Abstract
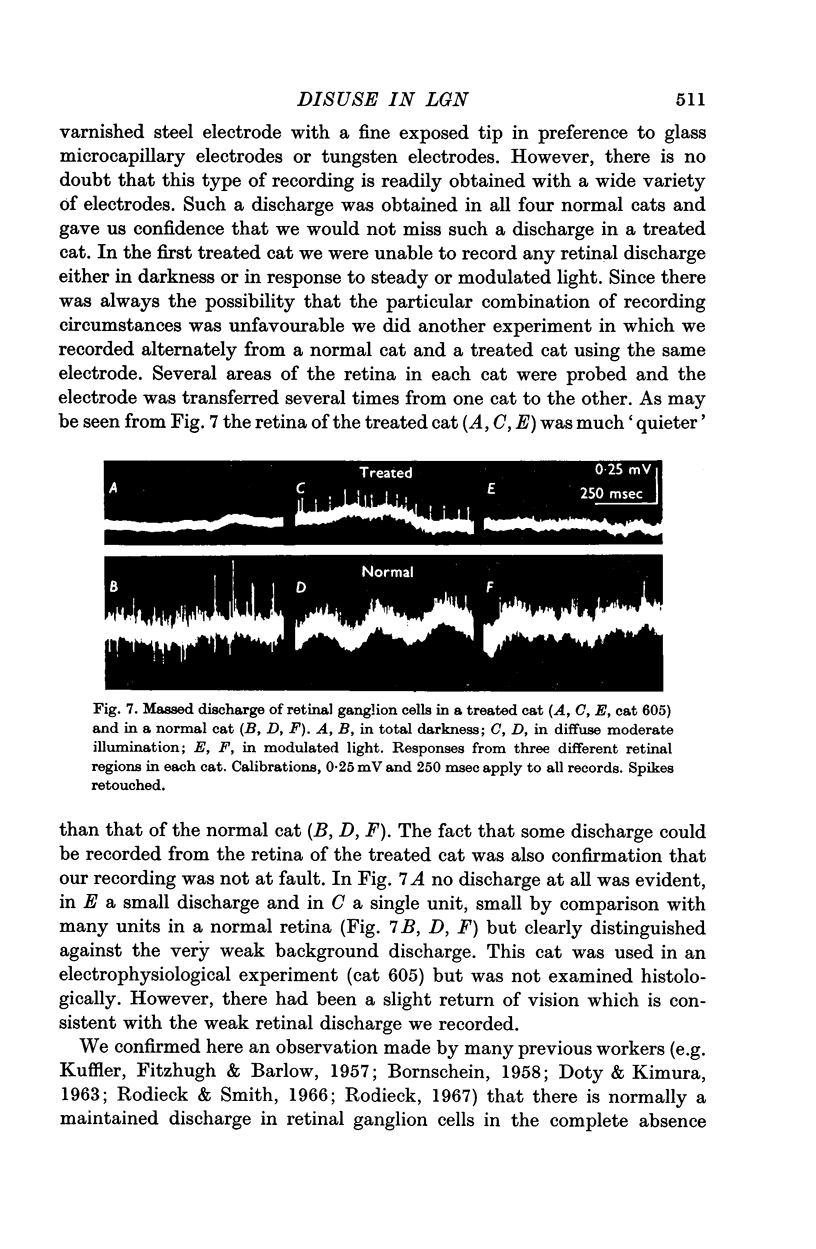
1. An attempt has been made to produce disuse in the lateral geniculate nucleus (LGN) of the cat, in the synapses between the optic nerve fibres and the principal cells of the nucleus. Evidence is produced that destruction of the visual receptor cells by iodoacetate or 1,5-di(p-aminophenoxy) pentane dihydrochloride effectively silences the optic nerve discharge and so achieves this result.
