Abstract
The C-type lectins DC-SIGN and DC-SIGNR efficiently bind human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) strains and can transmit bound virus to adjacent CD4-positive cells. DC-SIGN also binds efficiently to the Ebola virus glycoprotein, enhancing Ebola virus infection. DC-SIGN is thought to be responsible for the ability of dendritic cells (DCs) to capture HIV and transmit it to T cells, thus promoting HIV dissemination in vitro and perhaps in vivo as well. To investigate DC-SIGN function and expression levels on DCs, we characterized a panel of monoclonal antibodies (MAbs) directed against the carbohydrate recognition domain of DC-SIGN. Using quantitative fluorescence-activated cell sorter technology, we found that DC-SIGN is highly expressed on immature monocyte-derived DCs, with at least 100,000 copies and often in excess of 250,000 copies per DC. There was modest variation (three- to fourfold) in DC-SIGN expression levels between individuals and between DCs isolated from the same individual at different times. Several MAbs efficiently blocked virus binding to cell lines expressing human or rhesus DC-SIGN, preventing HIV and SIV transmission. Interactions with Ebola virus pseudotypes were also blocked efficiently. Despite their ability to block virus-DC-SIGN interactions on cell lines, these antibodies only inhibited transmission of virus from DCs by approximately 50% or less. These results indicate that factors other than DC-SIGN may play important roles in the ability of DCs to capture and transmit HIV.
For human immunodeficiency virus type 1 (HIV-1) to infect a cell, the viral envelope protein (Env) must interact with CD4 and a coreceptor, thereby inducing conformational changes in Env that mediate fusion between the viral and cellular membranes (1, 8, 9, 11, 12, 15). The efficiency of virus infection can be modulated by receptor density, by the inherent fusogenicity of the Env protein, and by cellular factors that enhance virus attachment to the cell surface (10, 13, 27). Attachment of HIV-1 to the cell surface can occur independently of CD4, and attachment can result in more efficient infection (36). Attachment can be due to interactions between Env and cell surface molecules. Alternatively, proteins that are incorporated into the viral membrane during budding can subsequently interact with their native ligands on the cell surface. For example, ICAM-1 incorporated into the viral membrane can bind to cell surface LFA-1, enhancing infection of both cell lines and lymphoid tissue by HIV-1 (6).
A cell surface molecule that can enhance virus infection by binding to the viral Env protein is DC-SIGN (3, 17, 28). DC-SIGN is a type II integral membrane protein that is expressed on dendritic cells (DCs) and on some types of tissue macrophages, including Hofbauer cells in human placenta (17, 33, 34). In addition, DC-SIGN expression can be induced on monocyte-derived macrophages by interleukin 13 (IL-13) treatment (34). DC-SIGN forms a homotetramer and contains a carbohydrate recognition domain that preferentially binds endogenous high-mannose oligosaccharides (14, 26). While the physiological ligands for DC-SIGN include ICAM-2 and ICAM-3 (16, 18), all HIV-1, HIV-2, and simian immunodeficiency virus (SIV) strains examined to date also bind DC-SIGN (17, 29). Binding of virus to DC-SIGN can enhance HIV infection both in cis (22) and in trans (17). Thus, virus bound to a DC-SIGN-positive cell can be transmitted to a cell that expresses CD4 and an appropriate coreceptor. It has been proposed that DC-SIGN may provide the molecular explanation for the efficiency with which DCs capture HIV and transmit it to T cells during the formation of an immunological synapse (7, 19, 32, 37). In addition, it has been posited that HIV may encounter and adhere efficiently to mucosal DCs by DC-SIGN at the site of entry and then be ferried to secondary lymphoid organs where infection can proceed (17). If this model is correct, then HIV interactions with DC-SIGN could represent a new molecular target for antiviral therapy, particularly in the context of microbicides. DC-SIGN also binds the Ebola virus glycoprotein, and cells expressing DC-SIGN are infected by Ebola virus more efficiently (G. Simmons et al., submitted for publication). Thus, other pathogens may interact with DC-SIGN in a manner that affects virus tropism or pathogenesis.
In this study, we have extended our characterization of a panel of monoclonal antibodies (MAbs) directed against the carbohydrate recognition domain of DC-SIGN (21). Using a MAb directly conjugated to a fluorochrome, we found that monocyte derived DCs (MDDCs) from normal human donors express high levels of DC-SIGN, often in excess of 250,000 copies per cell. Differences in expression between individuals rarely exceeded three- to fourfold and were similar to differences in expression seen on DCs isolated at different times from the same individual. A subset of the MAbs efficiently blocked HIV interactions with DC-SIGN on two different cell lines. Binding and infection of virus pseudotypes bearing the Ebola virus glycoprotein was also inhibited. However, the MAbs reduced transmission of HIV from DCs to CD4/coreceptor-positive cell lines by a maximum of only 50%. A competitive inhibitor of DC-SIGN (mannan) reduced virus transmission from DCs by a similar amount. Thus, we conclude that while DC-SIGN contributes to the ability of DCs to capture and transmit HIV, other molecules are also likely to play a significant role.
MATERIALS AND METHODS
Cells and cell lines.
To obtain MDDCs, we purified monocytes from peripheral blood mononuclear cells by discontinuous Percoll gradient centrifugation. The low-density fraction (monocyte enriched) was further depleted of lymphocytes by a 2-h plastic adhesion step at 37°C followed by extensive washing in prewarmed culture medium. This resulted in highly purified monocytes as determined by flow cytometry using anti-CD14 (90 to 95% positive) or anti-CD11c (95 to 98% positive) MAbs. To generate immature MDDC, purified monocytes were cultured in RPMI 1640 supplemented with granulocyte-macrophage colony-stimulating factor (50 ng/ml) and IL-4 (100 ng/ml) for 7 days. Each batch of immature MDDCs was analyzed by flow cytometry and had the following phenotype: HLA-DRhigh CD11chigh CD14− CD80+ CD83− (data not shown). Previously described stably DC-SIGN- or DC-SIGNR-transfected 293 cells were used for flow cytometry, ICAM-3 and virus binding, and for virus transmission experiments (29, 30). THP cells expressing DC-SIGN were kindly provided by Dan Littman (New York University) and have been described previously (17).
