazen, G. D., G. M. Pereira, G. LeGros, S. Gillis, and E. M. Shevach. 1989. Interleukin 7 is a T-cell growth factor. Proc. Natl. Acad. Sci. USA 86:5923-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 10.Chun, T. W., D. Engel, S. B. Mizell, L. A. Ehler, and A. S. Fauci. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83-91. (Erratum, 188:614.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun, T. W., D. Engel, S. B. Mizell, C. W. Hallahan, M. Fischette, S. Park, R. T. Davey, Jr., M. Dybul, J. A. Kovacs, J. A. Metcalf, J. M. Mican, M. M. Berrey, L. Corey, H. C. Lane, and A. S. Fauci. 1999. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat. Med. 5:651-655. [DOI] [PubMed] [Google Scholar]
- 12.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dardalhon, V., S. Jaleco, S. Kinet, B. Herpers, M. Steinberg, C. Ferrand, D. Froger, C. Leveau, P. Tiberghien, P. Charneau, N. Noraz, and N. Taylor. 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:9277-9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Benedetti, F., M. Massa, P. Pignatti, M. Kelley, C. R. Faltynek, and A. Martini. 1995. Elevated circulating interleukin-7 levels in patients with systemic juvenile rheumatoid arthritis. J. Rheumatol. 22:1581-1585. [PubMed] [Google Scholar]
- 15.Ducrey-Rundquist, O., M. Guyader, and D. Trono. 2002. Modalities of interleukin-7-induced human immunodeficiency virus permissiveness in quiescent T lymphocytes. J. Virol. 76:9103-9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 17.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, A. G., C. Burdet, C. Bunce, M. Merkenschlager, and R. Ceredig. 1995. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int. Immunol. 7:415-423. [DOI] [PubMed] [Google Scholar]
- 19.Fry, T. J., B. L. Christensen, K. L. Komschlies, R. E. Gress, and C. L. Mackall. 2001. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 97:1525-1533. [DOI] [PubMed] [Google Scholar]
- 20.Fry, T. J., E. Connick, J. Falloon, M. M. Lederman, D. J. Liewehr, J. Spritzler, S. M. Steinberg, L. V. Wood, R. Yarchoan, J. Zuckerman, A. Landay, and C. L. Mackall. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 21.Geiselhart, L. A., C. A. Humphries, T. A. Gregorio, S. Mou, J. Subleski, and K. L. Komschlies. 2001. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J. Immunol. 166:3019-3027. [DOI] [PubMed] [Google Scholar]
- 22.Giacalone, B., L. D'Auria, C. Bonifati, C. Ferraro, E. Riccardi, A. Mussi, G. D'Agosto, P. Cordiali-Fei, and F. Ameglio. 1998. Decreased interleukin-7 and transforming growth factor-beta1 levels in blister fluids as compared to the respective serum levels in patients with bullous pemphigoid. Opposite behavior of TNF-alpha, interleukin-4 and interleukin-10. Exp. Dermatol. 7:157-161. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson, B. D., D. C. Douek, S. Killian, L. E. Hultin, D. Scripture-Adams, J. V. Giorgi, D. Marelli, R. A. Koup, and J. A. Zack. 1999. Generation of functional thymocytes in the human adult. Immunity 10:569-575. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson, B. D., and J. A. Zack. 1998. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J. Virol. 72:6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komschlies, K. L., T. A. Gregorio, M. E. Gruys, T. C. Back, C. R. Faltynek, and R. H. Wiltrout. 1994. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J. Immunol. 152:5776-5784. [PubMed] [Google Scholar]
- 26.Korin, Y. D., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161.-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llano, A., J. Barretina, A. Gutierrez, J. Blanco, C. Cabrera, B. Clotet, and J. A. Este. 2001. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J. Virol. 75:10319-10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackall, C. L., T. J. Fry, C. Bare, P. Morgan, A. Galbraith, and R. E. Gress. 2001. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 97:1491-1497. [DOI] [PubMed] [Google Scholar]
