Abstract
Objective:
To identify the factors that increase mortality for either open or laparoscopic Roux-en-Y gastric bypass.
Summary Background Data:
Perioperative mortality is the most feared outcome of bariatric surgery, reported to occur in between 0.5% and 1.5% of patients.
Methods:
The bariatric database at Virginia Commonwealth University was queried for patients who had undergone either an open gastric bypass (O-GBP) or a laparoscopic gastric bypass (L-GBP). A multivariate logistic regression analysis to identify factors related to perioperative mortality was performed. Factors examined included age, gender, body mass index, preoperative weight, hypertension, diabetes mellitus, sleep apnea, obesity hypoventilation syndrome, venous stasis ulcers, intestinal leak, small bowel obstruction, and pulmonary embolus.
Results:
Since 1992, more than 2000 patients had either an O-GBP (n = 1431) or a L-GBP (n = 580). Of the O-GBP, 547 patients had a proximal GBP (P-GBP) and 884 superobese (body mass index > 50 kg/m2) patients had a long-limb GBP (LL-GBP). The differences in patient demographics, complications, and perioperative mortality rates between L-GBP and O-GBP and P-GBP and LL-GBP patients were examined. Overall, the independent risk factors associated with perioperative death included leak, pulmonary embolus, preoperative weight, and hypertension.
Conclusions:
The risk factors for perioperative death can be separated into patient characteristics and complications. The access method, open versus laparoscopic, was not independently predictive of death, but the operation type, proximal versus long limb, was predictive. The data do not suggest that superobese patients should not undergo surgery, as they are high risk for early death due to their body weight and comorbidities without surgery. Surgery should not be reserved as a desperate last measure for weight loss.
The bariatric database at Virginia Commonwealth University was queried for patients who had undergone either an open gastric bypass or a laparoscopic gastric bypass. A multivariate logistic regression analysis to identify factors related to mortality was performed. The independent risk factors associated with death for the overall series included leak, pulmonary embolism, preoperative weight, and hypertension.
More than half of Americans are overweight, and more than 1 in 5 are obese.1 The prevalence of obesity has tripled in the last 30 years. This has resulted in significant costs to society both in lost productivity and increased health expenditures. It is estimated that 300,000 deaths a year are related to obesity and close to $100 billion are spent on obesity-related health care costs.2 Diet and exercise therapy are frequently associated with weight loss failure.3 Currently, surgery offers the only effective long-term weight loss therapy for morbidly obese patients. Increased media attention in the United States as well as the newer option of laparoscopic treatment has led patients and surgeons to embrace surgical options in unprecedented numbers, particularly the option of laparoscopic Roux-en-Y gastric bypass (L-GBP).
The reported incidence of perioperative mortality varies between 0% and 1.5% in series of open Roux-en-Y gastric bypass (O-GBP)4–6 and L-GBP.7–10 With the increasing popularity and performance of the GBP, it is clear that the operative mortality for this procedure will attract greater public scrutiny. No prior population-based study has been able to delineate independent predictors of death. Two large series have defined risk factors for complications but were unable to do the same for mortality.11,12 Livingston et al did show a significantly higher mortality in patients older than 55 years, but he was unable to show that age was ultimately predictive of mortality.11
It is important to define predictors of mortality so that surgeons can give potential patients better risk information, obtain more accurate informed consent, and possibly avoid unacceptably high-risk operations. Death after GBP is infrequent, and accurate risk assessment requires a large series of patients. We used a large prospective database of more than 2000 gastric bypass procedures over a 10-year experience, including O-GBP and L-GBP, to define independent predictors for early death using a multivariate logistic regression analysis. The results should benefit surgeons, patients, and the general public in understanding the mortality risk for this operation.
MATERIALS AND METHODS
The database of 2011 patients who had undergone either O-GBP or L-GBP at Virginia Commonwealth University hospitals from 1992 to February 2003 was analyzed. Since the database was started in 1987, it has been prospectively maintained and updated based on the patients' in-hospital and clinic records. Institutional Review Board approval was obtained for collecting the data in a secure database and reporting on its analyses. Patients were considered eligible for surgery for obesity according to the 1991 NIH Consensus Conference guidelines13 if their body mass index (BMI, kg/m2) was ≥ 35 kg/m2 associated with obesity comorbidity or ≥ 40 kg/m2 with or without comorbidity.