Antibodies.
A phycoerythrin (PE)-conjugated goat anti-mouse Fab fragment (Caltag, Burlingame, Calif.) was used as a secondary antibody for standard fluorescence-activated cell sorter (FACS) assays. PE-conjugated DC11 was used for all quantitative FACS measurements (4). We used a previously characterized panel of MAbs specific for the lectin domain of DC-SIGN and/or DC-SIGNR (21). These MAbs were obtained by immunizing mice with murine 3T3 cells expressing human DC-SIGN or DC-SIGNR, and MAbs were produced as previously described (21). The name and isotypes of the antibodies are as follows: 506 (clone 120506, immunoglobulin G2a [IgG2a]), 507 (clone 120507, IgG2b), 516 (clone 120516, IgG2a), 518 (clone 120518, IgG2a), 526 (clone 120526 IgG2a), 531 (clone 120531, IgG1), 604 (clone 120604, IgG2b), and 612 (clone 120612, IgG2a). Alophycocyanin-conjugated HLA-DR, fluorescein isothiocyanate-conjugated CD14, fluorescein isothiocyanate-conjugated CD80, and tri-color-conjugated CD83 were all obtained from Caltag. Cy-chrome-conjugated CD11c was purchased from Pharmingen (San Diego, Calif.).
Generation of virus stocks.
Stocks of replication-competent luciferase reporter viruses were generated as described previously (29, 30). In brief, 293T cells were calcium phosphate-transfected with the proviral genomes, and the culture medium was replaced by fresh Dulbecco's modified Eagle medium (DMEM) 18 h after transfection. The culture supernatant was harvested 48 h after transfection, sterile filtered using 0.45-μm-pore-size filters, divided into aliquots, and frozen at −80°C. The amount of p24/p27 viral antigen in the supernatants was quantified using commercially available antigen capture enzyme-linked immunosorbent assay (ELISA) kits. HIV pseudotypes bearing the Ebola Zaire strain glycoprotein (EboZ-GP) were generated in a similar manner. However, 293T cells were cotransfected with an Env-defective proviral HIV genome harboring the luciferase gene in place of nef and an expression vector encoding the glycoprotein of the EboZ-GP.
Cell culture.
293T cells were maintained in DMEM supplemented with 10% fetal calf serum. T-REX cells (Invitrogen) expressing DC-SIGN or DC-SIGNR upon induction with doxycycline (Sigma, St. Louis, Mo.) were maintained in DMEM supplemented with 10% fetal calf serum, 100 μg of Zeocin (Invitrogen)/ml, and 5 μg of blasticidin (Invitrogen)/ml. Parental T-REX cells were cultivated in the same medium, but without Zeocin. CEMx174, C8166, and THP DC-SIGN as well as parental THP cells were cultured in RPMI 1640 medium containing 10% fetal calf serum and penicillin-streptomycin.
Flow cytometry and quantification.
Stably DC-SIGN- or DC-SIGNR-transfected 293 cells, treated overnight with doxycycline, or MDDCs were stained in FACS buffer (phosphate-buffered saline supplemented with 3% fetal calf serum and 0.02% sodium azide) for 30 min on ice with MAbs at a final concentration of 10 μg/ml. The samples were washed and incubated with PE-conjugated goat anti-mouse Fab fragments (Caltag) (1/100) for 30 min on ice and then washed and resuspended in FACS buffer containing 2% paraformaldehyde. The samples were analyzed with a FACScan (Becton Dickinson, San Jose, Calif.) cell analyzer using the CellQuest software for data evaluation. Dead cells were excluded on the basis of their forward- and side-scatter characteristics. For quantification we used the Quantum Simply Cellular microbead kit from Sigma according to the manufacturer's instructions and as previously described (24, 30). Briefly, quantification was performed by converting the geometrical mean channel fluorescence (GMCF) into antibody-binding sites (ABS). The kit contains a mixture of five microbead populations of uniform size, coated with goat anti-mouse antibodies, that have differing abilities to bind mouse antibodies (i.e., ranging from 0 to ∼250,000 molecules). PE-conjugated CD11 was added at saturating amounts to cells and separately to 100,000 beads. All samples including the beads were processed and analyzed identically. The binding capacities of the stained microbeads were then regressed against the corresponding GMCF of each bead population, and the GMCF of the antigen analyzed on the sample cells was converted to ABS per cell by comparison with the regression curve generated. The GMCF of the isotype control for each experiment was converted to ABS and subtracted from the ABS value obtained with the experimental sample.
ICAM-3 binding assays.
We used the assay we have previously described (2). Briefly, soluble Fc-ICAM-3 protein (R&D Systems, Minneapolis, Minn.) was iodinated by using Iodogen (Pierce). Specific activities of 500 to 2,000 Ci/mmol were obtained by using 5 μg of protein with 500 μCi of Na 125I for 20 min in 5-ml glass tubes precoated with 10 μg of Iodogen by chloroform evaporation. Radiolabeled proteins were purified from free Na 125I by separation through a 0.3-ml Dowex column prepared in a 1-ml syringe and preequilibrated in a mixture containing 50 mM HEPES (pH 7.4), 5 mM MgCl2, 1 mM CaCl2, 1% bovine serum albumin (BSA), and 150 mM NaCl. Protein fractions were eluted in the void volume of the column, and the fractions containing peaks of labeled protein were combined. Stably DC-SIGN- or DC-SIGNR-transfected cells were induced overnight with doxycycline, washed once with phosphate-buffered saline, and resuspended in binding buffer (50 mM HEPES [pH 7.4], 2 mM magnesium chloride, 2 mM calcium chloride,; 0.5% BSA). Cells (106) were incubated with 50,000 cpm of Fc-ICAM-3 for 60 min at room temperature. Cells were collected onto Brandel grade GF/B filters with wash buffer (same as binding buffer plus 150 mM sodium chloride and no BSA) using a cell harvester. Filters were counted using a Wallac Wizard 1470 automatic gamma counter. Percent binding was determined by dividing the counts from the filters by the input radioactivity after deduction of the background counts obtained on parental 293 T-REX cells.