- 29.McBreen, S., S. Imlach, T. Shirafuji, G. R. Scott, C. Leen, J. E. Bell, and P. Simmonds. 2001. Infection of the CD45RA+ (naive) subset of peripheral CD8+ lymphocytes by human immunodeficiency virus type 1 in vivo. J. Virol. 75:4091-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, Y., D. C. Douek, R. D. McFarland, and R. A. Koup. 2002. Effects of exogenous interleukin-7 on human thymus function. Blood 99:2851-2858. [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski, M. A., T. W. Chun, S. J. Justement, I. Motola, M. A. Spinelli, J. Adelsberger, L. A. Ehler, S. B. Mizell, C. W. Hallahan, and A. S. Fauci. 1999. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallard, C., A. P. Stegmann, T. van Kleffens, F. Smart, A. Venkitaraman, and H. Spits. 1999. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity 10:525-535. [DOI] [PubMed] [Google Scholar]
- 34.Peschon, J. J., P. J. Morrissey, K. H. Grabstein, F. J. Ramsdell, E. Maraskovsky, B. C. Gliniak, L. S. Park, S. F. Ziegler, D. E. Williams, C. B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180:1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierson, T., T. L. Hoffman, J. Blankson, D. Finzi, K. Chadwick, J. B. Margolick, C. Buck, J. D. Siliciano, R. W. Doms, and R. F. Siliciano. 2000. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J. Virol. 74:7824-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich, B. E., J. Campos-Torres, R. I. Tepper, R. W. Moreadith, and P. Leder. 1993. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J. Exp. Med. 177:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenzweig, M., E. M. Bunting, R. L. Damico, D. P. Clark, and G. N. Gaulton. 1994. Human neonatal thymic organ culture: an ex vivo model of thymocyte ontogeny and HIV-1 infection. Pathobiology 62:245-251. [DOI] [PubMed] [Google Scholar]
- 38.Schmid, I., S. W. Cole, Y. D. Korin, J. A. Zack, and J. V. Giorgi. 2000. Detection of cell cycle subcompartments by flow cytometric estimation of DNA-RNA content in combination with dual-color immunofluorescence. Cytometry 39:108-116. [DOI] [PubMed] [Google Scholar]
- 39.Soares, M. V., N. J. Borthwick, M. K. Maini, G. Janossy, M. Salmon, and A. N. Akbar. 1998. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J. Immunol. 161:5909-5917. [PubMed] [Google Scholar]
- 40.Tan, J. T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K. I. Weinberg, and C. D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98:8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uittenbogaart, C. H., D. J. Anisman, B. D. Jamieson, S. Kitchen, I. Schmidt, J. A. Zack, and E. F. Hays. 1996. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS 10:F9-F16. [DOI] [PubMed] [Google Scholar]
- 42.Uittenbogaart, C. H., D. J. Anisman, J. A. Zack, A. Economides, I. Schmid, and E. F. Hays. 1995. Effects of cytokines on HIV-1 production by thymocytes. Thymus 23:155-175. [PubMed] [Google Scholar]
- 43.Uittenbogaart, C. H., W. J. Boscardin, D. J. Anisman-Posner, P. S. Koka, G. Bristol, and J. A. Zack. 2000. Effect of cytokines on HIV-induced depletion of thymocytes in vivo. AIDS 14:1317-1325. [DOI] [PubMed] [Google Scholar]
- 44.Unutmaz, D., F. Baldoni, and S. Abrignani. 1995. Human naive T cells activated by cytokines differentiate into a split phenotype with functional features intermediate between naive and memory T cells. Int. Immunol. 7:1417-1424. [DOI] [PubMed] [Google Scholar]
- 45.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson, J. D., P. J. Morrissey, A. E. Namen, P. J. Conlon, and M. B. Widmer. 1989. Effect of IL-7 on the growth of fetal thymocytes in culture. J. Immunol. 143:1215-1222. [PubMed] [Google Scholar]
- 47.Welch, P. A., A. E. Namen, R. G. Goodwin, R. Armitage, and M. D. Cooper. 1989. Human IL-7: a novel T cell growth factor. J. Immunol. 143:3562-3567. [PubMed] [Google Scholar]
- 48.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]