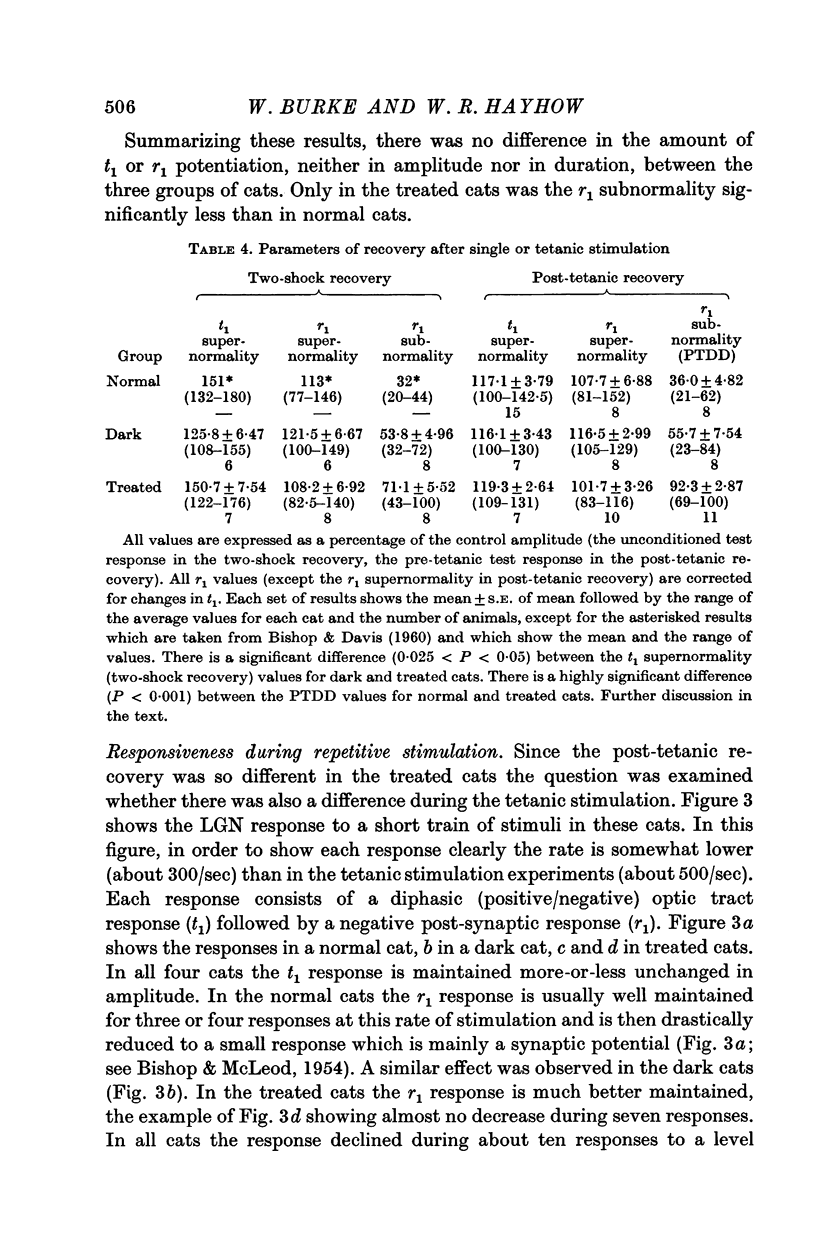
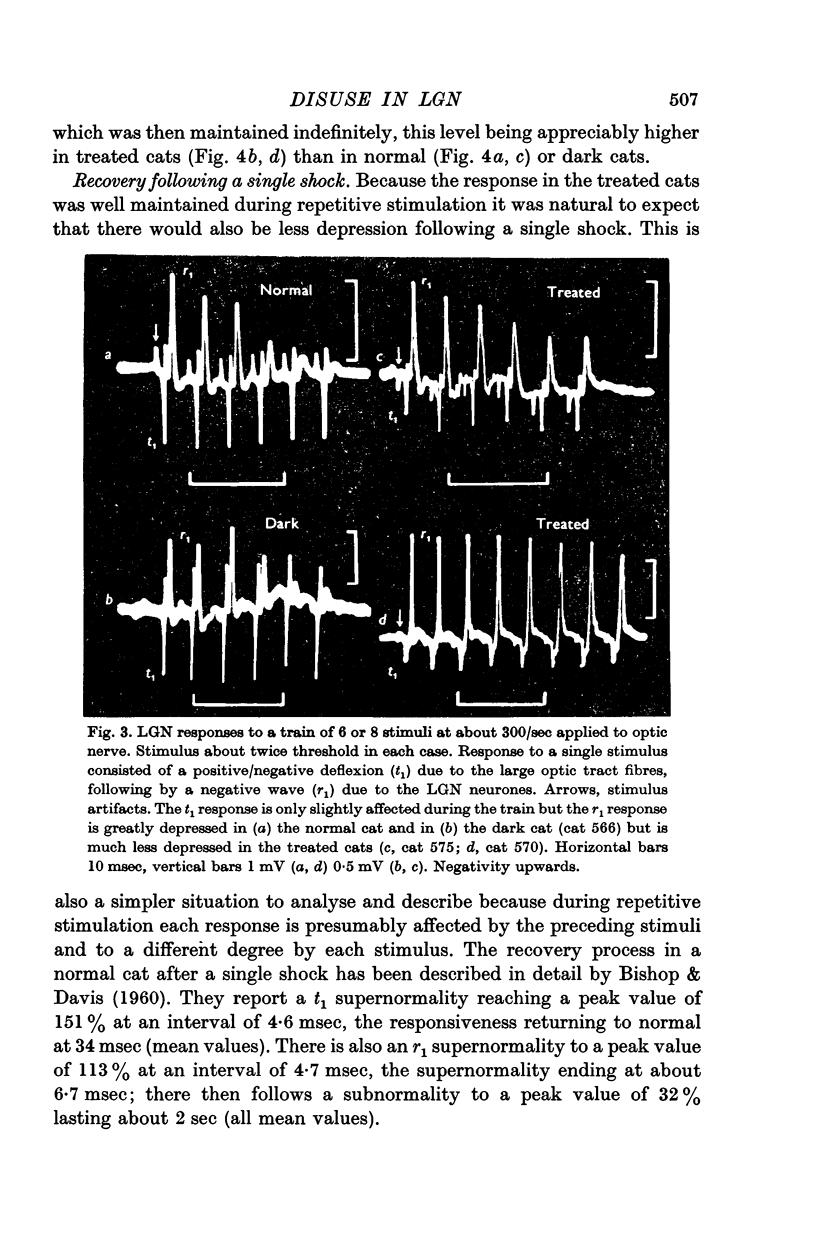
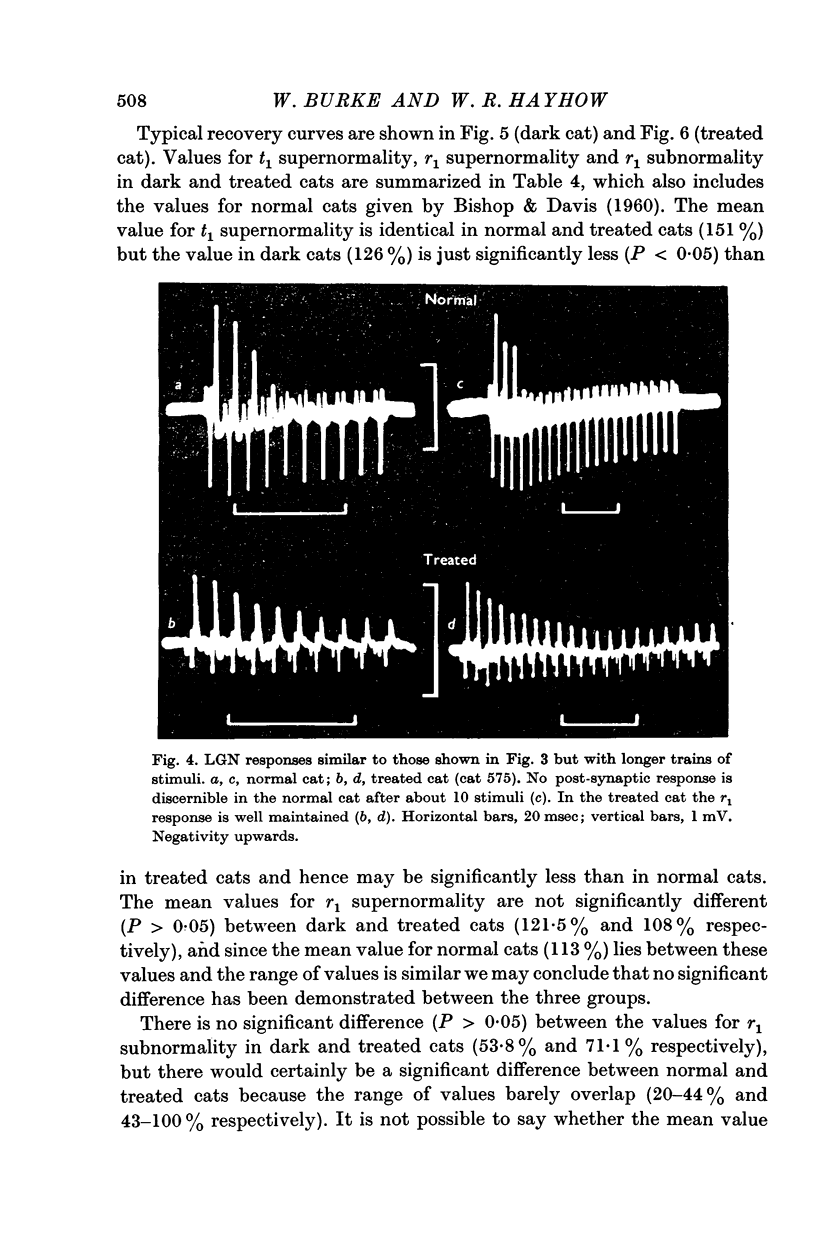
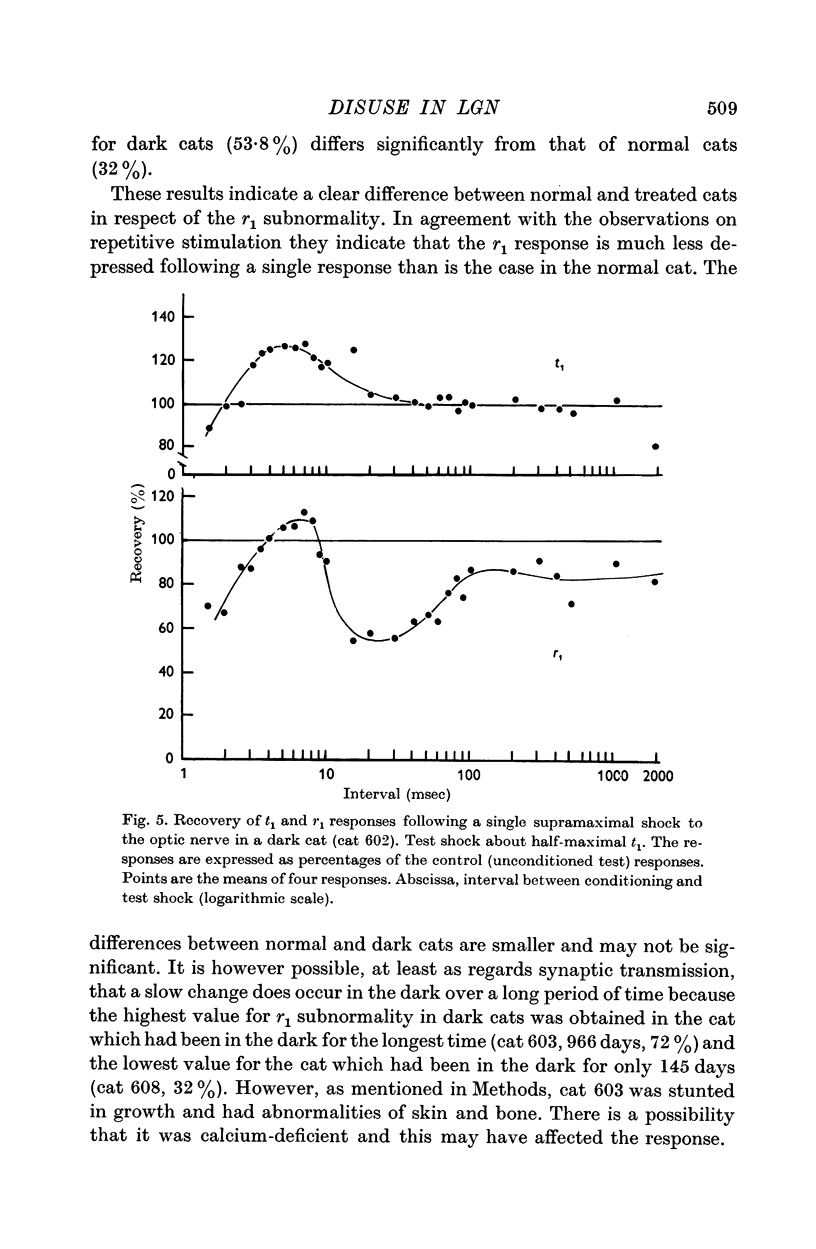