Inhibition of DC-SIGN/DC-SIGNR engagement by HIV and SIV particles and EboZ-GP-pseudotyped HIV particles.
DC-SIGN/DC-SIGNR-expressing cells were seeded in 96-well plates and preincubated with 20 μg of the DC-SIGN/DC-SIGNR-specific MAbs or mannan/ml for 30 min. Thereafter the cells were typically pulsed with 15 ng of p24-normalized or 5 ng of p27-normalized luciferase reporter virus stocks/well for 3 h at 37°C. Unbound virus was removed by washing the cells three times with DMEM culture medium, and virus binding was quantified by lysing the cells in 1% Triton X-100 and assessing the amount of bound viral antigen by antigen capture ELISA. To determine transmission, the virus-pulsed cells were cocultivated with CEMx174 target cells and the luciferase activity in the cultures was measured 3 days after the start of the cocultivation. Blocking of EboZ-GP-mediated binding to DC-SIGN/DC-SIGNR was determined in a cis infection assay. T-REX cells were seeded in 96-well plates, induced with doxycycline to express the respective lectins, and preincubated with the MAbs as described above. Subsequently the cells were challenged with 5 ng of EboZ-GP-bearing HIV luciferase pseudotypes/well, and luciferase activity in the cultures was quantified 3 days after infection.
RESULTS
Affinities of lectin domain-specific MAbs for DC-SIGN.
We previously described the generation of eight MAbs directed against the lectin domain of DC-SIGN (21). One is specific for DC-SIGNR, four are specific for DC-SIGN, and three recognize both DC-SIGN and DC-SIGNR (Table 1). The DC-SIGN-specific MAbs also recognize rhesus macaque DC-SIGN by FACS and by immunofluorescent staining of frozen tissue sections (21). To further characterize this panel of MAbs, we determined their respective affinities for DC-SIGN or, in the case of the DC-SIGNR-specific MAb 604, DC-SIGNR. Stably transfected 293 cells were stimulated overnight with doxycycline to induce DC-SIGN or DC-SIGNR expression (29, 30), stained with different concentrations of each MAb, and processed for FACS analysis. We found that all MAbs exhibited similar affinities for DC-SIGN and/or DC-SIGNR (Table 1), with approximately 1.5 μg of each MAb/ml being needed to obtain 50% of the maximum staining intensity, and that there were no significant differences in maximum staining intensities between the MAbs (2, 23). MAbs that recognized both DC-SIGN and DC-SIGNR bound to both proteins with similar affinities (data not shown).
TABLE 1.
Affinity measurements for the DC-SIGN/DC-SIGNR monoclonal antibodiesa
| MAb | Isotype | Specificity | nEC50 (μg/ml) |
|---|---|---|---|
| DC11 | IgG1 | S/R | 1.5 ± 0.3 |
| 506 | IgG2a | S | 1.7 ± 0.4 |
| 507 | IgG2b | S | 1.3 ± 0.4 |
| 516 | IgG2a | S | 1.3 ± 0.3 |
| 518 | IgG2a | S/R | 1.3 ± 0.2 |
| 526 | IgG2a | S/R | 1.4 ± 0.4 |
| 531 | IgG1 | S | 1.6 ± 0.3 |
| 604 | IgG2b | R | 1.5 ± 0.2 |
| 612 | IgG2a | S/R | 1.5 ± 0.2 |
The specificity of the monoclonal antibodies was assessed using DC-SIGN or DC-SIGNR stably transfected 293 cells (30). The normalized 50% effective concentrations (nEC50s) were determined as previously described (2, 23) and are shown ± standard deviations derived from three independent experiments. S, DC-SIGN; R, DC-SIGNR.
DC-SIGN quantification on monocyte-derived DCs reveals very high copy numbers of DC-SIGN.
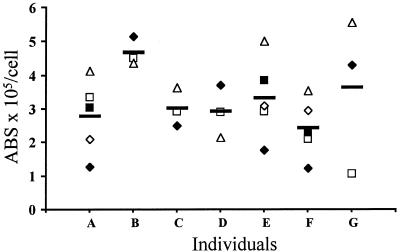
The ability of DC-SIGN to bind and transmit HIV is related in part to DC-SIGN expression levels (29). To determine if there are significant differences in DC-SIGN expression on MDDCs that could impact virus transmission efficiency, we isolated monocytes from seven different donors three to five times each. MDDCs were derived by GM-CSF and IL-4 treatment for 7 days, and the MDDCs were shown by FACS to have the expected phenotype of immature DCs, i.e., HLA-DRhigh CD11chigh CD14− CD80+ CD83− (data not shown). Maturation of MDDCs as judged by CD83 upregulation (data not shown) was achieved by exposure to 100ng of lipopolysaccharides (LPS)/ml. DC-SIGN expression levels were measured using quantitative FACS, which provides a reproducible means for converting mean channel fluorescence values into ABS through the use of synthetic beads that bind fixed numbers of antibody molecules (24). To eliminate errors associated with the use of secondary antibodies, we used MAb DC11 directly conjugated to PE at saturating levels. However, similar results were also obtained with a MAb directed to the carbohydrate recognition domain of DC-SIGN (data not shown). While there was variation in DC-SIGN expression on MDDCs from the same donor over time as well as between donors (Fig. 1), DC-SIGN was always expressed in excess of 100,000 copies per cell, and often at much higher levels. On cell lines, we have found that approximately 60,000 copies of DC-SIGN are needed for efficient virus transmission activity (29). It is important to note that the quantitative FACS assay can accurately measure ABS values to approximately 250,000. Values in excess of this are derived from projecting the standard curve beyond the last standard and so should not be deemed as definitive. These experiments show that for the individuals examined, DC-SIGN is expressed at high levels on MDDCs, with some variability over time and between individuals.
