Abstract
The cellular inhibitor of apoptosis 2 (cIAP2/HIAP1) is a potent inhibitor of apoptotic death. In contrast to the other members of the IAP family, cIAP2 is transcriptionally inducible by nuclear factor-κB in response to multiple triggers. We demonstrate here that cIAP2−/− mice exhibit profound resistance to lipopolysaccharide (LPS)-induced sepsis, specifically because of an attenuated inflammatory response. We show that LPS potently upregulates cIAP2 in macrophages and that cIAP2−/− macrophages are highly susceptible to apoptosis in a LPS-induced proinflammatory environment. Hence, cIAP2 is critical in the maintenance of a normal innate immune inflammatory response.
The activation of the nuclear factor-κB (NF-κB) family of transcription factors rapidly induces the upregulation of inflammatory and antiapoptotic genes including the cellular inhibitor of apoptosis 2 (cIAP2; also known as HIAP1 or BIRC3) (25). The ciap2 gene was first identified as a member of the evolutionarily conserved IAP family of proteins (14) that are critical repressors of apoptosis. In addition, cIAP2 is a highly inducible gene that, along with cIAP1, is a component of the tumor necrosis factor receptor 2 (TNFR2) complex and, therefore, is a constituent of the TNF alpha (TNF-α) signaling pathway (19). cIAP2 has been demonstrated to inhibit cell death by directly repressing the proapoptotic activity of a family of cysteine proteases (25), caspases, as well as targeting proapoptotic components of the TNF-α signaling pathway for ubiquitin degradation (17). Despite these findings, the precise antiapoptotic mechanisms, as well as a pathophysiological role for cIAP2 has yet to be determined.
The extent of cytokine response to inflammatory agents, such as lipopolysaccharide (LPS; the biologically active element of the bacterial gram-negative membrane component endotoxin), is regulated by NF-κB. Under normal conditions an inflammatory response is beneficial in controlling invading pathogens and in clearing debris. However, a systemic activation of host macrophages by LPS can induce a hyperinflammatory response resulting in pathogenic endotoxic shock (23). LPS-induced activation of macrophages is typically associated with the production of inflammatory mediator cytokines such as TNF-α and interleukin-1β (IL-1β) (11). These cytokines act synergistically in the initiation of the inflammatory cascade of sepsis (2), resulting in hypotension, tachycardia, systemic edema, disseminated intravascular coagulation, and finally multiple organ system failure.
LPS specifically binds the macrophage cell surface receptor, CD14 (6), which subsequently interacts with the Toll-like receptor 4 (TLR4) (1). TLR4 next recruits the Toll-adaptor protein, myeloid differentiation factor 88 (MyD88) (26), to activate NF-κB and thereby induce the upregulation of proinflammatory cytokines. Activation by LPS of a macrophage results in enhanced phagocytosis of bacteria and the release of cytokines, prompting other macrophages, phagocytes, and T cells to the site of infection. This initiates a proinflammatory response and thereby influences the nature of the adaptive immune response. Macrophages are now well recognized to be the primary mediators for the lethal effects caused by bacterium- or LPS-induced septic shock (10).
LPS activation is known to impart a macrophage with an increased resistance against apoptotic triggers. An inflammatory response produces nitric oxide, reactive oxygen intermediates, and the upregulation of Fas ligand on immune-regulating lymphocytes, all of which are detrimental to both invading pathogens and resident cells. This LPS-induced apoptotic resistance is essential for macrophages to function within an inherently hostile, antimicrobial proinflammatory environment. Considerable interest in the function of cIAP2 has arisen from its role as a major NF-κB-regulated survival factor. cIAP2 has been suggested to be the essential component chiefly responsible for protecting rat hepatocytes from an LPS-induced lethal assault (22).
Given that cIAP2 is a potential key survival factor induced via NF-κB activation in many cells including macrophages, we investigated whether cIAP2 could be an essential component during an innate proinflammatory response. Surprisingly, our studies demonstrate that cIAP2−/− mice display profound resistance to LPS-induced endotoxic shock, specifically via an attenuated inflammatory response. We show that cIAP2−/− macrophage cells are highly susceptible to apoptosis in an LPS-induced proinflammatory environment, indicating that cIAP2 is a critical factor in maintaining a normal innate immune inflammatory response.
MATERIALS AND METHODS
Generation of germ line chimeras and homozygous mice.
129/sv genomic clones (13) spanning the mouse ciap2 gene were used to construct a replacement-type targeting vector in which an internal ribosome entry site (IRES)-lacZ and phosphoglycerate kinase (PGK)-neomycin (neo) cassette (SA-IRES-βgeo; (16) replaced exons 2 to 5 in the plasmid pKO (M. Holcik and R. G. Korneluk, unpublished). The resultant targeting vector (pKO.hiap1) was comprised of a 4.1-kb 5′ arm and a 5.5-kb 3′ arm bracketing the IRES-lacZ/PGK-neo insertion. RW4 embryonic stem (ES) cells were electroporated as described previously (28), and DNA from neomycin-resistant clones was extracted and analyzed. Disruption of the ciap2 allele was confirmed by Southern blot analysis of EcoRV-digested genomic DNA after hybridization with a probe corresponding to exon 1 of the ciap2 gene. Chimeric mice were produced by morula aggregation (27) with targeted RW-4 cells. Chimeric male progeny were mated with 129/SvJ females and heterozygous progenies were backcrossed to C57BL/6 mice for at least 10 generations. Heterozygous mice were then crossed to produce homozygous cIAP−/− mice. Both the electroporation of ES cells and the generation of chimeric animals were performed at the Genome Systems, Inc., facility (St. Louis, MO). Mice were housed in a specific-pathogen-free environment, and all experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care and protocols approved by the University of Ottawa Animal Care Committee.
Southern blot analysis.