The database maintained information on age, gender, preoperative weight, preoperative BMI, patient comorbidities (hypertension, diabetes mellitus, sleep apnea, obesity hypoventilation syndrome, and venous stasis ulcers), complications (intestinal leak, small bowel obstruction, pulmonary embolus, and early death), and the type of surgery (O-GBP, L-GBP, proximal GBP [P-GBP], or long-limb GBP [LL-GBP]). The diagnosis of diabetes mellitus required an elevated fasting blood sugar (≥ 150 mg/dL) and either a “diabetic diet” recommended by their primary care physician, oral hypoglycemic medications, or insulin treatment. Hypertension required a sitting blood pressure at the time of their initial visit of ≥ 150 mm Hg systolic and/or ≥90 mm Hg diastolic (using a wide blood pressure cuff taken with an automatic sphygmomanometer) or use of antihypertensive medications. Sleep apnea required a respiratory disturbance index ≥ 10 hypopneic and/or apneic episodes/hour of sleep. Obesity hypoventilation syndrome was defined as a PaO2 ≤ 55 mm Hg and/or PaCO2 ≥ 65 mm Hg. Venous stasis ulcers required the presence or history of pretibial venous stasis ulcers. Leak was defined as any anastomotic disruptions (either at the gastrojejunostomy or the jejunojejunostomy), intestinal perforations, or staple line disruptions.
The data were evaluated to find independent factors related to early death. Early deaths were defined as deaths that occurred within 30 days of the initial procedure or as a direct result of a complication of the original procedure, if death occurred more than 30 days after the original procedure. The patient database was examined for patients that had undergone either O-GBP or L-GBP during the time period. The O-GBP patients had undergone either a P-GBP or LL-GBP procedure. The superobese O-GBP patients (BMI ≥ 50 kg/m2) underwent an LL-GBP with a 150-cm Roux limb.14 The P-GBP Roux limb measured 45 cm. Any open revisions were excluded. The L-GBP group was composed of all L-GBP regardless if they were de novo procedures or revisions from prior obesity or antireflux surgery. The Roux limb length in the L-GBP group was variable and depended on the attending surgeon's preference but was generally less than 100 cm.
Univariate analysis and multivariate logistic regression analysis were performed to identify risk factors for early death. Analysis of variance, Fisher exact t tests, and χ2 tests were performed. P < 0.05 were considered significant.
RESULTS
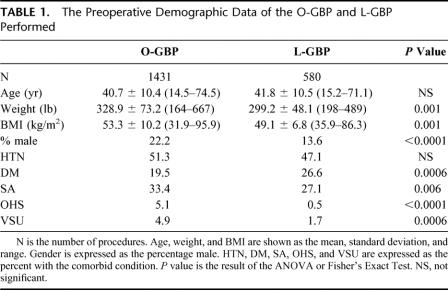
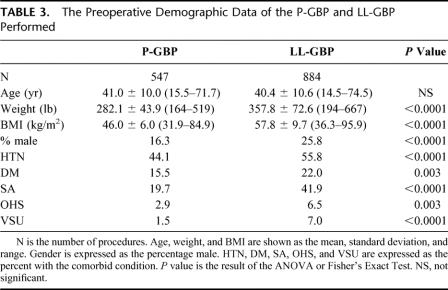
Table 1 compares the demographic information of the O-GBP and L-GBP groups. O-GBP has a higher incidence of male gender and heavier patients. The incidence of hypertension is comparable between the 2 groups. Except for diabetes mellitus, which is found more frequently in the L-GBP group, the incidence of the rest of the recorded comorbid conditions is significantly higher in the O-GBP group compared with L-GBP. Despite this, the rates of life-threatening postoperative complications (ie, leak, small bowel obstruction, and pulmonary embolus) are similar between the 2 groups (Table 2). Leak approaches, but falls just short, of a statistical difference between the 2 groups (P = 0.0506). Mortality is significantly higher in the O-GBP group.