FIG. 1.
Quantitative FACS measurements of DC-SIGN on MDDCs. Monocytes were purified from peripheral blood mononuclear cells by discontinuous Percoll gradient centrifugation, and MDDCs were derived by GMCSF and IL-4 treatment for 7 days (see Materials and Methods). MDDCs were obtained from seven donors three to five times each over a period of 3 months and phenotyped, i.e., MDDCs were HLA-DRhigh CD11chigh CD14− CD80+ CD83− (data not shown). For each batch of MDDCs we quantified the surface level of DC-SIGN using PE-coupled DC11 (4) in conjunction with the Quantum Simply Cellular microbead kit (Sigma). Shown are the numbers of ABS per cell for each individual (A to G). Each measuring is represented by a symbol, and a horizontal bar shows the average number of ABS for each individual. Because of the technical characteristics of the quantification (see the text), numbers above 250,000 ABS/cell should be considered with caution.
The lectin domain-specific MAbs do not efficiently inhibit the binding of ICAM-3.
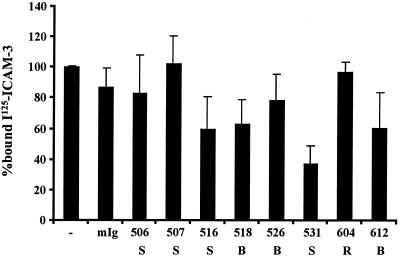
Having identified MAbs to the lectin domain of DC-SIGN and showing that all bind with similar affinities, we tested their abilities to block binding of soluble ICAM-3 to 293 cells expressing DC-SIGN or DC-SIGNR (Fig. 2). To average data between experiments, we normalized results to the positive control in each experiment. Thus, the amount of ICAM-3 or virus (Fig.2 through 6) bound to cells expressing DC-SIGN in the absence of inhibitors was set to 100%, making it possible to average results between experiments that used different ICAM-3 or virus stocks. Raw data values for representative experiments are included in the legends for Fig. 2 through 6. We found that only MAb 531 inhibited significantly the binding of ICAM-3 to DC-SIGN (by 63% ± 12%). MAbs 604 and 612 competed the binding of ICAM-3 to DC-SIGNR by 67% ± 8% and 52% ± 11%, respectively (data not shown). Thus, none of these MAbs potently inhibited binding of soluble ICAM-3 to DC-SIGN or DC-SIGNR.
FIG. 2.
Inefficient inhibition of ICAM-3 binding by the lectin domain-specific MAbs. The abilities of the lectin domain-specific MAbs to block binding of iodinated soluble, monomeric ICAM-3 to 293 cells expressing DC-SIGN is shown. Each measure represents the average from three independent experiments, each done in triplicate, ± the standard error of the mean. The number of each MAb is shown as well as its specificity. −, no MAb; mIg, mouse immunoglobulin; S, DC-SIGN specific; R, DC-SIGNR specific; B, DC-SIGN and DC-SIGNR specific. Within a representative experiment, DC-SIGN-expressing cells specifically bound 5.1% of the input material whereas preincubation with antibody 531 reduced specific binding to 1.7%. ICAM-3 was added in excess so that it would not be limiting for binding.
FIG. 6.
Inhibition of EboZ-GP engagement of DC-SIGN and DC-SIGNR. The capacity of the MAbs to inhibit the EboZ-GP-mediated infection of T-REX cells expressing either DC-SIGN (black bars) or DC-SIGNR (white bars) was assessed. T-REX cell lines were induced to express human DC-SIGN/DC-SIGNR, preincubated with 20 μg of the indicated MAbs or mannan/ml, and infected with a HIV luciferase reporter virus pseudotyped with EboZ-GP. Infection efficiency was determined by assessing the luciferase activity 3 days after infection. Infection relative to control cells is presented. The average ± standard error of the mean for three independent experiments is shown. In a single representative experiment infection of untreated T-REX DC-SIGN and DC-SIGNR cells resulted in luciferase activities of 5,135 and 8,797 cps, respectively. Preincubation with mannan diminished luciferase production to 323 cps in DC-SIGN T-REX cells and 1,424 cps in DC-SIGNR T-REX cells.
Identification of MAbs which inhibit HIV binding and transmission by DC-SIGN/DC-SIGNR.
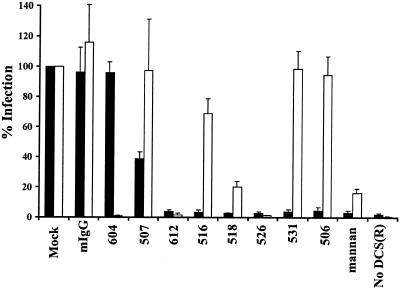
We investigated the ability of the MAbs to inhibit HIV binding and potentiation of infection in trans by DC-SIGN/DC-SIGNR-expressing cell lines in order to identify MAbs that could be used to investigate the role of DC-SIGN in virus transmission on MDDCs. T-REX cells were induced to express DC-SIGN or DC-SIGNR and were preincubated with the indicated MAbs or mannan, a carbohydrate that blocks virus interactions with both DC-SIGN and DC-SIGNR (17). The cells were subsequently pulsed with HIV-1 NL4-3 luciferase reporter virus, extensively washed, and either lysed with the amount of bound virus being quantified by p24 ELISA or cocultivated with target C8166 cells. The efficiency of virus transmission was then assessed by quantifying the luciferase activity in the cocultures 3 days later (Fig. 3A ).