Genomic DNA was isolated by standard methods and digested with EcoRV, separated on agarose gels, and transferred to Biodyne Nylon Paper (Life Technologies, Rockville, MD). Full-length 32P-labeled cIAP2 cDNA probes were prepared by using Rediprime (Amersham Pharmacia) and [32P]dCTP (Amersham Pharmacia) according to the manufacturer's directions. Membranes were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate at 65°C for 10 min and exposed to X-ray film (Kodak).
Western blot analysis.
Mouse tissue was weighed, lysed in 5 volumes (wt/vol) of lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM vanadate, 1% [vol/vol] Nonidet P-40, 0.25% [vol/vol] sodium deoxycholate, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml, and 1 μM phenylmethylsulfonyl fluoride), and then crushed and well mixed. The samples were then rotated for 45 min at 4°C. The samples were then centrifuged for 15 min at ∼14,000 rpm in a microcentrifuge. The supernatant was collected and assayed by using a BCA kit (Pierce); then, equal amounts of protein samples were loaded per lane, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by Western blotting with rabbit polyclonal α-cIAP2, α-cIAP1, or α-XIAP antibodies (1:2,500 dilution; Ægera), followed by anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham), and the immune complexes were visualized by using an enhanced chemiluminescence kit (Roche).
mRNA isolation and quantitative mRNA analysis.
mRNA was extracted from mouse embryonic fibroblasts or peritoneal macrophages by using QIAGEN RNeasy 96-well extraction kit (QIAGEN, Mississauga, Ontario, Canada) and tested with a TaqMan instrument (Perkin-Elmer, Foster City, CA) with specific DNA probes for murine xiap, ciap1, and ciap2 and the TaqMan EZ RT-PCR kit (QIAGEN).
Animal LPS models.
Adult 4- to 6-week-old mice (n = 6 to 10) were injected intraperitoneally (i.p.) with a range of LPS doses (10 to 200 mg of LPS/kg) from Escherichia coli (Sigma) in a total volume of 0.2 ml of nonpyrogenic saline. It should be noted that the LPS used (L4516; Sigma) might contain <1% protein and RNA content, and therefore some of the effects seen may be attributed to alternative signaling TLR pathways other than TLR4.
Adult 4- to 6-week-old mice (n = 3), cIAP2−/− and littermate control mice, were injected i.p. with a 100% lethal dose (LD100) of LPS (35 mg of LPS/kg). At the appropriate times mice were anesthetized with pentobarbital and killed by cervical dislocation. The plasma was then collected from these animals and used to determine concentrations of IL-1β, TNF-α, and IL-12 in serum with an ELISA kit (R&D Systems).
Adult 4- to 6-week-old mice (n = 6) were injected i.p. with LPS (35 mg of LPS/kg), and at time zero and 5 h the mice were euthanized and the cells from the peritoneum were collected and stained to determine macrophage, T-cell, and B-cell percentages. The macrophages were also stained with fluorescein isothiocyanate (FITC)-labeled annexin V (Immunotech, Marseille, France) to determine apoptotic status.
Flow cytometry.
T and B cells were isolated from mouse lymphoid tissue (spleen) by first mincing and then pressing the tissue through a 10-μm-pore-size metal mesh and were then counted by the trypan blue exclusion method. Macrophages were harvested from mice by using repeated i.p. lavage (three times with 10 ml of media: 5% fetal calf serum [FCS], 50 μM β-mercaptoethanol, and 125 mM l-glutamine, penicillin, and streptomycin) at 4°C and washed once (centrifuged at 800 × g for 15 min) and resuspended at ∼2 × 107 cells/ml for peritoneal macrophages. Subsequently, peritoneal macrophages were layered on 5 ml of room temperature Lympholyte-M (CedarLane, Canada) and centrifuged at 1,500 × g for 20 min at room temperature. For splenic macrophages, 3 ml of the minced and pressed tissue (∼2 × 107 splenocytes/ml) was layered on 5 ml of room temperature Lympholyte-M (CedarLane, Canada) and centrifuged at 1,500 × g for 20 min at room temperature. Subsequently, either peritoneum- or spleen-derived macrophages were collected from the interface layer, washed twice with complete Dulbecco modified Eagle medium (DMEM), and resuspended in 1 ml of complete DMEM. The resulting cells were then counted by using trypan blue exclusion and a DiffQuik stain kit (IMEB, Inc., San Marcos, CA). Cells (105 to 106) were incubated with the following conjugated monoclonal antibodies: α-CD3ɛ-FITC, CD4-phycoerythrin (PE), CD8a-Cy-Chrome, CD69-PE, B220-FITC, B220-PE, CD11b-Cy-Chrome (Pharmingen) F4/80-FITC and F4/80-PE (Cedarlane Laboratories, Hornby, Ontario, Canada). Flow cytometric analyses were performed on a Coulter XL cytometer (Coulter, Canada). Concentrations of TNF-α and IL-1β in primary tissue culture supernatants were determined by using an enzyme-linked immunosorbent assay kit (R&D Systems).
Primary tissue culture and death assays.
Primary cultures were maintained in DMEM supplemented with 10% FCS and 10 ng of granulocyte-macrophage colony-stimulating factor (R&D Systems)/ml for macrophages and with 5% FCS, 50 μM β-mercaptoethanol, and 125 mM l-glutamine, penicillin, and streptomycin (∼85% confirmed via a FITC-stained α-CD3 antibody using flow cytometry) for T cells, B cells, and thymocytes.
Confluent primary cultures of peritoneum-derived macrophages were either pretreated with LPS (10 μg/ml, 4 h) or not pretreated prior to exposure to α-Fas antibody (20 μg/ml, clone Jo2) and then TUNEL stained (Roche) to assess cell viability. Primary cultures of spleen-derived T cells were preincubated with a range of IL-7 concentrations (0, 5, and 10 ng/ml) and then exposed to dexamethasone (100 nM) and T-cell survival was monitored over a 12-h period.
RESULTS
Establishment of cIAP2−/− mice.