TABLE 1. The Preoperative Demographic Data of the O-GBP and L-GBP Performed
TABLE 2. The Rate of Postoperative Complications of the O-GBP Compared with the L-GBP
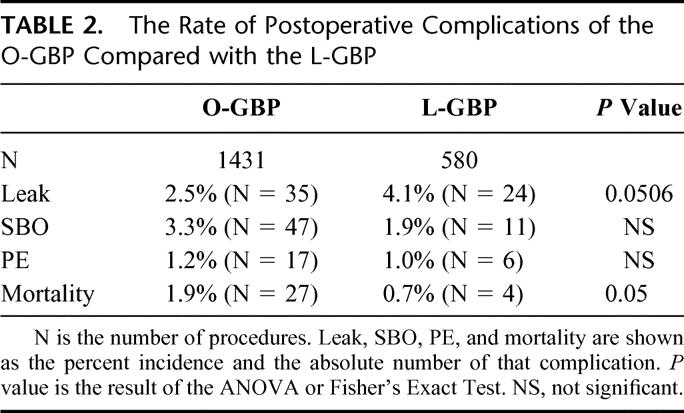
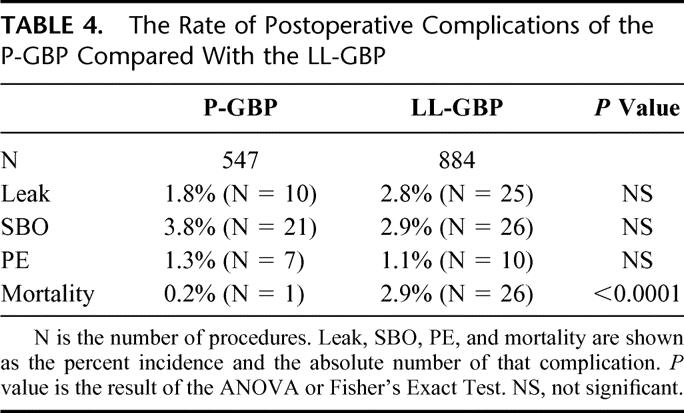
Within the O-GBP group, there are 2 distinct and separate groups. Table 3 shows the demographic data for the P-GBP and the LL-GBP patients. The LL-GBP patients were bigger (BMI 57.8 kg/m2 vs. 46.0 kg/m2, P < 0.0001), had a significantly higher proportion of males (25.8% vs. 16.3%, P < 0.0001), and had a significantly higher incidences of hypertension, diabetes mellitus, sleep apnea, obesity hypoventilation syndrome, and venous stasis ulcers when compared with the P-GBP patients. Surprisingly, there was no difference in the rate of complications between the 2 groups (Table 4). The mortality rates were statistically different (0.2% vs. 2.9%, P < 0.001). The P-GBP group had 1 death compared with 26 deaths in the LL-GBP group.
TABLE 3. The Preoperative Demographic Data of the P-GBP and LL-GBP Performed
TABLE 4. The Rate of Postoperative Complications of the P-GBP Compared With the LL-GBP
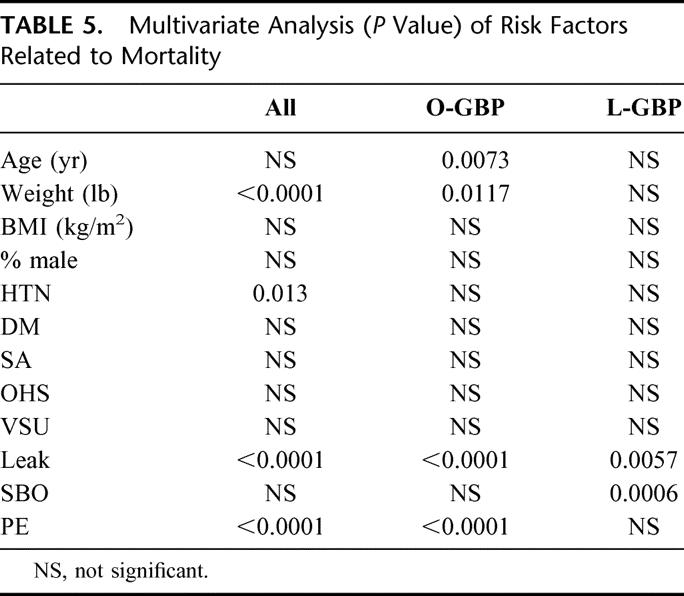
The overall mortality from all causes in the population was 1.5% (31 deaths); 0.7% (n = 4) in L-GBP, 1.9% (n = 27) in O-GBP, 0.2% (n = 1) in P-GBP, and 2.9% (n = 26) in the LL-GBP group. Multivariate logistic regression analysis identified the independent risk factors associated with early death as leak, pulmonary embolus, preoperative weight, and hypertension (Table 5). Analysis within the O-GBP group identified leak, pulmonary embolus, age, weight, and the operation type (ie, P-GBP vs. LL-GBP). Leak and pulmonary embolus were the independent predictors for death in the L-GBP group.
