Abstract
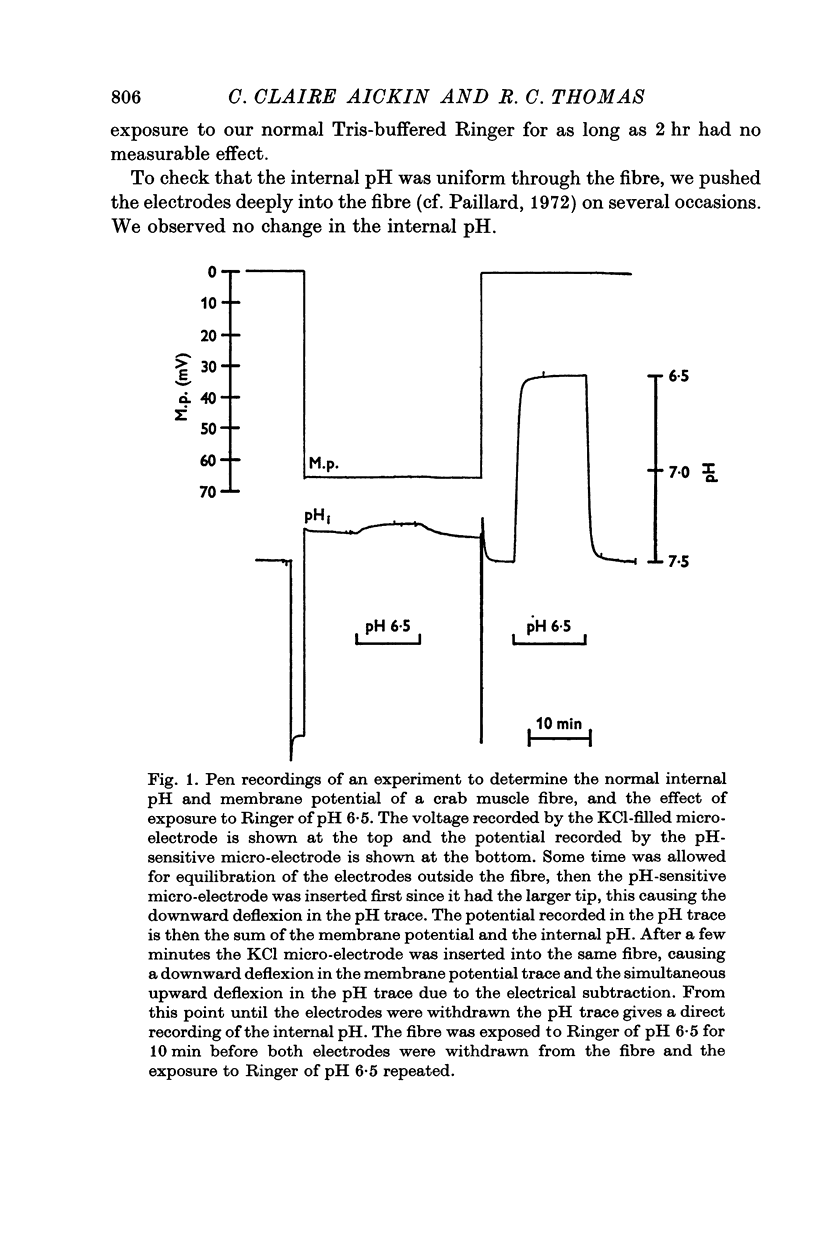
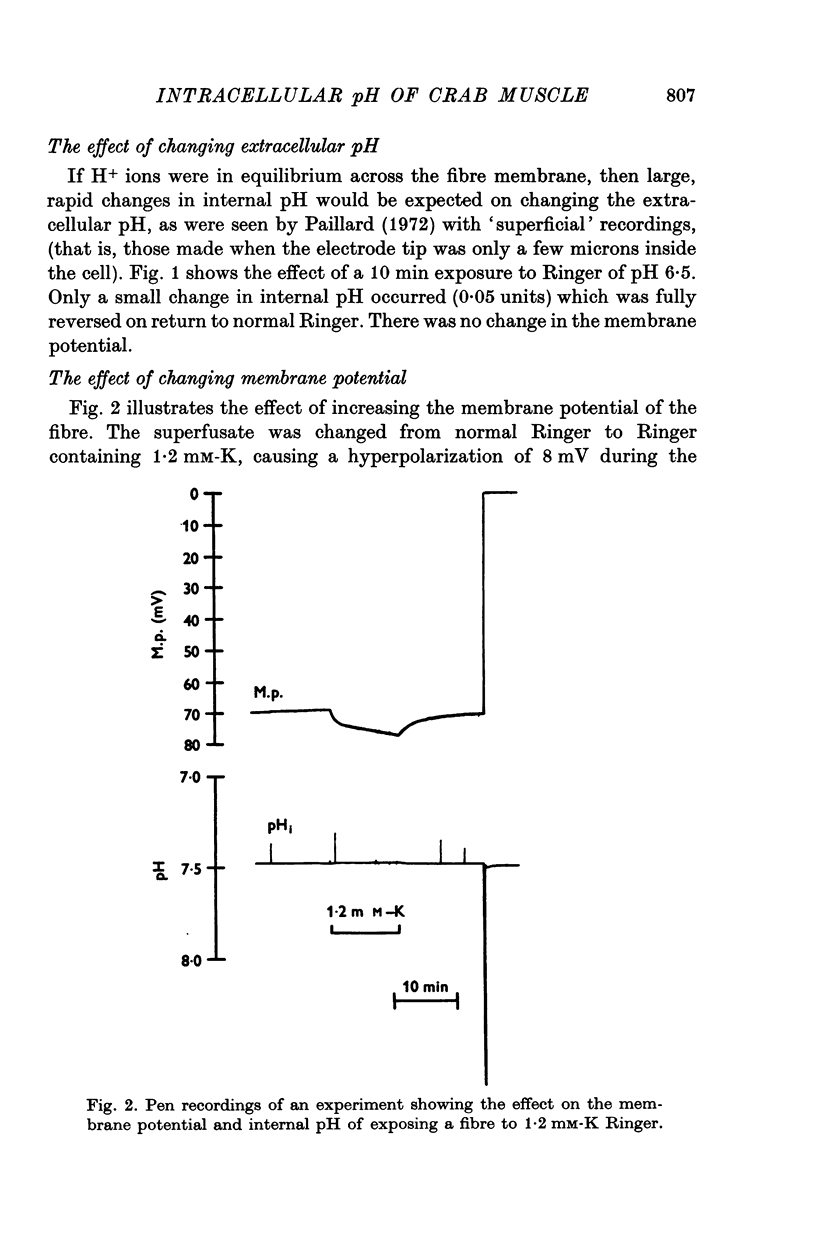
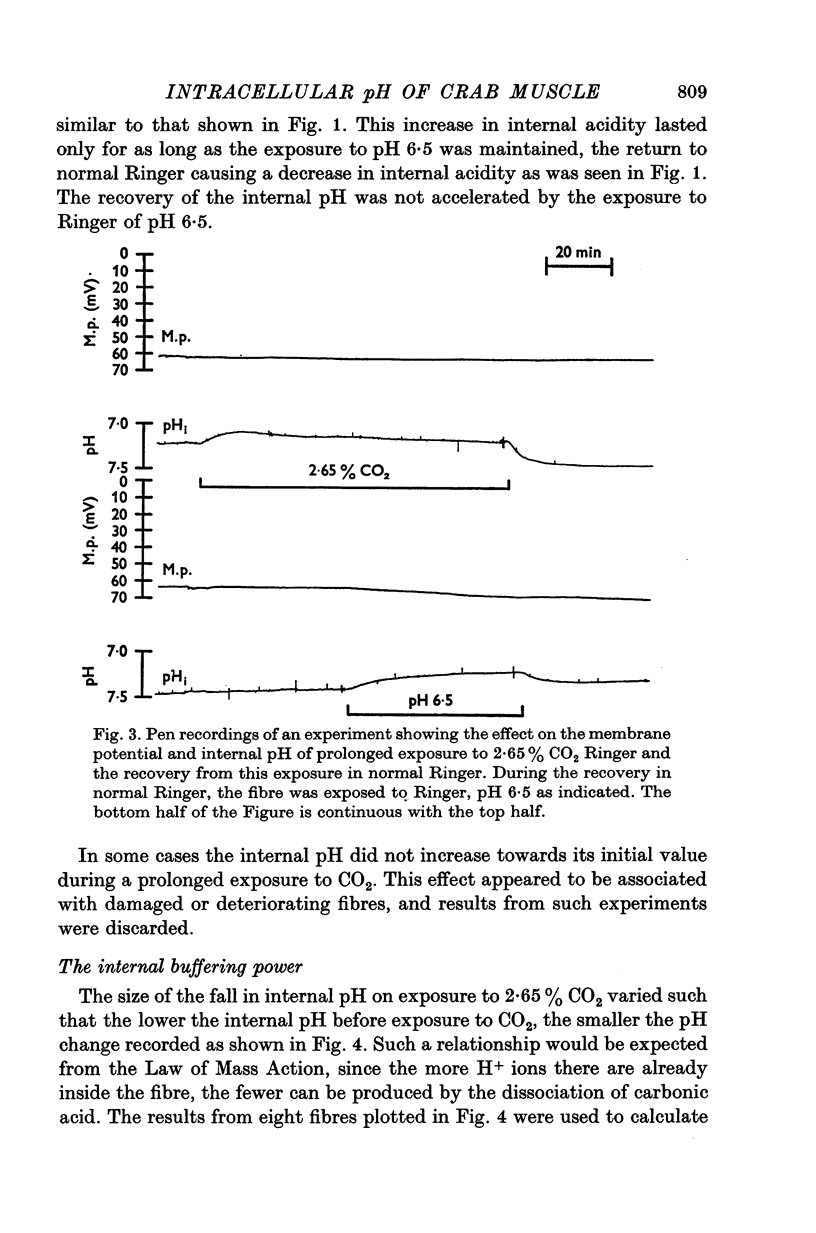
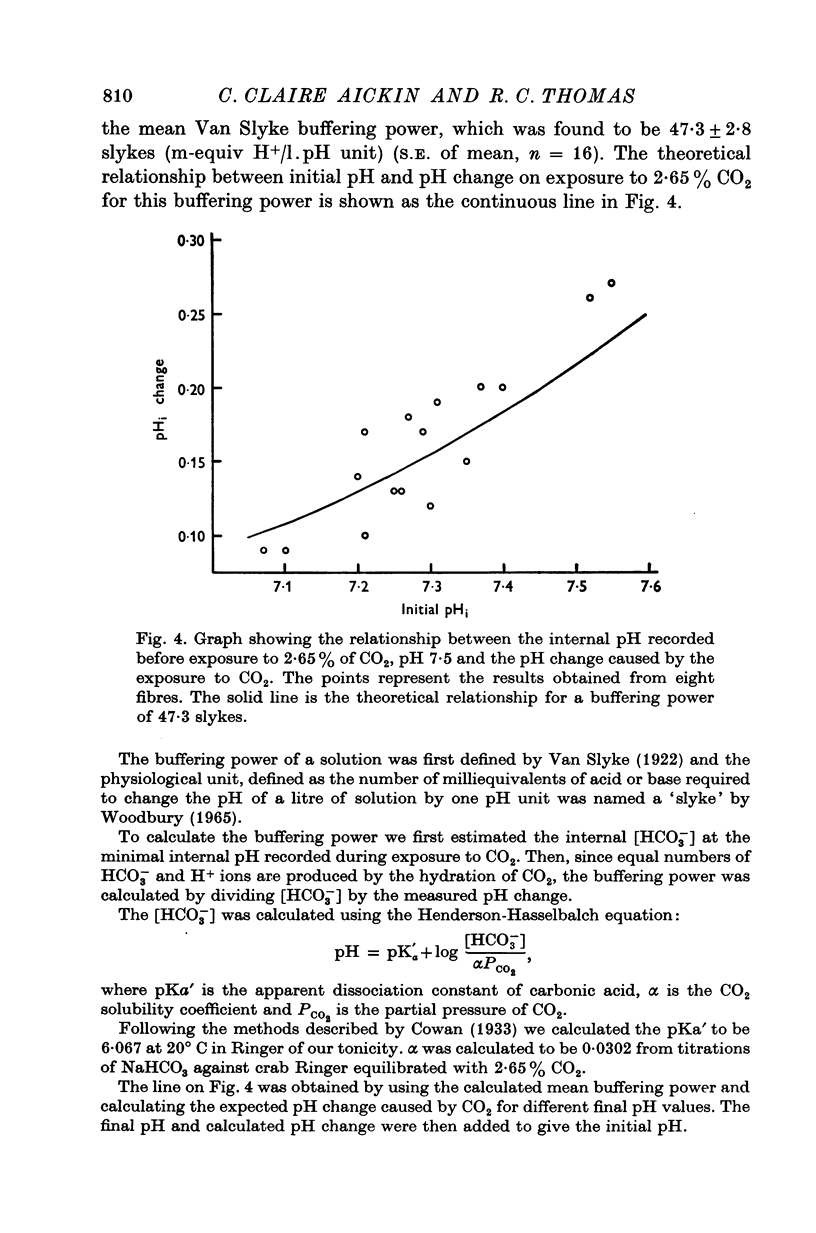
The internal pH of crab muscle fibres was measured using recessed-tip pH-sensitive micro-electrodes. Immediately following electrode penetration the mean internal pH was 7-21 +/- 0-02 (S.E. of mean) and the mean membrane potential was -64-9 +/- 0-6 mV (S.E. of mean). If H+ ions were passively distributed across the fibre membrane the internal pH would have been 6-39. 2. The internal pH tended to rise before stabilizing at a mean value of 7-27 +/- 0-02 (S.E. of mean). The difference between immediate and stabilized values is highly significant and suggests acid injury on electrode penetration. 