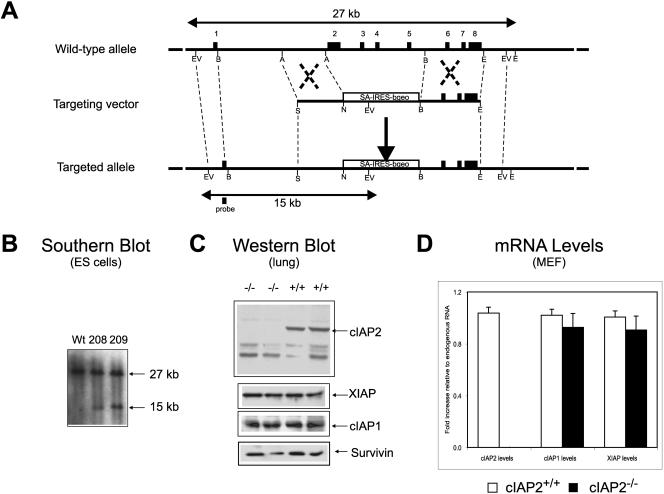
To study the function of cIAP2, we disrupted the murine ciap2 gene by homologous recombination in ES cells (Fig. 1A and B). cIAP2−/− mice showed no overt phenotype, were fertile, followed Mendelian frequency of inheritance, and appeared healthy up to 52 weeks of age. Whole-mouse and organ weights, as well as primary and secondary lymphoid compartment cell count numbers, for cIAP2−/− and control littermates, up to the age of 36 weeks, showed no significant difference (data not shown). Western blot analysis and quantitative reverse transcription-PCR showed no compensatory increase of IAP family members in cIAP2-ablated tissue (Fig. 1C and D).
FIG. 1.
Disruption of the ciap2 gene by homologous recombination. (A) Structure of the 5′ end of the mouse ciap2 locus, targeting vector, and the targeted ciap2 allele. The open black box shows the position of the probe used for the genomic Southern blot analysis. (B) A Southern blot analysis of EcoRV-digested genomic DNA from embryonic stem cells shows the presence of the targeted (15 kb) and the wild-type (27 kb) alleles. (C) A Western blot analysis of lung protein extracts indicates the absence of the 66-kDa cIAP2 full-length polypeptide and the presence of cIAP1 and Survivin protein. (D) Relative quantitative levels of cIAP2, cIAP1, and xiap mRNA, which was derived from mouse embryonic fibroblasts of cIAP2−/− and wild-type littermate mice. The results are means ± the standard deviations. n = 6, average of triplicate wells per mouse, P < 0.05.
cIAP2 is highly upregulated in macrophages treated with LPS.
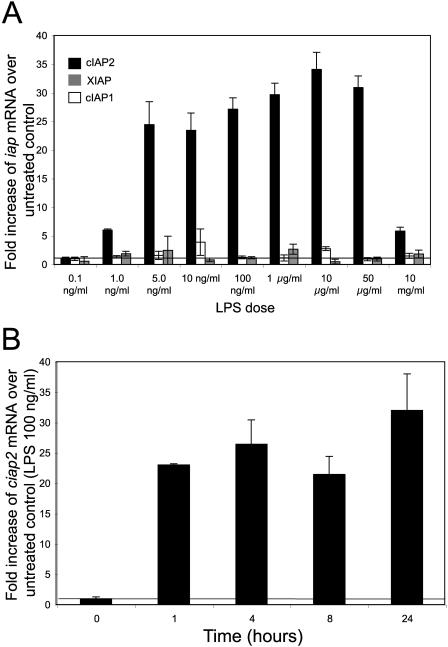
LPS-induced activation of macrophages causes the upregulation of a multitude of genes, including the production or release of inflammatory mediators and the upregulation of cell surface receptors and of cell survival proteins. Conflicting previous reports suggested that in macrophages cIAP2 is upregulated or remains unchanged in response to LPS treatment. Also, it has been suggested that either XIAP or cIAP1 levels would rise to compensate for the loss of cIAP2. Therefore, we assayed the iap mRNA levels of LPS-treated macrophages, derived from the peritoneal cavity, relative to untreated controls. Peritoneal macrophages were cultured at 105 cell/well (96 well, flat-bottom plate) and exposed to various concentrations of LPS for 18 h. xiap and ciap1 message levels remained relatively unchanged over the range of LPS doses (0.1 ng/ml to 10 mg/ml) (Fig. 2A). In contrast, ciap2 levels increased dramatically, up to 30-fold above untreated controls (P < 0.01 for all LPS doses) (Fig. 2A). The innate immune system provides protection within the first minutes to hours after a pathogenic challenge. Therefore, a LPS-ciap2 mRNA time course assay was performed (100 ng of LPS/ml). ciap2 mRNA was substantially upregulated, >20-fold above untreated controls, within 1 h, and this increased level was maintained for 24 h (Fig. 2B).
FIG. 2.
cIAP2 is highly upregulated in response to LPS. The iap mRNA levels of LPS-treated peritoneal macrophages derived from wild-type C57BL/6 mice relative to untreated controls were assayed. (A) xiap, ciap1, and ciap2 message levels of macrophages exposed to a range of LPS doses relative to untreated controls. (B) The ciap2 mRNA message of macrophages exposed to LPS was assayed over 24 h. The results are means ±thestandard deviation. n = 5, average of triplicate wells per mouse.
cIAP2−/− mice are resistant to LPS-induced endotoxic shock.