TABLE 5. Multivariate Analysis (P Value) of Risk Factors Related to Mortality
DISCUSSION
This study uses a multivariate statistical analysis of a large database from a tertiary care center, which has specialized in bariatric surgery for more than 2 decades. This patient set contains an unusually high percentage of high-risk patients, such as those with obstructive sleep apnea, obesity hypoventilation, and high body mass indexes.
Certain inherent selection biases are evident. For example, during the first half of the period of this study, all patients with BMI > 50 kg/m2 had an open LL-GBP. During the early years of the L-GBP, the superobese male was frequently excluded from this operation.
Livingston et al,11 reporting on a cohort of 1067 patients, found that male gender was the only factor independently predictive of “severe life-threatening adverse outcomes” by multistep logistic regression. Although noting that the mortality rate was threefold higher in patients older than 55 years, they did not report that age was independently predictive of mortality. This contrasts with our own series, in which age was predictive of a higher mortality risk in the open gastric bypass group despite not having any deaths in patients older than 60 years (n = 65, 3.2% of all patients).15
Liu et al16 recently reported that case volume loads, along with patient gender and comorbidity, significantly affect complication rates in their demographic study of bariatric surgery in California. Specifically, hospitals in which 0 to 50 or 50 to 99 gastric bypasses were performed annually were more likely to have complications (odds ratios, 2.72 and 2.70, respectively) than those in which more than 200 such operations were performed.
In a similar study in Pennsylvania, Courcoulas et al17 noted a 0.6% overall mortality rate in 4685 gastric bypass operations between 1999 and 2001. They reported an operative mortality rate of 5% in surgeons performing fewer than 10 procedures per year, compared with a 0.3% mortality rate for high-volume surgeons (P = 0.06). Adverse outcomes were 17.4% and 14%, respectively (P < 0.05) for these two groups of surgeons.
Our own study, based on more than 2000 patients at a single institution, found that preoperative weight, hypertension, postoperative leak, and pulmonary embolism were independently predictive of death across the entire series. In the open gastric bypass cohort, preoperative weight and age were predictive, as well as postoperative leak and pulmonary embolism. The apparent predictive nature of short-limb versus long-limb bypass in the open group is suspect, since the choice of operation was based entirely upon preoperative BMI. Only postoperative leak and pulmonary embolism were predictive of operative mortality in the laparoscopic group.
This information is important in providing prospective patients with accurate risk information. It may also be useful to surgeons in the “learning curve” phase of gastric bypass surgery in avoiding operating on those patients with excessive mortality risks. The finding that risks in open gastric bypass are significantly higher for older patients and for those with higher preoperative weights and hypertension is an argument for offering gastric bypass at younger ages and for patients with lower weights and less comorbidity. Whether laparoscopic gastric bypass, which has now been expanded to nearly all patient categories, will result in lower operative mortality rates in the future remains to be seen.
Discussions
Dr. Ken G. MacDonald, Jr. (Greenville, North Carolina): This paper looks at risk factors for perioperative mortality by analyzing a large group of patients over a fairly long period of time. And this isn't an easy task, because we have recently tried it. The analysis is complicated by different techniques which have evolved over that decade, different instrumentation for sure, and probably some significant differences in pre- and postoperative care, although it tried to limit the time period somewhat to minimize those effects.
The results are similar to our own experience at ECU, as a matter of fact within a 10th of a percentage point, that heavier patients have increased co-morbidity and therefore increased perioperative mortality. Further, this paper showed that the open gastric bypass group with even less leaks and equivalent rates of pulmonary emboli had over twice the mortality of the laparoscopic gastric bypass.
My first question for you, Dr. DeMaria, is, can you—you did somewhat, but can you stratify the possible explanations for this in terms of importance? In other words, is this due more to the fact that many of the open operations were obviously done in the earlier years of this analysis or that open operations included the higher percentage of super obese which they did and the higher percentage of males? And do they still tend to be more technically challenging patients, those who have had upper abdominal surgery or other things? Can you in any way stratify the relative importance of these variables?
The difference in mortality between the long limb group and the proximal gastric bypass in the open group was very significant with a 0.2% mortality for the proximal and a 2.9% for the long limb. These seem easy to explain. The long limb was significantly heavier, with a mean BMI of 58 versus 46 in the proximal group. It had significantly more males and had a significant higher percentage of all the co-morbidities.