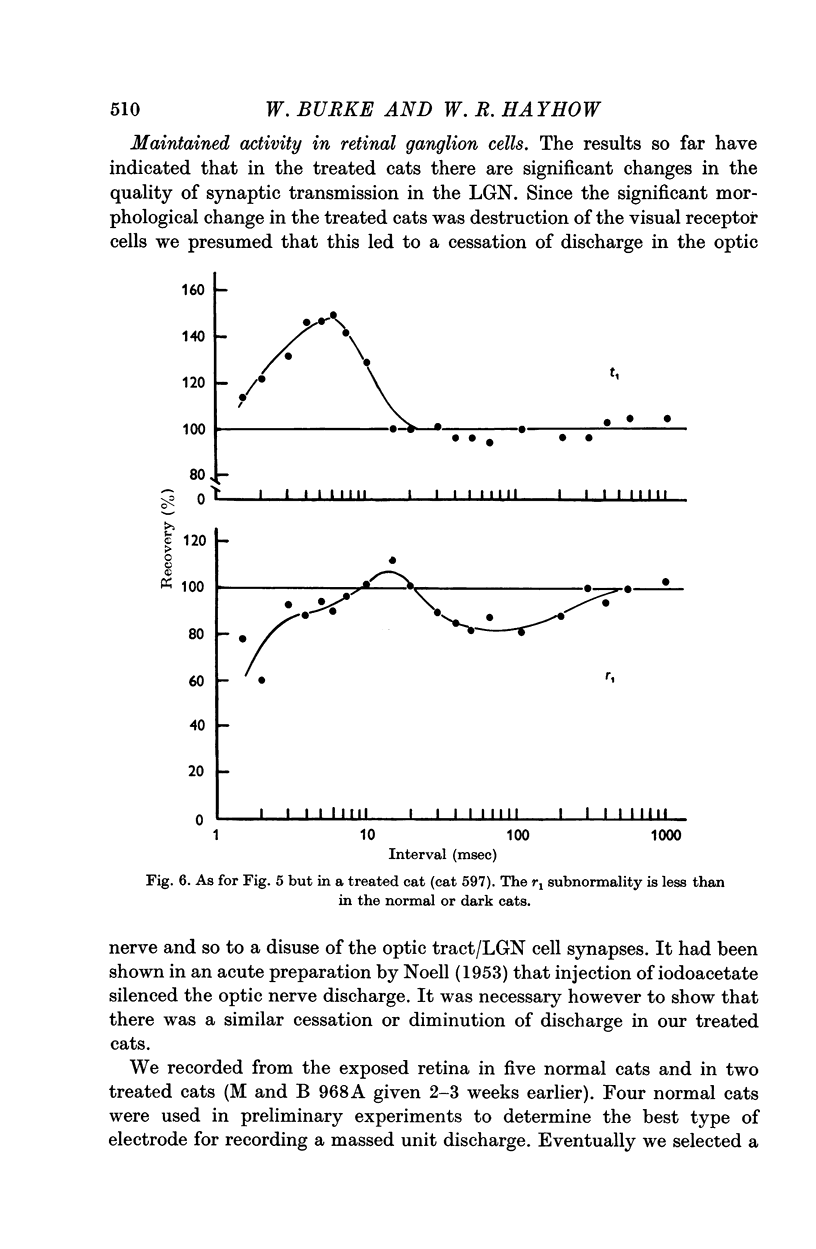
2. Disuse of the LGN synapses does not cause any decrease in synaptic efficiency. The LGN response to a single stimulus to the optic nerve was not appreciably altered, and the depression which normally follows single or repeated stimuli was much reduced or absent. This increased responsiveness was unaffected by prolonged tetanization of the optic nerve during the experiment.