FIG. 3.
Inhibition of virus binding and transfer by DC-SIGN/DC-SIGNR-specific antibodies. (A) Blocking of HIV-1 NL4-3 interaction with DC-SIGN expressed on T-REX cells. T-REX cells were seeded in 96-well plates and induced to express the indicated lectins by an overnight incubation in medium containing 0.1 μg of doxycycline/ml. The cells were preincubated in 20 μg of the indicated MAbs or mannan/ml and then pulsed with NL4-3 luciferase reporter virus for 3 h. Unbound virus was washed away and to determine binding, the cells were lysed, and the amount of bound viral antigen was quantified by p24-ELISA (solid bars). Alternatively, the virus-bearing cells were cocultivated with CEMx174 target cells, and the efficiency of virus transmission was assessed by quantifying the luciferase activity in the cultures 3 days after the addition of the target cells (white bars). Virus binding and transmission are shown relative to the values obtained for the mock control. The data represent the average ± SEM of at least three independent experiments performed in quadruplicates. In separate, representative experiments, T-REX DC-SIGN cells recovered 7.4% of the input virus in the absence of inhibitors, and a luciferase activity of 1,232 cps was measured after transmission to target cells. In contrast, T-REX cells that did not express DC-SIGN bound 0.71% of the input virus, and transmission to target cells resulted in a luciferase activity of 71 cps. (B) Blocking of HIV-1 NL4-3 interaction with DC-SIGNR expressed on T-REX cells. The experiment was performed as for panel A except that DC-SIGNR-expressing cells were used. Binding is shown by solid bars; virus transmission is shown by white bars. In a representative experiment, mock-treated DC-SIGNR cells bound 4.2% of the input virus, and transmission to target cells resulted in a luciferase activity of 1,965 cps. Control cells bound 0.71% of the input virus, and after transmission to target cells a luciferase activity of 387 cps was measured. (C) inhibition of HIV-1 engagement of DC-SIGN ex-pressed on THP cells. THP-DC-SIGN cells and parental THP cells (no DC-SIGN) were used in this experiment. Antibody blocking of NL4-3 binding and transmission was assessed as described for panel A. A representative binding experiment resulted in 8.8% of virus recovery by mock-treated THP DC-SIGN cells compared to 0.65% recovered by control cells. After THP DC-SIGN-mediated virus transmission to target cells, a luciferase activity of 7,962 cps was measured whereas transmission from control cells resulted in 251 cps.
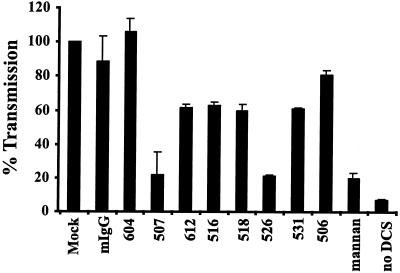
We found that five out of the eight antibodies that were tested efficiently blocked virus binding and transmission (Fig. 3A) from DC-SIGN-expressing cells, inhibiting both functions by at least 70% compared to the mock control. Preincubation with mannan inhibited binding and transmission to a comparable degree. In contrast, a control mouse immunoglobulin as well as the DC-SIGNR-specific MAb 604 and the DC-SIGN-specific MAb 507 had no significant effect on HIV binding to DC-SIGN. The MAb 518 exhibited detectable blocking activity in this cellular context, inhibiting virus binding to about 50% and transmission to about 30% relative to the mock control. HIV interactions with DC-SIGNR were efficiently blocked by the DC-SIGNR-specific MAb 604 and the dual-specific MAb 612, both of which reduced binding and transmission by at least 85% compared to the mock and mouse IgG (mIgG) controls (Fig. 3B). MAb 526 also reduced virus binding and transmission via DC-SIGNR, though less efficiently than was observed with MAbs 604 and 612.
Finally we examined HIV binding and transmission from THP cells stably expressing DC-SIGN as well as from DC-SIGN-negative, parental THP cells. DC-SIGN-positive THP cells transmitted bound virus four- to eightfold more efficiently than 293 cells despite similar expression levels. Despite this, the MAbs inhibited HIV binding to and transmission from DC-SIGN-positive THP cells more efficiently than from the inducible 293 cell lines (Fig. 3C). Seven out of eight MAbs reduced binding and transmission to background levels. Even MAb 507, which did not significantly inhibit DC-SIGN engagement on T-REX cells, reduced binding and transmission from THP cells to about 10%, whereas the MAbs 516 and 526 diminished both functions to about 3% compared to the mock control.
Antibody inhibition of SIV transmission by rhesus macaque DC-SIGN.
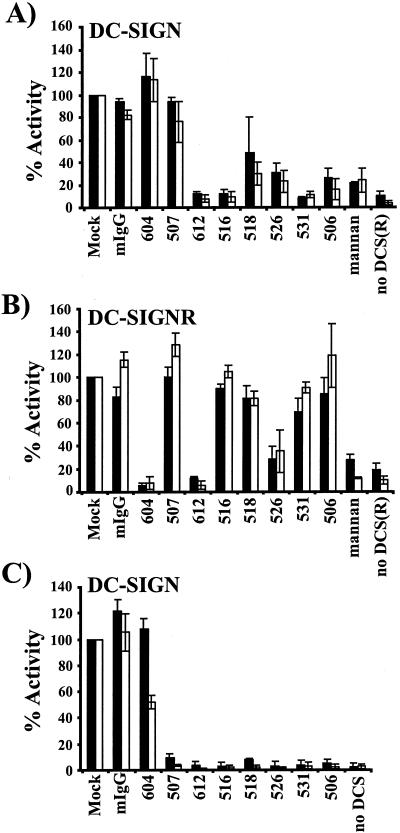
The experimental inoculation of macaques with SIV is one of the most commonly used animal models for the HIV infection in humans. DCs in the rectal and vaginal mucosa of rhesus macaques express DC-SIGN (21). The importance of DC-SIGN for virus transmission in vivo could thus be evaluated by investigating whether interference with DC-SIGN function inhibits viral spread in macaques after a vaginal or rectal challenge. We have previously shown that MAb 507 inhibits binding and transmission of SIV to and from rhesus macaque DC-SIGN (21). Here, we tested the entire panel of MAbs for the ability to inhibit SIV transmission by rhesus macaque DC-SIGN (Fig. 4). T-REX cells expressing rhesus DC-SIGN were preincubated with the indicated MAbs, challenged with SIV luciferase reporter virus bearing the M-tropic 239 MER Env protein, vigorously washed, and cocultivated with target cells. Virus transmission was assessed by quantifying the luciferase activity in the cocultures 3 days later. We found that only MAbs 507 and 526 efficiently inhibited virus transmission by rhesus macaque DC-SIGN (Fig. 4).