3. Changing the membrane potential or external pH had only small, slow effects on internal pH. 4. External CO2 caused a large and rapid decrease in internal pH. With low concentrations of CO2, the effect was dependent on the initial pH as predicted by the Law of Mass Action. During a long exposure to 2-65% CO2 at pH 7-5, the internal pH returned slowly to its previous value, suggesting active transport of H+ (or OH- or HCO3-) ions across the fibre membrane. 5. The internal buffering power calculated from the response to 2-65% CO2 was 47-3 +/- 2-8 slykes (m-equiv H+/pH unit per l.) (S.E. of mean).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH of skeletal muscle with pH-sensitive glass microelectrodes. J Clin Invest. 1967 Jun;46(6):920–933. doi: 10.1172/JCI105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. L., Brown E. B., Jr In vivo CO-2 buffer curves of skeletal and cardiac muscle. Am J Physiol. 1966 Dec;211(6):1309–1312. doi: 10.1152/ajplegacy.1966.211.6.1309. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler N., Piiper J. Determination of intracellular buffering properties in rat diaphragm muscle. Am J Physiol. 1972 Mar;222(3):747–753. doi: 10.1152/ajplegacy.1972.222.3.747. [DOI] [PubMed] [Google Scholar]
- LIGOU J. C., NAHAS G. G. Comparative effects of acidosis induced by acid infusion and carbon dioxide accumulation. Am J Physiol. 1960 Jun;198:1201–1206. doi: 10.1152/ajplegacy.1960.198.6.1201. [DOI] [PubMed] [Google Scholar]
- Paillard M. Direct intracellular pH measurement in rat and crab muscle. J Physiol. 1972 Jun;223(2):297–319. doi: 10.1113/jphysiol.1972.sp009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIESJO B. K. The bicarbonate/carbonic acid buffer system of the cerebral cortex of cats, as studied in tissue homogenates. II. The pKI'of carbonic acid at 37.5 degrees C, and the relation between carbon dioxide tension and pH. Acta Neurol Scand. 1962;38:121–141. doi: 10.1111/j.1600-0404.1962.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]