3. Two possible explanations of the increased responsiveness are suggested: a post-synaptic `decentralization'-hypersensitivity and an increased output of transmitter per impulse. The relevance of these results to theories of learning is discussed.
4. The LGN response of adult cats kept in complete darkness for periods of up to 966 days was not appreciably different from that in the normal cat and there was little or no increased responsiveness. This suggests that many retinal ganglion cells continue to discharge in total darkness for long periods. There is a possibility that disuse may develop after a long time in the dark.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER B. L., RISSEN A. H. Electroretinogram of the visually deprived cat. Science. 1961 Nov 17;134(3490):1626–1627. doi: 10.1126/science.134.3490.1626. [DOI] [PubMed] [Google Scholar]
- BECH K. The basophilic substances in the retinal ganglion cells and the physiological activity changes in these cells; an experimental histological study. Acta Ophthalmol Suppl. 1957;(Suppl 46):1–105. [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., HAYHOW W. R. Repetitive stimulation of optic nerve and lateral geniculate synapses. Exp Neurol. 1959 Dec;1:534–555. doi: 10.1016/0014-4886(59)90016-0. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., DAVIS R. The recovery of responsiveness of the sensory synapses in the lateral geniculate nucleus. J Physiol. 1960 Jan;150:214–238. doi: 10.1113/jphysiol.1960.sp006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., EVANS W. A. The refractory period of the sensory synapses of the lateral geniculate nucleus. J Physiol. 1956 Dec 28;134(3):538–557. doi: 10.1113/jphysiol.1956.sp005664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., JEREMY D., LANCE J. W. The optic nerve; properties of a central tract. J Physiol. 1953 Aug;121(2):415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., McLEOD J. G. Nature of potentials associated with synaptic transmission in lateral geniculate of cat. J Neurophysiol. 1954 Jul;17(4):387–414. doi: 10.1152/jn.1954.17.4.387. [DOI] [PubMed] [Google Scholar]
- BROWN G. L., DAVIES B. N., FERRY C. B. The effect of neuronal rest on the output of sympathetic transmitter from the spleen. J Physiol. 1961 Dec;159:365–380. doi: 10.1113/jphysiol.1961.sp006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., HAYROW W. R. Disuse of a central synapse and spontaneous activity in the optic neve. Nature. 1960 Nov 19;188:668–669. doi: 10.1038/188668a0. [DOI] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Discharge patterns of principal cells and interneurones in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):201–212. doi: 10.1113/jphysiol.1966.sp008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Inhibitory mechanisms in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):231–246. doi: 10.1113/jphysiol.1966.sp008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Recovery of responsiveness of cells of lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):213–229. doi: 10.1113/jphysiol.1966.sp008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W. Neuronal models for conditioned reflexes. Nature. 1966 Apr 16;210(5033):269–271. doi: 10.1038/210269a0. [DOI] [PubMed] [Google Scholar]
- CHOW K. L., RIESEN A. H., NEWELL F. W. Degeneration of retinal ganglion cells in infant chimpanzees reared in darkness. J Comp Neurol. 1957 Feb;107(1):27–42. doi: 10.1002/cne.901070103. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E. Submicroscopic morphology and function of the synapse. Exp Cell Res. 1958;14(Suppl 5):347–369. [PubMed] [Google Scholar]