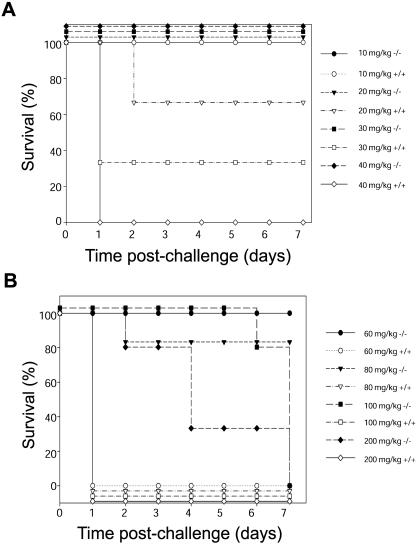
Schoemaker et al. proposed an important role of cIAP2 in LPS-induced apoptotic death of liver cells sensitized to endotoxic shock by d-galactosamine (DGLN) (22). This work also predicted that cIAP2-null mice would therefore be highly susceptible to LPS-induced endotoxic shock. Thus, to investigate the role of cIAP2 in an innate immune response the cIAP2−/− mice were treated with LPS. Surprisingly, contrary to the predicted outcome, an i.p.-injected administration of LPS (0 to 40 mg/gm) proved fatal to both wild-type (Fig. 3A) and cIAP2−/+ (data not shown) mice but not to cIAP2-null mice (Fig. 3A). All cIAP2−/− mice treated with 40 mg of LPS/kg or less survived, whereas littermate controls succumbed in a dose-dependent manner. In fact, the LD100 dose for cIAP2−/− mice was found to be approximately threefold greater (100 mg of LPS/kg) compared to that of control littermates (35 mg of LPS/kg). Moreover, cIAP2−/− mice survived 2 to 7 days even at an LPS dose of 200 mg of LPS/kg, in contrast to control littermates that all died within 24 h, even at the lower dose of 40 mg of LPS/kg (Fig. 3B).
FIG. 3.
cIAP2−/− mice are resistant to LPS-induced endotoxic shock. Mice were injected i.p. with a range of indicated LPS doses (n = 15). (A) cIAP2−/− mice (solid shapes; all cIAP2−/− mice survived for up to 7 days at an LPS dose of 40 mg/kg and lower) and littermate controls (open shapes) were treated with a range of LPS doses. (B) cIAP2−/− mice (solid shapes) and littermate controls (open shapes; all littermate control mice did not survive past the first day at an LPS dose of 60 mg/kg and above) were given a higher range of LPS doses (P < 0.001).
cIAP2−/− mice are susceptible to Fas-, platelet-activating factor (PAF)-, and DGLN/LPS-induced death.
In order to determine the sensitivity of cIAP2−/− mice to other lethal insults and inflammatory mediators, additional triggers were tested. The response of these animals to an i.p. injection of α-Fas antibodies (100 μg/mouse) (Table 1) and the effect of treatment with PAF, an inflammatory mediator that acts downstream of the LPS activation of macrophages (24) (Table 2), were examined. In addition, we exposed cIAP2−/− mice to a second mode of LPS-induced toxicity, where treatment with DGLN sensitizes mice to endotoxic shock. In contrast to LPS alone, LPS with DGLN caused a rapid demise of the mice (<3 h, Table 3). In all cases, cIAP2−/− and control littermates demonstrated similar sensitivity and died at identical rates.
TABLE 1.
Survival of cIAP2−/− and control mice after treatment with α-Fas antibodya
| Mouse type | Survival (no. surviving/ no. tested) | Avg survival time (h) ± SD |
|---|---|---|
| cIAP2−/− | 0/6 | 3.3 ± 0.9 |
| cIAP2−/+ | 0/6 | 3.7 ± 0.9 |
| cIAP2+/+ | 0/6 | 3.1 ± 0.8 |
Mice (4 to 6 weeks of age) were injected i.p. with α-Fas antibody (100 μg, 0.2ml, clone Jo2).
TABLE 2.
Survival of cIAP2−/− and control mice after treatment with PAFa
| Mouse type | No. surviving/no. tested at PAF concn (μg/kg):
|
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 2.5 | 3 | 5 | |
| cIAP2−/− | 6/6 | 6/6 | 6/6 | 6/6 | 0/6 | 0/6 |
| cIAP2+/+ | 6/6 | 6/6 | 6/6 | 6/6 | 0/6 | 0/6 |
Mice (4 to 6 weeks of age) were injected intravenously with the indicated doses of PAF in 0.2 ml of nonpyrogenic saline. All deaths occurred within 24 h.
TABLE 3.
Survival of cIAP2−/− and control mice after treatment with DGLN and LPS
| Mouse type | No. surviving/no. tested at LPS concn (μg/kg):
|
|||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 20 | |
| cIAP2−/− | 6/6 | 6/6 | 6/6 | 2/6 | 0/6 | 0/6 |
| cIAP2+/+ | 6/6 | 6/6 | 6/6 | 2/6 | 0/6 | 0/6 |
Mice (4 to 6 weeks of age) were injected i.p. with the indicated doses of LPS (E. coli K235) with DGLN (0.6 g/kg) in 0.2 ml of nonpyrogenic saline. All deaths occurred within 6 h.
cIAP2−/− mice display an attenuated inflammatory response.
LPS directly activates macrophages to produce large amounts of IL-1β and TNF-α and to mediate a cascade of events leading to endotoxic shock. We therefore assayed the levels of these proinflammatory cytokines in serum from cIAP2−/− mice treated with LPS (35 mg of LPS/kg). In cIAP2−/− mice, IL-1β levels in serum peaked at 4 h and then markedly dropped off (Table 4). This was in contrast to littermate controls, where IL-1β levels decreased much later. Likewise, comparable initial TNF-α serum levels were observed in both groups; however, the TNF-α levels dropped off to approximately 10 pg/ml in cIAP2−/− mice by 10 h, whereas in littermate controls the TNF-α serum levels stabilized to approximately 400 pg/ml and were maintained until death (Table 4). As a further study we also investigated the concentrations of IL-12 (Table 4) in serum after an injection of LPS (35 g/kg). Yet again, we observed an attenuation of a macrophage cytokine, IL-12, by the 6-h time point. Nevertheless, cIAP2−/− mice did display early outward signs of sepsis, such as eye exudates and ruffled fur; however, their condition quickly ameliorated, corresponding to the observed waning of the LPS-induced inflammatory cytokines seen within the cIAP2−/− mice.