The second question, can you tell by looking at the characteristics of each mortality group which variables are more important as predictors of mortality? Is BMI more significant in the male sex or is the presence of sleep apnea or obesity hyperventilation more important than some of the other co-morbidities? Again the hypertension is an interesting new variable. And I wonder if this is just an aberrancy or if that is going to turn out to be as important as the pulmonary diseases, which has not been our experience.
Finally, did you look at the effects of race as an independent factor in predicting mortality? When we looked at our long-term experience at ECU since 1979, we found surprisingly that African-American males had greater than twice the mortality of white males and that African-American females had three times the mortality of white females. We haven't yet been able to show definitively whether this is due to increased PMI or co-morbidities in the these groups, or in the African-American group or due to other less obvious differences.
We need to be as specific as possible in determining risk factors for mortality, like Dr. DeMaria said, to obtain accurate and honest informed consent, and also to choose appropriate candidates for surgery. I predict that we are going to find out that the patient groups with increased operative mortality are also at correspondingly increased risk for mortality if they don't have surgery. I think that we need further analyses to determine if the benefits of surgery in terms of future increased survival in these high risk groups justify the increased risk of surgery. Intrinsically that appears to be so, and it is my bias. But we need to show it better. Dr. DeMaria, I would appreciate any comments you may have about this. And do you have any preliminary opinions based on your data? Like all good papers, this work probably raises more questions than it answers.
Dr. Michael L. Hawkins (Augusta, Georgia): I ask about hypertension, whether this was controlled or uncontrolled type hypertension, and whether there was any relationship with the medical management and/or compliance of the patients that have hypertension.
Dr. J. Patrick O'Leary (New Orleans, Louisiana): Dr. DeMaria, I want to congratulate you and Dr. Sugerman and the rest of your group.
You have showed that the strongest predictor of mortality was the presence of a complication. We all know that once you get on that “slippery slope” of a complication, especially a septic complication in the seriously obese individual, that their chance of survival goes substantially down. I wonder if you looked at your data as to what intrinsic characteristics would predict that a patient would get a complication thereby placing them at an increased risk of death.
Finally, smoking wasn't listed. Dr. Leonard Furlow, an avid antitobaccoist, asked me, “Wouldn't you think that smoking in these patients might be a predictor of a less than an ideal outcome?” Your comments, please.
Dr. J. Bradley Aust (San Antonio, Texas): What were the albumin levels?
Dr. Achilles A. Demetriou (Los Angeles, California): This is one of the most commonly performed general surgical operations at our institutions today. I have two questions.
One, have you gone back and looked at the use of beta blockers in these patients and were the hypertensive patients identified pre-op, optimized before undergoing the operation?
Two, how do you manage, based on your data now, your new patients you treat with obesity surgery surgery when they are hypertensive? Do you send them to the cardiologist or an internist? Is there a protocol that you are following? Because this is a very important issue that came out of this initial trial.
Dr. Gregory A. Timberlake (Jackson, Mississippi): Dr. DeMaria, I enjoyed your presentation very much. I have been struck, as you said that when reading the newspapers, listening to TV, etc., everyone is talking about this epidemic of obesity. We as surgeons have talked in the past about the epidemic of trauma and the need for surgeons to be involved in prevention. What is the role of the surgeon in this epidemic in the future? Are we going to just be treating the disease after it occurs? Or is there some role we should be taking now in preventing this new epidemic? Because, to be honest, if your experience and the national news sources are correct, I don't want to end my career as a bariatric surgeon, overwhelmed by the tidal wave of obesity.
Dr. Eric J. Demaria (Richmond, Virginia): Dr. MacDonald mentioned looking at the mortality stratified over the entire ten years and whether we see a difference over time. Clearly there is a decrease. But we have been a program that has shifted from 100% open gastric bypass to 95% laparoscopic over the last four or five years. That is clearly a confounding matter and one of the reasons that we limited our experience review to ten years, so that we could try to rule out some of the changes in critical care. But obviously we feel very strongly that a good solid critical care program is part of a comprehensive obesity surgery program.
We have not independently evaluated race and the influence of the African-American patient, although it would not surprise me that they are somehow involved in higher mortality risks. We have also seen problems with long-term weight loss outcomes in that group.