FIG. 4.
Inhibition of SIV transmission mediated by rhesus macaque DC-SIGN. The ability of the MAbs to inhibit transmission of a SIVmac239 MER Env reporter virus was assessed as described in the legend to Figure 3; however, T-REX cells expressing rhesus macaque DC-SIGN were used and were preincubated with 30 μg of the MAbs/ml. The infection efficiency is presented relative to mock-treated cells. The average ± standard error of the mean for three independent experiments, each performed in quadruplicate, is shown. In a representative experiment transmission from rhesus macaque DC-SIGN cells in the absence of inhibitors resulted in a luciferase activity of 1,920 cps whereas pretreatment with mannan reduced luciferase activity to 531 counts.
Virus transfer by MDDCs does not solely depend on DC-SIGN.
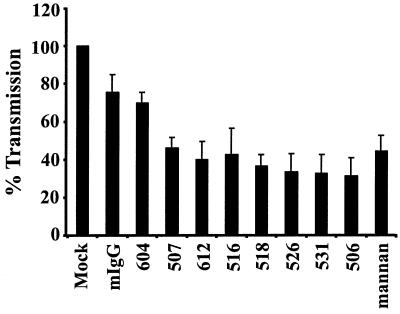
Dendritic cells express DC-SIGN, CD4, and perhaps other molecules that could serve as attachment factors for HIV. To examine the role of DC-SIGN in this process, MDDCs were matured by exposure to LPS and preincubated with the indicated MAbs or mannan. The cells were pulsed with the virus strain NL4-3. After binding for 2 h, the cells were washed extensively and cocultured with CEMssR5 target cells. Luciferase activity in the cultures was quantified 3 days later. Mannan reduced transmission of NL4-3 by only 56% compared to results with the mIgG controls (Fig. 5). MAb 506 blocked transmission with the greatest efficiency, reducing infection in trans by 69% for HIV-1 NL4-3 relative to the mIgG controls. Direct addition of virus to DCs did not result in efficient infection (data not shown), indicating that the luciferase activity in the cocultures was due to infection of the target cells. When immature MDDCs were used, inhibition of virus transmission by the MAbs and by mannan was somewhat less efficient and more variable (data not shown). Thus, even though mannan treatment as well as some DC-SIGN-specific MAbs efficiently blocked DC-SIGN-mediated virus binding and transmission to and from THP and 293 cells, inhibition of virus transmission from either mature or immature MDDCs was inefficient. These results indicate that other molecules play important roles in virus transmission from MDDCs or that the MAbs and mannan fail to inhibit virus interactions with DC-SIGN in the context of dendritic cells.
FIG. 5.
Transmission of virus from MDDCs is partially inhibited by anti-DC-SIGN MAbs. MDDCs were prepared from blood monocytes and matured by overnight incubation in medium containing LPS. The cells were pulsed with NL4-3 luciferase reporter virus. After a 1.5-h incubation the cells were washed and cocultivated with CEMssR5 cells, and luciferase activity in the cultures was determined 3 days after the start of the cocultures. CEMssR5 cells express both major HIV coreceptors, CXCR4 and CCR5. Transmission efficiency is presented relative to the mock control; the values represent the average ± standard error of the mean for three independent experiments performed in quadruplicate. Within a representative experiment a luciferase activity of 585 cps was detected after virus transmission by mock-treated cells. Pretreatment with mannan reduced luciferase activity in target cells to 245 cps.
Antibody blocking of EboZ-GP engagement of DC-SIGN and DC-SIGNR.
We recently found that DC-SIGN/DC-SIGNR bind to the glycoprotein (GP) of Ebola virus and strongly enhance infection of target cells in cis (Simmons et al., submitted). The expression of these lectins on critical targets of Ebola infection suggests that DC-SIGN/DC-SIGNR might play an important role in Ebola virus dissemination in and between hosts. We investigated whether the MAbs inhibited EboZ-GP mediated engagement of these lectins. T-REX cells expressing the indicated lectins were preincubated with the panel of MAbs or mannan and challenged with Env-defective HIV luciferase reporter viruses bearing the EboZ-GP, and luciferase activity was determined 3 days after infection (Fig. 6). Expression of DC-SIGN/DC-SIGNR rendered the cells 50- to 200-fold more permissive than parental T-REX control cells. Addition of mannan reduced infection to background levels, whereas mIgG did not significantly inhibit viral entry. Similar to the situation observed for HIV transmission, MAb 604 blocked binding to DC-SIGNR, while MAbs 612 and 526 inhibited EboZ-GP usage of DC-SIGN and DC-SIGNR. MAb 507 weakly inhibited entry into DC-SIGN-expressing T-REX cells and had no effect on infection of DC-SIGNR-positive cells. These results indicate that Ebola GP and HIV Env can efficiently interact with DC-SIGN/DC-SIGNR, leading to enhanced infection of target cells, and viral engagement of these lectins seems to require similar determinants in the respective lectin domains.