TABLE 4.
cIAP2−/− mice serum cytokine levels are attenuated compared to cIAP2+/+ mice after both received an i.p. LPS dose of 35 mg/kga
| Cytokine | Mean serum cytokine level (pg/ml) ± SD at:
|
|||||
|---|---|---|---|---|---|---|
| 2 h
|
6 h
|
10 h
|
||||
| cIAP2−/− mice | cIAP2+/+ mice | cIAP2−/− mice | cIAP2+/+ mice | cIAP2−/− mice | cIAP2+/+ mice | |
| IL-1β | 314 ± 153 | 427 ± 24 | 476 ± 78 | 993 ± 158 | 162 ± 21 | 669 ± 48 |
| TNF-α | 1,589 ± 413 | 1,623 ± 348 | 202 ± 89 | 502 ± 56 | 8 ± 7 | 371 ± 64 |
| IL-12 | 152 ± 24 | 210 ± 17 | 11 ± 5 | 260 ± 31 | ||
n = 6.
Macrophage cytokine production and cell counts are not impaired in cIAP2−/− mice.
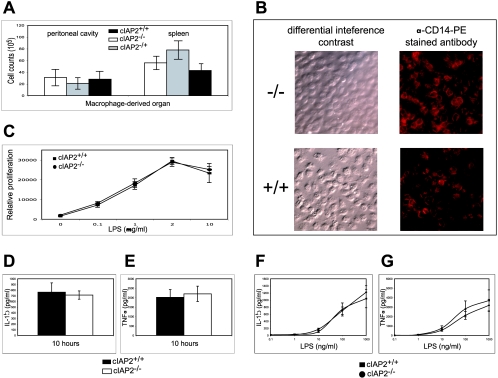
The inability of the cIAP2−/− mice to sustain IL-1β and TNF-α levels in serum in response to LPS suggests a dysfunction of the macrophages. This dysfunction may be correlated to a reduced initial number of macrophages, to a block in the LPS-induced signaling pathway, or to an increased apoptotic susceptibility of the cIAP2−/− macrophages. However, cIAP2−/− mice have comparable initial cell count numbers of peritoneum- and spleen-derived macrophages relative to control littermates, as assessed by trypan blue exclusion and the Diff Quik stain kit and flow cytometry (Fig. 4A). The cIAP2−/− and wild-type macrophages also stained similarly for the LPS-binding receptor, CD14 (Fig. 4B). Moreover, isolated splenic B cells derived from cIAP2−/− mice proliferated normally in response to various concentrations of LPS (0.1 to 100 μg/ml) (Fig. 4C). In addition, primary cultures of cIAP2−/− peritoneal macrophages generated comparable levels of TNF-α and IL-1β as littermate controls when exposed to various doses of LPS (0.1 to 1,000 ng/ml) at 10 and 24 h in vitro (Fig. 4D to G).
FIG. 4.
Macrophage cell counts and function is not impaired in cIAP2−/− mice. (A) Spleen- and peritoneum-derived macrophage cell count numbers for cIAP2−/−, cIAP2+/−, and cIAP2+/+ mice (n = 10). (B) Both cIAP2−/− mouse- and littermate control-derived macrophages stained for the LPS-binding surface receptor CD14. (C) Proliferation of B cells for cIAP2−/− mice and littermate controls cIAP2+/+ after culture with the indicated range of LPS doses (n = 6). (D and E) Cultured macrophages from cIAP2−/− or littermate control cIAP2+/+ mice were exposed to LPS for 10 h, and the IL-1β (D) or TNF-α (E) levels were measured by enzyme-linked immunosorbent assay (n = 6). (F and G) In addition, macrophages were exposed to the indicated range of LPS doses for 24 h, and the IL-1β (F) or TNF-α (G) levels were determined (n = 6). The results are means ± the standard deviations in triplicate per mouse (P < 0.01).
cIAP2−/− macrophages and T cells are unresponsive to antiapoptotic LPS and IL-7 signals.
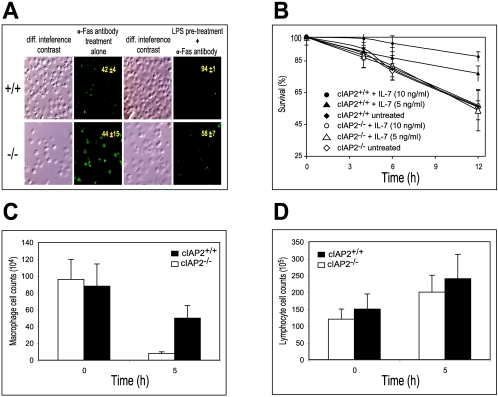
Despite the demonstrated antiapoptotic properties of cIAP2, purified B cells, T cells, and mouse embryonic fibroblasts from cIAP2−/− mice displayed no significant differences in susceptibility to a variety of apoptotic triggers (α-Fas antibody, C2-ceramide, and dexamethasone) in vitro compared to wild-type cells (data not shown). However, since cIAP2 is strongly inducible by NF-κB activation, it could be argued that the antiapoptotic properties of cIAP2 could be observed only under appropriate conditions. Macrophages exposed to LPS normally show an increased vigor and resistance toward various apoptotic triggers (3). Indeed, macrophages derived from wild-type mice and pretreated with LPS showed the expected resistance to Fas-induced death compared to wild-type-derived macrophages that were not preexposed to LPS (Fig. 5A) as observed by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining. In sharp contrast, macrophages derived from cIAP2−/−mice displayed no difference in their ability to resist Fas-mediated killing with or without preexposure to LPS (Fig. 5A). This suggests that LPS-induced upregulation of cIAP2, within a macrophage, was the essential protective component against Fas mediated death and is in agreement with the findings of Cui et al. (3), who demonstrated that cIAP2 upregulation in vitro was at least in part responsible for the general antiapoptotic resistance displayed by an LPS-induced macrophage. A similar dependency on cIAP2 was also observed with another key regulatory immune cell, the T cell (20). T cells derived from wild-type littermates and preexposed to IL-7 (5 or 10 ng/ml) showed a dose-dependent resistance against dexamethasone-induced death (Fig. 5B). In contrast, T cells derived from cIAP2−/− mice with or without IL-7 pretreatment displayed no difference in the resistance to apoptosis (Fig. 5B).