- DOTY R. W., KIMURA D. S. OSCILLATORY POTENTIALS IN THE VISUAL SYSTEM OF CATS AND MONKEYS. J Physiol. 1963 Aug;168:205–218. doi: 10.1113/jphysiol.1963.sp007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K., MILEDI R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959 Jan 28;145(1):204–220. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., McINTYRE A. K. The effects of disuse and of activity on mammalian spinal reflexes. J Physiol. 1953 Sep;121(3):492–516. doi: 10.1113/jphysiol.1953.sp004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGE N. D., MASON D. F., WIEN R. Pharmacological effects of certain diaminodiphenoxy alkanes. Nature. 1956 Oct 13;178(4537):806–807. doi: 10.1038/178806a0. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V., HUGHES J. R. Effects of prolonged optic nerve tetanization on lateral geniculate potentials. Am J Physiol. 1957 Feb;188(2):245–248. doi: 10.1152/ajplegacy.1957.188.2.245. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V., HUGHES J. R., MARSHALL W. H. Post-tetanic potentiation in the visual system of cats. Am J Physiol. 1956 Sep;186(3):483–487. doi: 10.1152/ajplegacy.1956.186.3.483. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V., HUGHES J. R. Relation of posttetanic potentiation to subnormality of lateral geniculate potentials. Am J Physiol. 1957 Feb;188(2):238–244. doi: 10.1152/ajplegacy.1957.188.2.238. [DOI] [PubMed] [Google Scholar]
- GYLLENSTEN L. Postnatal development of the visual cortex is darkness (mice). Acta Morphol Neerl Scand. 1959;2:331–345. [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPFER C., PALMER P. LATERAL GENICULATE NUCLEUS: HISTOLOGICAL AND CYTOCHEMICAL CHANGES FOLLOWING AFFERENT DENERVATION AND VISUAL DEPRIVATION. Exp Neurol. 1964 May;9:400–409. doi: 10.1016/0014-4886(64)90074-3. [DOI] [PubMed] [Google Scholar]
- LEVICK W. R., WILLIAMS W. O. MAINTAINED ACTIVITY OF LATERAL GENICULATE NEURONES IN DARKNESS. J Physiol. 1964 Apr;170:582–597. doi: 10.1113/jphysiol.1964.sp007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORLOCK N. L., PEARLMAN A. L., MARSHALL W. H. SINGLE UNIT STUDY OF POST-TETANIC POTENTIATION AND SECOND SUBNORMALITY IN THE LATERAL GENICULATE BODY OF CATS. Exp Neurol. 1965 Jan;11:38–47. doi: 10.1016/0014-4886(65)90021-x. [DOI] [PubMed] [Google Scholar]
- NOELL W. K. The impairment of visual cell structure by iodoacetate. J Cell Physiol. 1952 Aug;40(1):25–55. doi: 10.1002/jcp.1030400104. [DOI] [PubMed] [Google Scholar]
- RASCH E., SWIFT H., RIESEN A. H., CHOW K. L. Altered structure and composition of retinal cells in darkreared mammals. Exp Cell Res. 1961 Nov;25:348–363. doi: 10.1016/0014-4827(61)90285-3. [DOI] [PubMed] [Google Scholar]
- RIESEN A. H., KURKE M. I., MELLINGER J. C. Interocular transfer of habits learned monocularly in visually naive and visually experienced cats. J Comp Physiol Psychol. 1953 Jun;46(3):166–172. doi: 10.1037/h0057003. [DOI] [PubMed] [Google Scholar]
- Riesen A. H. The Development of Visual Perception in Man and Chimpanzee. Science. 1947 Aug 1;106(2744):107–108. doi: 10.1126/science.106.2744.107. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Smith P. S. Slow dark discharge rhythms of cat retinal ganglion cells. J Neurophysiol. 1966 Sep;29(5):942–953. doi: 10.1152/jn.1966.29.5.942. [DOI] [PubMed] [Google Scholar]
- SHARPLESS S. K. REORGANIZATION OF FUNCTION IN THE NERVOUS SYSTEM--USE AND DISUSE. Annu Rev Physiol. 1964;26:357–388. doi: 10.1146/annurev.ph.26.030164.002041. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J., RAJKOVITS K. Die Rückwirkung der spezifischen Funktion auf die Struktur der Nervenelemente. Acta Morphol Acad Sci Hung. 1955;5(3-4):253–274. [PubMed] [Google Scholar]
- WEISKRANTZ L. Sensory deprivation and the cat's optic nervous system. Nature. 1958 Apr 12;181(4615):1047–1050. doi: 10.1038/1811047b0. [DOI] [PubMed] [Google Scholar]
- WENDELL-SMITH C. P. EFFECT OF LIGHT DEPRIVATION ON THE POSTNATAL DEVELOPMENT OF THE OPTIC NERVE. Nature. 1964 Nov 14;204:707–707. doi: 10.1038/204707a0. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]