DISCUSSION
Intraepithelial and mucosal dendritic cells are among the first cells encountered by HIV during sexual transmission. Studies with rhesus macaques show that DCs in the vaginal mucosa can be infected by virus, though other infected cell types can be found as well (20, 35, 39). Virus has been found in draining lymph nodes within 18 h of intravaginal SIV exposure, indicating that virus is disseminated rapidly within the host (20). These and other studies are consistent with a model in which DCs interact with virus, migrate to draining lymph nodes, and transmit virus to CD4+ T lymphocytes (20, 25, 35). In vitro studies support the idea that DCs play an important role in the transmission and dissemination of HIV in vivo. DCs are particularly effective at capturing HIV and transferring it to adjacent CD4+ T cells, even when the DCs are not infected themselves (7, 19, 32, 37). Furthermore, virus captured by DC-SIGN can remain infectious for a prolonged period of time (17), again consistent with a model in which DCs ferry virus from a distal, mucosal site to a more proximally located secondary lymphoid organ.
The mechanisms by which DCs capture and transmit HIV are incompletely understood. However, the discovery that the C-type lectin DC-SIGN is expressed on DCs and binds to HIV-1 gp120 with high affinity represents an important first step in understanding this process (17). DC-SIGN binds to endogenous high-mannose oligosaccharides that are present on the HIV-1 Env protein as well as on ICAM-2 and ICAM-3, the normal cellular ligands for DC-SIGN (14, 16, 18, 26). The tetrameric nature of DC-SIGN may also play an important role in binding and mediating specificity, since several binding events between a ligand and DC-SIGN may be needed to confer a high-avidity interaction (26). When expressed on cell lines, DC-SIGN functions as a universal attachment factor for primate lentiviruses, binding to all HIV-1, HIV-2, and SIV strains studied to date (17, 29). Once bound to a DC-SIGN-positive cell, virus can be transmitted to cocultured cells that express CD4 and an appropriate coreceptor, recapitulating at least in part the ability of DCs to propagate virus infection in vitro.
DC-SIGN is expressed on MDDCs, a proportion of human alveolar macrophages, and decidual macrophages and Hofbauer cells in human placenta (17, 33, 34). In addition, its expression can be induced on monocyte-derived macrophages by IL-13 treatment (34). The in vivo distribution of DC-SIGN in intestinal and genital mucosae has also been examined (17, 21, 34). In Peyer's patches, DC-SIGN-positive cells are found in the interfollicular regions and on clusters of cells in the subepithelial dome regions. Numerous DC-SIGN-positive cells are found throughout the entire thickness of the rectal mucosa, while in the vaginal epithelium DC-SIGN is found on subepithelial DCs in the lamina propria (21). Thus, DC-SIGN is expressed on DCs at the major sites of HIV transmission, and it binds virus efficiently in vitro and mediates its transmission to other cells. A related molecule, termed DC-SIGNR or L-SIGN, functions in a similar manner but has a different distribution, being found on endothelial cells in the placenta, intestine, liver, and lymph nodes (5, 31).
While DC-SIGN can clearly bind and transmit virus in vitro, to what degree is it responsible for the ability of DCs to potentiate infection of T-cells? Since DC-SIGN function is related to its surface expression, with approximately 60,000 copies of DC-SIGN being needed for efficient transmission of virus from DC-SIGN-positive 293 cells (29), we measured DC-SIGN expression on MDDCs using quantitative FACS (24). If expression levels of DC-SIGN on DCs varied markedly between individuals, it could be determined whether differences in DC-SIGN expression levels correlate with differences in virus transmission efficiencies. However, at least with the small group of normal human donors we examined here, DC-SIGN was invariably expressed at high levels, with a minimum of 100,000 copies of DC-SIGN per MDDC. Variability in expression levels rarely exceeded a factor of 3 to 4, and there was as much variability in DC-SIGN expression levels between individuals as there was between cells isolated at different times from the same individual. The uniformly high levels of DC-SIGN expression on the MDDCs derived from our donor pool precluded us from drawing conclusions about DC-SIGN expression levels and virus transmission activity on this cell type.
As an alternative approach to assessing the role of DC-SIGN in binding of virus to DCs, infection of DCs, and transmission from DCs to adjoining receptor-positive cells, we screened MAbs for their ability to block virus interactions with DC-SIGN. The first panel of MAbs to DC-SIGN that we produced were generated against a bacterial fusion protein, with MAbs binding to epitopes in the repeat region predominating (4). None of these MAbs inhibited virus interactions with DC-SIGN, even though the repeat region itself is important for DC-SIGN interactions with HIV (29, 30). However, the MAbs generated against murine cells expressing human DC-SIGN or DC-SIGNR bind determinants in the carbohydrate recognition domain and so are more likely to interfere with its function as a lectin. Indeed, some of the MAbs efficiently blocked interactions between HIV and DC-SIGN when it was expressed on either 293 or THP cells. Inhibition of virus binding to DC-SIGN-positive THP cells was particularly efficient. The MAbs also prevented binding of the heavily glycosylated EboZ-GP to DC-SIGN. Interestingly, none of the MAbs efficiently blocked binding of soluble ICAM-3 to DC-SIGN, suggesting that ICAM-3 and Env interact differently with DC-SIGN. Whether this is due to differences in affinity or more substantial differences in binding interactions remains to be determined.