FIG. 5.
cIAP2−/−-mouse-derived macrophages display an increased sensitivity to apoptosis. (A) Peritoneum-derived macrophages from cIAP2−/− mice (lower panels) and cIAP2+/+ mice (upper panels) were either pretreated with LPS for 4 h or not pretreated prior to exposure to α-Fas antibody and then TUNEL stained to assess cell viability. The percentages of viable cells (n = 5; average of triplicate wells per mouse, P < 0.01) are shown within each TUNEL-stained panel along with the standard deviation. (B) cIAP2−/−-derived (open shapes) and cIAP2+/+-derived (solid shapes) T cells were preincubated with a range of IL-7 concentrations and then exposed to dexamethasone, and T-cell survival was monitored over a 12-h period (n = 3, average of triplicate wells per mouse; results are means ± the standard deviation, P12 h < 0.05). (C) Peritoneum-derived cell numbers are shown for macrophages of cIAP2−/− mice and littermate control cIAP2+/+ mice injected i.p. with LPS. (D) Combined number of T and B cells of cIAP2−/− mice and littermate control mice that had been i.p. injected with LPS.
cIAP2−/− macrophages display an increased sensitivity to apoptosis.
We have demonstrated that macrophages derived from cIAP2−/− mice (i) had typical initial cell count numbers compared to control littermates, (ii) produced normal levels of proinflammatory cytokines in response to LPS in vitro, and (iii) were highly susceptible to apoptotic triggers relative to control littermates when activated by LPS. It was also found that both cIAP2−/− and control littermates were equally sensitive to PAF, an inflammatory mediator that acts downstream of the LPS-induced activation of macrophages. Given these observations, we predicted that cIAP2−/− mice were resistant to endotoxic shock due to the inability of the cIAP2−/−-mouse-derived macrophages to upregulate cIAP2, thus leading to a loss of viability and hence a loss of the ability of the cIAP2−/− mice to produce a lethal inflammatory response. Therefore, in cIAP2−/− mice injected with a normal lethal dose of LPS (35 mg/kg) the expected results would be either a rapid loss of the macrophage populations and/or an increased apoptotic state of macrophages from cIAP2−/− mice relative to control littermates.
To determine the apoptotic sensitivity of cIAP2−/− macrophages within an LPS-induced proinflammatory environment, the peritoneal macrophage cell numbers before and during an endotoxin-elicited response were assessed by trypan blue exclusion, Diff Quik staining, and flow cytometry (using the macrophage marker PE-conjugated α-F4/80 antibody). In addition, the apoptotic status of the peritoneal and splenic macrophages derived from LPS-injected cIAP2−/− and littermate control mice was also assessed via flow cytometry (using the macrophage marker PE conjugated α-F4/80 antibody and FITC-conjugated annexin V). cIAP2−/− mice demonstrated a markedly reduced number of peritoneum-derived macrophages at 5 h after LPS injection relative to littermate controls (Fig. 5C). However, total lymphocyte cell count numbers (B and T cells) were comparatively unaffected in both animal types (Fig. 5D). In addition, at 5 h after LPS injection both the peritoneal and the splenic macrophages from cIAP2−/− mice stained ∼100% positive for annexin V (Table 5). Therefore, peritoneal and splenic macrophages from cIAP2−/− mice undergoing LPS-induced endotoxic shock are highly sensitive to apoptotic stimuli compared to control littermates in vivo.
TABLE 5.
cIAP2−/−-mouse-derived macrophages are highly sensitive to apoptotic stimuli during endotoxic shock in vivo
| Location and time (h) post-LPS | Mean % ± SDa in:
|
|||
|---|---|---|---|---|
| cIAP2−/− mice
|
cIAP2+/+ mice
|
|||
| Macrophage | Apoptotic | Macrophage | Apoptotic | |
| Peritoneal cavity | ||||
| 0 | 41 ± 24 | 15 ± 3 | 54 ± 29 | 10 ± 2 |
| 5 | 8 ± 11 | 100 ± 3 | 31 ± 17 | 66 ± 8 |
| Spleen | ||||
| 0 | 24 ± 11 | 2 ± 5 | 33 ± 15 | 5 ± 3 |
| 5 | 24 ± 8 | 98 ± 4 | 28 ± 5 | 46 ± 8 |
The percentage of apoptotic peritoneum- and spleen-derived macrophages from either cIAP2−/− or cIAP2+/+ mice (n = 6) that were injected i.p. with LPS is given.
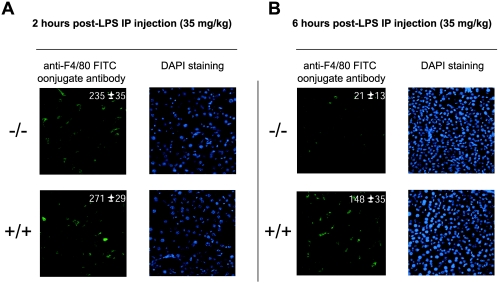
Liver-derived macrophages are key mediators of LPS-induced endotoxic shock (7, 12). Therefore, to further investigate the survival status of macrophages within LPS-treated cIAP2−/− mice, we examined cell count levels of liver-derived macrophages within liver sections. Macrophage cell count numbers within both cIAP2−/− and control mice were similar at 2 h after i.p. LPS treatment (Fig. 6A): 235 ± 35 and 271 ± 29, respectively (n = 5, average of three sections [average of five fields per section] per mouse; P < 0.01). However, at the 6-h time point (Fig. 6B) there was a precipitous drop of liver-derived macrophages of the cIAP2−/− mice (21 ± 13) versus that of the control littermates (148 ± 19; n = 5, average of three sections [average of five fields per section] per mouse; P < 0.01]). Therefore, like peritoneal and splenic macrophages, the liver-derived macrophages from cIAP2−/− mice are highly sensitive to LPS in vivo.
FIG. 6.