As far as other complications of obesity, co-morbidities rather, we see that some of these correlate with increased complications. For example, we have a paper in press in Surgical Endoscopy looking at a multivariate analysis of intestinal leak in our overall 20-year experience. And in this group, for example, obstructive sleep apnea increases the risk of intestinal leak. The mechanism of that, we also do not know whether it is a pressure-related phenomenon in the digestive system or again the same type of patient characteristic which comes out, which is male patients and higher body mass index patients have higher complications. Part of this is clearly technical in nature. For example, the enlarged liver of the massively obese male patient, the amount of visceral fat and so forth.
Questions were asked about hypertension and how it was managed. This was preoperatively identified in the patient's evaluation. We would often refer patients for cardiologic evaluation with this co-morbidity. However, we must recognize the limitations of that specialty and optimization of the patients. Many patients who weigh more than 350–400 pounds can't have a cardiac catheterization because they will break the equipment. And many times we are the last resort for patients like this, and we operate with the famous recommendation, “Avoid hypotension, please,” from the cardiologist. So it is not a very satisfying situation for us.
The comment by Dr. O'Leary about smoking is a very good one and brings up the point that for many years now we have mandated a smoking cessation for all patients undergoing obesity surgery. With that intervention we noticed a dramatic decrease in postoperative pneumonia in our open as well as laparoscopic patients. I think we have had maybe one pneumonia in our entire laparoscopic experience with that. So that is part of our usual protocol.
Unfortunately, I don't have albumin levels to share in this population of patients. We don't often notice an abnormal albumin preoperatively.
Finally, in regards to future of obesity treatment and what role surgery may have, I think we would all like to believe that ultimately the cure for obesity is not going to be an operation on the stomach of the patient. We do not know the future as far as how many years away we may be from specific therapy for this heterogeneous disease, which includes important genetic factors as well as behavioral factors. But at least at this point in the history of obesity treatment we have an epidemic out of control and an operation that provides value and cure for patients, and I think that is meaningful at this point in time.
Footnotes
Reprints: Eric J. DeMaria, MD, Department of Surgery, Virginia Commonwealth University, Minimally Invasive Surgery Center, Gateway Building, Basement B-217, P.O. Box 980428, Richmond, VA 23298. E-mail: edemaria@hsc.vcu.edu.
REFERENCES
- 1.Flegal KM, Carroll MD, Kuczmarsski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes. 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. [DOI] [PubMed] [Google Scholar]
- 3.Johnson D, Drenick EJ. Therapeutic fasting in morbid obesity. Arch Intern Med. 1977;137:1381–1382. [PubMed] [Google Scholar]
- 4.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass. Am J Surg. 1996;171:74–79. [DOI] [PubMed] [Google Scholar]
- 5.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult onset diabetes mellitus. Ann Surg. 1995;222:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-en-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93–100. [DOI] [PubMed] [Google Scholar]
- 7.Champion JK, Hunt T, Delisle N. Laparoscopic vertical banded gastroplasty and Roux en-Y gastric bypass in morbid obesity. Obes Surg. 1999;9:123–130.10391724 [Google Scholar]
- 8.DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer P, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittgrove AC, Clark WC. Laparoscopic gastric bypass, Roux en-Y–500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–239. [DOI] [PubMed] [Google Scholar]
- 11.Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason EE, Renquist KE, Jiang D. Perioperative risks and safety of surgery for severe obesity. Am J Clin Nutr. 1992;55(suppl):73–76. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health. Gastrointestinal surgery for severe obesity: National Institutes of Health consensus development conference statement. Am J Clin Nutr. 1992;55(suppl):615–619. [DOI] [PubMed] [Google Scholar]
- 14.Brolin RE, Kenler HA, Gorman JH, et al. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg. 1992;215:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugerman HJ, DeMaria EJ, Kellum JK, et al. Effects of bariatric surgery in older patients. Ann Surg. (in press). [DOI] [PMC free article] [PubMed]
- 16.Liu JH, Zingmond D, Etzioni DA, et al. Characterizing the performance and outcome of obesity surgery in California. Am Surg. 2003;69:823–828. [PubMed] [Google Scholar]
- 17.Courcoulas A, Schuchert M, Gatti G, et al. The relationship of surgeon and hospital volume to outcome after gastric bypass surgery in Pennsylvania: a 3-year summary. Surgery. 2003;134:613–621. [DOI] [PubMed] [Google Scholar]