Despite their ability to efficiently block virus interactions with DC-SIGN on cell lines, none of the MAbs efficiently blocked transmission of virus from MDDCs to cocultured T-cell lines. At best, approximately 50% inhibition was obtained when mature MDDCs were used. Inhibition of virus transmission from immature MDDCs by the MAbs was somewhat less efficient and more variable. We also used mannan, a carbohydrate that competitively inhibits ligand binding to DC-SIGN, and found that it too reduced virus transmission activity of MDDCs by approximately 50%. We consider the most likely interpretation of these results to be that DC-SIGN, expressed at high levels on MDDCs, does indeed play an important role in HIV binding and transmission, but that other cell surface molecules that have yet to be identified are also likely to participate in these interactions. Thus, the failure of mannan to completely block virus transfer from MDDCs suggests that one or more nonlectins, or lectins with different carbohydrate specificities, account for a significant portion of the ability of MDDCs to transmit HIV.
That molecules other than DC-SIGN can mediate virus transmission from MDDCs was recently shown by KewalRamani and colleagues, who showed that rhesus macaque MDDCs efficiently transmit SIV to T-cell lines (38). Interestingly, the MDDCs expressed only low levels of DC-SIGN—probably below the levels needed to support virus transmission. Indeed, MAbs to rhesus DC-SIGN had no effect on the ability of rhesus MDDCs to transmit virus. Thus, molecules other than DC-SIGN must account for the ability of rhesus MDDCs to transmit virus to T cells. This should not, however, be taken to mean that rhesus DC-SIGN is not relevant for virus transmission in vivo. While DC-SIGN is not expressed on macaque MDDCs under the differentiation conditions examined thus far, tissue staining has shown that DC-SIGN is expressed in rhesus vaginal and rectal mucosae in a pattern similar to that seen in humans (21). It must be admitted that our understanding of the factors that control DC-SIGN expression in vitro, and its pattern of expression on specific types of DCs in vivo, is far from complete.
Regardless of the role that DC-SIGN plays in HIV transmission in vitro and in vivo, its ability to efficiently bind virus and either boost infection in cis or mediate efficient infection in trans requires a reassessment of the role virus attachment plays in virus tropism and pathogenesis in general, and not just for HIV. Mucosal DCs are likely to express an array of proteins that enable them to recognize common structural motifs (like high-mannose carbohydrate chains) present on a wide variety of endogenous molecules and pathogens. It will be interesting to determine if pathogens other than HIV take advantage of the adhesive and migratory properties of DCs to enhance their infectivity and dissemination. As for HIV and DC-SIGN, the MAbs used here represent tools that may be used in attempts to determine the relevance of this interaction for virus infection and transmission in the rhesus macaque model.
Acknowledgments
Frédéric Baribaud and Stefan Pöhlmann contributed equally to this work.
We thank Dan Littman for providing both THP and THP-DC-SIGN-positive cell lines. We thank Elizabeth Soilleux for reading the manuscript and for helpful advice. We thank Mike Malim for the CEMssR5 cells.
This work was supported by NIH R01 35383 and 40880 to R.W.D. and by the Virus and Molecular Core of the Centers for AIDS Research at the University of Pennsylvania. This work was also supported by a Burroughs Wellcome Fund Translational Research Award and an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation to R.W.D. S.P. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG). Support was also provided by the Penn Center for AIDS Research under NIH grant P30 AI45008.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Baribaud, F., T. G. Edwards, M. Sharron, A. Brelot, N. Heveker, K. Price, F. Mortari, M. Alizon, M. Tsang, and R. W. Doms. 2001. Antigenically distinct conformations of CXCR4. J. Virol. 75:8957-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baribaud, F., S. Pöhlmann, and R. W. Doms. 2001. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Baribaud, F., S. Pöhlmann, T. Sparwasser, M. T. Kimata, Y.-K.Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. Edwards, G. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashirova, A. A., T. B. Geijtenbeek, G. C. V. Duijnhoven, S. J. V. Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. V. Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (dc-sign)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 8.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 9.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. D. Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 10.Doms, R. W. 2000. Beyond receptor expression: the influence jof receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 11.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 12.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 13.Etemad-Moghadam, B., Y. Sun, E. K. Nicholson, M. Fernandes, K. Liou, R. Gomila, J. Lee, and J. Sodroski. 2000. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J. Virol. 74:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 15.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane domain, G-protein coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., D. J. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B. H., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, J. Middel, I. L. M. H. A. Cornelissen, H. S. L. M. Nottet, V. N. Kewalramani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B. H., R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T-cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jameson, B., F. Baribaud, S. Pöhlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 24.Lee, B., M. Sharron, L. Montaner, D. Weissman, and R. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96:5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, D. A., A. J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939-28945. [DOI] [PubMed] [Google Scholar]
- 27.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pöhlmann, S., F. Baribaud, and R. W. Doms. 2001. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 22:643-646. [DOI] [PubMed] [Google Scholar]
- 29.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pöhlmann, S., G. J. Leslie, T. G. Edwards, T. Macfarlan, J. D. Reeves, K. Hiebenthal-Millow, F. Kirchhoff, F. Baribaud, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus: virus binding and transfer are dissociable functions. J. Virol. 75:10523-10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pöhlmann, S., E. J. Soilleux, F. Baribaud, G. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 in cutaneous dendritic cells initiate a productive infection upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soilleux, E. J., L. S. Morris, B. Lee, S. Pöhlmann, J. Trowsdale, R. W. Doms, and N. Coleman. 2001. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 195:586-592. [DOI] [PubMed] [Google Scholar]
- 34.Soilleux, E. J., L. S. Morris, G. Leslie, J. Chehimi, J. Trowsdale, L. J. Montaner, R. W. Doms, D. Weissman, N. Coleman, and B. Lee. 2002. DC-SIGN is expressed on subsets of dendritic cells and specialized macrophages in tissue, and on a sub-population of plasmacytoid blood dendritic cells. J. Leukoc. Biol. 71:445-457. [PubMed]
- 35.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 37.Weissman, D., Y. Li, J. M. Orenstein, and A. S. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expressed CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 155:4111-4117. [PubMed] [Google Scholar]
- 38.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Z.-Q., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of, SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]