Reduced liver-derived macrophage cell counts in cIAP2−/− mice treated with LPS. (A and B) Liver tissue derived from cIAP2−/− (upper panels) and cIAP2+/+ mice (lower panels) pretreated with LPS (35 mg/kg) for 2 h (A) or 6 h (B). Cell counts (n = 5; average of three sections [average of five fields per section] per mouse, P < 0.01]) are shown within each anti-F4/80 FITC-conjugated antibody-stained panel, along with the standard deviation.
DISCUSSION
Our data clearly demonstrate that upon a bolus i.p. injection of LPS, macrophage survival is dependent upon rapid upregulation of the cIAP2 protein. LPS activation of peritoneum-derived macrophages induced a rapid and dramatic increase of ciap2 message 20 times greater than that of untreated controls in less than 1 h. Moreover, in contrast to published reports of the XIAP−/− mice (5), there was no observed general compensatory increases in either mRNA or protein levels of the other IAP family members, cIAP1 and XIAP.
LPS confers apoptotic resistance to macrophages via induction of cIAP2 protein.
The observed rapid induction of cIAP2 in macrophages in response to LPS activation suggested that, at least in part, cIAP2 might be a key resistance component for maintaining macrophage viability under apoptotic conditions. Indeed, the in vivo findings presented here clearly demonstrate that cIAP2 has a critical antiapoptotic role in sustaining macrophage viability. Peritoneal macrophages derived from either cIAP2−/− or control littermates displayed similar sensitivity to Fas-induced death; however, when pretreated with LPS, only macrophages from cIAP2+/+ mice displayed an increase resistance to Fas-induced apoptosis.
Ablation of cIAP2 renders macrophages susceptible to apoptosis during endotoxic shock in vivo.
We have shown that LPS activation of macrophages induces a prompt and robust induction of cIAP2. Moreover, loss of cIAP2 protects mice from acute endotoxic shock, and this is associated with the effects of cIAP2 loss on the deaths of macrophages that normally produce large amounts of proinflammatory cytokines. In support of this notion, peritoneal macrophage numbers from cIAP2−/− mice are reduced considerably by the 5-h time point after LPS injection relative to cIAP2−/− mice at time zero hours. More importantly, peritoneal macrophage numbers from cIAP2−/− mice are lower by the 5-h time point after LPS injection relative to wild-type mice at the same time point time. In addition, the peritoneal and splenic macrophages from cIAP2−/− mice 5 h after LPS injection were ∼100% apoptotic for both macrophage populations.
cIAP2−/− mouse-derived macrophage cytokine levels are normal in response to LPS treatment in vitro.
The loss of cytokine production in cIAP2−/− mice treated with a lethal dose of LPS may be due to a signaling dysfunction of the macrophages. Similar to the results obtained for cIAP2−/− mice, TLR4-null (8) and MyD88-deficient mice (9) have also been found to be resistant to LPS-induced endotoxic shock. However, B cells isolated from these animals failed to proliferate in response to LPS, whereas B cells from cIAP2−/− mice responded normally to LPS. Furthermore, cultured macrophages derived from either TLR4- or MyD88-null mice were unable to produce proinflammatory cytokines. Clearly, in the case of TLR4 and MyD88 deficiency, the observed resistance to endotoxic shock is due to a block of the LPS-induced activation pathway of the macrophage. In contrast, cIAP2−/− mouse macrophages exposed to LPS generate normal levels of TNF-α and IL-1β, suggesting that the classical LPS-induced NF-κB pathway is intact in macrophages lacking cIAP2.
Proposed mechanism of action: cIAP2−/− mice resist endotoxic shock.
LPS challenge of mice causes the activation of multiple types of genes, including the upregulation of the survival genes. The proinflammatory response generates an inherently hostile environment that can be lethal to both pathogen and host immune cell. Therefore, expression of prosurvival genes is probably vital to maintain macrophage viability during an immune response. The inability to upregulate cIAP2 renders LPS-activated macrophages highly susceptible to apoptotic triggers, thereby quickly eliminating the resident macrophage population soon after the initiation of a systemic inflammatory response. This leads to the loss of the principal source of proinflammatory cytokines and subsequently to the attenuation of the immune response, preventing the development of multiple organ failure.
Unlike cIAP1 and XIAP, the results presented here indicate that cIAP2 regulation is dependent upon signal transduction pathways. An upregulation of cIAP2 mRNA was elicited in T cells upon exposure to IL-7, and a dramatic increase was observed in macrophages treated with LPS. There is a possibility that the cIAPs and XIAP may be able to functionally “stand in” for one another. Although the caspases inhibited by the IAPs coincide, cIAP1 and -2 bind caspases with significantly lower affinities than XIAP. In addition, each IAP has unique properties and cellular localizations. XIAP is involved in the TAK1/JNK1 signaling cascade (21), whereas the cIAPs associate with TRAFs (19). In addition, and more importantly, the observed differences in IAP regulation, demonstrated here, serve to underscore the nonredundant physiological functions of the IAPs and indicate that these proteins cannot completely functionally substitute for each other.
Possible therapeutic applications.
The results presented here suggest that antagonizing cIAP2 expression and/or function may have therapeutic benefit in patients with sepsis. Neither IL-1β- nor TNF-α-deficient mice alone are resistant to LPS-induced endotoxic shock (4, 15, 18). The ablation of cIAP2 expression results not only in a loss of sustained IL-1β production but also in a loss of TNF-α. Therefore, a pharmacological ablation of cIAP2 will potentially limit the severity of inflammatory diseases by transiently abolishing IL-1β- and TNF-α-producing macrophages. These findings may be extended to other macrophage-dependent inflammatory disorders such as colitis.
Our results suggest that cIAP2 is a highly regulated protein whereby its apoptotic inhibitory properties can be observed only under a suitable situation. In addition, the cIAP2-inducing agents, LPS and IL-7, imbue their target cells, macrophages and T cells, respectively, with an increased apoptotic resistance. More importantly, cIAP2-null macrophages and T cells are unable to respond to these protective signals, indicating that cIAP2 is the crucial protective component.
Acknowledgments
We thank L. Kelly, C. McRoberts, and M. St. Jean for technical support.
This study was supported by grants from the Canada Foundation for Innovation, Ontario Research and Development Challenge Fund, Canadian Institutes of Health Research (CIHR), the Canadian Networks of Centers of Excellence, Muscular Dystrophy Association and the Howard Hughes Medical Institute (HHMI). M.H. is a CIHR New Investigator. R.G.K. is a recipient of an MRC Senior Scientist Award, a Fellow of the Royal Society of Canada, and an HHMI International Research Scholar.
REFERENCES
- 1.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 2.Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, B. Chernow, et al. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 3.Cui, X., T. Imaizumi, H. Yoshida, K. Tanji, T. Matsumiya, and K. Satoh. 2000. Lipopolysaccharide induces the expression of cellular inhibitor of apoptosis protein-2 in human macrophages. Biochim. Biophys. Acta 1524:178-182. [DOI] [PubMed] [Google Scholar]
- 4.Fantuzzi, G., H. Zheng, R. Faggioni, F. Benigni, P. Ghezzi, J. D. Sipe, A. R. Shaw, and C. A. Dinarello. 1996. Effect of endotoxin in IL-1β-deficient mice. J. Immunol. 157:291-296. [PubMed] [Google Scholar]
- 5.Harlin, H., S. B. Reffey, C. S. Duckett, T. Lindsten, and C. B. Thompson. 2001. Characterization of XIAP-deficient mice. Mol. Cell. Biol. 21:3604-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haziot, A., S. Chen, E. Ferrero, M. G. Low, R. Silber, and S. M. Goyert. 1988. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 141:547-552. [PubMed] [Google Scholar]
- 7.Hirano, K., Y. Shimizu, Y. Nakayama, M. Minemura, S. Yasumura, and T. Sugiyama. 2005. Overexpression of granulocyte-macrophage colony-stimulating factor in mouse liver enhances the susceptibility of lipopolysaccharide leading to massive apoptosis of hepatocytes. Liver Int. 25:1027-1035. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 9.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 10.Koay, M. A., X. Gao, M. K. Washington, K. S. Parman, R. T. Sadikot, T. S. Blackwell, and J. W. Christman. 2002. Macrophages are necessary for maximal nuclear factor-κB activation in response to endotoxin. Am. J. Respir. Cell Mol. Biol. 26:572-578. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, A., V. Thota, L. Dee, J. Olson, E. Uretz, and J. E. Parrillo. 1996. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 183:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumins, N. H., J. Hunt, R. L. Gamelli, and J. P. Filkins. 1996. Partial hepatectomy reduces the endotoxin-induced peak circulating level of tumor necrosis factor in rats. Shock 5:385-388. [DOI] [PubMed] [Google Scholar]
- 13.Liston, P., C. Lefebvre, W. G. Fong, J. Y. Xuan, and R. G. Korneluk. 1997. Genomic characterization of the mouse inhibitor of apoptosis protein 1 and 2 genes. Genomics 46:495-503. [DOI] [PubMed] [Google Scholar]
- 14.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J. E. Ikeda, A. MacKenzie, and R. G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349-353. [DOI] [PubMed] [Google Scholar]
- 15.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountford, P., B. Zevnik, A. Duwel, J. Nichols, M. Li, C. Dani, M. Robertson, I. Chambers, and A. Smith. 1994. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc. Natl. Acad. Sci. USA 91:4303-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park, S.-M., J.-B. Yoon, and T. H. Lee. 2004. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 566:151-156. [DOI] [PubMed] [Google Scholar]
- 18.Riedemann, N. C., R. F. Guo, and P. A. Ward. 2003. The enigma of sepsis. J. Clin. Investig. 112:460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothe, M., M. G. Pan, W. J. Henzel, T. M. Ayres, and D. V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83:1243-1252. [DOI] [PubMed] [Google Scholar]
- 20.Sade, H., and A. Sarin. 2003. IL-7 inhibits dexamethasone-induced apoptosis via Akt/PKB in mature, peripheral T cells. Eur. J. Immunol. 33:913-919. [DOI] [PubMed] [Google Scholar]
- 21.Sanna, M. G., C. S. Duckett, B. W. Richter, C. B. Thompson, and R. J. Ulevitch. 1998. Selective activation of JNK1 is necessary for the antiapoptotic activity of hILP. Proc. Natl. Acad. Sci. USA 95:6015-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoemaker, M. H., J. E. Ros, M. Homan, C. Trautwein, P. Liston, K. Poelstra, H. van Goor, P. L. Jansen, and H. Moshage. 2002. Cytokine regulation of pro- and antiapoptotic genes in rat hepatocytes: NF-κB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J. Hepatol. 36:742-750. [DOI] [PubMed] [Google Scholar]
- 23.Sriskandan, S., and J. Cohen. 1995. The pathogenesis of septic shock. J. Infect. 30:201-206. [DOI] [PubMed] [Google Scholar]
- 24.Sun, X. M., and W. Hsueh. 1991. Platelet-activating factor produces shock, in vivo complement activation, and tissue injury in mice. J. Immunol. 147:509-514. [PubMed] [Google Scholar]
- 25.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 26.Weighardt, H., S. Kaiser-Moore, R. M. Vabulas, C. J. Kirschning, H. Wagner, and B. Holzmann. 2002. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 169:2823-2827. [DOI] [PubMed] [Google Scholar]
- 27.Wood, S. A., N. D. Allen, J. Rossant, A. Auerbach, and A. Nagy. 1993. Non-injection methods for the production of embryonic stem cell-embryo chimaeras. Nature 365:87-89. [DOI] [PubMed] [Google Scholar]
- 28.Wurst, W., J. A. L. 1993. Production of targeted embryonic stem cell clones, p. 33-62. In J. A. L (ed.), Gene targeting. Oxford University Press, Oxford, United Kingdom.